Abstract
Background
Loeffler endocarditis is a rare restrictive cardiomyopathy, characterized by hypereosinophilia and fibrous thickening of the endocardium causing progressive onset of heart failure and appearance of thrombi on the walls of the heart chambers.
Case summary
A 72-year-old man known for hypertension and dyslipidaemia consults for progressive dyspnoea up to New York Heart Association (NYHA) Classes 2–3 over 3 weeks. The biological balance sheet shows a high eosinophil level and an echocardiography shows a mild echodensity fixed to the left apex. After exclusion of a secondary cause of hypereosinophilia, diagnosis of endomyocardial fibrosis in the context of a hypereosinophilic syndrome (HES) is therefore retained. The patient’s clinical presentation with cardiac involvement leads us to start a treatment with corticosteroids. The patient is then regularly followed every 6 months with an initially stable course without complications. Two years later, he develops progressive signs of heart failure. Transthoracic echocardiography shows a left ventricular (LV) dilatation with a normal ejection fraction, but decreased volume due to a large echodense mass in the apex, and moderate aortic regurgitation caused by myocardial infiltration. In view of this rapid evolution, resection of the LV mass with concomitant aortic valve replacement is performed. Pathology confirms eosinophilic infiltration. The clinical course is very good with a patient who remains stable with dyspnoea NYHA Classes 1–2, and echocardiography at 1 year shows a normalization of LV filling pressure.
Discussion
HES represents a heterogeneous group of disorders characterized by overproduction of eosinophils. One of the major causes of mortality is associated cardiac involvement. Endocardial fibrosis and mural thrombosis are frequent cardiac findings. Echocardiography plays a crucial role in initial diagnosis of endomyocardial fibrosis, and for regular follow-up in order to adapt medical treatment and monitor haemodynamic evolution of the restrictive physiology and of valvular damage caused by the disease’s evolution. This case also shows that surgery can normalize filling pressure and allow a clear improvement on the clinical condition even at the terminal fibrotic state.
Keywords: Loeffler endocarditis, Restrictive cardiomyopathy, Hypereosinophilia, Filling pressure, LV mass, Case report
For the podcast associated with this article, please visit https://academic.oup.com/ehjcr/pages/podcast
Learning points
Endomyocardial fibrosis is a rare cause of restrictive cardiomyopathy.
Main echocardiographic signs are elevated filling pressure, progressive endomyocardial thickening, valve regurgitation, and possible intracardiac thrombus formation.
Regular follow-up is important and surgery can normalize filling pressure and allow a clear improvement on the clinical condition.
Introduction
Loeffler endocarditis is a rare restrictive cardiomyopathy, characterized by hypereosinophilia and fibrous thickening of the endocardium causing progressive onset of heart failure and appearance of thrombi on the walls of the heart chambers. We report the case of a patient illustrating gradual change of diastolic function after surgery, suggesting significant fall in left ventricular (LV) filling pressure, going from a restrictive to an impaired relaxation pattern over a 1-year follow-up.
Timeline
| 11 December 2016 |
| Discovery of a hypereosinophilia |
| Cardiac magnetic resonance imaging (MRI) and echocardiography show moderate endomyocardial fibrosis |
| Start of rivaroxaban and prednisone treatment |
| 2017–2018 |
| 6 months clinical follow-up with echocardiography |
| November 2018 |
| Progressive exertional dyspnoea with clinical and biological signs of cardiac failure due to a large echodense mass in the apex |
| Surgical resection of the left ventricular mass with concomitant aortic valve replacement |
| December 2018 |
| Cardiac rehabilitation with gradual improvement in dyspnoea (New York Heart Association II) |
| Cardiac MRI and transthoracic echocardiogram show normalized filling pressure without recurrence of the mass in the left ventricle |
Case presentation
A 72-year-old man known for hypertension and dyslipidaemia consults for progressive dyspnoea up to New York Heart Association (NYHA) Classes 2–3 over 3 weeks. At physical examination, the patient’s heart rate is 85 beats per minute, respiratory rate is 19 breaths per minute, and the blood pressure is 135/75 mmHg. The electrocardiogram (ECG) shows a sinus rhythm with no disturbance in conduction or repolarization. The biological balance sheet shows a standard haemoglobin of 145 g/L, a C-reactive protein level of 31 mg/L, an N-terminal prohormone of brain natriuretic peptide (NT-proBNP) of 600 ng/L, and surprisingly a high eosinophil level. Echocardiography shows a normal LV function with a mild echodensity fixed to the left apex (20 mm × 13 mm) and mild aortic regurgitation. After exclusion of a secondary cause of hypereosinophilia, diagnosis of endomyocardial fibrosis in the context of a hypereosinophilic syndrome (HES) is therefore made. Following contemporary consensus of 2012, the combination of hypereosinophilia and the patient’s clinical presentation with cardiac involvement leads us to start a treatment with corticosteroids (prednisone 0.75 mg/kg once daily) and antithrombotics (rivaroxaban 20 mg once daily).1–3
The patient is then followed through clinical, echocardiographic, and cardiac magnetic resonance imaging (MRI) monitoring every 6 months with an initially stable course without complications (Figure 1).3
Figure 2.
Apical four-chamber view: (A) large echodense mass in the apex of the left ventricle (white arrow head) and (B) left ventricle after surgery. (C) Left ventricle 1 year after surgery.
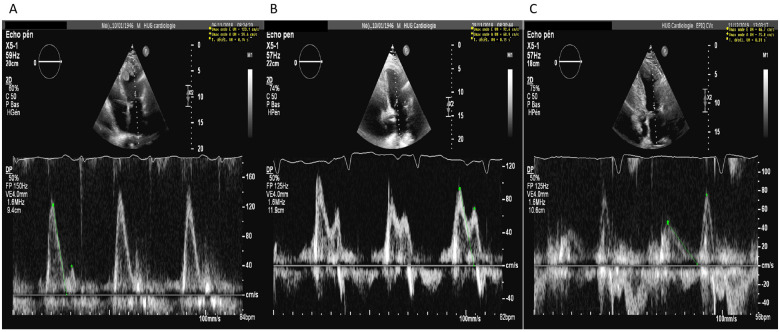
Two years after diagnosis, the patient develops progressive exertional dyspnoea reaching NYHA Class 3, with concomitant peripheral oedema. The ECG shows a sinus rhythm with the appearance of QS complex from V1 to V3, and NT-proBNP rises to 2000 ng/L. Echocardiography shows a preserved LV ejection fraction, but more marked dilatation (left ventricular end-systolic dimension 3.8 cm,left ventricular end-diastolic dimension 6 cm) with decrease in LV volume due to a large echodense fixed mass in the apex (60 mm × 43 mm) (Figure 2 and video 2-3). The transmitral flow is restrictive (E/A 3.1, DT 0.31 s, E/e′ 20.9) (Figure 3), and the aortic regurgitation is moderate (vena contracta 4 mm, pressure halt time 366 ms, effective regurgitation orificae area 20 mm2).
Figure 3.
(A) Restrictive diastolic function (Grade III) with image of mitral inflow with large E wave and small A wave, E/A ratio 3.1 and deceleration time 140 ms. (B) Pseudonormal diastolic function (Grade II) with E/A ratio 1.3 and deceleration time 150 ms. (C) Diastolic dysfunction Grade I with E/A ratio 0.62 and deceleration time 310 ms.
Figure 4.
Perioperative images of the large resected mass (white arrow head).
In view of this rapid evolution reflecting unsatisfactory response to corticosteroids, and after a ‘Heart Team’ meeting and consensus, resection of the LV mass with concomitant aortic valve replacement is performed.
An aortic bioprosthetic valve (Perimount Magna Ease 25 mm) is implanted with a good final result. Upon inspection of the LV cavity, the surgeons find a fibrotic mass, well bonded to the endocardium, which they are able to remove (cardiopulmonary bypass time 2 h, aortic clamping 1 h 32 min). Histopathology confirms eosinophilic infiltration with chronic inflammation of the myocardial wall and in the aortic valve tissue (Figure 4).
Figure 1.
Cardiac magnetic resonance imaging demonstrating left ventricle mass before surgery (white arrow head). Increase in T2 signal involving the same walls suggesting oedema.
Six days later, the clinical course is gradually favourable with an improvement of dyspnoea (NYHA Class 2), so the patient is moved to cardiac rehabilitation before returning home in excellent condition (Figures 2-3 and video 1).
During the 1-year clinical follow-up, the patient is improving under a maintaining dose of prednisone and anticoagulation. He is in good overall condition without increase of dyspnoea and with an improvement of functional capacity. An echocardiography suggests a significant fall in LV filling pressure, with an impaired relaxation pattern of the transmitral flow (E/A 0.62 and DT 0.31 s), without recurrence of the LV mass (Figures 2-3 and video 1).
Discussion
First described in 1975, HES is a rare pathology that is defined as an absolute eosinophil count (>1.5 G/L) in the peripheral blood, with eosinophil-mediated organ damage and/or dysfunction.1,4 Prevalence and overall incidence have not been well defined. In the USA, incidence is estimated ∼0.035 per 100 000 person-years.5 We know three main categories: primary (or neoplastic), secondary (or reactive), and idiopathic. Assessing for organ involvement is fundamental for establishing diagnosis of HES.
Asymptomatic patient or hypereosinophilia of undetermined significance requires no specific treatment. For newly diagnosed symptomatic patient with evidence of involving vital organs, initiation of therapy is crucial. High-dose systemic glucocorticoids (prednisone 0.5–1 mg/kg/day) are normally first-line agent for treatment of most patients who present with life-threatening or potentially disabling manifestations. Hydroxyurea is the most commonly used second-line agent for treating HES (0.5–2 g/day) generally in combination with another molecule. Interferon-α has recently been considered an additional agent against HES. Immunomodulatory and cytotoxic agents, like methotrexate or cyclosporine, and agents that target eosinophils (tyrosine kinase inhibitors and monoclonal antibodies) have been used as maintenance therapy, but reports on their administration are scarce. Once clinical and biological remission has been achieved, treatment must be tapered so that disease control is maintained using the lowest dose possible.1–3 Sometimes, surgical treatment is the only option.6,7 Specific treatments are required for patient who have HES due to a secondary cause.
Cardiac involvement could be present approximately in up to 50% of HES8 and it was first described in 1936 by Wilhelm Loeffler. Endomyocardial fibrosis and eosinophilic myocarditis, also known as Loeffler’s endocarditis, are major causes of morbidity and mortality among patients with HES.9 Cardiac injury is variable and it does not clearly correlate with degree of peripheral eosinophilia. It should be suspected in case of cardiorespiratory symptoms or ECG changes, even if not specific for this pathology, like, for example, T-wave inversions, first-degree atrioventricular block, or left ventricle hypertrophy.10
Heart tissue damage evolves through three stages (necrotic, intermediate, and fibrotic). Thrombotic risk is important during the intermediate phase, which is characterized by thrombus formation along damaged endocardium, eventually causing distant embolization. Systemic anticoagulation is, however, not usually started empirically in the absence of thrombotic event. Finally, the fibrotic stage induces restrictive cardiomyopathy and/or valve regurgitation, predominantly of atrioventricular valves, eventually leading to heart failure.
Echocardiography plays a crucial role in the evaluation of cardiac involvement in HES, even if its capacity to evaluate severity of endomyocardial fibrosis has never clearly been systematically assessed due to lack of standardized classification. Although it could be normal during the first stage of the disease, in the thrombotic stage, thrombi can be seen within the apices (fixed mass embedding left/right ventricle or both with texture and margins distinct from the endocardium). The diagnosis of endomyocardial fibrosis requires myocardial wall thickening, and signs of restriction (mitral inflow with large E wave and small A wave, E/A ratio >2, short deceleration time <150 ms).11 Valve regurgitation is another possible complication due to entrapment of the chordae tendinae and/or leaflets during the fibrotic stage of this disease. Valvular abnormalities with or without the resulting endomyocardial fibrosis can cause a congestive heart failure which explains progressive dyspnoea. Our case illustrates well how echocardiography can allow a follow-up and guide therapy of this often unpredictable disease.
Cardiac MRI is another important diagnostic tool for precising cardiac involvement and it is the gold standard for non-invasive monitoring of disease progression and assessing prognosis.12 It allows tissue characterization and it could identify thrombi with a higher degree of sensitivity and specificity than echocardiography. Contrast-enhanced cardiac MRI can also detect inflammation and fibrosis.6,13 Despite of steadily improving non-invasive diagnostic imaging methods, endomyocardial biopsy still remains the gold standard exam for the diagnosis of endomyocardial fibrosis.
The prognosis of HES is generally poor, even if with the new treatments may improve survival time. In recently reported cohorts, the median survival was 30–48 months.14,15 Cardiac involvement (especially Loeffler endocarditis), male sex >60 years old, and haemoglobin <10 g/dL were associated with inferior overall survival. Early detection and treatment of the disease is therefore essential.
Conclusion
Endomyocardial fibrosis is a rare cause of restrictive cardiomyopathy characterized by echocardiographic signs of elevated filling pressure, progressive endomyocardial thickening, valve regurgitation, and possibly intracardiac thrombus formation.
This case underlines the importance of echocardiography in initial diagnosis and regular follow-up of this type of patients, in order to adapt medical treatment and monitor haemodynamic evolution of the restrictive physiology and of valvular damage. It also shows that surgery can normalize filling pressure and allow a clear improvement on the clinical condition even at the terminal fibrotic state.
Lead author biography

Andrea Carcaterra is a graduate of the University of Geneva in Switzerland in 2013. After completing his specialty in internal medicine between 2014 and 2018, he is undertaking his residency in Cardiology in Geneva University Hospital since 2018.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
The authors are grateful to their colleagues (Dr T. Sologahsvili and Dr G. Giannakopoulos) who contributed invaluable clinical information.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
Contributor Information
Andrea Carcaterra, Department of Cardiology, University Hospital of Geneva, Rue Gabrielle-Perret-Gentil 4, 1205 Geneva, Switzerland.
Stéphane Mock, Department of Cardiology, University Hospital of Geneva, Rue Gabrielle-Perret-Gentil 4, 1205 Geneva, Switzerland.
Hajo Müller, Department of Cardiology, University Hospital of Geneva, Rue Gabrielle-Perret-Gentil 4, 1205 Geneva, Switzerland.
Ariane Testuz, Department of Cardiology, University Hospital of Geneva, Rue Gabrielle-Perret-Gentil 4, 1205 Geneva, Switzerland.
References
- 1.Cogan E, Roufosse F.. Clinical management of the hypereosinophilic syndromes. Expert Rev Hematol 2012;5:275–290. [DOI] [PubMed] [Google Scholar]
- 2.Podjasek HC, Butterfield JH.. Mortality in hypereosinophilic syndrome: 19 years of experience at Mayo Clinic with a review of the literature. Leuk Res 2013;37:392–395. [DOI] [PubMed] [Google Scholar]
- 3.Pardanani A, Lasho T, Wassie E, Finke C, Zblewski D, Hanson CA. et al. Predictors of survival in WHO-defined hypereosinophilic syndrome and idiopathic hypereosinophilia and the role of next-generation sequencing. Leukemia 2016;30:1924–1926. [DOI] [PubMed] [Google Scholar]
- 4.Weller PF, Bubley GJ.. The idiopathic hypereosinophilic syndrome. Blood 1994;83:2759. [PubMed] [Google Scholar]
- 5.Ogbogu PU, Rosing DR, Horne MK 3rd. Cardiovascular manifestations of hypereosinophilic syndromes. Immunol Allergy Clin North Am 2007;27:457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plastiras SC, Economopoulos N, Kelekis NL, Tzelepis GE.. Magnetic resonance imaging of the heart in a patient with hypereosinophilic syndrome. Am J Med 2006;119:130–132. [DOI] [PubMed] [Google Scholar]
- 7.Mankad R, Bonnichsen C, Mankad S.. Hypereosinophilic syndrome: cardiac diagnosis and management. Heart 2016;102:100–106. [DOI] [PubMed] [Google Scholar]
- 8.Parrillo JE, Borer JS, Henry WL, Wolff SM, Fauci AS.. The cardiovascular manifestations of the hypereosinophilic syndrome. Prospective study of 26 patients, with review of the literature. Am J Med 1979;67:572–582. [DOI] [PubMed] [Google Scholar]
- 9.Gotlib J. World Health Organization-defined eosinophilic disorders: 2017 update on diagnosis, risk stratification, and management. Am J Hematol 2017;92:1243–1259. [DOI] [PubMed] [Google Scholar]
- 10.Ommen SR, Seward JB, Tajik AJ.. Clinical and echocardiographic features of hypereosinophilic syndromes. Am J Cardiol 2000;86:110–113. [DOI] [PubMed] [Google Scholar]
- 11.Ries LAG, Melbert D, Krapcho M, Mariotto A, Miller BA, Feuer EJ. et al. SEER Cancer Statistics Review, 1975-2004. Bethesda, MD: National Cancer Institute; 2007. http://seer.cancer.gov/csr/1975_2004/. [Google Scholar]
- 12.Deb K, Djavidani B, Buchner S, Poschenrieder F, Heinicke N, Feuerbach S. et al. Time course of eosinophilic myocarditis visualized by CMR. J Cardiovasc Magn Reson 2008;10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valent P, Klion AD, Horny HP, Roufosse F, Gotlib J, Weller PF. et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol 2012;130:607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T. et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;277–314. [DOI] [PubMed] [Google Scholar]
- 15.Caudron J, Arous Y, Fares J, Lefebvre V, Dacher J.. Endomyocardial fibrosis in the context of hypereosinophilic syndrome: the contribution of cardiac MRI. Diagn Interv Imaging 2012;93:790–792. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.