Abstract
Background
Recessive dystrophic epidermolysis bullosa (RDEB) is a severe, complicated inherited blistering skin disease with few treatment options currently available. Recently, haematopoietic stem cell transplantation (HCT) has been used as an alternative therapy that can improve skin integrity, but it is not known if the HCT preparative regimen also might be contributing to the therapeutic response.
Objectives
To determine whether chemotherapy drugs used in the HCT preparative regimen influence type VII collagen expression, which is inherently reduced or absent in RDEB skin, and to explore the pathomechanisms of such responses, if present.
Methods
Drugs from the HCT preparative regimen (busulfan, cyclophosphamide, cyclosporine A, fludarabine, and mycophenolate) with inhibitors (PD 98059, U0126, LY 294002, SR 11302, SIS3 and N-acetyl-L-cysteine) were added to normal human dermal and human RDEB fibroblasts. Type VII collagen expression was measured using reverse-transcription PCR and immunoblotting.
Results
We uncovered a previously unknown consequence of fludarabine whereby dermal fibroblasts exposed to fludarabine upregulate type VII collagen. This effect is mediated in part through activation of the MAPK/ERK, PI3K/AKT, and TGF-β pathways. Activation of these pathways leads to activation of downstream transcription factors, including AP-1 and SMAD. Subsequently, both AP-1 and SMAD bind the COL7A1 promoter and increase COL7A1 expression.
Conclusions
Fludarabine influences the production of type VII collagen in RDEB fibroblasts.
Keywords: epidermolysis bullosa, MAPK/ERK, PI3/AKT, TGF-β, AP-1, fludarabine
INTRODUCTION
Recessive dystrophic epidermolysis bullosa (RDEB) is a life-threatening genetic skin disorder that results in skin fragility and severe blistering. RDEB patients suffer from painful symptoms including esophageal strictures, mitten deformities, corneal abrasions, and severe itching.1,2 The severity of RDEB is compounded by chronic infections and early development of an aggressive form of squamous cell carcinoma that typically develops later in life.3,4 There is currently no cure for RDEB, although significant progress in treating this disorder has been made using cellular, genetic, and protein therapies.5–8 Specifically, haematopoietic stem cell transplantation (HCT) has recently been used as an alternative therapy and may be effective in treating the systemic nature of the disease. However, much about the use of HCT to treat RDEB is unknown, including potential side effects specific to RDEB.
RDEB is an inherited disorder where loss-of-function mutations in the gene COL7A1, which encodes for type VII collagen (C7), result in structural deficiency in the skin. C7 is the main component of anchoring fibrils, which are required for the epidermis to adhere to the dermis. In the case of RDEB, absence or impaired function of C7 leads to absent or structurally defective anchoring fibrils and extreme fragility in skin architecture.9 C7 is produced by keratinocytes in the epidermis and fibroblasts in the dermis, respectively, where C7 is then deposited at the dermal-epidermal junction (DEJ). Recently studies have shown that other cell types, such as mesenchymal stem cells and other haemogenic cells, may be capable of homing to injured skin and depositing C7 at the DEJ as well.6,10–12 During inflammation, dermal fibroblasts respond to signals such as IL-1β and TNFα by increasing COL7A1 expression.13 Fibroblasts, keratinocytes, and mesenchymal stem cells also respond to TGF-β signaling by increasing COL7A1 expression at both a transcriptional and post-transcriptional level.14–16 This is mediated through downstream transcription factors of the TGF-β pathway, specifically SMAD3 and SMAD4. Furthermore, dermal fibroblasts have been shown to upregulate COL7A1 upon exposure to ultraviolet (UV) light. This upregulation is mediated through activation of the AP-1 transcription factor, which binds to an AP-1 response element in the COL7A1 promoter.17 Most studies investigating the regulation of COL7A1 have focused on skin responses to injury or inflammation; however, COL7A1 regulation in response to drugs, including those used in chemotherapy, remains only partially understood. This is of utmost importance in the context of HCT due both to the ability to effectively measure the presence of functional versus non-functional C7 and to the potential for non-functional, truncated C7 to compete with functional C7 at the DEJ and disrupt the benefits of the therapy.
Initial studies using HCT to treat RDEB have shown promising results and demonstrated the ability of engrafted stem cells to produce long-term, systemic distribution of functional C7; however, the process also requires immune suppression, which can be risky for RDEB patients.6,18 The HCT preparatory regimen uses cytotoxic drugs including busulfan, cyclophosphamide, and fludarabine that may have effects on RDEB skin, beneficial or otherwise, that are not currently known.19–21 Fludarabine’s primary function in the HCT preparative regimen is to induce apoptosis in haematopoietic cells. However, other cell types have shown a wide variety of responses—including activation, differentiation, and growth arrest—as well as altered gene expression.22–24 Fludarabine treatment has previously been shown to result in increased expression of both ICAM-1 and IL-8 in U937 cells.25 This increased expression was shown to be partially regulated through activation of the MAPK/ERK pathway and subsequent increase in AP-1 transcriptional activity. Similar studies have demonstrated an increase in TGFβ1 expression in cells exposed to fludarabine in vitro. Other studies have demonstrated that fludarabine increased levels of reactive oxygen species (ROS) production, which is also associated with an increase in MAPK/ERK activation.26 Side effects from the response of non-haematopoietic cells to chemotherapy may play an important role in both clinical outcomes of newer therapies and the biomarkers by which we measure them.
Here, we demonstrate that fludarabine treatment results in increased COL7A1 expression in dermal fibroblasts. We identify that this increased C7 production is due in part to activation of the MAPK/ERK, PI3K/AKT, and TGF-β pathways and subsequent activity of the downstream AP-1 and SMAD transcription factors, and suggest that it is mediated through ROS from exposure through fludarabine.
RESULTS
Fludarabine treatment results in increased COL7A1 expression in dermal fibroblasts
Initial results of the clinical trial utilizing HCT to treat RDEB demonstrated a link between successful engraftment and an increase in functional C7 deposition at the DEJ following transplant.6 However, little was known about the impact of the transplant process on endogenous, non-functional C7 expression from the recipient in non-haematopoietic cells. Specifically, drugs found in the transplant preparative regimen might artificially alter COL7A1 expression in the transplant recipient, confounding initial clinical assessment. Furthermore, initial staining with one RDEB patient who underwent HCT demonstrated an increase in C7 staining at the DEJ despite engraftment failure following the transplant (Fig. 1a–b). Therefore, we examined drugs found in the HCT preparative regimen to see if any of the drugs in the HCT preparative regimen modulated endogenous COL7A1 expression independent of functional C7 produced from the graft (Fig. 1c). To determine if any drug found in the HCT regimen was capable of upregulating COL7A1, we exposed both fibroblasts and keratinocytes to the drugs associated with the HCT regimen (Fig. 1d–e). Following 24h exposure to fludarabine, both RDEB and normal dermal fibroblasts showed a significant increase in COL7A1 expression; with COL7A1 reaching its maximum expression levels at 48h following the exposure (Fig. 1f–g). Dermal fibroblasts respond to fludarabine in a dose-dependent matter (Fig. 1h). Furthermore, there was an increase in C7 protein in both normal and RDEB fibroblasts following exposure to fludarabine (Fig. 1i). This effect was also seen in vivo, following fludarabine expression in an RDEB mouse model (Fig. 1j). This led us to determine the mechanism by which fludarabine exposure leads to an increase in COL7A1 expression in dermal fibroblasts.
Figure 1. RDEB Patient exhibits increase in C7 deposition despite engraftment failure.

Immunofluorescent staining of skin samples taken (a) the day before and (b) 28 days post HCT of an RDEB patient in the initial clinical trial utilizing HCT to treat RDEB. C7 staining (red) and 4,6-diamidino-2-phenylindole staining (blue) of the dermal-epidermal junction (20× magnification). (c) Diagram of HCT preparative regimen. Prior to transplant, patients receive busulfan, fludarabine, and cyclophosphamide. Following transplant patients are also given immunosuppressive drugs including cyclosporine and mycophenolate. (d) Fibroblasts and (e) keratinocytes were screened using drugs from the chemotherapy preparative regimen. Quantitative RT-PCR analysis of COL7A1 expression relative to GAPDH expression following 24h of exposure. The results shown correspond to the mean ± SEM of 3 independent experiments. (f) Normal and (g) RDEB dermal fibroblasts were exposed to fludarabine and subsequently analyzed for COL7A1 expression. Quantitative RT-PCR analysis of COL7A1 expression relative to GAPDH expression following 24, 48, or 72h of exposure. The results shown correspond to the mean ± SEM of 3 independent experiments. (h) Dermal fibroblasts were exposed to various doses of fludarabine and subsequently analyzed for COL7A1 expression. Quantitative RT-PCR analysis of COL7A1 expression relative to GAPDH expression following 24h of exposure. Normal and (i) RDEB dermal fibroblasts were exposed to fludarabine and subsequently analyzed for C7 protein expression at 48, 72, and 96h post exposure. (j) 10 μg of protein was loaded per well, and protein blot was stained for both C7 and beta actin as a loading control. RNA was isolated from skin samples from both COL7A1flNeo/flNeo mice and WT controls 48h post-fludarabine injection. Quantitative RT-PCR analysis of COL7A1 relative to GAPDH. *Statistically significant (p < 0.05) in comparison to untreated cells at matched time points. HCT, haematopoietic stem cell transplant; RDEB, recessive dystrophic epidermolysis bullosa; RT-PCR, reverse transcriptase PCR.
PI3K/AKT and MAPK/ERK activation following fludarabine exposure
Fludarabine treatment has been shown to activate stress pathways, in particular the MAPK/ERK pathway.25,26 To determine whether fludarabine activates MAPK family members in normal and RDEB fibroblasts, we utilized the Human Phospho-MAPK Array (R&D Systems, Minneapolis, MN, USA). Following 15 minutes of exposure to fludarabine, there was a relative increase in phosphorylation of kinases associated with the PI3K/AKT pathway (AKT1, AKT2, GSK3α/β, HSP27), as well as kinases associated with the MAPK/ERK pathway (ERK1/ERK2) (Fig. 2a–b).
Figure 2. Fludarabine exposure results in activation of the PI3K/AKT and MAPK/ERK pathways in normal and RDEB fibroblasts.

(a) Proteome Profiler arrays were used to identify changes in MAPK family activity following exposure to fludarabine. Densitometry analysis identified changes in relative phosphorylation between both untreated and fludarabine-treated normal and RDEB fibroblasts. (b) Averages between duplicate spots were then compared between untreated and fludarabine-treated blots and graphed accordingly. RDEB, recessive dystrophic epidermolysis bullosa; MAPK, mitogen-activated protein kinases.
To confirm that AKT and ERK were activated following exposure to fludarabine, we utilized immunoblots with antibodies specific to the phosphorylated forms of AKT and ERK. Following 15 minutes of exposure to fludarabine, we saw a relative increase in the phosphorylation of AKT (Fig. 3a), as well as an increase in the relative phosphorylation of ERK1/2 (Fig. 3b). This demonstrates that both the PI3K/AKT and MAPK/ERK pathways are activated following fludarabine exposure.
Figure 3. Fludarabine exposure results in activation of the PI3K/AKT and MAPK/ERK pathways in normal and RDEB fibroblasts.
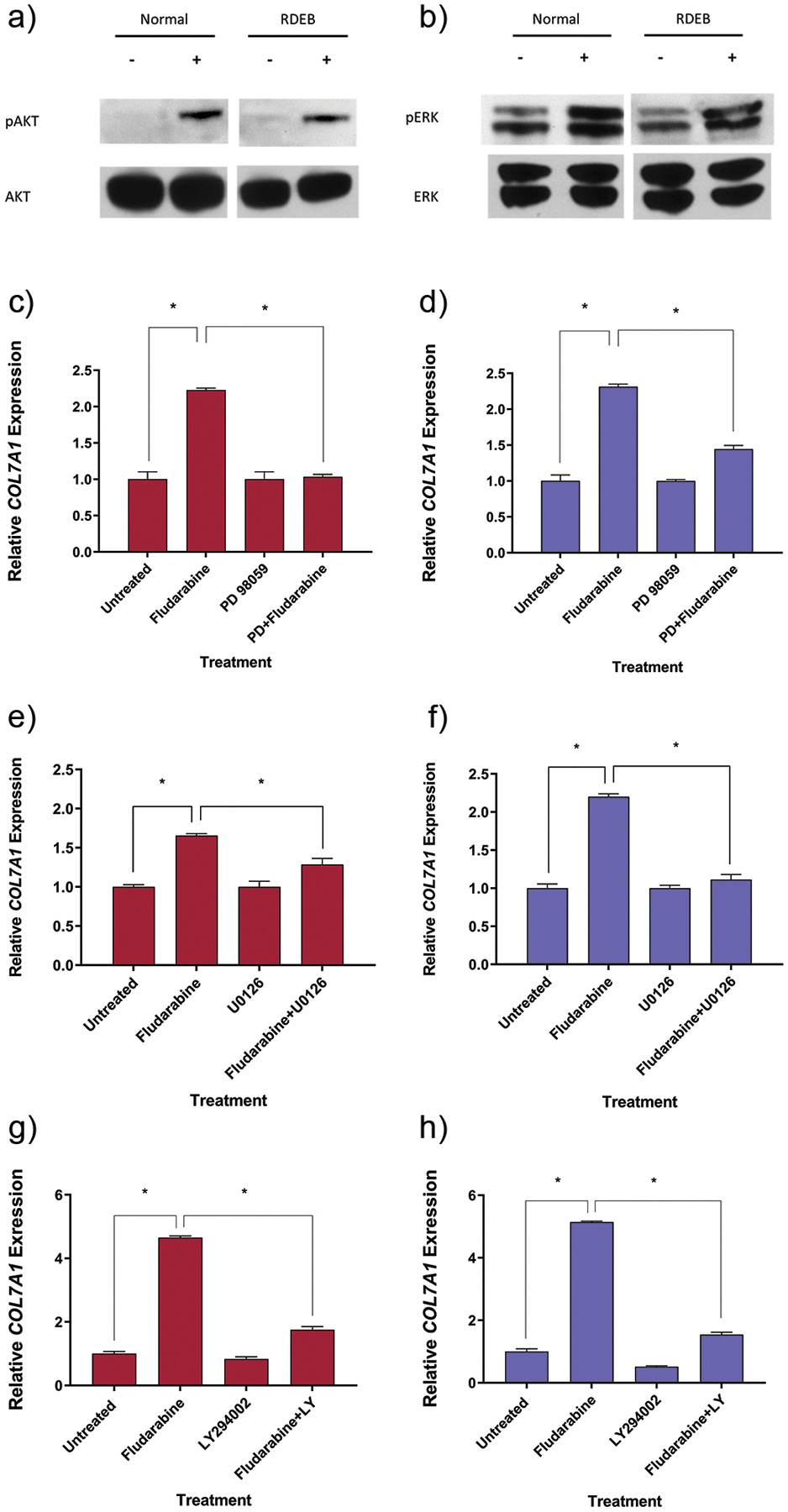
(a) AKT and (b) ERK phosphorylation was analyzed via western blot in normal and RDEB fibroblasts. Fibroblasts were incubated with or without fludarabine for 15 minutes prior to cell lysis. Western blot was performed and antibodies against total AKT/phospho-AKT or total ERK/phospho-ERK were used during immunoblotting. Inhibition of the MAPK/ERK pathway attenuates COL7A1 upregulation following fludarabine exposure. (c) Normal fibroblasts or (d) RDEB fibroblasts were initially incubated with an ERK inhibitor (PD98095) for 1h prior to being treated with fludarabine and the inhibitor for 24h. Quantitative RT-PCR analysis was performed of COL7A1 expression relative to GAPDH expression following fludarabine exposure. Inhibition of the MAPK/ERK pathway attenuates COL7A1 upregulation following fludarabine exposure. (e) Normal human fibroblasts or (f) RDEB fibroblasts were initially incubated with an ERK inhibitor (U0126) for 1h prior to being treated with fludarabine and the inhibitor for 24h. Quantitative RT-PCR analysis was performed of COL7A1 expression relative to GAPDH expression following fludarabine exposure. Inhibition of the AKT pathway attenuates COL7A1 upregulation following fludarabine exposure. (g) Normal human fibroblasts or (h) RDEB fibroblasts were initially incubated with an AKT inhibitor (LY 294002) for 1h prior to being treated with fludarabine and the inhibitor for 24h. Quantitative RT-PCR analysis was performed of COL7A1 expression relative to GAPDH expression following fludarabine exposure. RDEB, recessive dystrophic epidermolysis bullosa; RT-PCR, reverse transcriptase PCR. The results shown correspond to the mean ± SEM of 3 independent experiments. *Statistically significant (p < 0.05) in comparison to untreated cells or cells treated with fludarabine and inhibitor.
Fludarabine modulates COL7A1 expression via PI3K/AKT and MAPK/ERK activity
Previous studies have demonstrated that COL7A1 is upregulated in fibroblasts in response to inflammation and UV light.15–17,27 To determine if activation of the MAPK/ERK pathways contributed to COL7A1 upregulation, we utilized MAPK/ERK pathway inhibitors (PD98059 and U0126) in combination with fludarabine. Fibroblasts that were pre-treated with either of the MAPK inhibitors and then sustained treatment with the inhibitor throughout fludarabine exposure showed less of an increase in COL7A1 expression in comparison to fibroblasts treated with fludarabine alone (Fig. 3c–f). We also sought to determine if the PI3K/AKT pathway contributed to COL7A1 upregulation in a similar fashion. Similarly, fibroblasts pre-treated with an AKT pathway inhibitor (LY294002) and treated with the inhibitor throughout fludarabine exposure showed less of an increase in COL7A1 expression in comparison to fibroblasts treated with fludarabine alone (Fig. 3g–h). This demonstrates that fludarabine is capable of activating the PI3K/AKT and MAPK/ERK pathways, and that an increase in COL7A1 upon fludarabine exposure is mediated in part through both PI3K/AKT and MAPK/ERK activity.
Fludarabine modulates COL7A1 expression via TGF-β signaling
Beyond ERK and AKT, fludarabine has also been shown to regulate other pathways associated with COL7A1 regulation, including the TGF-β pathway.28 To determine if TGF-β signaling was involved in regulating COL7A1 expression, we used a TGF-β signaling inhibitor (SIS3) in combination with fludarabine. Fibroblasts that were pre-treated with the TGF-β inhibitor and that sustained treatment with the inhibitor throughout fludarabine exposure showed less of an increase in COL7A1 expression in comparison to fibroblasts treated with fludarabine alone (Fig. 4a–b). To verify that TGF-β signaling is involved in the increased expression of COL7A1 following fludarabine exposure, we also targeted TGFβ1 as well as SMAD3 with siRNA in fibroblasts prior to fludarabine exposure. Fibroblasts that were transfected with either TGFβ1 or SMAD3 siRNA prior to fludarabine treatment showed less of an increase in COL7A1 expression in comparison to fibroblasts transfected with an siRNA control (Fig. 4c). This demonstrates that TGF-β signaling is at least in part responsible for mediating an increase in COL7A1 expression following fludarabine treatment.
Figure 4. Inhibition of TGF-β signaling attenuates COL7A1 upregulation following fludarabine exposure.

Dermal fibroblasts were initially incubated with a TGF-β signaling inhibitor (SIS3) for 1h prior to being treated with fludarabine and SIS3 for 24h. Quantitative RT-PCR analysis was performed of COL7A1 expression relative to GAPDH expression following fludarabine exposure in (a) normal and (b) RDEB fibroblasts. (c) Normal fibroblasts were transfected with siRNA against TGFB1 and SMAD3 24h prior to fludarabine exposure. Quantitative RT-PCR analysis was performed of COL7A1 expression relative to GAPDH expression following 24h fludarabine exposure. RDEB, recessive dystrophic epidermolysis bullosa; RT-PCR, reverse transcriptase PCR. The results shown correspond to the mean ± SEM of 3 independent experiments. *Statistically significant (p < 0.05) in comparison to untreated cells or cells treated with fludarabine and SIS3 or siRNA.
Fludarabine modulates COL7A1 expression through AP-1
The MAPK/ERK and PI3K/AKT pathways have a host of downstream transcription factors that mediate upregulation of genes following particular stimuli. AP-1, a transcription factor typically composed of the canonical FOS/JUN heterodimer, mediates transcription of many genes following both PI3K/AKT and MAPK/ERK activation.29–33 Dermal fibroblasts increase production of C7 upon exposure to UVA light, which is mediated through the transcription factor AP-1.17 To determine if an increase in COL7A1 expression following fludarabine exposure is mediated through AP-1, we utilized an AP-1 inhibitor (SR-11302) in combination with fludarabine. Fibroblasts pre-treated with SR-11302 and treated with the inhibitor throughout fludarabine exposure showed a smaller increase in COL7A1 expression in comparison to fibroblasts treated with fludarabine alone (Fig. 5a–b). To verify that AP-1 was in part responsible for the increase in COL7A1 expression following the exposure to fludarabine, we utilized siRNA to target the canonical components of the AP-1 transcription factor, cFos and cJun. Fibroblasts transfected with siRNA towards cFos, cJun, or both combined demonstrated a lower increase in COL7A1 than with fludarabine treatment alone (Fig. 5c). This demonstrates that an increase in COL7A1 expression following fludarabine exposure is mediated, in part, through AP-1 activity.
Figure 5: Inhibition of the AP-1 attenuates COL7A1 upregulation following fludarabine exposure.

(a) Normal dermal fibroblasts or (b) RDEB fibroblasts were initially incubated with an AP-1 inhibitor (SR 11302) for 1h prior to being treated with fludarabine for 24h. Quantitative RT-PCR analysis was performed of COL7A1 expression relative to GAPDH expression following fludarabine exposure. (c) Normal fibroblasts were transfected with siRNA against FOS, JUN, and the combination of FOS and JUN 24h prior to fludarabine exposure. Quantitative RT-PCR analysis was performed of COL7A1 expression relative to GAPDH expression following 24h fludarabine exposure. (d) Dermal fibroblasts were initially incubated with N-acetyl-L-cysteine for 1h prior to being treated with fludarabine and NAC for 24h. Quantitative RT-PCR analysis was performed of COL7A1 expression relative to GAPDH expression following fludarabine exposure. (e) A schematic diagram illustrating the proposed model by which fludarabine treatment leads to upregulated C7 fludarabine treatment leads to activation of the P13K/AKT, MAPK/ERK, and TGF-β pathways. Subsequently, AKT and ERK activity leads to downstream activation of the transcription factor, AP-1. Furthermore, TGF-β activity leads to downstream activation of SMAD3. AP-1 and SMAD3 then binds to the COL7A1 promoter, which leads to an increased transcription of COL7A1. RDEB, recessive dystrophic epidermolysis bullosa; RT-PCR, reverse transcriptase PCR. The results shown correspond to the mean ± SEM of 3 independent experiments. *Statistically significant (p < 0.05) in comparison to untreated cells or cells treated with fludarabine and inhibitor, siRNA or NAC.
Fludarabine modulates COL7A1 expression through Reactive Oxygen Species
Previous reports have demonstrated that fludarabine treatment results in generation of ROS, and that some of fludarabine’s effects may be mediated through ROS.34,35 Specifically, the MAPK/ERK pathway is activated by ROS, and the increase in COL7A1 expression in fibroblasts exposed to fludarabine may be a result of the MAPK/ERK pathway being activated by ROS.36 To determine if an increase in COL7A1 expression in fibroblasts exposed to fludarabine is mediated through ROS, we utilized N-acetyl-L-cysteine (NAC). NAC is a free radical scavenger capable of reducing ROS levels in culture.17,25 Upon co-administration of fludarabine and NAC, we saw a reduction in COL7A1 expression levels in comparison to fibroblasts exposed to fludarabine alone (Fig. 5d). This demonstrates that fludarabine-induced upregulation of COL7A1 in dermal fibroblasts may be partly mediated through ROS generation. These findings highlight a potential mechanism by which fludarabine treatment leads to activation of PI3K/AKT, MAPK/ERK and TGF-β pathways and subsequent activation of the downstream AP-1 and SMAD transcription factors, and ultimately increased COL7A1 expression (Fig. 5e).
DISCUSSION
Since cellular therapies are increasingly used to treat genetic disorders, it is critical to understand how the treatment process influences the outcomes and benchmarks of success for each particular disorder. In the particular case of utilizing HCT to treat RDEB, we hereby demonstrate that fludarabine treatment results in a substantial increase in COL7A1 expression in both wild-type and RDEB fibroblasts. This increase in COL7A1 expression is partially mediated through activation of the PI3K/AKT, MAPK/ERK and TGF-β pathways and subsequent increase in downstream AP-1 and SMAD transcriptional activity, which is likely mediated in part through ROS generation. This finding is consistent with the various side effects of chemotherapy, specifically fludarabine, on non-haematopoietic cells.22,25
Cellular responses to injury or stress have been well characterized, including the MAPK/ERK and PI3K/AKT responses to ROS and DNA damage.32,36 C7 and other extracellular matrix proteins are required for tissue repair following injury to the skin, and so it is reasonable that cellular responses to stress or DNA damage similar to UV exposure or tissue injury lead to an increase in C7.27,37 Furthermore, the tissue-specific response to fludarabine in fibroblasts versus keratinocytes is consistent with previous studies investigating fibroblast and keratinocyte responses to UV radiation, IL-1β, and TNF-α.13,38 While fludarabine influences fibroblasts to increase COL7A1 expression, it remains to be seen if there are other side effects in RDEB skin due to the preparative regimen.
In using HCT to treat RDEB, it appears that the transplant regimen itself may confound initial testing based on COL7A1 expression alone. Testing functional C7 as well as donor chimerism may be necessary to determine the efficacy of the transplant. In addition, followup beyond the period in which the preparative regimen can influence COL7A1 expression may be necessary to determine whether the newly produced C7 originated from the transplanted donor cells or from an increase in C7 expression derived from the patient’s cells as a side effect of the therapy. This is dependent on the half-life of the functional and non-functional COL7A1 being expressed from the donor and the recipient, respectively.39 Current and future studies focusing on cellular therapies where preparatory regimens or drugs are involved should consider this.
Furthermore, it is unclear if an increase in non-functional C7 production would be beneficial, detrimental, or inconsequential in the context of HCT for treatment of RDEB. RDEB is a complex disease with a large degree of variation in both severity and clinical presentation. Additionally, confounding factors such as genetic modifiers can have a profound effect on RDEB phenotype.40,41 A small overall increase in the amount of C7 produced may reduce the severity of the disease for patients with milder forms of RDEB.6,11 However, an increase in non-functional C7 deposited at the DEJ might impede the deposition of incoming functional C7 from the HCT graft. If the preparative regimen is determined to be detrimental to outcomes, it may be beneficial to use a reduced conditioning regimen, or include other types of stem/progenitor cells.18,42
MATERIALS AND METHODS
Cell culture
Normal and RDEB human dermal fibroblasts were maintained in Dulbecco’s modified Eagle media supplemented with 10% fetal bovine serum, 100 U/ml nonessential amino acids, and 0.1 mg/ml each of penicillin and streptomycin (Invitrogen). Normal and RDEB keratinocytes were maintained in Epilife medium supplemented with 60 μM calcium, 1% Epilife Defined Growth Supplement, and 1% gentamicin/amphotericin solution (Invitrogen).
RDEB mouse model fludarabine injection
The RDEB hypomorphic mouse model, C57Bl/6-TgH(COL7A1flNeo)288LBT, was used for fludarabine injection and subsequent COL7A1 expression analysis. Intraperitoneal injection of 100mg/kg of fludarabine in PBS (as well as PBS alone as a control) was injected into RDEB mice and WT neonatal littermates. 48h post injection, full-thickness skin samples were isolated from RDEB hypomorphic pups and wild-type littermate controls. Samples were placed in RNAlater (Qiagen) prior to small RNA extraction. Skin samples were first processed using a Tissue-Tearor rotor/stator homogenizer for 1 minute in RNA lysis buffer prior to RNA isolation.
siRNA Transfection
Normal human dermal fibroblasts were transiently transfected utilizing the Neon Transfection System (Invitrogen). 2 × 105 fibroblasts were transfected with 12.5 pmol of siRNA select (TGFβ1:s14056, SMAD3:s8402, and FOS:s5339, JUN:s7658) using the following settings: 1,500V, 20 ms pulse width, 1 pulse.
Drugs and Inhibitors
Drugs from the HCT preparative regimen, including busulfan (60 μg/ml, B2635, Sigma-Aldrich), cyclophosphamide (100 μg/ml, C0768, Sigma-Aldrich), cyclosporine A (10 μg/ml, 9973, Cell Signaling Technology, Inc.), fludarabine (5 μg/ml, F2773, Sigma-Aldrich), and mycophenolate (10 μg/ml, M3536, Sigma-Aldrich), were added directly to the media. Inhibitors including PD 98059 (5 μM, P215, Sigma-Aldrich), U0126 (10 μM, 9903, Cell Signaling Technology), LY 294002 (50 μM, L9908, Sigma-Aldrich), SR 11302 (10 μM, A8185, Apex Bio) and SIS3 (10 μM, Tocris, 5291), as well as N-acetyl-L-cysteine (10 mM, A7250, Sigma-Aldrich), were added alone or 1h prior in combination with fludarabine. All drugs and inhibitors were resuspended in dimethyl sulfoxide (D2650, Sigma-Aldrich).
RT-PCR
RNA was isolated using the RNeasy Plus Mini Kit (QIAGEN). Subsequent first strand cDNA synthesis was performed using the SuperScript Vilo cDNA Synthesis Kit (Invitrogen). RT-PCR was performed using Taqman Gene Expression Assays and Taqman Universal Master Mix II, no UNG (Invitrogen). RT-PCR was performed on the StepOnePlus Real-Time PCR System (Applied Biosystems). The Taqman Gene Expression assays used for qRT-PCR experiments on human samples were: Hs00164310_m1 (COL7A1) and Hs02758991_g1 (GAPDH) as an endogenous control. The Taqman Gene Expression assays used for qRT-PCR experiments on mouse samples were: Mm01227938_m1 (COL7A1) and Mm99999915_g1 (GAPDH) as an endogenous control.
Immunoblot
Normal and RDEB fibroblasts were treated with fludarabine prior to cell lysis. Cells were lysed in RIPA buffer containing a protease/phosphatase inhibitor cocktail (Cell Signaling Technology). Lysates were clarified and measured for protein concentration using a BCA protein assay prior to loading. Equal quantities of protein were loaded onto 3–8% tris-acetate gel using a Novex™ Sharp Pre-stained Protein Standard, LC5800 (Thermo Fisher Scientific) to determine protein size. Following electrophoresis, protein was transferred onto a nitrocellulose membrane and incubated with primary antibodies overnight. For staining of type VII collagen, primary antibodies were either anti-C7 antibody (a kind gift provided by Drs. David Woodley and Mei Chen) or anti-β-Actin (A2228, Sigma-Aldrich). For staining of AKT/pAKT, antibodies were either anti-Phospho-AKT (9271, Cell Signaling Technology) or AKT (pan) (4691, Cell Signaling Technology). For staining of ERK/pERK, antibodies were either anti-phospho-p44/42-MAPK (4370, Cell Signaling Technology) or anti-p44/42 MAPK (4695, Cell Signaling Technology). The following day, membranes were incubated with goat anti-rabbit HRP-conjugated secondary antibody (sc-2005, Santa Cruz Biotechnology). After incubation with secondary antibody, blots were developed using SuperSignal West Pico Chemiluminescent Substrate (Thermo Fischer Scientific) and developed on X-ray film for imaging.
Proteome Profiler Array
Normal and RDEB dermal fibroblasts were treated with fludarabine for 15 minutes prior to cell lysis. Cell lysates were then used with the Human Phospho-MAPK Array (ARY002B, R&D Systems). Optical density was analyzed using AlphaImager EC software (Alpha Innotech). The average between duplicate spots was then compared between untreated and fludarabine-treated blots and graphed in descending order, starting with the highest relative average in comparison to untreated control.
Statistics
All data are presented as mean ± SD of three or more independent biological replicates. Student’s t-test was used to determine the significance between two groups or between an experimental variable and a control. P-values ≤ 0.05 were considered to be statistically significant.
What’s known/what’s new.
Recessive dystrophic epidermolysis bullosa has few therapies.
Haematopoietic cell transplantation has recently been used with some success in treating recessive dystrophic epidermolysis bullosa, but the effects of the preparative regimen are not well understood.
Fludarabine influences the production of type VII collagen in RDEB fibroblasts
Funding:
This research was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number R01AR063070. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- C7
type VII collagen
- DEJ
dermal-epidermal junction
- RDEB
recessive dystrophic epidermolysis bullosa
- HCT
haematopoietic stem cell transplantation
- ROS
reactive oxygen species
- NAC
N-acetyl-L-cysteine
Footnotes
Disclosures: None declared.
Data Availability
The datasets (including quantitative RT-PCR and proteome profiler array analysis) used and generated in this study are available from the corresponding author upon request.
REFERENCES
- 1.Kirkorian AY, Weitz NA, Tlougan B et al. Evaluation of wound care options in patients with recessive dystrophic epidermolysis bullosa: a costly necessity. Pediatr Dermatol 2014; 31: 33–7. [DOI] [PubMed] [Google Scholar]
- 2.Mack MR, Wendelschafer-Crabb G, McAdams BD et al. Peripheral neuro-immune pathology in recessive dystrophic epidermolysis bullosa. J Invest Dermatol 2015; 135: 1193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng YZ, Pourreyron C, Salas-Alanis JC et al. Fibroblast-derived dermal matrix drives development of aggressive cutaneous squamous cell carcinoma in patients with recessive dystrophic epidermolysis bullosa. Cancer Res 2012; 72: 3522–34. [DOI] [PubMed] [Google Scholar]
- 4.Pourreyron C, Cox G, Mao X et al. Patients with recessive dystrophic epidermolysis bullosa develop squamous-cell carcinoma regardless of type VII collagen expression. J Invest Dermatol 2007; 127: 2438–44. [DOI] [PubMed] [Google Scholar]
- 5.Osborn MJ, Starker CG, McElroy AN et al. TALEN-based gene correction for epidermolysis bullosa. Mol Ther 2013; 21: 1151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner JE, Ishida-Yamamoto A, McGrath JA et al. Bone marrow transplantation for recessive dystrophic epidermolysis bullosa. N Engl J Med 2010; 363: 629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webber BR, Osborn MJ, McElroy AN et al. CRISPR/Cas9-based genetic correction for recessive dystrophic epidermolysis bullosa. In: NPJ Regen Med, 2016/12/08 edn., Vol. 1. 2016; 16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodley DT, Wang X, Amir M et al. Intravenously injected recombinant human type VII collagen homes to skin wounds and restores skin integrity of dystrophic epidermolysis bullosa. J Invest Dermatol 2013; 133: 1910–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jarvikallio A, Pulkkinen L, Uitto J. Molecular basis of dystrophic epidermolysis bullosa: mutations in the type VII collagen gene (COL7A1). Hum Mutat 1997; 10: 338–47. [DOI] [PubMed] [Google Scholar]
- 10.Tamai K, Yamazaki T, Chino T et al. PDGFRalpha-positive cells in bone marrow are mobilized by high mobility group box 1 (HMGB1) to regenerate injured epithelia. Proc Natl Acad Sci U S A 2011; 108: 6609–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tolar J, Ishida-Yamamoto A, Riddle M et al. Amelioration of epidermolysis bullosa by transfer of wild-type bone marrow cells. Blood 2009; 113: 1167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conget P, Rodriguez F, Kramer S et al. Replenishment of type VII collagen and re-epithelialization of chronically ulcerated skin after intradermal administration of allogeneic mesenchymal stromal cells in two patients with recessive dystrophic epidermolysis bullosa. Cytotherapy 2010; 12: 429–31. [DOI] [PubMed] [Google Scholar]
- 13.Takeda H, Kon A, Ito N et al. Keratinocyte-specific modulation of type VII collagen gene expression by pro-inflammatory cytokines (tumor necrosis factor-alpha and interleukin-1beta). Exp Dermatol 2005; 14: 289–94. [DOI] [PubMed] [Google Scholar]
- 14.Vanden Oever M, Muldoon D, Mathews W et al. miR-29 Regulates Type VII Collagen in Recessive Dystrophic Epidermolysis Bullosa. J Invest Dermatol 2016; 136: 2013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verrecchia F, Vindevoghel L, Lechleider RJ et al. Smad3/AP-1 interactions control transcriptional responses to TGF-beta in a promoter-specific manner. Oncogene 2001; 20: 3332–40. [DOI] [PubMed] [Google Scholar]
- 16.Vindevoghel L, Lechleider RJ, Kon A et al. SMAD3/4-dependent transcriptional activation of the human type VII collagen gene (COL7A1) promoter by transforming growth factor beta. Proc Natl Acad Sci U S A 1998; 95: 14769–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakano H, Gasparro FP, Uitto J. UVA-340 as energy source, mimicking natural sunlight, activates the transcription factor AP-1 in cultured fibroblasts: evidence for involvement of protein kinase-C. Photochem Photobiol 2001; 74: 274–82. [DOI] [PubMed] [Google Scholar]
- 18.Geyer MB, Radhakrishnan K, Giller R et al. Reduced toxicity conditioning and allogeneic hematopoietic progenitor cell transplantation for recessive dystrophic epidermolysis bullosa. J Pediatr 2015; 167: 765–9 e1. [DOI] [PubMed] [Google Scholar]
- 19.Bonin M, Pursche S, Bergeman T et al. F-ara-A pharmacokinetics during reduced-intensity conditioning therapy with fludarabine and busulfan. Bone Marrow Transplant 2007; 39: 201–6. [DOI] [PubMed] [Google Scholar]
- 20.Bornhauser M, Storer B, Slattery JT et al. Conditioning with fludarabine and targeted busulfan for transplantation of allogeneic hematopoietic stem cells. Blood 2003; 102: 820–6. [DOI] [PubMed] [Google Scholar]
- 21.Reimer J, Bien S, Ameling S et al. Antineoplastic agent busulfan regulates a network of genes related to coagulation and fibrinolysis. Eur J Clin Pharmacol 2012; 68: 923–35. [DOI] [PubMed] [Google Scholar]
- 22.Eissner G, Multhoff G, Gerbitz A et al. Fludarabine induces apoptosis, activation, and allogenicity in human endothelial and epithelial cells: protective effect of defibrotide. Blood 2002; 100: 334–40. [DOI] [PubMed] [Google Scholar]
- 23.Jiang H, Su Z, Datta S et al. Fludarabine phosphate selectively inhibits growth and modifies the antigenic phenotype of human glioblastoma-multiforme cells expressing a multidrug resistance phenotype. Int J Oncol 1992; 1: 227–39. [DOI] [PubMed] [Google Scholar]
- 24.Nishioka C, Ikezoe T, Togitani K et al. Fludarabine induces growth arrest and apoptosis of cytokine- or alloantigen-stimulated peripheral blood mononuclear cells, and decreases production of Th1 cytokines via inhibition of nuclear factor kappaB. Bone Marrow Transplant 2008; 41: 303–9. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez-Calotti P, Gamberale R, Costas M et al. Fludarabine induces pro-inflammatory activation of human monocytic cells through a MAPK/ERK pathway. Int Immunopharmacol 2006; 6: 715–23. [DOI] [PubMed] [Google Scholar]
- 26.Maggio SC, Rosato RR, Kramer LB et al. The histone deacetylase inhibitor MS-275 interacts synergistically with fludarabine to induce apoptosis in human leukemia cells. Cancer Res 2004; 64: 2590–600. [DOI] [PubMed] [Google Scholar]
- 27.Nyström A, Velati D, Mittapalli VR et al. Collagen VII plays a dual role in wound healing. J Clin Invest 2013; 123: 3498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epperly MW, Rhieu B-H, Franicola D et al. Induction of TGF-β by Irradiation or Chemotherapy in Fanconi Anemia (FA) Mouse Bone Marrow Is Modulated by Small Molecule Radiation Mitigators JP4–039 and MMS350. In Vivo 2017; 31: 159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bishnupuri KS, Luo Q, Murmu N et al. Reg IV activates the epidermal growth factor receptor/Akt/AP-1 signaling pathway in colon adenocarcinomas. Gastroenterology 2006; 130: 137–49. [DOI] [PubMed] [Google Scholar]
- 30.Frigo DE, Tang Y, Beckman BS et al. Mechanism of AP-1-mediated gene expression by select organochlorines through the p38 MAPK pathway. Carcinogenesis 2004; 25: 249–61. [DOI] [PubMed] [Google Scholar]
- 31.Silvers AL, Bachelor MA, Bowden GT. The role of JNK and p38 MAPK activities in UVA-induced signaling pathways leading to AP-1 activation and c-Fos expression. Neoplasia 2003; 5: 319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang CM, Lin CC, Lee IT et al. Japanese encephalitis virus induces matrix metalloproteinase-9 expression via a ROS/c-Src/PDGFR/PI3K/Akt/MAPKs-dependent AP-1 pathway in rat brain astrocytes. J Neuroinflammation 2012; 9: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu HS, Lin TH, Tang CH. Involvement of intercellular adhesion molecule-1 upregulation in bradykinin promotes cell motility in human prostate cancers. Int J Mol Sci 2013; 14: 13329–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosato RR, Almenara JA, Maggio SC et al. Role of histone deacetylase inhibitor-induced reactive oxygen species and DNA damage in LAQ-824/fludarabine antileukemic interactions. Mol Cancer Ther 2008; 7: 3285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu HM, Chi KH, Lin WW. Proteasome inhibitors stimulate activator protein-1 pathway via reactive oxygen species production. FEBS Lett 2002; 526: 101–5. [DOI] [PubMed] [Google Scholar]
- 36.Frank GD, Eguchi S, Yamakawa T et al. Involvement of reactive oxygen species in the activation of tyrosine kinase and extracellular signal-regulated kinase by Angiotensin II. Endocrinology 2000; 141: 3120–6. [DOI] [PubMed] [Google Scholar]
- 37.Watt FM, Fujiwara H. Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harb Perspect Biol 2011; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen M, Petersen MJ, Li HL et al. Ultraviolet A irradiation upregulates type VII collagen expression in human dermal fibroblasts. J Invest Dermatol 1997; 108: 125–8. [DOI] [PubMed] [Google Scholar]
- 39.Kühl T, Mezger M, Hausser I et al. Collagen VII Half-Life at the Dermal-Epidermal Junction Zone: Implications for Mechanisms and Therapy of Genodermatoses. J Invest Dermatol 2016; 136: 1116–23. [DOI] [PubMed] [Google Scholar]
- 40.Küttner V, Mack C, Rigbolt KT et al. Global remodelling of cellular microenvironment due to loss of collagen VII. Mol Syst Biol 2013; 9: 657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Odorisio T, Di Salvio M, Orecchia A et al. Monozygotic twins discordant for recessive dystrophic epidermolysis bullosa phenotype highlight the role of TGF-β signalling in modifying disease severity. Hum Mol Genet 2014; 23: 3907–22. [DOI] [PubMed] [Google Scholar]
- 42.Perdoni C, McGrath JA, Tolar J. Preconditioning of mesenchymal stem cells for improved transplantation efficacy in recessive dystrophic epidermolysis bullosa. Stem Cell Res Ther 2014; 5: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]


