Supplemental Digital Content is available in the text.

Abstract
Background.
The impact of ABO incompatibility (ABO-I) on hematopoietic stem cell transplant outcomes is still debated.
Methods.
We retrospectively investigated 432 consecutive transplants performed at our center (2012–2020). All patients but 6 were affected by hematologic malignancies. The effect of different ABO match combinations on engraftment rate, transfusion support, acute and chronic graft-versus-host disease incidences, nonrelapse mortality (NRM), disease-free survival, and overall survival was assessed in univariate and multivariate analysis. Significance was set at P < 0.05.
Results.
ABO match distribution among transplants was as follows: 223 ABO-compatible, 94 major ABO-I, 82 minor ABO-I, and 33 bidirectional ABO-I. At univariate analysis, major ABO-I delayed the engraftment of neutrophils, platelets, and erythroid cells. At multivariate analysis, major ABO-I transplants displayed delayed erythroid engraftment (odds ratio [OR], 0.51; 95% confidence intervals [CIs], 0.38-0.70; P < 0.0001) and hindered transfusion independence for both red blood cells (OR, 0.52; 95% CI, 0.37-0.72; P = 0.0001) and platelets (0.60; 95% CI, 0.45-0.86; P = 0.0048). Moreover, major ABO-I transplants received greater amounts of blood products (P < 0.0001 for red blood cells and P = 0.0447 for platelets). In comparison with other ABO matches, major ABO-I was associated with an increased NRM (OR, 1.67; 95% CI, 1.01-2.75; P = 0.0427). No effects of ABO-mismatch were found on graft-versus-host disease, disease-free survival, and overall survival.
Conclusions.
Major ABO mismatch delays multilineage engraftment hinders transfusion independence and increases NRM. The prognostic impact of transfusion burden in hematopoietic stem cell transplantation deserves to be explored.
INTRODUCTION
Allogeneic hematopoietic stem cell transplant (HSCT) involves the balance between the transient coexistence of donor and recipient immunologic and hematologic systems. Limitations in donor availability require that ABO-incompatible donors be used. Since the inheritance of ABO group antigens does not parallel that of HLA, HLA-matched allogeneic HSCT frequently shows some degree of ABO incompatibility (ABO-I). Blood type frequencies in the general population vary in different racial/ethnic groups: in general, 30% of HSCT from HLA-matched related donors and up to 50% of those from HLA-matched unrelated donors are ABO-incompatible so that different immune-hematologic issues must be carefully considered and appropriately managed.1-3 The ABO-I is classified as major (recipient natural isoagglutinins to donor red cells), minor (donor natural isoagglutinins to recipient red cells), and bidirectional (major and minor coexist). The impact of ABO-I on long-term patients’ survival is widely debated; nonetheless, the main clinical concerns in ABO-incompatible HSCT still relate to massive hemolysis at transplant, delayed engraftment, and pure red cell aplasia.
In general, publications concerning the impact of ABO-I on allogeneic HSCT outcomes involve multicentric datasets of patients transplanted in different periods, receiving different grafts, conditioning regimens, and immunosuppressive therapies.4-8 Moreover, the strategy to remove incompatible isoagglutinins by plasmapheresis, to enable the engraftment of major ABO-I transplants and prevent pure red cell aplasia, is not uniformly adopted among transplant centers.9,10 The ensemble of all these variables makes conclusions of different studies sometimes conflicting with different results depending on dissimilar clinical settings and even transplant period.8,11,12
In this study, we assessed the impact of ABO-I on major transplant outcomes in patients receiving an allogeneic HSCT at our center. We principally aimed at investigating a homogeneously treated population. Therefore, all patients included in the study population were managed according to the same decisional algorithms for conditioning regimen, graft-versus-host disease (GVHD) prophylaxis, and ABO-I management.
MATERIALS AND METHODS
Study Population
Four-hundred thirty-two consecutive allogeneic HSCTs performed between January 2012 and August 2020 at Hematology Transplant Unit of Fondazione Policlinico A. Gemelli IRCCS of Rome (Italy) were retrospectively investigated. The study followed the tenets of the Declaration of Helsinki and received approval from the Ethics Committee of Fondazione Policlinico A. Gemelli IRCCS (prot.0030921/20; July 23, 2020).
Patient, Donor, and Graft Data
Patients’ variables included demographics, diagnosis and date of diagnosis, date of transplant, disease status (complete remission or not), disease risk index (DRI),13 hematopoietic cell transplantation comorbidity index (HCT-CI),14 cytomegalovirus (CMV) serology, date of neutrophil, platelet, and reticulocyte engraftment, donor-recipient chimerism at day +30,15 date of acute or chronic GVHD (aGVHD and cGVHD),16-18 number of red blood cell (RBC) or platelet transfusions before relapse or death, date of relapse, date of death, or last follow-up. Donor variables included HLA match (HLA identical sibling, haploidentical, 8/8, or 7/8 mismatch unrelated donor), age, and gender. Graft variables included source (bone marrow [BM] or peripheral blood stem cells [PBSCs]), total nucleated cell (TNC) content, CD34+ cell content, and CD3+ cell content. Cell contents were expressed as cell dose (ie, the number of cells per kilogram of the recipient’s body weight) and were obtained as previously reported.19
Study Outcomes and Definitions
We investigated the impact of ABO-I on the following outcomes: cumulative incidence of neutrophil, platelet, and reticulocyte engraftment; cumulative incidence of acute (grade II-IV) and chronic (moderate-severe) GVHD; transfusion requirements, that is, the number of RBC and platelet (PLT) units, either as apheresis or pool PLT products, received after transplant), RBC, and PLT transfusion discontinuation (TD, ie, the probability to discontinue transfusions at any time after transplant for at least 30 consecutive d, with transfused patients censored at death, relapse, or second transplant), overall survival (OS, ie, the probability of being alive at any time, calculated from transplant until death for any cause, with surviving patients censored at last follow up); disease free-survival (DFS, calculated from transplant until death for any cause or relapse with surviving patients censored at last follow up); nonrelapse mortality (NRM, ie, death without prior relapse). Neutrophil and platelet engraftments were defined as the achievement of an absolute neutrophil count ≥ 0.5 × 109/L and a PLT count ≥ 20 × 109/L unsupported by transfusion, respectively; erythrocyte engraftment was defined as a reticulocyte count ≥2%. DRI was defined according to Armand et al.13 Hematopoietic cell transplantation comorbidity index (HCT-CI) was defined according to Sorror et al.14 Diagnosis and grading of aGVHD and cGVHD were made according to standard criteria.16-18 Regimens were classified as myeloablative conditioning or nonmyeloablative (NMA) conditioning, including reduced-intensity conditioning.19 In particular, myeloablative conditioning regimens consisted of fludarabine and total body irradiation (fludarabine 120 mg/m2, followed by 9–12 Gy TBI) or thiotepa, busulfan, fludarabine (thiotepa 5 mg/kg on d 6 and 5 total; intravenous busulfan 3.2 mg/kg on d 4, 3, 2; and fludarabine 50 mg/m2 on d 4, 3, 2). Reduced and NMA regimens consisted of busulphan or fludarabine/TBI 2Gy-based regimens. GVHD prophylaxis included cyclosporine A (from d 0 to +20, 3 mg/kg with target blood levels of 200–400 ng/mL, and then orally until d +180), mycophenolate (15 mg/kg every 12 h from d +1 to +28), and cyclophosphamide (50 mg/kg on d +3 and +5). In major and bidirectional ABO-I transplants, grafts were subjected to RBC depletion with a target RBC residual volume of 1 mL/kg of the recipient’s body weight. Moreover, patients with an isoagglutinin title >1:32 underwent 2 consecutive plasmaphereses before transplantation. In minor ABO-I, grafts were subjected to plasma removal, with a target residual plasma volume of 200 mL. Transfusion support in ABO-I transplants was carried out according to Rowley et al.3 Criteria for transfusions remained unchanged over the whole study period and aimed at maintaining a hemoglobin level >8 g/dL and a platelet count >10,000/μL or 20,000/μL in patients without or with hemorrhage, respectively.
Statistical Analysis
Continuous variables were expressed as median with relative interquartile range (IQR) and categorical variables as n (%). Univariate analysis of continuous variables was performed by the Mann-Whitney U test or the Wilcoxon matched-pairs rank test, as appropriate. For categorical variables, Fisher’s exact test or χ2 test was used as appropriate. Cumulative incidence of neutrophil, platelet, and reticulocyte engraftment was calculated with death in absence of the engraftment as a competing event. Cumulative incidence of TD was calculated with death in absence of TD as a competing event. Cumulative incidence of aGVHD and cGVHD was calculated considering death in the absence of aGVHD or cGVHD as competing event. NRM was calculated with relapse as a competing event. Probabilities of OS and DFS were calculated using the Kaplan-Meier estimate. Comparisons between curves were performed according to the Grey’s method or log-rank test and expressed as hazard ratio, with relative 95% confidence intervals (CIs). The multivariate Cox logistic regression analysis was performed incorporating all the variables with a plausible effect on the outcome. The results were expressed as odds ratio (OR) with the relative 95% CI. All tests were 2-sided, and a P < 0.05 was considered statistically significant. Analyses were performed using the IBM SPSS Statistics 25.0 and NCSS 10 v 10.0.19. The data that support the findings of this study are available from the corresponding author upon reasonable request.
RESULTS
On the whole, 432 transplants were performed in 411 patients (235 males and 176 females) and were included in the analysis. Among them, 223 were ABO-compatible, 94 displayed major ABO-I, 82 minor ABO-I, and 33 bidirectional ABO-I. Diagnoses were acute myeloid leukemia (AML, 221 patients), acute lymphoid leukemia (53 patients), primary or postmyeloproliferative neoplasm myelofibrosis (52 patients), myelodysplastic syndromes (MDSs, 47 patients), Hodgkin’s and non-Hodgkin’s lymphomas and chronic lymphocytic leukemia (43 patients), multiple myeloma (10 patients), and severe aplastic anemia (6 patients). Donors were related in 273 cases (126 haploidentical) and unrelated in 159. Grafts consisted of PBSC in 285 transplants and BM in 147. There was a slightly different distribution of ABO matches combinations in BM and PBSC transplants, with a higher proportion of bidirectional ABO mismatch in patients receiving PBSC grafts (P = 0.0065; Table S1, SDC, http://links.lww.com/TXD/A341).
Tables 1 and 2 illustrate the characteristics of BM and PBSC transplants, respectively, and the univariate analysis of differences among the 4 ABO match groups. Overall, no significant disparities among ABO match groups were observed regarding all baseline characteristics potentially affecting the investigated outcomes (patients’ age, comorbidities, diagnosis, disease severity, conditioning type, and graft cell content) (Tables 1 and 2). No episodes of acute hemolysis were recorded.
TABLE 1.
Characteristics of patients and donors in 147 bone marrow hematopoietic stem cell transplants
| BM transplants, N = 147 | ABO-matched, N = 83 | Major ABO-I, N = 39 | Minor ABO-I, N = 21 | Bidirectional ABO-I, N = 4 | P |
|---|---|---|---|---|---|
| Follow-up, mo | 13.8 (3.9-34.4) | 19.4 (4.0-41.6) | 15.2 (10.1-36.8) | 31.9 (6.3-42.0) | 0.6627 |
| Age, y | 52.3 (38.8-62.7) | 56.0 (45.4-63.2) | 53.3 (30.7-61.3) | 36.3 (20.4-65.0) | 0.4636 |
| Males/females | 50 (60.2)/33 (39.8) | 19 (48.7)/20 (51.3) | 11 (52.4)/10 (47.6) | 3 (75.0)/1 (25.0) | 0.5449 |
| Diagnosis | |||||
| Acute myeloid leukemia | 47 (56.6) | 19 (48.7) | 9 (42.9) | 2 (50.0) | 0.4832 |
| Acute lymphoid leukemia | 10 (12.0) | 7 (17.9) | 4 (19.0) | 0 | |
| HL/NHL/CLL | 6 (7.2) | 2 (5.1) | 3 (14.3) | 1 (25.0) | |
| Myelodysplastic syndromes | 8 (9.6) | 3 (7.7) | 0 | 1 (25.0) | |
| Myeloproliferative neoplasms | 7 (8.4) | 8 (20.5) | 3 (14.3) | 0 | |
| Severe aplastic anemia | 3 (3.6) | 0 | 2 (9.5) | 0 | |
| Multiple myeloma | 2 (2.4) | 0 | 0 | 0 | |
| Mo from diagnosis | 8.8 (5.7-18.3) | 8.8 (6.3-26.8) | 11 (6.4-42.0) | 22.8 (9.9-30.6) | 0.5995 |
| High/very high DRI | 29 (35.0) | 19 (48.7) | 9 (42.9) | 1 (25.0) | 0.4644 |
| HCT-CI score >2 | 45 (54.2) | 18 (47.4) | 13 (61.9) | 0 (0.0) | 0.1300 |
| Complete remission | 40 (48.2) | 16 (41.0) | 11 (52.3) | 3 (75.0) | 0.5551 |
| HLA matcha | |||||
| HLA-identical sibling | 16 (19.3) | 10 (25.6) | 1 (4.7) | 0 (0.0) | 0.7293 |
| Haploidentical sibling | 64 (77.1) | 27 (69.2) | 18 (85.7) | 3 (100.0) | |
| 8/8 MUD | 1 (1.2) | 1 (2.6) | 1 (4.8) | 0 (0.0) | |
| 7/8 MUD | 2 (2.4) | 1 (2.6) | 1 (4.8) | 0 (0.0) | |
| MA/NMA conditioning | 44 (53.0)/39 (47.0) | 21 (53.9)/18 (46.1) | 13 (61.9)/8 (38.1) | 2 (50.0)/2 (50.0) | 0.9016 |
| Female donor to male | 14 (16.9) | 7 (17.9) | 5 (23.8) | 1 (25.0) | 0.8822 |
| TNC × 108/kg | 4.1 (3.1-5.2) | 3.3 (2.3-4.1) | 4.3 (2.8-4.9) | 3.7 (3.3-3.8) | 0.0974 |
| CD34+ cells × 106/kg | 3.6 (2.5-4.6) | 3.5 (2.4-5.2) | 3.1 (2.0-5.2) | 4.4 (3.8-4.9) | 0.6802 |
| CD3+ cells × 106/kg | 35.2 (24.0-42.4) | 26.8 (21.9-35.0) | 28.9 (19.9-45.2) | 37.4 (35.4-43.5) | 0.0566 |
Data are shown according to the ABO match. Continuous variables are given as median (interquartile range). Categorical variables are given as a number (%).
a1 missing value. ABO-I, ABO incompatibility; BM, bone marrow; CLL, chronic lymphocytic leukemia; DRI, disease-related index; HCT-CI, hematopoietic cell transplantation comorbidity index; HL, Hodgkin lymphoma; MA, myeloablative; MUD, matched unrelated donor; NHL, non-Hodgkin lymphoma; NMA, nonmyeloablative; TNC, total nucleated cell.
TABLE 2.
Characteristics of patients and donors in 285 peripheral blood stem cell transplants
| PBSC transplants, N = 285 | ABO-matched, N = 140 | Major ABO-I, N = 55 | Minor ABO-I, N = 61 | Bidirectional ABO-I, N = 29 | P |
|---|---|---|---|---|---|
| Follow-up, mo | 16.1 (3.9-32.6) | 7.9 (3.3-23.1) | 6.4 (3.2-17.8) | 14.5 (8.4-30.9) | 0.0923 |
| Age, y | 50.8 (41.7-58.2) | 50.8 (42.3-59.8) | 48.6 (41.4-57.9) | 54.8 (39.6-60.4) | 0.7410 |
| Males/females | 76 (54.3)/64 (45.7) | 40 (72.7)/15 (27.3) | 31 (50.8)/30 (49.2) | 15 (51.7)/14 (48.3) | 0.0646 |
| Diagnosis | |||||
| Acute myeloid leukemia | 72 (51.4) | 22 (40.0) | 36 (59.0) | 14 (48.3) | 0.7502 |
| Acute lymphoid leukemia | 15 (10.7) | 6 (10.9) | 7 (11.5) | 4 (13.8) | |
| HL/NHL/CLL | 17 (12.1) | 6 (10.9) | 4 (6.5) | 4 (13.8) | |
| Myelodysplastic syndromes | 15 (10.7) | 10 (18.2) | 6 (9.8) | 4 (13.8) | |
| Myeloproliferative neoplasms | 16 (11.4) | 7 (12.7) | 8 (13.1) | 3 (10.3) | |
| Severe aplastic anemia | 1 (0.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Multiple myeloma | 4 (2.9) | 4 (7.3) | 0 (0.0) | 0 (0.0) | |
| Mo from diagnosis | 7.8 (5.4-20.8) | 10.1 (5.9-42.6) | 7.3 (4.8-15.7) | 7.8 (5.9-14.6) | 0.2276 |
| High/very high DRI | 39 (27.9) | 18 (32.7) | 15 (24.6) | 6 (20.7) | 0.6371 |
| HCT-CI score > 2 | 62 (44.6) | 25 (45.5) | 28 (45.9) | 14 (48.2) | 0.9866 |
| Complete remission | 67 (47.8) | 25 (45.4) | 28 (45.9) | 18 (62.1) | 0.4699 |
| HLA matcha | |||||
| HLA-identical sibling | 76 (56.3) | 16 (34.0) | 23 (39.7) | 9 (31.0) | 0.1249 |
| Haploidentical sibling | 5 (3.7) | 3 (6.4) | 1 (1.7) | 1 (3.5) | |
| 8/8 MUD | 41 (30.4) | 21 (44.7) | 26 (44.8) | 15 (51.7) | |
| 7/8 MUD | 13 (9.6) | 7 (14.9) | 8 (13.8) | 4 (13.8) | |
| MA/NMA conditioning | 69 (49.6)/70 (50.4) | 25 (45.4)/30 (54.6) | 30 (49.2)/31 (50.8) | 14 (48.3)/15 (51.7) | 0.9624 |
| Female donor to male | 22 (15.8) | 11 (20.7) | 6 (9.8) | 4 (14.8) | 0.4491 |
| TNC × 108/kg | 9.3 (7.2-11.9) | 7.9 (6.5-10.3) | 9.1 (7.2-11.1) | 9.2 (7.9-11.0) | 0.1441 |
| CD34+ cells × 106/kg | 6.7 (5.1-8.7) | 6.7 (4.8-8.8) | 6.9 (5.1-8.8) | 6.6 (4.6-9.0) | 0.9376 |
| CD3+ cells × 106/kg | 209.6 (159.2-269.4) | 210.1 (145.4-297.3) | 199.8 (149.9-284.4) | 269.6 (201.7-328.6) | 0.0581 |
Data are shown according to the ABO match. Continuous variables are given as median (interquartile range). Categorical variables are given as a number (%).
a16 missing values (5 ABO-matched, 8 major ABO-Is, 3 minor ABO-Is). ABO-I, ABO incompatibility; CLL, chronic lymphocytic leukemia; DRI, disease-related index; HCT-CI, hematopoietic cell transplantation comorbidity index; HL, Hodgkin lymphoma; MA, myeloablative; MUD, matched unrelated donor; NHL, non-Hodgkin lymphoma; NMA, nonmyeloablative; PBSC, peripheral blood stem cell; TNC, total nucleated cell.
Engraftment
Graft failure was observed in 16 patients (5 BM and 11 PBSC transplants; P = 0.8111). The graft failure rate was similar across the different ABO matches: 10 cases (4.5%) occurred in the ABO-matched group, 4 (4.2%) in the major ABO-I group, 1 (1.2%) in the minor ABO-I group, and 1 (3.0%) in the bidirectional ABO-I group (P = 0.5887). Among these patients, 13 received a subsequent transplant and 3 died. Day +30 donor-recipient chimerism was available in 377 patients. Full donor chimerism (≥98%) was observed in 67.8% of ABO match transplants, in 62.1% of major ABO-I transplants, in 59.1% of minor ABO-I transplants, and in 64.0% of bidirectional ABO-I transplants (P = 0.5921). Figure 1 shows the cumulative incidence of neutrophil, platelet, and reticulocyte engraftment in patients grouped according to the ABO match at transplant. The day-30 neutrophil engraftment rate was 83% (95% CI, 78-88) in ABO-matched transplants, 85% (95% CI, 78-92) in major ABO-I transplants, 95% in minor ABO-I transplants (95% CI, 90-99), and 90% in bidirectional ABO-I transplants (95% CI, 81-100) (P = 0.0022). Regarding platelet recovery, we observed a day-30 engraftment rate of 71% (95% CI, 65-77) in ABO-matched transplants, 63% (95% CI, 54-73) in major ABO-I transplants, 80% (95% CI, 72-89) in minor ABO-I transplants, and 88% (95% CI 77-99) in bidirectional ABO-I transplants (P = 0.0017). The reticulocyte engraftment was significantly impaired in major ABO-I transplants. The day-100 engraftment rate in these patients was 62% (95% CI, 52-73), in comparison with 82% (95% CI, 77-87) in ABO-matched, 96% (95% CI, 92-100) in minor ABO-I, and 87% (95% CI, 76-99) in bidirectional ABO-I transplants (P < 0.0001). We further assessed the engraftment according to graft type (Figure S1, SDC, http://links.lww.com/TXD/A341). A significant delay of reticulocyte engraftment was confirmed in major ABO-I, either in BM or PBCS transplants. Moreover, minor ABO-I showed a faster neutrophil, platelet, and reticulocyte engraftment in BM but not in PBSC transplants (Figure S1 and Table S2, SDC, http://links.lww.com/TXD/A341).
FIGURE 1.

Cumulative incidence of neutrophil, platelet, and reticulocyte engraftment in different ABO match groups.
We then included in a multivariate regression logistic model the ABO match, CD34+ cell dose, conditioning regimen, graft source, HLA match, type of disease, complete remission at transplant, discordant donor-recipient CMV serology, and time interval from diagnosis. We found that major ABO-I was the main detrimental factor for reticulocyte engraftment (OR, 0.51; 95% CI, 0.38-0.70; P < 0.0001; Table 3). In addition, BM graft was associated with delayed reticulocyte engraftment, whereas NMA conditioning negatively affected all types of engraftment. Finally, CD34+ cell dose, HLA match (haploidentical and 8/8 matched unrelated donor [MUD]), and myeloid neoplasms all carried a higher risk for late platelet count recovery (Table 3).
TABLE 3.
Multivariate analysis of the effect of ABO incompatibility on the engraftment
| Neutrophil engraftment OR (95% CI) | Platelet engraftment OR (95% CI) | Reticulocyte engraftment OR (95% CI) | |
|---|---|---|---|
| Major ABO-Ia | 0.83 (0.63-1.09)P = 0.1805 | 0.75 (0.56-1.00)P = 0.0526 | 0.51 (0.38-0.70)P < 0.0001 |
| Minor ABO-Ia | 1.19 (0.89-1.58)P = 0.2160 | 1.12 (0.84-1.50)P = 0.4328 | 1.15 (0.86-1.54)P = 0.3317 |
| Bidirectional ABO-Ia | 1.07 (0.71-1.61)P = 0.7395 | 1.17 (0.77-1.78)P = 0.4449 | 0.84 (0.55-1.29)P = 0.4406 |
| NMA conditioning | 0.78 (0.62-0.99)P = 0.0456 | 0.63 (0.49-0.80)P = 0.0002 | 0.65 (0.51-0.83)P = 0.0007 |
| BM graft | 0.97 (0.68-1.38)P = 0.8763 | 0.70 (0.49-1.01)P = 0.0593 | 0.63 (0.43-0.91)P = 0.0143 |
| CD34+ cells × 106/kg | 1.01 (0.97-1.04)P = 0.5562 | 1.03 (1.00-1.07)P = 0.0420 | 1.02 (0.99-1.06)P = 0.1276 |
| Haploidentical donorb | 0.84 (0.59-1.19)P = 0.3443 | 0.69 (0.48-0.99)P = 0.0462 | 0.98 (0.67-1.42)P = 0.9177 |
| 8/8 MUDb | 0.93 (0.70-1.25)P = 0.6595 | 0.70 (0.52-0.95)P = 0.0225 | 0.95 (0.70-1.30)P = 0.7929 |
| 7/8 MUDb | 1.04 (0.69-1.55)P = 0.8361 | 1.04 (0.70-1.56)P = 0.8229 | 1.51 (0.99-2.28)P = 0.0501 |
| Myeloid neoplasm | 0.90 (0.70-1.16)P = 0.4442 | 0.69 (0.53-0.89)P = 0.0050 | 0.68 (0.60-1.01)P = 0.0680 |
| Complete remission | 1.09 (0.86-1.37)P = 0.4393 | 0.97 (0.77-1.23)P = 0.8069 | 0.93 (0.73-1.18)P = 0.5714 |
| Discordant CMV serology | 0.83 (0.66-1.05)P = 0.1258 | 0.91 (0.72-1.16)P = 0.4833 | 1.03 (0.81-1.32)P = 0.7838 |
| Mo from diagnosis | 1.00 (0.99-1.00)P = 0.9998 | 0.99 (0.99-1.00)P = 0.6841 | 0.99 (0.99-1.00)P = 0.5858 |
Significant P values are highlighted in bold.
avs ABO-matched.
bvs HLA-identical donor.
ABO-I, ABO incompatibility; BM, bone marrow; CI, confidence interval; CMV, cytomegalovirus; MUD, matched unrelated donor; NMA, nonmyeloablative; OR, odds ratio.
Transfusion Discontinuation
A more prolonged transfusion dependence was recorded in major ABO-I transplants than in others (Figure 2). After the transplant, patients in the major ABO-I group received RBC transfusions for an average period of 7.6 wk (IQR, 2.1–16), in comparison with 2.8 (IQR, 1.4–5.8), 3 (IQR, 1.7–7.4), and 2.4 (IQR, 1.8–5.4) wk in ABO-matched, minor and bidirectional ABO-I groups, respectively (P < 0.0001). At 6 mo from transplants, fewer patients in the major ABO-I than in other groups discontinued RBC transfusions (Figure 2). Major ABO-I transplants also showed a trend for delayed platelet transfusion independence. Patients in the major ABO-I group received PLT transfusions for an average time of 2.2 wk (IQR, 1.7–7.6), whereas transfusion dependence lasted 1.8 (IQR, 1.4–3.7), 1.8 (IQR, 1.6–4.8), and 1.9 (IQR, 1.4–2.8) wk in ABO-matched, minor, and bidirectional ABO-I groups, respectively (P = 0.0429). Also, the major ABO-I group showed a trend for delayed platelet transfusion discontinuation (Figure 2A). A further subanalysis by graft type confirmed the negative impact of major ABO-I on RBC transfusion discontinuation in BM and PBSC transplants and a trend for delaying PLT transfusion discontinuation in PBSC transplants (Figure S2, SDC, http://links.lww.com/TXD/A341).
FIGURE 2.

Cumulative incidence of transfusion discontinuation according to the ABO match. A, Discontinuation of red blood cell and platelet transfusions. B, Transfusion requirements in different ABO match groups. PLT, platelet; RBC, red blood cell.
The effect of ABO match on transfusion discontinuation was then investigated in the same multivariate model used for the engraftment analysis. The negative impact of major ABO-I on reaching the transfusion independence was confirmed for RBC (OR, 0.52; 95% CI, 0.37-0.72; P = 0.0001) and PLT (0.60; 95% CI, 0.45-0.86; P = 0.0048). A similar negative effect was detected also for the NMA regimen (OR 0.62, 95% CI, 0.47-0.81, P = 0.0005; OR 0.60, 95% CI, 0.46-0.87, P = 0.0001, for RBC and PLT units, respectively), whereas BM graft seemed to delay the transfusion discontinuation of PLT (OR, 0.62; 95% CI, 0.42-0.92; P = 0.0179). As expected, the delayed transfusion discontinuation resulted in a larger need for RBC and PLT units in major ABO-I transplants (Figure 2B).
Graft-versus-host Disease
Overall, in our study population, the 3-y cumulative incidences were 19% (95% CI, 15-23) for grade II-IV aGVHD and 29% (95% CI, 25-34) for moderate-severe cGVHD. We found no differences among ABO match groups (evaluated either among different ABO matches combinations or as major ABO-I versus others) regarding both aGVHD and cGVHD. When ABO match groups were evaluated in a multivariate analysis, no significant effect was found for any of the ABO-mismatch types regarding either acute or chronic forms of GVHD (Table 4). We observed a higher risk for aGVHD in patients with 7/8 MUD (P = 0.0225). Moreover, a higher risk for cGVHD was associated with PBCS graft (P = 0.0125). Finally, a slight effect of CD34+ cell dose on cGVHD risk was also observed (Table 4).
TABLE 4.
Multivariate analysis of the effect of ABO incompatibility on acute (grade II–IV) and chronic (moderate-severe) graft-vs-host disease
| Acute GVHD OR (95% CI) | Chronic GVHD OR (95% CI) | |
|---|---|---|
| Major ABO-Ia | 1.18 (0.66-1.10)P = 0.5674 | 0.84 (0.48-1.49)P = 0.5684 |
| Minor ABO-Ia | 0.67 (0.33-1.33)P = 0.2596 | 0.83 (0.474-1.44)P = 0.5188 |
| Bidirectional ABO-Ia | 1.68 (0.82-3.44)P = 0.1489 | 1.13 (0.58-2.18)P = 0.7140 |
| NMA conditioning | 1.14 (0.72-1.82)P = 0.5557 | 0.87 (0.58-1.32)P = 0.5328 |
| Haploidentical donorb | 1.77 (0.67-4.63)P = 0.2422 | 1.60 (0.66-3.87)P = 0.2982 |
| 8/8 MUDb | 1.48 (0.80-2.74)P = 0.2099 | 0.84 (0.49-1.45)P = 0.5434 |
| 7/8 MUDb | 2.39 (1.13-5.06)P = 0.0225 | 0.52 (0.21-1.24)P = 0.1426 |
| Female donor to male recipient | 0.89 (0.45-1.78)P = 0.7590 | 1.12 (0.66-1.90)P = 0.6603 |
| Bone marrow graft | 0.58 (0.20-1.65)P = 0.3081 | 0.28 (0.10-0.76)P = 0.0125 |
| CD34+ cells × 106/kg | 1.04 (0.97-1.12)P = 0.1815 | 0.91 (0.84-0.99)P = 0.0383 |
| CD3+ cells × 106/kg | 0.99 (0.99-1.00)P = 0.7880 | 1.00 (0.99-1.00)P = 0.2066 |
| Discordant CMV serology | 1.06 (0.65-1.74)P = 0.7929 | 0.81 (0.50-1.30)P = 0.3916 |
Cell doses were considered as continuous variables.
Significant P values are highlighted in bold.
avs ABO-matched.
bvs HLA identical donor.
ABO-I, ABO incompatibility; CI, confidence interval; CMV, cytomegalovirus; GVHD, graft-vs-host disease; MUD, matched unrelated donor; NMA, nonmyeloablative; OR, odds ratio.
Nonrelapse Mortality, Disease-free Survival, and Overall Survival
Considering the entire series of patients, 5-y NRM was 22% (95% CI, 18-27), 5-y DFS was 52% (95% CI, 47-58), and 5-y OS was 54% (95% CI, 47-60). Figure 3 illustrates NRM, DFS, and OS in patients grouped according to the ABO match. No significant effect of any ABO mismatches was found at univariate analysis (Figure 3A). We then investigated the effect of major ABO-I in comparison with the other ABO matches merged in 1 group, and we found that major ABO-I significantly increased NRM (P = 0.0254) (Figure 3B). We therefore used the same approach to investigate the effect of major ABO-I in multivariate analysis (Table 5). We found that major ABO-I (OR, 1.67; 95% CI, 1.01-2.75) and patient age (OR 1.25 for each decade; 95% CI, 1.02-1.53) conveyed an increased NRM risk. In contrast, we observed no effect of ABO mismatch on DFS and OS (Table 5). High/very high DRI and absence of complete remission at transplant predicted poorer DFS and OS, whereas a discordant CMV serostatus was associated with a lower OS.
FIGURE 3.
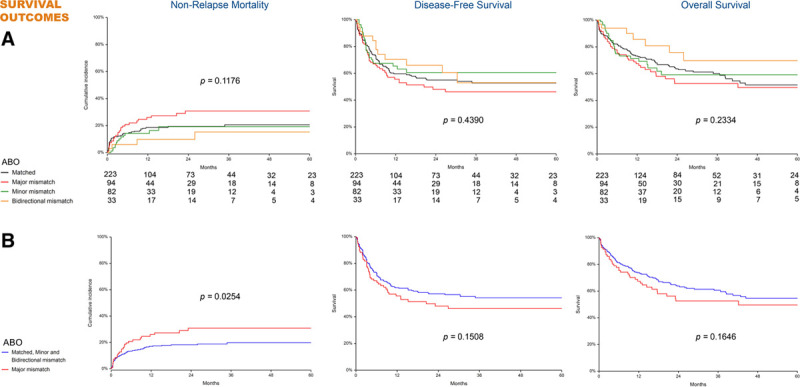
Nonrelapse mortality, disease-free survival, and overall survival in 432 transplants. A, Comparison among various ABO match combinations. B, Comparison between major ABO-mismatched transplants and other ABO matches merged in 1 single group.
TABLE 5.
Multivariate analysis of the effect of ABO incompatibility on transplant outcomes
| Nonrelapse mortality OR (95% CI) | Disease-free survival OR (95% CI) | Overall survival OR (95% CI) | |
|---|---|---|---|
| Major ABO-Ia | 1.67 (1.01-2.75)P = 0.0427 | 1.15 (0.79-1.68)P = 0.4418 | 1.33 (0.89-1.97)P = 0.4736 |
| Female donorto male recipient | 1.30 (0.74-2.29)P = 0.3503 | 0.99 (0.65-1.49)P = 0.9695 | 1.10 (0.71-1.69)P = 0.6625 |
| Patient age(10 y increment) | 1.25 (1.02-1.53)P = 0.0306 | 1.10 (0.96-1.26)P = 0.1679 | 1.13 (0.97-1.31)P = 0.1037 |
| HCT-CI score >2 | 1.34 (0.90-2.26)P = 0.2444 | 0.91 (0.65-1.28)P = 0.6055 | 1.01 (0.70-1.44)p 0.9434 |
| High/very high DRI | 1.48 (0.81-2.19)P = 0.3885 | 2.58 (1.77-3.76)P < 0.0001 | 1.87 (1.26-2.79)P = 0.0018 |
| NMA conditioning | 1.28 (0.76-2.17)P = 0.3461 | 1.06 (0.75-1.49)P = 0.7134 | 1.18 (0.82-1.72)P = 0.3598 |
| Myeloid neoplasm | 0.85 (0.48-1.50)P = 0.5927 | 0.85 (0.58-1.23)P = 0.4037 | 0.90 (0.61-1.35)P = 0.6431 |
| Bone marrow graft | 1.54 (0.72-3.28)P = 0.2885 | 0.60 (0.33-1.08)P = 0.0935 | 0.79 (0.42-1.48)P = 0.4736 |
| Haploidentical donorb | 0.66 (0.30-1.42)P = 0.2885 | 0.91 (0.49-1.68)P = 0.7741 | 0.79 (0.41-1.53)P = 0.4939 |
| 8/8 MUDb | 0.97 (0.48-1.93)P = 0.9372 | 0.96 (0.62-1.49)P = 0.8832 | 0.97 (0.60-1.56)P = 0.9144 |
| 7/8 MUDb | 0.97 (0.39-2.42)P = 0.9645 | 1.11 (0.63-1.97)P = 0.7074 | 1.15 (0.62-2.12)p 0.6526 |
| Complete remission | 0.58 (0.32-1.03)P = 0.0676 | 0.66 (0.44-0.99)P = 0.0465 | 0.62 (0.40-0.96)P = 0.0329 |
| Discordant CMV serology | 1.55 (0.92-2.51)P = 0.0946 | 1.10 (0.96-1.26)P = 0.1806 | 1.57 (1.08-2.27)P = 0.0163 |
| Mo from diagnosis | 1.00 (0.99-1.00)P = 0.9934 | 1.00 (0.99-1.00)P = 0.6868 | 1.00 (0.99-1.00)P = 0.9065 |
Significant P values are highlighted in bold.
bvs HLA identical donor.
avs other ABO matches merged in 1 single group.
bvs HLA identical donor.
ABO-I, ABO incompatibility; CI, confidence interval; CMV, cytomegalovirus; DRI, disease risk index; GVHD, graft-vs-host disease; HCT-CI, hematopoietic cell transplantation comorbidity index; MUD, matched unrelated donor; NMA, nonmyeloablative; OR, odds ratio.
Death Causes
In our series of 432 transplants, 156 death events (34.2%) were recorded. In total, 67 patients died of relapse, 52 of infection, 25 of GVHD, and 12 of other causes. Causes and timing of death according to major ABO-I are detailed in Table S3, SDC, http://links.lww.com/TXD/A341. Figure 4 illustrates the cumulative incidence of mortality related to relapse, infection, and GVHD in the same groups. The major ABO-I group showed a higher, although not statistically significant, infection mortality compared with other transplants. The most frequent pathogens were Klebsiella pneumoniae (9 cases), Pseudomonas aeruginosa (7 cases), Streptococcus spp. (5 cases), Staphylococcus spp. (4 cases), Acinetobacter baumannii (2 cases), Candida spp. (7 cases), and Aspergillus spp. (4 cases).
FIGURE 4.

Cumulative incidence of mortality related to relapse, GVHD and infections in 432 transplants. Curves show the comparison between major ABO-mismatched transplants and other ABO matches merged in 1 single group. P values refer to log-rank test. GVHD, graft-vs-host disease.
DISCUSSION
This study investigated the impact of ABO-I in our series of 432 consecutive BM and PBSC transplants. The results show that major ABO mismatch delays multilineage engraftment, hinders transfusion independence, and increases the risk for mortality not related to relapse. Moreover, our analysis suggests that, in bidirectional ABO-mismatch, the unfavorable outcome due to the major ABO-I is to some extent counteracted by the coexistence of the minor ABO-I, producing a clinical condition with different characteristics than the single major ABO-I, in terms of engraftment and transfusion needs.
Pure red cell aplasia is a frequent complication of major ABO-I transplants.20 The hampering effect on the erythropoiesis recovery is due to residual lymphocytes and plasma cells of recipient’s origin, which may persist for several weeks after the transplant.21,22 ABO blood group antigens are also expressed on additional hematologic and nonhematologic cells, including epithelial and endothelial cells.23 A role for ABO-mismatch in hindering platelet engraftment has been reported in other series of patients.4,24 In this study, we found that major ABO-I not only delays the erythroid engraftment but also affects platelet and neutrophil recovery at univariate analysis. At multivariate analysis, we observed only a trend for delayed platelet recovery in the major ABO-I group (P = 0.0526), but other authors reported that pancytopenia frequently accompanies pure red cell aplasia and resolves with erythropoiesis recovery.25
In our study, patients receiving major ABO-I transplants were transfusion-dependent significantly longer than others. On average, they required 7.6 (IQR, 2.1–16) wk and 2.2 (IQR, 1.7–7.6) wk to achieve RBC and platelet transfusion independence, respectively. The same figures in different ABO matches were significantly lower: for example, in the ABO-matched group, transfusion independence was reached in 2.8 (IQR, 1.4–5.8) wk for RBC and 1.8 (IQR, 1.4–3.7) wk for PLT, respectively. These findings are in agreement with data recently reported in a series of 800 HLA-matched sibling HSCT transplants at National Institute of Health Clinical Center (1993–2010).24 Interestingly, as found in the above-mentioned study, also our analysis confirmed the detrimental influence of NMA conditioning on transfusion dismissal.24 As a result, major ABO-I transplants in both NIH and our experience required a greater amount of platelet and RBC transfusions than other ABO matches.24 Previous authors have suggested that BM, more than PBSC, affects red blood cell engraftment in major ABO-I transplants.26 In our patients, BM graft predicted the delay of erythroid recovery at multivariate analysis. In addition, when analyzed by graft type, the major ABO-I group had longer RBC transfusion dependence in BM but not PBSC transplant. Indeed, also our data support the hypothesis that graft type may influence the engraftment in major ABO-I transplants. Interestingly, bidirectional ABO-I behaved very differently than major ABO-I and was not accompanied by delayed engraftment or protracted transfusion dependence. As a result, patients receiving bidirectional ABO-I transplants were given similar amounts of blood products as those in ABO-matched or minor ABO-I transplant groups. Although this observation lacks a documented biologic rationale, we could hypothesize that donor’s isoagglutinins may exert an inhibitory effect on the anti-A/anti-B antibody production by the recipient’s lymphocytes, which carry the targeted A/B antigen. This explanation might also support the earlier multilineage engraftment of BM grafts in minor ABO-I transplants. Similarly, an analogous mechanism has been hypothesized to explain the mitigating effect of major ABO-I on severe aGVHD.6,27
An additional result of the present study is that major ABO-I transplants have a significantly higher NRM. Logan et al12 explored the association of ABO-I mismatch and mortality in a seminal study involving various transplant datasets. The authors found that minor ABO-I increased early NRM in a series of 1737 patients receiving BM grafts at Stanford University and in an independent dataset of 435 lymphoma patients gathered at the Center for International Bone and Marrow Transplant Research (CIBMTR).12 However, another analysis of an additional CIBMTR dataset including 5179 AML/MDS patients, revealed that major ABO-I exerted the preeminent negative impact on survival.12 Regarding our patients, the vast majority were affected by AML or MDS and, accordingly to Logan et al,12 we could confirm the increased NRM risk in a multivariate analysis adjusted for several additional confounders related to donors (gender, HLA match, CMV serostatus),28 recipients (age, comorbidities, time interval from diagnosis), graft type, conditioning, and disease (DRI, myeloid neoplasm, complete remission at transplant).
Many studies ascribed the higher NRM of ABO-mismatched transplants to an increased incidence of severe aGVHD.7,12,27,29 First, Bacigalupo et al27 found a higher risk for severe aGVHD in minor ABO-I transplants. A subsequent meta-analysis, including 709 related and 184 unrelated HSC transplants, showed that bidirectional ABO-I carries a higher risk for aGVHD-related mortality.29 This finding was subsequently confirmed in the haploidentical setting.7 In contrast, Damodar et al5 reported no effect of ABO-mismatch on aGVHD and survival of 1502 patients analyzed by graft sources. Regarding our patients, we failed to observe any effect of ABO mismatch on aGVHD so that the higher NRM in our series conceivably relies on other causes.
In general, more severely ill patients are likely to receive a greater number of blood products so that the causality between transfusions and negative outcomes is difficult to be ascertained in retrospective analyses. However, transfusion burden has been identified as an independent predictor for poor outcome in several prospective studies carried out in cancer and noncancer patients.30-33 Number of transfusions at transplant is also an independent predictor for graft failure and survival in the liver transplant setting.34 The immunomodulatory effect by blood products has been largely evoked as the mechanism underlying this association.35,36 For example, markers of endothelial activation and inflammatory cytokine release have been clearly documented in neonatal patients.37 Moreover, it deserves to be emphasized that transfusions themselves carry many types of infectious risks.38 We observed a trend for higher infection-related mortality in major ABO-I transplants, suggesting a higher susceptibility to severe infections in these patients. In this regard, the plausible relation between major ABO-I and higher NRM may reside in the iron overload consequent to the high-transfusion burden. Numerous studies have shown that pre-HSCT ferritin levels correlate with long-term transplant outcomes.39 Of note, also posttransplant ferritin levels have emerged as predictors of decreased OS and increased NRM, independently of pre-HSCT values.40,41
Our study also exhibits some limitations. First, the retrospective design does not allow to reach definite conclusions. Second, the study population includes patients with different underlying diseases and dissimilar relapse risk. Third, the effect of various immunosuppressive regimens was not considered. Finally, the occurrence of delayed hemolysis episodes was not included among the investigated outcomes. Nonetheless, our study population encompassed consecutive patients homogeneously treated, accrued over an 8-y period, which is relatively short in comparison with that of many previous publications on this topic. Of note, at variance with other analyses,7 we assessed the role of ABO-mismatch on transplant outcomes including in multivariate models several additional confounders with an acknowledged risk effect on survival.
In conclusion, we report that major ABO mismatch delays multilineage engraftment and extends transfusion dependence. Conceivably, the high transfusion burden might have a role in increasing nonrelapse mortality of patients receiving ABO-mismatched grafts. These findings prompt further studies exploring the prognostic impact of transfusion burden.
ACKNOWLEDGMENTS
The authors are in debt to Claudia Frau for her valuable work in managing relations with patients, donor registry, and familial donors.
Supplementary Material
Footnotes
Published online 9 July, 2021.
The authors declare no conflict of interests.
The study was funded by “Università Cattolica del Sacro Cuore”—Linea D1 2020 to L.T.
C.G.V., P.C., and L.T. participated in conception and design of the study, acquisition of data, analysis and interpretation of data, article writing, and final approval of the submitted version. N.O., S.G., E.M., M.B., and L.G. did acquisition of data, analysis and interpretation of data, article writing, and final approval of the submitted version. A.B. and S.S. participated in critical revision for important intellectual content, article writing, and final approval of the submitted version. L.T. and P.C. equally contributed to the study as last authors.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Staley EM, Schwartz J, Pham HP. An update on ABO incompatible hematopoietic progenitor cell transplantation. Transfus Apher Sci. 2016; 54:337–344. [DOI] [PubMed] [Google Scholar]
- 2.Booth GS, Gehrie EA, Bolan CD, et al. Clinical guide to ABO-incompatible allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2013; 19:1152–1158. [DOI] [PubMed] [Google Scholar]
- 3.Rowley SD, Donato ML, Bhattacharyya P. Red blood cell-incompatible allogeneic hematopoietic progenitor cell transplantation. Bone Marrow Transplant. 2011; 46:1167–1185. [DOI] [PubMed] [Google Scholar]
- 4.Hefazi M, Litzow M, Hogan W, et al. ABO blood group incompatibility as an adverse risk factor for outcomes in patients with myelodysplastic syndromes and acute myeloid leukemia undergoing HLA-matched peripheral blood hematopoietic cell transplantation after reduced-intensity conditioning. Transfusion. 2016; 56:518–527. [DOI] [PubMed] [Google Scholar]
- 5.Damodar S, Shanley R, MacMillan M, et al. Donor-to-recipient ABO mismatch does not impact outcomes of allogeneic hematopoietic cell transplantation regardless of graft source. Biol Blood Marrow Transplant. 2017; 23:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canaani J, Savani BN, Labopin M, et al. ABO incompatibility in mismatched unrelated donor allogeneic hematopoietic cell transplantation for acute myeloid leukemia: a report from the acute leukemia working party of the EBMT. Am J Hematol. 2017; 92:789–796. [DOI] [PubMed] [Google Scholar]
- 7.Canaani J, Savani BN, Labopin M, et al. Impact of ABO incompatibility on patients’ outcome after haploidentical hematopoietic stem cell transplantation for acute myeloid leukemia—a report from the Acute Leukemia Working Party of the EBMT. Haematologica. 2017; 102:1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura F, Kanda J, Ishiyama K, et al. ; donor/source working group of the Japan Society for Hematopoietic Cell Transplantation. ABO blood type incompatibility lost the unfavorable impact on outcome in unrelated bone marrow transplantation. Bone Marrow Transplant. 2019; 54:1676–1685. [DOI] [PubMed] [Google Scholar]
- 9.Stussi G, Halter J, Bucheli E, et al. Prevention of pure red cell aplasia after major or bidirectional ABO blood group incompatible hematopoietic stem cell transplantation by pretransplant reduction of host anti-donor isoagglutinins. Haematologica. 2009; 94:239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marco-Ayala J, Gómez-Seguí I, Sanz G, et al. Pure red cell aplasia after major or bidirectional ABO incompatible hematopoietic stem cell transplantation: to treat or not to treat, that is the question. Bone Marrow Transplant. 2021; 56:769–778. [DOI] [PubMed] [Google Scholar]
- 11.Ma YR, Wang WJ, Cheng YF, et al. Impact of ABO incompatibility on outcomes after haploidentical hematopoietic stem cell transplantation for severe aplastic anemia. Bone Marrow Transplant. 2020; 55:1068–1075. [DOI] [PubMed] [Google Scholar]
- 12.Logan AC, Wang Z, Alimoghaddam K, et al. ABO mismatch is associated with increased nonrelapse mortality after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015; 21:746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the disease risk index for allogeneic stem cell transplantation. Blood. 2014; 123:3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005; 106:2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiusolo P, Bregante S, Giammarco S, et al. Full donor chimerism after allogeneic hematopoietic stem cells transplant for myelofibrosis: the role of the conditioning regimen. Am J Hematol. 2021; 96:234–240. [DOI] [PubMed] [Google Scholar]
- 16.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995; 15:825–828. [PubMed] [Google Scholar]
- 17.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980; 69:204–217. [DOI] [PubMed] [Google Scholar]
- 18.Lee SJ, Vogelsang G, Flowers MED. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003; 9:215–233. [DOI] [PubMed] [Google Scholar]
- 19.Teofili L, Chiusolo P, Valentini CG, et al. Bone marrow haploidentical transplant with post-transplantation cyclophosphamide: does graft cell content have an impact on main clinical outcomes? Cytotherapy. 2020; 22:158–165. [DOI] [PubMed] [Google Scholar]
- 20.Bolan CD, Leitman SF, Griffith LM, et al. Delayed donor red cell chimerism and pure red cell aplasia following major ABO-incompatible nonmyeloablative hematopoietic stem cell transplantation. Blood. 2001; 98:1687–1694. [DOI] [PubMed] [Google Scholar]
- 21.Mielcarek M, Leisenring W, Torok-Storb B, et al. Graft-versus-host disease and donor-directed hemagglutinin titers after ABO-mismatched related and unrelated marrow allografts: evidence for a graft-versus-plasma cell effect. Blood. 2000; 96:1150–1156. [PubMed] [Google Scholar]
- 22.Blin N, Traineau R, Houssin S, et al. Impact of donor-recipient major ABO mismatch on allogeneic transplantation outcome according to stem cell source. Biol Blood Marrow Transplant. 2010; 16:1315–1323. [DOI] [PubMed] [Google Scholar]
- 23.Ravn V, Dabelsteen E. Tissue distribution of histo-blood group antigens. APMIS. 2000; 108:1–28. [DOI] [PubMed] [Google Scholar]
- 24.Griffith LM, VanRaden M, Barrett AJ, et al. Transfusion support for matched sibling allogeneic hematopoietic stem cell transplantation (1993–2010): factors that predict intensity and time to transfusion independence. Transfusion. 2019; 59:303–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aung FM, Lichtiger B, Rondon G, et al. Pure red cell aplasia in major ABO-mismatched allogeneic hematopoietic stem cell transplantation is associated with severe pancytopenia. Biol Blood Marrow Transplant. 2016; 22:961–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blin N, Traineau R, de Latour R, et al. Impact of donor/recipient major ABO mismatch on allogeneic transplantation outcome according to stem cell source: results from an 809-patient retrospective study. Blood. 2008; 112:2157–2157. [Google Scholar]
- 27.Bacigalupo A, Van Lint MT, Occhini D, et al. ABO compatibility and acute graft-versus-host disease following allogeneic bone marrow transplantation. Transplantation. 1988; 45:1091–1094. [DOI] [PubMed] [Google Scholar]
- 28.Kasamon YL, Luznik L, Leffell MS, et al. Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Transplant. 2010; 16:482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanda J, Ichinohe T, Matsuo K, et al. Impact of ABO mismatching on the outcomes of allogeneic related and unrelated blood and marrow stem cell transplantations for hematologic malignancies: IPD-based meta-analysis of cohort studies. Transfusion. 2009; 49:624–635. [DOI] [PubMed] [Google Scholar]
- 30.Petrelli F, Ghidini M, Ghidini A, et al. Red blood cell transfusions and the survival in patients with cancer undergoing curative surgery: a systematic review and meta-analysis. Surg Today. 2021:1–23. doi: 10.1007/s00595-020-02192-3. [DOI] [PubMed] [Google Scholar]
- 31.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013; 368:11–21. [DOI] [PubMed] [Google Scholar]
- 32.Patel NN, Avlonitis VS, Jones HE, et al. Indications for red blood cell transfusion in cardiac surgery: a systematic review and meta-analysis. Lancet Haematol. 2015; 2:e543–e553. [DOI] [PubMed] [Google Scholar]
- 33.Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999; 340:409–417. [DOI] [PubMed] [Google Scholar]
- 34.Avolio AW, Franco A, Schlegel A, et al. Development and validation of a comprehensive model to estimate early allograft failure among patients requiring early liver retransplant. JAMA Surg. 2020; 155:e204095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neal MD, Raval JS, Triulzi DJ, et al. Innate immune activation after transfusion of stored red blood cells. Transfus Med Rev. 2013; 27:113–118. [DOI] [PubMed] [Google Scholar]
- 36.Aguilar-Nascimento JE, Zampieri-Filho JP, Bordin JO. Implications of perioperative allogeneic red blood cell transfusion on the immune-inflammatory response. Hematol Transfus Cell Ther. 2021; 43:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crawford TM, Andersen CC, Stark MJ. Effect of repeat transfusion exposure on plasma cytokine and markers of endothelial activation in the extremely preterm neonate. Transfusion. 2020; 60:2217–2224. [DOI] [PubMed] [Google Scholar]
- 38.Gilliss BM, Looney MR, Gropper MA. Reducing noninfectious risks of blood transfusion. Anesthesiology. 2011; 115:635–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan Z, Chen X, Wang H, et al. Effect of pre-transplantation serum ferritin on outcomes in patients undergoing allogeneic hematopoietic stem cell transplantation. Medicine (Baltimore). 2018; 97:e10310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer SC, O’Meara A, Buser AS, et al. Prognostic impact of posttransplantation iron overload after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2013; 19:440–444. [DOI] [PubMed] [Google Scholar]
- 41.Fingrut W, Law A, Lam W, et al. Post-transplant ferritin level predicts outcomes after allogeneic hematopoietic stem cell transplant, independent from pre-transplant ferritin level. Ann Hematol. 2021; 100:789–798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


