Abstract
Background:
Multiple techniques exist to monitor free flap viability postoperatively, varying with practical and personal preference, yet the limitations of each technique remain unquantified. This systematic review aims to identify the most commonly reported limitations of these techniques in clinical practice.
Methods:
A systematic review was conducted according to PRISMA guidelines using MEDLINE, EMBASE, and Web of Science with search criteria for postoperative free flap monitoring techniques. Search results were independently screened using defined criteria by two authors and a senior clinician. Limitations of the techniques found in the discussion section of eligible articles were recorded and categorized using thematic analysis.
Results:
A total of 4699 records were identified. In total, 2210 articles met the eligibility criteria and were subsequently reviewed, with 195 papers included in the final analysis. The most frequently reported limitations of clinical monitoring were interpretation requiring expertise (25% of related papers), unsuitability for buried flaps (21%), and lack of quantitative/objective values (19%). For noninvasive technologies, the limitations were lack of quantitative/objective values (21%), cost (16%), and interpretation requiring expertise (13%). For invasive technologies, the limitations were application requiring expertise (25%), equipment design and malfunction (13%), and cost (13%).
Conclusions:
This is the first systematic review to quantify the limitations of different flap monitoring techniques, as reported in the literature. This information may enhance the choice in monitoring strategy for a reconstructive service, and inform the development and refinement of new flap monitoring technologies.
INTRODUCTION
Microvascular free tissue transfer is a well-established method for reconstruction of large and complex defects, owing to its superior functional and aesthetic outcomes.1 Despite success rates of around 95% or more,2–4 flap failure secondary to vascular compromise remains a potentially devastating complication, leading to patient morbidity, prolonged hospital stay, and increased healthcare costs.5
Although salvage rates of failing free flaps vary with etiology, flap type, and center experience,6 the literature has consistently shown that early detection of vascular compromise and swift surgical re-exploration significantly increases the likelihood of successful free flap salvage.7 Vascular compromise leading to flap failure occurs due to disturbances in either the flap’s arterial inflow, capillary microcirculation, or venous outflow.5 As such, monitoring methods to aid the recognition of one or more disturbances in flap vasculature are critical in maximizing the success of free flap reconstruction. Numerous flap monitoring strategies and technologies exist, which vary in complexity, invasiveness, and cost.8 In 1975, Creech and Miller succinctly outlined the desirable properties of the ideal free flap monitoring technique and concluded that it should be inexpensive; harmless to both patient and flap; produce rapid, reliable and objective results; simple to interpret by relatively inexperienced personnel; and applicable to all types of free flaps.9
Despite the availability of various monitoring techniques, a universally accepted single monitoring technique (or a combination of monitoring techniques) that satisfies the ideal criteria is still lacking.10 Although clinical studies often comment on the effectiveness of monitoring techniques in terms of sensitivity and specificity of identifying flap failure, their technical and practical limitations have thus far received little to no objective evaluation.11–15 Thus, there is no universal consensus regarding the ideal flap monitoring technique and local flap monitoring protocols are often influenced by surgeon preference and availability of technologies.16 An objective analysis of the limitations of free flap monitoring techniques would aid clinicians in deciding on the best single or combined flap monitoring technique on an individual case basis. This systematic review aims to collate the anecdotal evidence of flap monitoring limitations reported by authors in the literature, and provide a novel quantifiable report using thematic analysis.
METHODS
A systematic review of the literature was performed to identify the discussed limitations of postoperative free flap monitoring techniques using thematic analysis. This review was conducted in accordance with the Cochrane Handbook of Systematic Reviews of Interventions,17 and reported as per the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement18 (Fig. 1). Thematic analysis of the data was carried out by following the methodological steps outlined by Braun and Clarke.19
Fig. 1.

PRISMA diagram demonstrating the study selection process for inclusion in the review.
Eligibility Criteria
Studies reporting on the use of postoperative free flap monitoring were eligible for inclusion. Eight commonly used flap monitoring techniques eligible for inclusion were selected following an initial literature search carried out by two reviewers. The flap monitoring techniques for inclusion included five noninvasive techniques: “Clinical monitoring,” “Handheld Doppler,” “Near-infrared spectroscopy,” “Color duplex ultrasonography,” “Laser Doppler flowmetry,” and three invasive techniques: “Implantable Doppler,” “Flow coupler,” and “Microdialysis.” All article types were included. No language or publication status restrictions were imposed.
Search Strategy
Studies reporting on postoperative free flap monitoring were identified using electronic database searches of EMBASE, MEDLINE, and Web of Science published up to February 1, 2020. A combination of keywords and MeSH terms relating to postoperative free flap monitoring were developed by two independent reviewers, with the aid of a medical librarian search strategist to identify relevant articles. (See appendices, Supplemental Digital Content 1, which displays (a) MeSH and search terms used within our PRISMA search for all databases, (b) Inclusion criteria checklist, (c) Structure of the data extraction that was performed for each article within the database, (d) Organization of recurrent themes into metathemes, with the included original themes and a definition of the metatheme, and (e) Response to reviewers Table A and Table B. http://links.lww.com/PRSGO/B691.) No language restrictions were applied. Reference lists of included articles were screened to identify further relevant articles not captured by the original search criteria. The Refworks (Ex Libris Ltd) reference manager tool and Rayyan QRCI were used to record and filter duplicate articles.
Eligibility Assessment
Eligibility assessment of articles identified through the original search strategy was carried out independently by two reviewers according to a predefined inclusion criteria checklist. (See appendices, Supplemental Digital Content 1, which displays (a) MeSH and search terms used within our PRISMA search for all databases, (b) Inclusion criteria checklist, (c) Structure of the data extraction that was performed for each article within the database, (d) Organisation of recurrent themes into metathemes, with the included original themes and a definition of the metatheme, and (e) Response to reviewers Table A and Table B. http://links.lww.com/PRSGO/B691.) Abstracts were initially screened for “free flap surgery” or equivalent as well as mention of one or more included postoperative monitoring techniques. Following successful abstract screening, full-text publication was screened for limitations reported by the authors in the discussion section of the article.
Data Extraction
Data were extracted using Microsoft Excel (Microsoft Corp., Redmond, Wash.) using a data extraction form agreed upon by three reviewers, following an initial pilot on a proportion of eligible articles. (See appendices, Supplemental Digital Content 1, which displays (a) MeSH and search terms used within our PRISMA search for all databases, (b) Inclusion criteria checklist, (c) Structure of the data extraction that was performed for each article within the database, (d) Organisation of recurrent themes into metathemes, with the included original themes and a definition of the metatheme, and (e) Response to reviewers Table A and Table B. http://links.lww.com/PRSGO/B691). Information was extracted from each article on general article characteristics and characteristics of intervention, and limitations were discussed.
Thematic Analysis
Thematic analysis is a qualitative research tool used for the identification, evaluation, and presentation of recurring patterns (or themes) within a specified dataset.19 Thematic analysis has been previously used in medical research to appraise health policy,20 evaluate the effectiveness of currently available medical devices,21,22 and aid the innovation of novel medical products.23
Limitations of monitoring techniques were recorded and analyzed using thematic analysis. Following the guidelines proposed by Braun and Clarke, articles eligible for inclusion were initially read by two reviewers to gain familiarity with the data. Statements relating to the limitations of monitoring techniques reported by the authors in the articles were recorded and coded into primary themes. If any discrepancies arose, a third reviewer’s opinion was sought to ensure correct coding. Metathemes were developed, which encompassed multiple similar primary themes and reflected the practical and technical limitations of flap monitoring techniques (Table 1).
Table 1.
Organization of Recurrent Themes into Metathemes, with the Definition of the Metatheme (See Appendix 4, SDC1)
| Metatheme | Description |
|---|---|
| Interpretation requires expertise | Includes discussions about the requirement for training or expertise to accurately use the monitoring technique. |
| Complex operative technique | Includes discussions about the operative time required to apply the monitoring technique and the variability in outcomes depending on the application skill of the surgeon. |
| Lack of quantitative/objective values | Includes discussion about a lack of definitive, quantitative cut off values that indicate a requirement to return the free flap to theatre. |
| Unsuitable for buried flaps | Includes discussion about the inability to monitor buried free flaps with the mentioned monitoring technique. |
| Cost | Includes discussion about the high cost of postoperative techniques. |
| Cannot identify offending vessel | Includes discussion about the difficulty to identify the specific vessel that the technique is measuring (identify the cause of the measurement changes of the monitoring techniques). |
| External artifact | Includes discussion of the interference of external artifacts leading to measurement inaccuracies. |
| Probe contact limitations | Includes discussion of any limitations that relate to the contact of the postoperative technique to the patient. This includes poor probe to patient connections and lack of multiple probes. |
| Local tissue trauma | Includes discussion of local damage caused by the monitoring technique. |
| Variation with physiological factors | Includes discussion of patient systemic or local changes of physiology, including pathology, that lead to measurements changes that are not associated with any changes in the free flap. |
| Lack of continuous monitoring | Includes discussion relating to the inability for the monitoring technique to provide continuous measurements. |
| Equipment design and malfunction | Includes discussion relating to the design of the monitoring technique that makes the device less suitable for clinical practice. Discussions also include designs that lead to device malfunction and lack of robustness. |
| Labor-intensive monitoring | Includes discussions relating to an increase of time used by clinical staff to perform the monitoring technique. |
| Disruption to patient | Includes discussion of any occasions which the monitoring technique led to disturbance of the patient, over normal procedure. |
| Damage to vessels | Includes discussion of any damage to the vessels involved in the anastomosis following free flap transfer caused by the monitoring technique. |
| Late recognition of flap failure | Includes discussions relating to a delay between free flap changes and changes in the measurements of the monitoring technique. |
Categorizing Techniques
The metathemes were reviewed by all three reviewers to ensure that they reflected the ideal characteristics of flap monitoring techniques as presented by Creech and Miller for identifying limitations in context of these well-established criteria. Limitations were analyzed firstly for all monitoring techniques together and then analyzed for the limitations that came from each individual technique separately. Techniques were then grouped into invasive and noninvasive monitoring techniques. Techniques that cannot monitor buried free flaps, in the majority of circumstances (ie, without a skin paddle or a very superficial buried flap) include: NIRS, ultrasound Doppler, handheld Doppler and laser Doppler, which were grouped into noninvasive monitoring techniques. All remaining techniques that have the ability to monitor buried flaps were grouped into invasive techniques. Their limitations were collated within these respective groups and were compared with the “gold standard” monitoring technique of clinical monitoring as a further analysis.
Evidence Stratification
The quality of the evidence of reported limitations was evaluated and stratified to aid the interpretation of data collected in this study. All limitations were categorized into one of the four tiers of evidence: (1a) limitation identified through primary study objective, (1b) limitation identified as part of a secondary study objective along with direct observation by authors (both 1a and 1b require the study to achieve a level 4 or greater Center for Evidence Based Medicine score), (2) not directly observed, but specific papers cited to justify claims of limitations by the authors, or (3) authors’ opinion without citation or reported direct observation. Two reviewers assessed each limitation according to the agreed criteria mentioned above, and limitations were cross-checked between reviewers to identify inter-reviewer disagreements and increase the reliability of the results.
Statistical Analysis
Statistical analysis of data was performed using Microsoft Excel. Percentage frequency of metathemes of reported limitations were calculated for each monitoring technique by contrasting against the total number of reported limitations for each monitoring technique.
RESULTS
Using our search criteria, 4699 articles were identified for consideration: 1941 from Medline, 1617 from Embase, and 1111 from Web of Science. Of these 4699 articles, 2489 articles were duplicates. No new appropriate articles were found in the bibliographies of considered papers that were not included via our search criteria. Following title and abstract screening, and free text assessment, 195 articles were found to be eligible for inclusion. (Fig. 1)
Data Characteristics
Techniques were searched individually; the frequency of articles discussing each technique within the original 4699 articles was documented and analyzed (Table 2). Ultrasound Doppler returned the most results with 2806, whereas Microdialysis returned the least at 154. Of the 195 papers included in the statistical analysis, the frequency of reference to each specific technique was clinical monitoring (29%), laser Doppler flowmetry (24%), NIRS (23%), implantable Doppler (23%), duplex ultrasound (12%), handheld Doppler (10%), microdialysis (10%), and flow coupler (3%). Within the 195 papers, there were 505 limitations mentioned, which produced 89 themes. These 89 themes were then collated into the 12 metathemes that have been previously shown. (See appendices, Supplemental Digital Content 1, which displays (a) MeSH and search terms used within our PRISMA search for all databases, (b) Inclusion criteria checklist, (c) Structure of the data extraction that was performed for each article within the database, (d) Organisation of recurrent themes into metathemes, with the included original themes and a definition of the metatheme, and (e) Response to reviewers Table A and Table B. http://links.lww.com/PRSGO/B691.)
Table 2.
Number of Articles Returned when Searching Each Database for Each Technique.
| Method | Medline | Embase | Web of Science | Total | Included |
|---|---|---|---|---|---|
| Colour duplex ultrasonography | 1432 | 1240 | 134 | 2806 | 43 |
| Laser Doppler | 567 | 813 | 124 | 1504 | 47 |
| Clinical monitoring | 383 | 480 | 394 | 1257 | 57 |
| NIRS | 126 | 112 | 158 | 396 | 45 |
| Implantable Doppler | 84 | 93 | 161 | 338 | 44 |
| Flow coupler | 59 | 80 | 21 | 160 | 6 |
| Microdialysis | 54 | 51 | 49 | 154 | 20 |
Most Frequent Limitations
For all articles collectively the three most common limitations that were reported were a lack of quantitative/objective values by the techniques (16.2% of all limitations), interpretation of results requiring expertise (14.3%), and high cost (10.9%).
Invasive versus Noninvasive Monitoring Techniques
The most common limitations of clinical monitoring reported in the included articles were interpretation requiring expertise (25% of clinical monitoring limitations), unsuitability for buried flap measurement (21%), and a lack of quantitative/objective values (19%). For noninvasive technology, a lack of quantitative/objective values (21% of noninvasive limitations), high cost (16%), and interpretation requiring expertise (13%) were the most common limitations; invasive technology ranked interpretation requiring expertise (25% of invasive limitations), unsuitable equipment design and malfunction (13%), and then high cost (13%) as the most frequent limitations.
Individual Technique Analysis
Analysis for postoperative monitoring of free flaps was done for all individual techniques to show the most frequent limitations of each technique. The most common limitation of clinical monitoring is that its interpretation requires expertise (25%). For NIRS, the most common limitation is the lack of quantitative/objective values provided (21%) and its high cost (21%). Laser Doppler flowmetry have similarly been reported to provide a lack of objective/quantitative values as its most common limitation (26%). The most common limitation for handheld Doppler is its difficulty in identifying the exact vessel causing the flap failure (58%). For color duplex ultrasonography, the most commonly reported limitation was that interpretation requires expertise (38%). Microdialysis causes local tissue trauma, which is its most commonly reported limitation (23%). The flow coupler’s most commonly reported limitation was damage to vessels (58%), whereas the most commonly reported limitation for the implantable doppler was its associated complex operative technique (46%) (Table 3).
Table 3.
Metathemes that Scored >10% in Frequency for Each Technique
| Monitoring Technique | Most Common Themes | Proportion % |
|---|---|---|
| NIRS | Lack of quantitative/objective values | 23.86 |
| Cost | 23.86 | |
| Probe contact limitations | 14.77 | |
| Variation with physiological factors | 12.50 | |
| Clinical monitoring | Interpretation requires expertise | 25.25 |
| Unsuitable for buried flaps | 21.21 | |
| Lack of quantitative/objective values | 19.19 | |
| Lack of continuous monitoring | 11.11 | |
| Handheld Doppler | Cannot identify offending vessel | 57.58 |
| Unsuitable for buried flaps | 18.18 | |
| Duplex Doppler | Interpretation requires expertise | 38.10 |
| Lack of continuous monitoring | 14.29 | |
| Cannot identify offending vessel | 14.29 | |
| Cost | 11.90 | |
| Laser Doppler | Lack of quantitative/objective values | 26.09 |
| External artifact | 15.22 | |
| Variation with physiological factors | 11.96 | |
| Cost | 10.87 | |
| Microdialysis | Local tissue trauma | 23.08 |
| Cost | 17.31 | |
| Equipment design and malfunction | 15.38 | |
| Interpretation requires expertise | 15.38 | |
| Flow Coupler | Damage to vessels | 58.33 |
| Equipment design and malfunction | 25.00 | |
| Implantable Doppler | Complex operative technique | 43.68 |
| Lack of quantitative/objective values | 12.65 | |
| Cost | 10.34 |
Evidence Stratification
Evidence levels were established for each limitation and were then grouped into their respective metathemes to be analyzed. Of all reported limitations, 21.92% were directly observed in original research and identified either through the primary study objective, or as part of a secondary study objective (ie Type 1a or 1b). The remaining 78.08% came from cited claims or from authors’ opinion. The only technology to have a larger portion of type 1 evidence was flow coupler with 55.56% type 1b evidence, with the 44.44% remaining being type 2 and 3.
DISCUSSION
A systematic review was performed to collate and quantify the reported limitations of postoperative monitoring techniques for free flaps. Although anecdotal reports of limitations have been previously recorded, this is the first article to quantify the frequency of these limitations relative to each monitoring technique, using thematic analysis. All monitoring techniques were found to have relative strengths and limitations, with none completely satisfying the criteria presented by Creech and Miller. Limitations such as cost and the lack of objective values produced by the technologies were found to be shared amongst multiple technologies, whilst various technology-specific limitations also emerged.
The key limitations to the use of clinical monitoring include that expertise is needed to interpret clinical signs, its unsuitability for use in buried flaps, and the lack of qualitative/objective values that it produces. These limitations may be explained by a combination of operator and patient-dependent factors. There is an inherent subjective nature of parameters that are being assessed during clinical monitoring, necessitating the need for reliable and experienced personnel.24 Temperature, color, skin turgor, and capillary refill of the flap25 are usually all assessed manually by varying members of the healthcare team. This introduces subjectivity and potential for human error during the evaluation and documentation of a flap’s viability. When experienced personnel monitor flaps, outcomes of clinical monitoring in the early detection of flap compromise have been favorable13; however, this may not always be achieved, given that flaps require continuous monitoring for at least 72 hours26; out-of-hours ward staffing levels are often low, and training may not be easy to achieve. Overcoming this experience shortfall requires training or hiring staff, which is costly and time intensive. The development of reproducible and uniform clinical flap monitoring protocols in centers have allowed more objective recording of flap status.27–29 The review and refinement of these local protocols mitigate the operator-dependent subjectivity associated with clinical monitoring. Technology adjuncts may enable the availability of consistency and experience, decreasing observer error and reducing interpretation training costs.30 Digital photographic images of flaps may be securely sent to clinicians to be reviewed remotely. Temperature monitoring may also be standardized via the use of temperature probes or temperature sensitive tapes.31,32
The unsuitability of clinical monitoring to assess buried flaps is a limitation that is often cited, particularly in head and neck surgery, where flaps in esophageal or pharyngeal reconstruction are often interiorized and not visible externally.33 The inability of clinical monitoring to accurately assess buried flaps has been partially circumvented by the use of an exteriorized segment of the flap supplied by the same vascular pedicle, which can be visualized easily to check for vascular compromise. This may, however, compromise the aesthetic result.34
When considering noninvasive flap monitoring technologies, the frequently occurring limitations shared by NIRS, laser Doppler, handheld Doppler and color duplex ultrasonography emerged as high cost; interpretation requiring experienced personnel; and the overall subjective nature of the values that they produce. The lack of quantitative/objective values produced by noninvasive technologies and the need for expert interpretation originates from the type of signal that is being processed by the monitoring techniques. In the case of NIRS and laser Doppler, the signal that is being recorded is selective light refraction by hemoglobin, and the Doppler shift of laser light due to blood flow respectively.35,36 These parameters may vary with systemic and external factors, including the location of the probe, motion, temperature changes, flap type, and minor device setting changes.37 As a result, a reliable cut off value indicating flap failure in NIRS has been difficult to establish and standardize for a large set of heterogeneous patient cohorts.38 Individual trends in flow seem to be more suggestive of flap failure; this may lead to the development of criteria that are based on a percentage decline in initial signal levels.39
Handheld Doppler and color duplex ultrasonography both rely on the Doppler shift of ultrasound waves reflected from moving blood flow within vessels to evaluate the vascular status of the flap. This, therefore, requires detailed knowledge of both the recipient site and flap specific to the patient, and an understanding of the use of the device.40 To aid the evaluation of the vascular anatomy, the location of the vessels and anastomoses may be marked on the skin with ink or a stitch.41 Despite this, the presence of a trained radiologist and the operating surgeon may both be required to fully evaluate the viability of a flap using ultrasound.40 The requirement for expertise was the most widely shared limitation related to duplex ultrasound. Both an understanding of vessel anatomy and interpretation expertise come with training and a cost to employ staff with these qualities to monitor the free flap.
The cost of noninvasive flap monitoring devices, except handheld Doppler, poses a major limitation to their use in reconstructive centers. Devices such as NIRS and laser doppler traditionally have costs associated with both the monitoring device unit and the disposable probes.42 Novel technology has overcome a large proportion of these costs through the use of smartphone displays or via a unified monitoring and interpretation device.43 Recently, cost benefit analyses of these techniques have been published; however, most have been inconclusive.44 More evidence is, therefore, needed regarding the cost-effectiveness of available flap monitoring techniques.
The main limitations of invasive flap monitoring technologies include the need for complex operative technique, unsuitable equipment design and malfunction, and high cost. The limitation of unsuitable equipment design and malfunction is common to all invasive monitoring techniques. It can be explained by the use of intricate medical equipment, which needs to produce reliable readings from within tissues. In the case of the implantable Doppler, the cuff applied to the anastomosis may slip and the Doppler probe may become dislodged on movement, leading to a false loss or reduction of signal.45 Similar cases of accidental probe dislodgement have been reported with the use of the flow coupler.46 In the case of microdialysis, the catheter may become dislodged on movement or if not secured properly, leading to false readings.47,48 Strict perioperative care is required to limit the occurrence of equipment malfunction. The limitation of complex operative technique is almost exclusively associated with the use of the implantable Doppler device. As this device is fitted manually to the anastomosis at the time of operation, its incorrect placement can lead to ineffective probe position, dislodgement, increased operative time, and damage to the anastomosis upon removal.49 Overcoming the limitation of complex operative technique associated with the placement of the implantable Doppler probe has in fact led to the development of the venous flow coupler device.50 The Doppler device which is integral to the coupler reduced the technical skill associated with its application, but does bring about its own disadvantage of added cost.
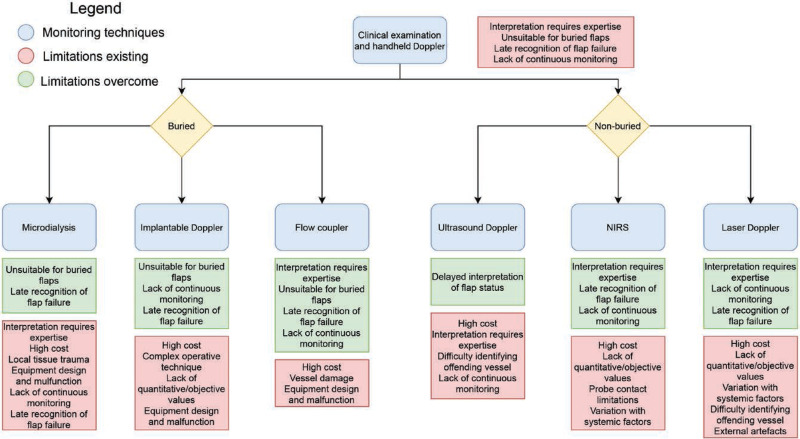
Through the identification of the most frequent limitations, it became clear that each technique had a unique combination of limitations. Therefore, a proposed pathway was developed by the authors for the selection of postoperative free flap monitoring technique (Fig. 2).
Fig. 2.
Flow diagram that outlines the limitations of using clinical monitoring with handheld Doppler, followed by the limitations overcome by using an adjunct technique and the limitations that exist despite adding this technique.
The pathway initially demonstrates the limitations associated with clinical monitoring, followed by the option to monitor buried or nonburied flaps. This will have clear implications on the most effective technology. Following this, the choice of adjunctive monitoring technique is accompanied by the limitations that are overcome by use of the selected technique, as well as the specific limitations associated with the selected technique. This allows clinicians to visualize the limitations of using these adjunctive techniques and decide which suits their needs depending on the clinical scenario. It may be that other strategies such as staffing, or training may help overcome some of these remaining challenges.
Limitations
The identification and thematic analysis of reported limitations of monitoring techniques in the literature was based on a structured methodology, but ultimately contained elements of subjectivity. Given that only a minor proportion of the limitations arose from data generated from the same study, the findings of this work are largely based on expert opinion. However, many limitations are not suited to being proven or disproven, and so this study may still provide the most useful basis for choosing a flap monitoring strategy and highlighting priority areas for development. Human error and researcher subjectivity cannot be overlooked in the identification and thematic grouping of limitations. Given the qualitative nature of this type of study, there is no one correct or accurate interpretation of the data, but by adhering to a stringent methodology and cross-referencing with multiple reviewers, reproducibility is ensured.
A limitation of the data itself is the discrepancy in reporting for each technique. For techniques that have a high number of articles reporting limitations, the proportion of limitations may be more accurate and all the limitations that exist are likely to be expressed in the article pool.
CONCLUSIONS
This is the first systematic review to objectively quantify and evaluate the limitations of flap monitoring techniques from the available literature. Current monitoring techniques play a vital role in the detection of flap failure following free flap surgery, but there remains a lack of general consensus and standardization regarding the optimum flap monitoring protocol. This study brings a quantitative evaluation to previously available qualitative evidence, allowing clinicians to objectively view the limitations of currently existing free flap monitoring techniques. This may help clinicians and service providers to make more informed decisions about postoperative flap monitoring strategies by viewing these limitations and applying a tailored, patient-centered monitoring approach. We recommend that clinicians, using the techniques mentioned in their studies, should publish the limitations they have found and how they mitigated them. This will allow the clinical and scientific community to identify and provide means to overcome the current shortfalls in free flap monitoring.
Supplementary Material
Footnotes
Published online 12 July 2021.
Disclosure: All the authors have no financial interest to declare in relation to the content of this article. No funding was received for this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Bellamy JL, Mundinger GS, Flores JM, et al. Do adjunctive flap-monitoring technologies impact clinical decision making? An analysis of microsurgeon preferences and behavior by body region. Plast Reconstr Surg. 2015; 135:883–892. [DOI] [PubMed] [Google Scholar]
- 2.Bui DT, Cordeiro PG, Hu QY, et al. Free flap reexploration: Indications, treatment, and outcomes in 1193 free flaps. Plast Reconstr Surg. 2007; 119:2092–2100. [DOI] [PubMed] [Google Scholar]
- 3.Pohlenz P, Blessmann M, Blake F, et al. Outcome and complications of 540 microvascular free flaps: The Hamburg experience. Clin Oral Investig. 2007; 11:89–92. [DOI] [PubMed] [Google Scholar]
- 4.Nakatsuka T, Harii K, Asato H, et al. Analytic review of 2372 free flap transfers for head and neck reconstruction following cancer resection. J Reconstr Microsurg. 2003; 19:363–8; discussion 369. [DOI] [PubMed] [Google Scholar]
- 5.Chiu YH, Chang DH, Perng CK. Vascular complications and free flap salvage in head and neck reconstructive surgery: Analysis of 150 cases of reexploration. Ann Plast Surg. 2017; 78(3 Suppl 2):S83–S88. [DOI] [PubMed] [Google Scholar]
- 6.Novakovic D, Patel RS, Goldstein DP, et al. Salvage of failed free flaps used in head and neck reconstruction. Head Neck Oncol. 2009; 1:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown JS, Devine JC, Magennis P, et al. Factors that influence the outcome of salvage in free tissue transfer. Br J Oral Maxillofac Surg. 2003; 41:16–20. [DOI] [PubMed] [Google Scholar]
- 8.Cervenka B, Bewley AF. Free flap monitoring: A review of the recent literature. Curr Opin Otolaryngol Head Neck Surg. 2015; 23:393–398. [DOI] [PubMed] [Google Scholar]
- 9.Creech B, Miller S. Evaluation of circulation in skin flaps. Skin Flaps Boston: Little, Brown. 1975; 21. [Google Scholar]
- 10.Whitaker IS, Karoo ROS, Oliver DW, et al. Current techniques in the post-operative monitoring of microvascular free-tissue transfers. Eur J Plast Surg. 2005; 27:315–321. [Google Scholar]
- 11.Kagaya Y, Miyamoto S. A systematic review of near-infrared spectroscopy in flap monitoring: Current basic and clinical evidence and prospects. J Plast Reconstr Aesthet Surg. 2018; 71:246–257. [DOI] [PubMed] [Google Scholar]
- 12.Cuthbert R, Deutsch C, Roy A, et al. Postoperative monitoring of the free jejunal flap: Use of colour duplex and systematic review of available techniques. Ann R Coll Surg Engl. 2018; 100:485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Disa JJ, Cordeiro PG, Hidalgo DA. Efficacy of conventional monitoring techniques in free tissue transfer: An 11-year experience in 750 consecutive cases. Plast Reconstr Surg. 1999; 104:97–101. [PubMed] [Google Scholar]
- 14.Chang TY, Lee YC, Lin YC, et al. Implantable doppler probes for postoperatively monitoring free flaps: Efficacy. A systematic review and meta-analysis. Plast Reconstr Surg Glob Open. 2016; 4:e1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Um GT, Chang J, Louie O, et al. Implantable Cook-Swartz Doppler probe versus synovis flow coupler for the post-operative monitoring of free flap breast reconstruction. J Plast Reconstr Aesthet Surg. 2014; 67:960–966. [DOI] [PubMed] [Google Scholar]
- 16.Patel UA, Hernandez D, Shnayder Y, et al. Free flap reconstruction monitoring techniques and frequency in the era of restricted resident work hours. JAMA Otolaryngol Head Neck Surg. 2017; 143:803–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JPT, Thomas J, Chandler J, et al. (eds). Cochrane Handbook for Systematic Reviews of Interventions. Chichester, U.K.: John Wiley & Sons; 2019. [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, et al.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Plos Med. 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006; 3:77–101. [Google Scholar]
- 20.Carroll C, Kaltenthaler E, FitzGerald P, et al. A thematic analysis of the strengths and weaknesses of manufacturers’ submissions to the NICE Single Technology Assessment (STA) process. Health Policy. 2011; 102:136–144. [DOI] [PubMed] [Google Scholar]
- 21.Campling NC, Pitts DG, Knight PV, et al. A qualitative analysis of the effectiveness of telehealthcare devices (i) are they meeting the needs of end-users? BMC Health Services Research. 2017; 17: article number 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geoghegan L, Kwasnicki RM, Kanabar S, et al. A systematic recurrent theme analysis of the reported limitations of facial electromyography. Ann Med Surg (Lond). 2018; 33:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dijkstra M, van der Bijl WM. Thematic framing: Creating healthcare innovations1. J Med Devices. 2016; 10:020962. [Google Scholar]
- 24.Khatri N, Zhang S, Kale SS. Current techniques for postoperative monitoring of microvascular free flaps. J Wound Ostomy Continence Nurs. 2017; 44:148–152. [DOI] [PubMed] [Google Scholar]
- 25.Numata T, Iida Y, Shiba K, et al. Usefulness of color Doppler sonography for assessing hemodynamics of free flaps for head and neck reconstruction. Ann Plast Surg. 2002; 48:607–612. [DOI] [PubMed] [Google Scholar]
- 26.Devine JC, Potter LA, Magennis P, et al. Flap monitoring after head and neck reconstruction: Evaluating an observation protocol. J Wound Care. 2001; 10:525–529. [DOI] [PubMed] [Google Scholar]
- 27.Mofikoya BO, Ugburo AO, Belie OM. Clinical assessment score for monitoring free flaps in the dark skin. Available at https://ajmhs.umed.edu.al/images/ahead-of-print/2018/Artikulli-3-Short-Communication.pdf. Accessed January 2, 2021.
- 28.Kruse AL, Luebbers HT, Grätz KW, et al. Free flap monitoring protocol. J Craniofac Surg. 2010; 21:1262–1263. [DOI] [PubMed] [Google Scholar]
- 29.Khan MA, Mohan A, Ahmed W, et al. Nursing monitoring and management of free and pedicled flaps–outcomes of teaching sessions on flap care. Plast Surg Nurs. 2010; 30:213–6; quiz 217. [DOI] [PubMed] [Google Scholar]
- 30.Varkey P, Tan NC, Girotto R, et al. A picture speaks a thousand words: The use of digital photography and the internet as a cost-effective tool in monitoring free flaps. Ann Plast Surg. 2008; 60:45–48. [DOI] [PubMed] [Google Scholar]
- 31.Khouri RK, Shaw WW. Monitoring of free flaps with surface-temperature recordings: Is it reliable? Plast Reconstr Surg. 1992; 89:495–9; discussion 500. [PubMed] [Google Scholar]
- 32.Chiu ES, Altman A, Allen RJ, Jr, et al. Free flap monitoring using skin temperature strip indicators: Adjunct to clinical examination. Plast Reconstr Surg. 2008; 122:144e–145e. [DOI] [PubMed] [Google Scholar]
- 33.Katsaros J, Banis JC, Acland RD, et al. Monitoring free vascularised jejunum grafts. Br J Plast Surg. 1985; 38:220–222. [DOI] [PubMed] [Google Scholar]
- 34.Bafitis H, Stallings JO, Ban J. A reliable method for monitoring the microvascular patency of free jejunal transfers in reconstructing the pharynx and cervical esophagus. Plast Reconstr Surg. 1989; 83:896–898. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Shen Z, Shao Z, et al. Free flap monitoring using near-infrared spectroscopy: A systemic review. Ann Plast Surg. 2016; 76:590–597. [DOI] [PubMed] [Google Scholar]
- 36.Yuen JC, Feng Z. Monitoring free flaps using the laser Doppler flowmeter: Five-year experience. Plast Reconstr Surg. 2000; 105:55–61. [DOI] [PubMed] [Google Scholar]
- 37.Chao AH, Lamp S. Current approaches to free flap monitoring. Plast Surg Nurs. 2014; 34:52–6; quiz 57. [DOI] [PubMed] [Google Scholar]
- 38.Keller A. A new diagnostic algorithm for early prediction of vascular compromise in 208 microsurgical flaps using tissue oxygen saturation measurements. Ann Plast Surg. 2009; 62:538–543. [DOI] [PubMed] [Google Scholar]
- 39.Hallock GG. A “True” false-negative misadventure in free flap monitoring using laser Doppler flowmetry. Plast Reconstr Surg. 2002; 110:1609–1611. [DOI] [PubMed] [Google Scholar]
- 40.Few JW, Corral CJ, Fine NA, et al. Monitoring buried head and neck free flaps with high-resolution color-duplex ultrasound. Plast Reconstr Surg. 2001; 108:709–712. [DOI] [PubMed] [Google Scholar]
- 41.Khundkar R, Malic C. Re: A simple adjunct to facilitate hand held Doppler perforator localisation following microsurgical breast reconstruction. J Plast Reconstr Aesthet Surg. 2009; 62:417–418. [DOI] [PubMed] [Google Scholar]
- 42.Smit JM, Zeebregts CJ, Acosta R, et al. Advancements in free flap monitoring in the last decade: A critical review. Plast Reconstr Surg. 2010; 125:177–185. [DOI] [PubMed] [Google Scholar]
- 43.Chen CM, Kwasnicki RM, Curto VF, et al. Tissue oxygenation sensor and an active in vitro phantom for sensor validation. IEEE Sens J. 2019; 19:8233–8240. [Google Scholar]
- 44.Lindelauf AAMA, Vranken NPA, Rutjens VGH, et al. Economic analysis of noninvasive tissue oximetry for postoperative monitoring of deep inferior epigastric perforator flap breast reconstruction: a review. Surg Innov. 2020; 27:534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rozen WM, Enajat M, Whitaker IS, et al. Postoperative monitoring of lower limb free flaps with the Cook-Swartz implantable Doppler probe: A clinical trial. Microsurgery. 2010; 30:354–360. [DOI] [PubMed] [Google Scholar]
- 46.Fujiwara RJT, Dibble JM, Larson SV, et al. Outcomes and reliability of the flow coupler in postoperative monitoring of head and neck free flaps. Laryngoscope. 2018; 128:812–817. [DOI] [PubMed] [Google Scholar]
- 47.Jyränki J, Suominen S, Vuola J, et al. Microdialysis in clinical practice: Monitoring intraoral free flaps. Ann Plast Surg. 2006; 56:387–393. [DOI] [PubMed] [Google Scholar]
- 48.Setälä L, Papp A, Romppanen EL, et al. Microdialysis detects postoperative perfusion failure in microvascular flaps. J Reconstr Microsurg. 2006; 22:87–96. [DOI] [PubMed] [Google Scholar]
- 49.Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997; 78:606–617. [DOI] [PubMed] [Google Scholar]
- 50.Chadwick SL, Khaw R, Duncan J, et al. The use of venous anastomotic flow couplers to monitor buried free DIEP flap reconstructions following nipple-sparing mastectomy. JPRAS Open. 2020; 23:50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.