Abstract
Purpose:
AALL0932 evaluated two randomized maintenance interventions to optimize disease-free survival (DFS) while reducing the burden of therapy in children with newly diagnosed NCI standard-risk (SR) B-acute lymphoblastic leukemia (B-ALL).
Methods:
AALL0932 enrolled 9,229 patients with B-ALL; 2,364 average-risk (AR) patients were randomly assigned (2 × 2 factorial design) at the start of maintenance therapy to vincristine/dexamethasone pulses every 4 (VCR/DEX4) or every 12 (VCR/DEX12) weeks, and a starting dose of weekly oral methotrexate of 20 mg/m2 (MTX20) or 40 mg/m2 (MTX40).
Results:
Five-year event-free survival and overall survival (OS) from enrollment (with 95% CIs), for all eligible and evaluable SR B-ALL patients (n = 9,226), were 92.0% (91.1% and 92.8%) and 96.8% (96.2% and 97.3%), respectively. The 5-year DFS and OS from the start of maintenance for randomly assigned AR patients were 94.6% (93.3% and 95.9%) and 98.5% (97.7% and 99.2%), respectively. The 5-year DFS and OS for patients randomly assigned to receive VCR/DEX4 (n = 1,186) versus VCR/DEX12 (n = 1,178) were 94.1% (92.2% and 96.0%) and 98.3% (97.2% and 99.4%) v 95.1% (93.3% and 96.9%) and 98.6% (97.7% and 99.6%), respectively (P = .86 and .69). The 5-year DFS and OS for AR patients randomly assigned to receive MTX20 versus MTX40 were 95.1% (93.3% and 96.8%) and 98.8% (97.9% and 99.7%) v 94.2% (92.2% and 96.1%) and 98.1% (97.0% and 99.2%), respectively (P = .92 and .89).
Conclusions:
The 0NCI-SR AR B-ALL who received VCR/DEX12 had outstanding outcomes despite receiving one third of the vincristine/dexamethasone pulses previously used as standard of care on Children's Oncology Group (COG) trials. The higher starting dose of MTX of 40 mg/m2/week did not improve outcomes when compared with 20 mg/m2/week. The decreased frequency of vincristine/dexamethasone pulses has been incorporated into frontline COG B-ALL trials to decrease the burden of therapy for patients and their families.
INTRODUCTION
Acute lymphoblastic leukemia (ALL) is the most common childhood malignancy.1 NCI standard-risk (SR) B-acute lymphoblastic leukemia (B-ALL) patients comprise approximately 55% of children with ALL, and those treated on Children's Oncology Group (COG) regimens between 2000 and 2005 achieved 5-year overall survival (OS) rates of 95.0% ± 0.4%.1 However, treatment-related morbidity can extend into adulthood.2
CONTEXT
Key Objective
Can maintenance therapy in patients with newly diagnosed National Cancer Institute (NCI) standard-risk (SR) B-acute lymphoblastic leukemia (B-ALL) be optimized to improve disease-free survival (DFS) and reduce the burden of therapy?
Knowledge Generated
In the context of modern Children's Oncology Group (COG) therapy, vincristine/dexamethasone pulses administered every 12 weeks demonstrated excellent outcomes. Increasing the starting dose of oral methotrexate from 20 to 40 mg/m2/wk in maintenance did not improve DFS.
Relevance
Excellent treatment outcomes in NCI SR B-ALL have been achieved with a decreased frequency of vincristine/dexamethasone pulses while decreasing the burden of therapy. COG has adopted this change in new B-ALL trials.
Historically, COG B-ALL therapy has included every 4-week vincristine/corticosteroid (steroid) pulses during maintenance. CCG-161, which enrolled patients from 1978 to 1983 and used less intensive premaintenance therapy than given today, showed improved event-free survival (EFS) in lower-risk patients randomly assigned to receive monthly vincristine/prednisone pulses.3 Contemporary therapy is more effective, and current risk classification based on leukemia cytogenetics and minimal residual disease (MRD) defines a more rigorous SR population.4 CCG-1891 demonstrated no advantage to every 3-week versus every 4-week maintenance vincristine/prednisone pulses in intermediate risk B-ALL patients treated with a single delayed intensification (DI) phase.5 A prospective trial conducted between 1995 and 2000 showed that intermediate-risk patients did not benefit from six versus no vincristine/dexamethasone maintenance pulses (each containing two vincristine doses) on a Berlin-Frankfurt-Muenster (BFM) backbone.6 By contrast, the European Organization for Research and Treatment of Cancer (EORTC) 58951 study showed superior outcomes in patients with average-risk (AR) B-ALL and non-Hodgkin lymphoma randomly assigned to receive six maintenance vincristine/steroid pulses with a 6-year disease-free survival (DFS) of 90.6% ± 2.1% versus 82.8% ± 2.8% (P = .027) with no pulses. Interestingly, some of the EORTC patients were included in the above BFM trial that showed no benefit to pulses in a much larger patient cohort.7 A Childhood ALL Collaborative Group meta-analysis demonstrated improved EFS with vincristine/prednisone pulses but not vincristine/dexamethasone pulses; however, neither improved OS.8 Given studies showing that dexamethasone was more effective than prednisone, the lack of benefit of vincristine/dexamethasone pulses was presumed to be related to the more intense premaintenance chemotherapy rather than the steroid used.8,9 Intensified premaintenance therapy, refinements in genetic and MRD-based risk stratification, and better supportive care have improved outcomes on COG ALL trials,1 making the optimal frequency of maintenance pulses in modern ALL therapy uncertain.
Maintenance therapy is based on antimetabolites including daily 6-mercaptopurine (6-MP) and weekly methotrexate.10-12 Methotrexate is usually administered orally, but is sometimes given intravenously or subcutaneously.13,14 Incomplete absorption occurs at all dose levels, with absorption saturation occurring at oral doses of approximately 40 mg/m2, suggesting that higher oral doses might deliver more methotrexate.15,16 This may enhance efficacy, either directly or through xanthine oxidase inhibition, which may decrease the first-pass effect on oral 6-MP and increase bioavailability.17-21 Weekly methotrexate 40 mg/m2 has been given intravenously and orally without excessive toxicity.14,22
COG ALL trials include daily 6-MP and weekly methotrexate during maintenance, with dosing guidelines designed to minimize severe myelosuppression and infectious complications because of the large intrapatient variability in drug absorption and metabolism, and genetic polymorphisms in TPMT or NUDT15, that increase phosphorylated 6-MP metabolites.23,24 Titrating doses based on maintenance absolute neutrophil (ANC) and platelet counts potentially maximizes antileukemic effect and reduces relapse.24-27 Poor compliance with maintenance therapy is associated with a 2.5-fold increased risk of relapse, highlighting the importance of optimizing maintenance chemotherapy.23,28
Following a three-drug induction, COG AALL0932 risk-stratified patients with SR B-ALL and those classified as AR then received a low-intensity 4-week consolidation phase without high-dose methotrexate or asparaginase intensification, and two interim maintenance phases separated by a single DI. Patients were then eligible for two maintenance random assignments examining the efficacy of reduced frequency of vincristine/dexamethasone pulses and starting oral methotrexate doses of 40 versus 20 mg/m2.
METHODS
Patients
Eligible B-ALL patients had newly diagnosed NCI SR B-ALL (age 1-9.99 years and initial WBC < 50,000/µL).29 Patients with CNS3 (≥ 5 WBC/µL CSF with blasts), testicular leukemia, or prior cytotoxic chemotherapy were excluded.
Institutional review board approval was obtained at participating institutions prior to patient enrollment. Written informed consent and assent (where appropriate) were obtained from each patient and their parent/guardian before starting therapy. AALL0932 was registered at ClinicalTrials.gov identifier NCT01190930.
Study Design
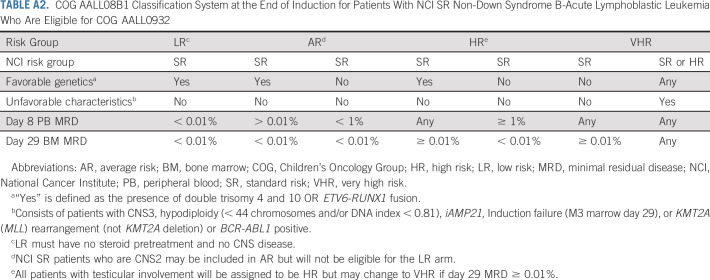
This phase III multicenter, randomized trial opened to accrual in August 2010. Enrollment on AALL08B1 (ClinicalTrials.gov identifier: NCT01142427), the risk-classification trial, was required. Patients received a three-drug induction (Appendix Table A1, online only).30 Patients without Down syndrome (DS) and who were Philadelphia chromosome (Ph)–negative were risk stratified at the end of induction (EOI) into four risk groups: low risk (LR), AR, high risk, or very high risk (VHR) based on induction day 8 peripheral blood (PB) flow cytometry-based MRD, day 29 bone marrow (BM) MRD, blast genetics, CNS status, and steroid pretreatment (Appendix Table A2). DS patients were classified as SR-DS or high-risk DS based on similar factors (Appendix Table A3). Ph-positive patients were not eligible for postinduction therapy. Favorable genetics included simultaneous trisomies of chromosome 4 and 10 (double trisomy; DT) or ETV6/RUNX1 fusion. Unfavorable genetics were intrachromosomal amplification of chromosome 21, KMT2A (MLL) rearrangements, or hypodiploidy (< 44 chromosomes and/or DNA index < 0.81). This report focuses on the primary objectives in AR SR B-ALL patients to determine (i) if vincristine/dexamethasone pulses could be given every 12 weeks versus every 4 weeks without adversely impacting DFS; and (ii) if a starting maintenance oral methotrexate dose of 40 mg/m2/week would improve DFS compared with 20 mg/m2/week.
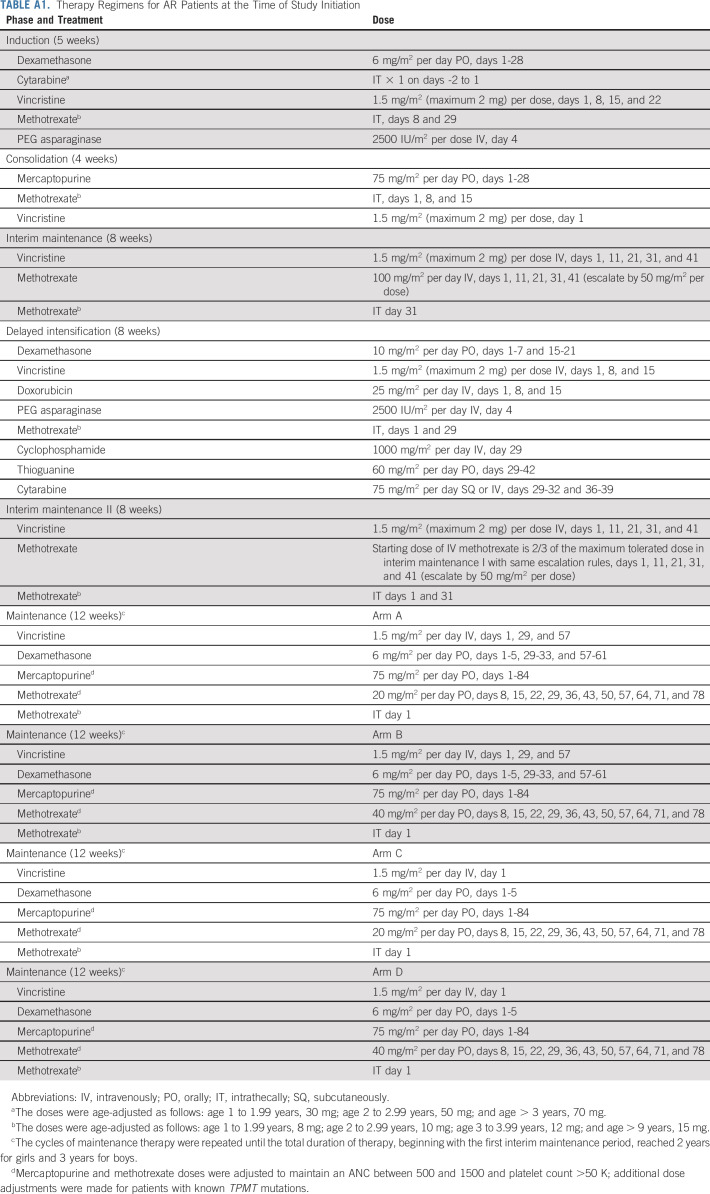
AR patients had day 29 BM MRD < 0.01% and either favorable genetics (without unfavorable genetics) and day 8 PB MRD ≥ 0.01%; steroid pretreatment; CNS2 (CSF WBC < 5/µL with blasts) status; or neutral cytogenetics with day 8 PB MRD < 1%. After completing premaintenance therapy (Fig 1), AR patients were randomly assigned using a 2 × 2 factorial design to one of four maintenance regimens as follows: (arm A) vincristine/dexamethasone pulses every 4 weeks (VCR/DEX4), and starting oral methotrexate dose 20 mg/m2/week (MTX20); (arm B) every 4 week pulses and methotrexate 40 mg/m2/week (MTX40); (arm C) every 12 week pulses and methotrexate 20 mg/m2/week; (arm D) every 12 week pulses and methotrexate 40 mg/m2/week. All arms included intrathecal methotrexate every 12 weeks, with oral methotrexate held that week. Maintenance cycles were repeated until completion of therapy, which was two (girls) or three (boys) years from starting interim maintenance I (Appendix Table A1). AALL0932 included guidelines (Data Supplement) for adjusting 6-MP and methotrexate based on ANC and platelet count, and TPMT and NUDT15 genotypes (incorporated mid-trial), which increased or decreased doses of 6-MP and methotrexate to target ANC 750-1,500/µL and platelets > 75,000/µL.
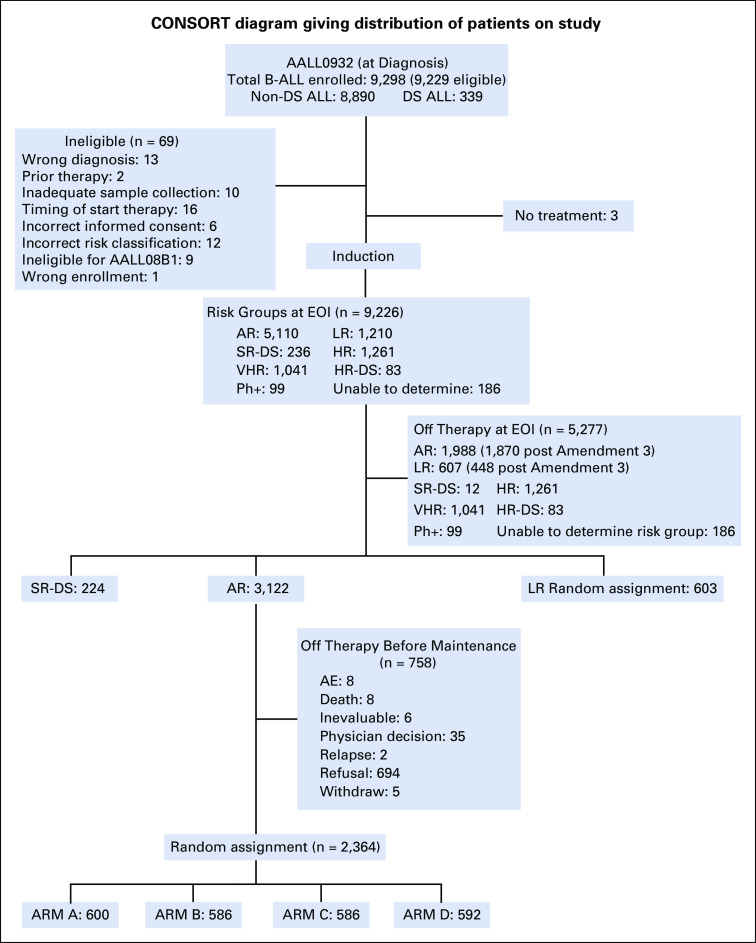
FIG 1.
CONSORT diagram for Children's Oncology Group AALL0932. AR, average risk; B-ALL, B-acute lymphoblastic leukemia; DS, Down syndrome; HR, hazard ratio; LR, low risk; Ph, Philadelphia chromosome; SR, standard risk; VHR, very high risk.
In January 2017, the Data Safety Monitoring Committee determined that a futility boundary had been crossed and that the study could not demonstrate that a 40 mg/m2/week methotrexate starting dose was superior to 20 mg/m2/week. Thus, all patients randomly assigned to MTX40 had their dose lowered to 20 mg/m2/week, the standard of care, and 6-MP and methotrexate doses were subsequently adjusted based on tolerability following protocol guidelines.
Statistical Analysis
Study data frozen from June 30, 2019, are included in this report. For the overall NCI SR B-ALL cohort, EFS and OS were calculated from study enrollment to first event (EFS: induction failure, relapse, second malignant neoplasm, or remission death; OS: death) or censored at last follow-up. For randomly assigned patients, DFS and OS were calculated from random assignment (start of maintenance) to first event (for DFS: relapse, second malignant neoplasm, or remission death) or censored at last follow-up. Median follow-up was calculated using the reverse Kaplan-Meier method.31 Survival rates were estimated by the method of Kaplan-Meier32 with 95% CIs calculated using SEs of Peto et al.33 The methotrexate dosing random assignment was powered (93% at one-sided α = .05) to detect a difference in 5-year DFS from 93% v 96% for the MTX20 and MTX40 groups, respectively. The VCR/DEX pulse frequency random assignment was powered (90% at one-sided α = .10) to detect a difference in 5-year DFS from 93% v 90% for VCR/DEX4 versus VCR/DEX every 12 weeks (VCR/DEX12), respectively. Both random assignments had interim monitoring for efficacy using an α*t2 spending function, and futility monitoring for the methotrexate dosing question was based on the method of Fleming et al34 with first interim analysis scheduled when 20% of expected DFS events were observed. A Cox proportional hazard model was fit to test for a possible interaction between the methotrexate dose and vincristine/dexamethasone pulse frequency random assignments using a two-sided 0.05 significance level threshold. Toxicities were graded according to NCI Common Toxicity Criteria Version 4 with targeted toxicity rates summarized by treatment arm and therapy period. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). Graphics were generated with R Version 3.0.1.34a
RESULTS
Patient Characteristics
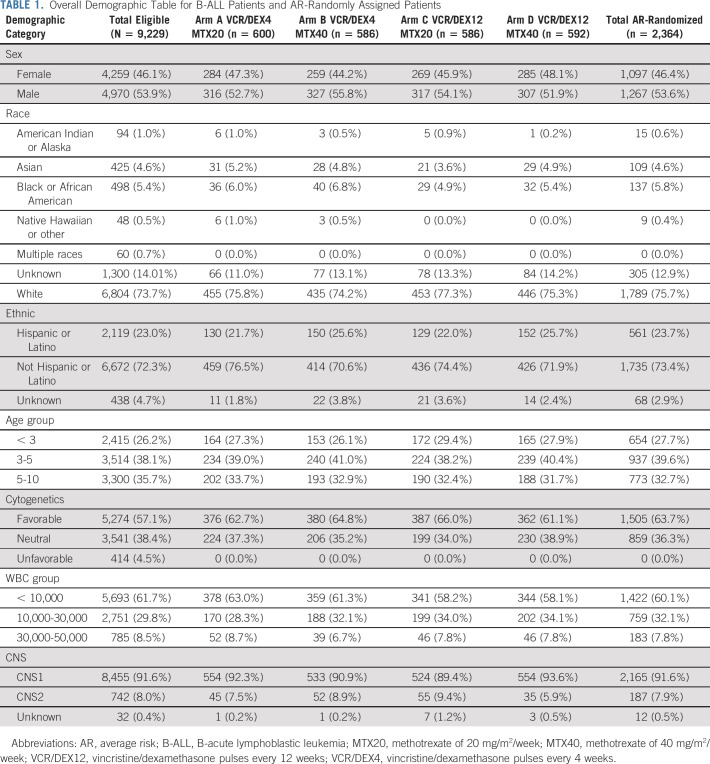
AALL0932 enrolled 9,298 B-ALL patients from 224 institutions between August 2010 and March 2018. Sixty-nine were ineligible because of incorrect risk classification (12), inadequate sample collection (10), incorrect informed consent (6), ineligibility for the AALL08B1 (9), prior therapy (2), timing of enrollment/start of therapy (16), wrong diagnosis (13), or wrong enrollment (1). Three of 9,229 eligible SR ALL patients did not receive any induction treatment and were deemed unevaluable. After completing induction, 1,210 patients were risk-classified as LR and 236 as SR-DS and will be reported separately. One thousand, two hundred and sixty-one patients were risk classified as high risk, 83 as high-risk DS, 1,041 as VHR, 99 as Ph-positive, and 186 as risk group unable to be determined, primarily because of lack of required MRD or cytogenetic data, and came off protocol therapy (Fig 1). Five thousand, one hundred and ten patients were classified as AR; 1,870 of these came off protocol therapy at the EOI because AR random assignment accrual goals had been met. An additional 118 patients were removed at EOI for other reasons including refusal or withdrawal of consent (n = 73) and physician determines it is in patient's best interest (n = 33). Those AR patients still on protocol therapy were approached before maintenance to consent to the AR maintenance random assignment, with 2,364 randomly assigned to one of the four maintenance arms: A [VCR/DEX4, MTX20] (n = 600), B [VCR/DEX4, MTX40] (n = 586), C [VCR/DEX12, MTX20] (n = 586), or D [VCR/DEX12, MTX40] (n = 592) (Fig 1). Consistent with the predicted 25% estimation of random assignment dropout, 22.3% of parents declined to participate in the maintenance random assignment. The 5-year DFS (P = .8291) and OS (P = .5201) of patients who declined random assignment were similar to those who participated. Presenting features of the four groups were similar (Table 1).
TABLE 1.
Overall Demographic Table for B-ALL Patients and AR-Randomly Assigned Patients
Treatment Outcomes
The 5-year EFS (CI) and OS from enrollment for the 9,226 eligible B-ALL patients were 92.0% (91.1% and 92.8%) and 96.8% (96.2% and 97.3%), respectively (Fig 2A). There was a significant difference in EFS and OS between boys and girls; 5-year EFS (hazard ratio [HR], 1.28 [1.08, 1.51], P = .003) and OS (HR, 1.38 [1.06, 1.80], P = .015) in boys were 91.2% (90.0% and 92.5%) and 96.4 (95.5% and 97.2%) v 92.8% (91.6% and 94.0%) and 97.2% (96.4% and 98.0%) in girls, respectively (Appendix Fig A1). The induction death and failure (M3) rates were 0.37% and 0.12%, respectively.
FIG 2.
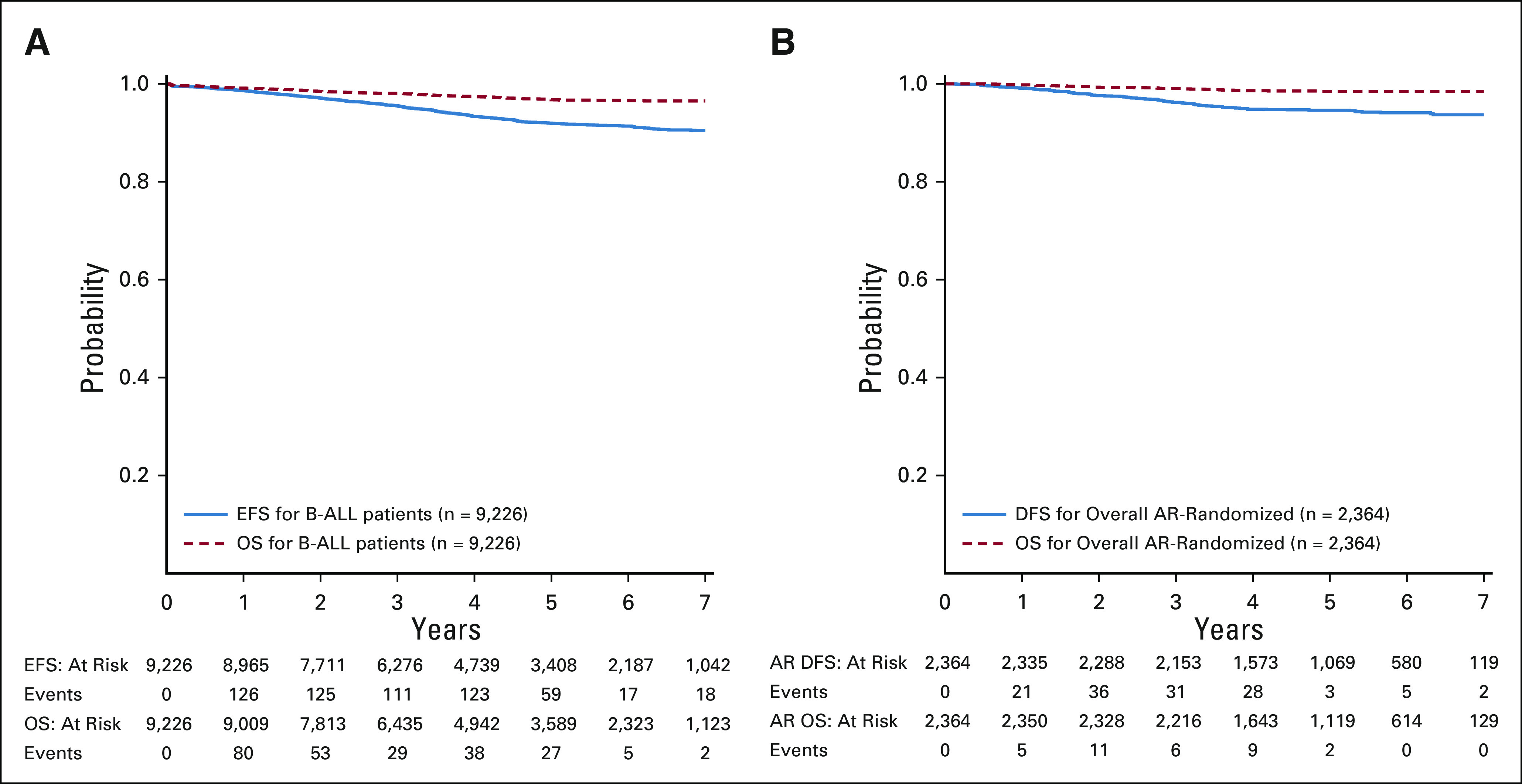
(A) Event-free survival (EFS) and overall survival (OS) from enrollment for all eligible B-ALL patients; (B) disease-free survival (DFS) and OS from start of maintenance for the randomly assigned average-risk patients. B-ALL, B-acute lymphoblastic leukemia.
The 5-year DFS and OS from the start of maintenance for the 2,364 randomly assigned AR patients were 94.6% (93.3% and 95.9%) and 98.5% (97.7% and 99.2%), respectively, with a median follow-up of 4.8 years (Fig 2B). There was no difference in outcome by sex; 5-year DFS (HR, 1.20 [0.84, 1.70], P = .31) and OS (HR, 1.32 [0.68, 2.65], P = .43) in boys were 94.2% (92.3% and 96.1%) and 98.3% (97.2% and 99.3%) versus 95.1% (93.3% and 96.9%) and 98.7% (97.7% and 99.7%) in girls, respectively (Appendix Fig A2).
Results of Randomized Maintenance Therapy Questions
Five-year DFS for AR patients randomly assigned to receive vincristine/dexamethasone pulses every 12 (n = 1,178) versus every 4 weeks (n = 1,186) was 95.1% (93.3% and 96.9%) and 94.1% (92.2% and 96.0%), respectively (HR, 0.83 [0.58, 1.17], P = .86; Fig 3A) and 5-year OS was 98.6% (97.7% and 99.6%) and 98.3% (97.2% and 99.4%), respectively (HR, 0.84 [0.42, 1.67], P = .69; Fig 3B).
FIG 3.
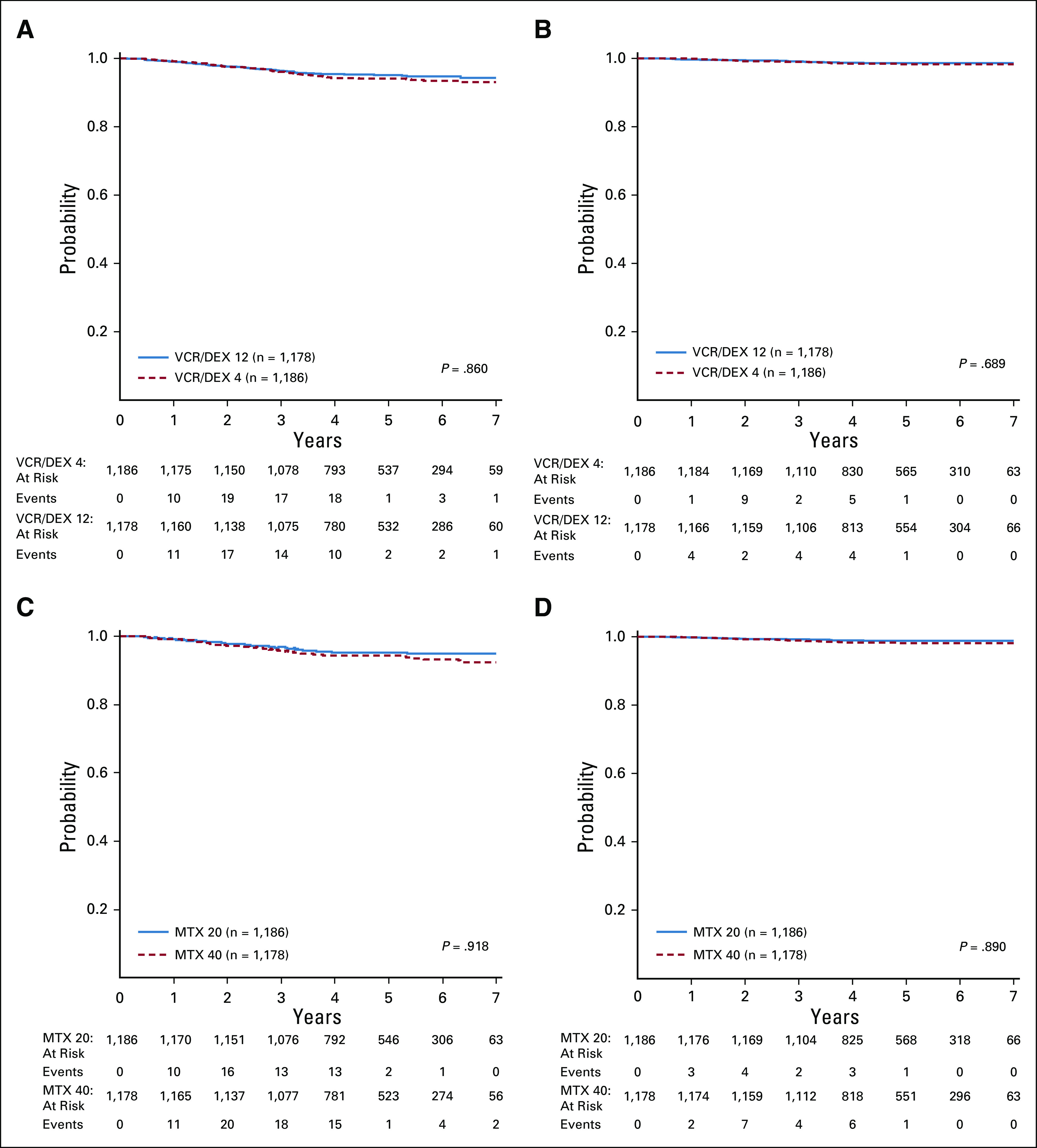
(A) Disease-free survival (DFS) for the average-risk (AR) subset of patients randomly assigned to receive VCR/DEX12 v VCR/DEX4; (B) overall survival (OS) for AR patients randomly assigned to receive VCR/DEX12 v VCR/DEX4; (C) DFS for the AR subset of patients randomly assigned to receive MTX20 v MTX40; (D) OS for the AR subset of patients randomly assigned to receive MTX20 v MTX40. MTX20, methotrexate of 20 mg/m2; MTX40; methotrexate of 40 mg/m2; VCR/DEX12, vincristine/dexamethasone pulses every 12 weeks; VCR/DEX4, vincristine/dexamethasone pulses every 4 weeks.
The 5-year DFS was 95.1% (93.3% and 96.8%) for MTX20 and 94.2% (92.2% and 96.1%) (HR, 0.78 [0.55, 1.11], P = .92; Fig 3C) for MTX40, with a 5-year OS of 98.8% (97.9% and 99.7%) and 98.1% (97.0% and 99.2%), respectively (HR, 0.65 [0.32, 1.30], P = .89; Fig 3D).
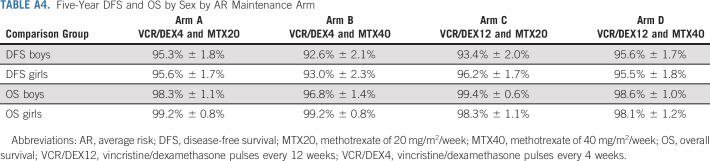
Five-year DFS for patients on maintenance arms A, B, C, and D was 95.4% (93.0% and 97.8%), 92.8% (89.7% and 95.8%), 94.7% (92.1% and 97.3%), and 95.6% (93.1% and 98.0%), respectively (P = .06; Fig 4A), and the 5-year OS was 98.7% (97.4% and 100%), 97.9% (96.2% and 99.5%), 98.9% (97.7% and 100%), and 98.4% (96.9% and 99.9%), respectively (P = .61; Fig 4B). The 5-year DFS and OS by sex were comparable for the four maintenance regimens (Appendix Table A4). Per trial design, a Cox proportional hazard model was used to examine possible significant interaction between the methotrexate starting dose and vincristine/dexamethasone pulse frequency random assignments. The result of P = .056, based on the prespecified criteria, indicated a lack of evidence; the dose level effect depends on pulse frequency and, conversely, that the pulse frequency effect depends on dose level. Exploratory simple effect comparisons (using contrasts in the Cox model) were made comparing factor levels controlling for the other factor and showed that arm B (MTX40 and VCR/DEX4) could have higher risk than arm A (MTX20 and VCR/DEX4; HR, 1.76 [1.09, 2.86], P = .02), and arm B (MTX40 and VCR/DEX4) could have higher risk than arm D (MTX40 and VCR/DEX12; HR, 1.66 [1.03, 2.68], P = .04). This is not clinically relevant as the higher methotrexate dose provided no advantage.
FIG 4.
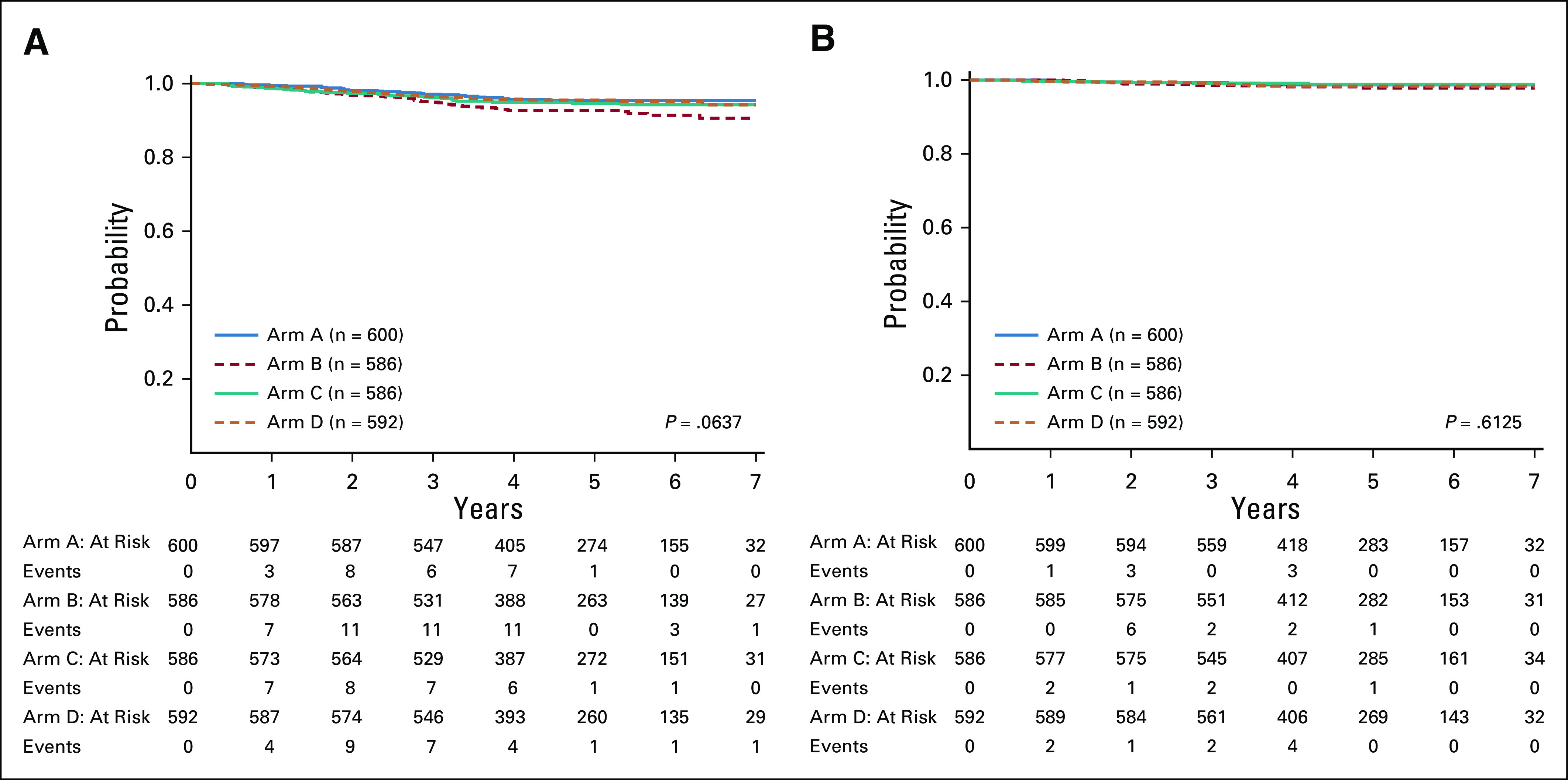
(A) Disease-free survival and (B) overall survival by average risk maintenance arm.
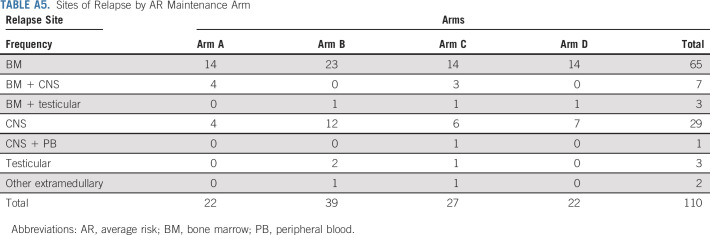
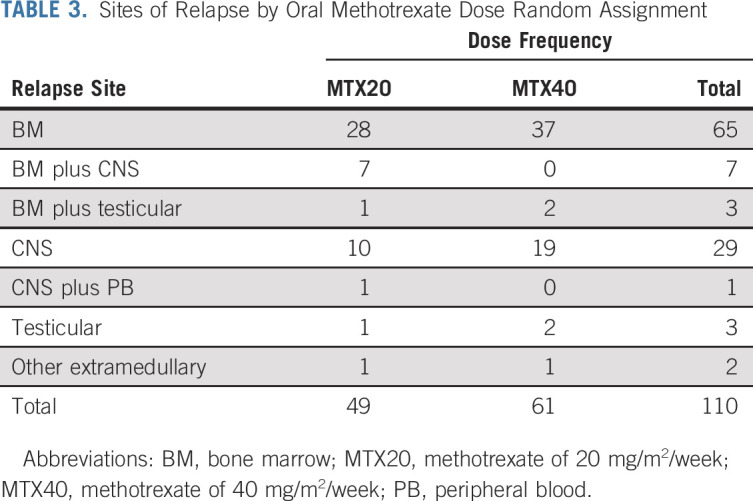
Sites of relapse are shown in Tables 2 and 3 and in the Appendix Table A5. There was no significant difference noted in the cumulative incidence of relapse by random assignments (Appendix Fig A3).
TABLE 2.
Sites of Relapse by Pulse Frequency Random Assignment
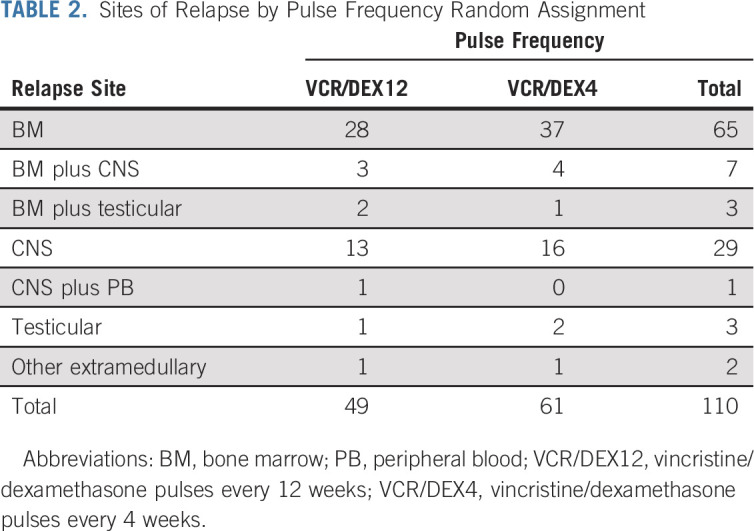
TABLE 3.
Sites of Relapse by Oral Methotrexate Dose Random Assignment
Adverse Events
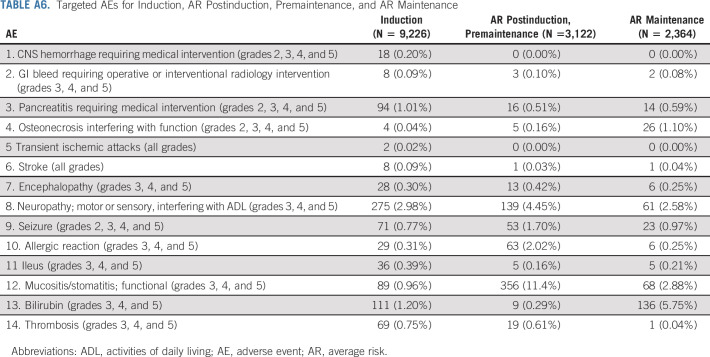
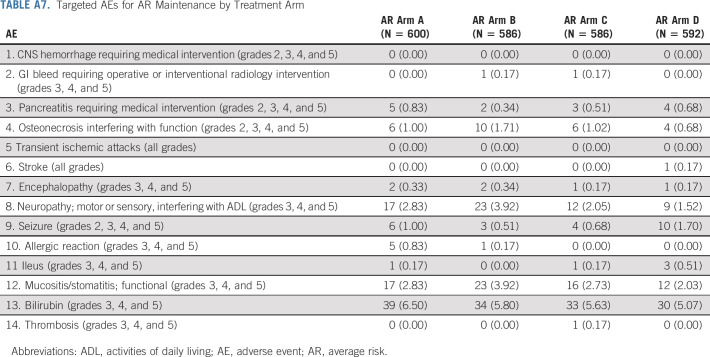
Therapy was well tolerated. The incidences of targeted toxicities were at expected rates (Appendix Tables A6 and A7). No differences were observed in targeted toxicities associated with vincristine (motor or sensory neuropathy, ileus) or methotrexate (mucositis/stomatitis, or elevated bilirubin) between maintenance arms. However, the rate of neuropathy was lower for patients receiving pulses every 12 v every 4 weeks, 1.8% v 3.4%, P = .015, respectively.
DISCUSSION
AALL0932 sought to improve outcomes and reduce toxicity and burden of therapy by optimizing maintenance therapy for the AR subset of NCI SR B-ALL, defined using a combination of NCI risk group, sentinel somatic genetic lesions, clinical variables, and early treatment response. These AR patients had projected 5-year DFS rates of 90%-95% at the time of study design, and AALL0932 demonstrated outstanding outcomes with vincristine/dexamethasone maintenance pulses given every 12 weeks, with 5-year DFS/OS rates of 95.1% and 98.6%. AALL0932 was not designed as a classic noninferiority trial, which would have required many more years to complete, but the lower 5-year 95% confidence limits of 93.3% (DFS) and 97.7% (OS) with every 12 week pulses were well within prestudy expectations. Our data do not address whether any maintenance pulses are needed. The AALL0932-reduced vincristine/dexamethasone pulses arm still provides more vincristine/steroid exposure than the plus-pulses arm of the BFM prospective meta-analysis study, which showed that six vincristine/steroid pulses in maintenance did not improve the outcome. However, that study used the BFM backbone, which included much more intensive premaintenance therapy with a four-drug induction, intensive Ib consolidation phase, and four courses of high-dose methotrexate.6,7
Reducing maintenance vincristine/dexamethasone pulses by two thirds lessens the burden of therapy and may translate to improved quality of life. Glucocorticoids cause significant toxicities: myalgias, myopathies, infections, hyperglycemia, osteonecrosis, obesity, metabolic sequelae, adrenal axis suppression, and neurocognitive late effects.35-38 Moreover, steroid exposure increases emotional lability and disruptive behaviors leading to missed days of school or daycare by patients and work by parents.39,40 Vincristine is also associated with declines in fine motor and sensory-perceptual performance.41,42 Early data regarding quality-of-life outcomes for AALL0932 have been reported, and longer-term data will be described separately.43
Because methotrexate doses of 40 mg/m2 were well tolerated in previous trials, AALL0932 also explored the optimal starting dose of oral methotrexate during maintenance based on its poor oral bioavailability and known interpatient variability in plasma methotrexate levels following oral doses.14-17,19,44 However, a futility boundary was crossed in January 2017, and patients who had been randomly assigned to receive a starting methotrexate dose of 40 mg/m2 had their dose lowered to 20 mg/m2/week, the standard of care therapy, and their doses of 6-MP and MTX were adjusted based on tolerability as per protocol guidelines for all arms. These data were confirmed with analysis of mature data, with 5-year DFS of 95.1% and 94.2% (P = .92), and 5-year OS of 98.8% and 98.1% (P = .89) for those receiving MTX20 and MTX40, respectively.
To investigate potential confounding impacts of the increased starting dose of oral MTX, we reviewed detailed data regarding cumulative maintenance doses of 6-MP and MTX administered by arm to assess whether the higher methotrexate dose might have led to lower doses of 6-MP or more frequent holding of oral chemotherapy, which per protocol guidelines occurred when ANC was < 500/µL or platelet count was < 50,000/µL. A comparison of 6-MP dosing showed nearly identical median doses administered on the MTX20 and MTX40 arms. For example, in the first month of maintenance cycle four, patients on MTX20 arms received a median (interquartile range [IQR]) of 95.2% (67.2%, 101.6%) of protocol 6-MP dosing compared with a median IQR of 95.2% (64.9%, 101.7%) on the MTX40 arms. As expected, the methotrexate dose administered on MTX40 arms was approximately double of that given on the MTX20 arms. However, the number of days chemotherapy was held was not collected; based on similar cumulative doses across arms, it seems unlikely that there were more interruptions in therapy on the MTX40 arms than on the MTX20 arms. As these doses were patient- or parent-reported and not confirmed by pill counts or MEMS cap, it is also possible that patients on the MTX40 arm were less compliant with oral chemotherapy.
In summary, AALL0932 achieved outstanding outcomes with a 5-year EFS of 92.0% and an OS of 96.8% > 9,000 SR B-ALL patients. The AR patients had a 5-year DFS of 94.6% and an OS of 98.5% from the time of random assignment, and outcomes with maintenance vincristine/dexamethasone pulses given every 12 weeks were not proven to be inferior to outcomes with pulses given every 4 weeks. Because the study was not designed as a noninferiority trial, it was not demonstrated statistically that decreased pulse frequency resulted in outcomes as good as those with the every 4 weeks group. In addition, a higher starting dose of maintenance oral methotrexate did not improve DFS. Together, these results establish that in the modern era, a relatively low-intensity premaintenance backbone with a three-drug induction, no intensive 1b phase, and no high-dose methotrexate results in outstanding outcomes and that additional intensifications of traditional maintenance cytotoxic chemotherapy do not improve outcome. Based on these results and those of the BFM with no maintenance pulses,6 COG B-ALL trials now use every 12 week vincristine/steroid pulses during maintenance, with efforts to improve survival focused on treatment interventions prior to starting maintenance therapy.
ACKNOWLEDGEMENT
We thank all the patients and their families as well as the participating institutions and study staff.
Appendix
TABLE A1.
Therapy Regimens for AR Patients at the Time of Study Initiation
TABLE A2.
COG AALL08B1 Classification System at the End of Induction for Patients With NCI SR Non-Down Syndrome B-Acute Lymphoblastic Leukemia Who Are Eligible for COG AALL0932
TABLE A3.
COG AALL08B1 Classification System at the End of Induction for Patients With NCI SR-DS Who Are Eligible for COG AALL0932
TABLE A4.
Five-Year DFS and OS by Sex by AR Maintenance Arm
TABLE A5.
Sites of Relapse by AR Maintenance Arm
TABLE A6.
Targeted Adverse Events for Induction, AR Postinduction, Premaintenance, and AR Maintenance
TABLE A7.
Targeted Adverse Events for AR Maintenance by Treatment Arm
FIGURE A1.
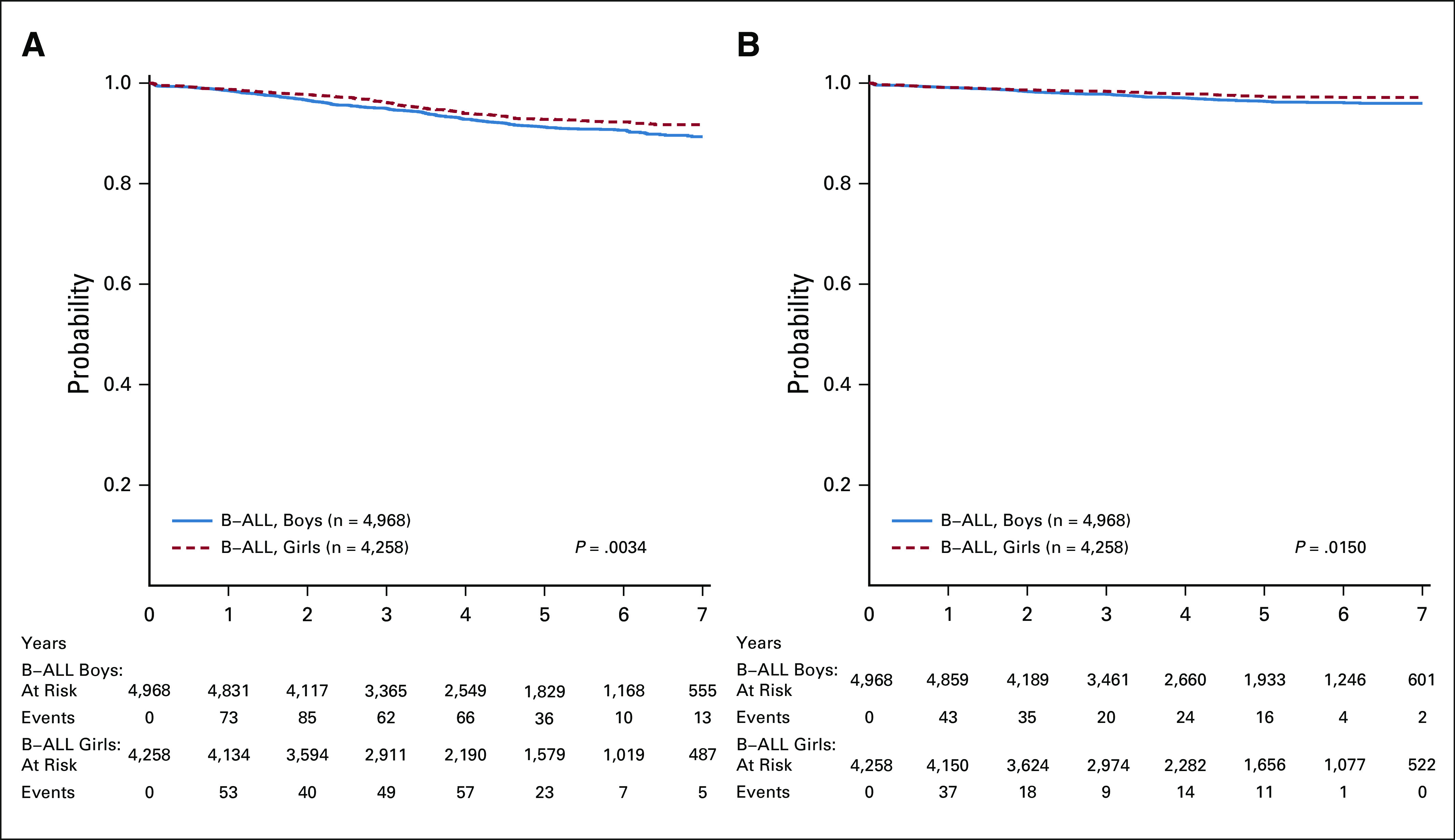
(A) EFS and (B) OS from enrollment for eligible B-ALL patients by sex. (B) OS from enrollment for the eligible B-ALL patients by sex. B-ALL, B-acute lymphoblastic leukemia; EFS, event-free survival; OS, overall survival.
FIGURE A2.
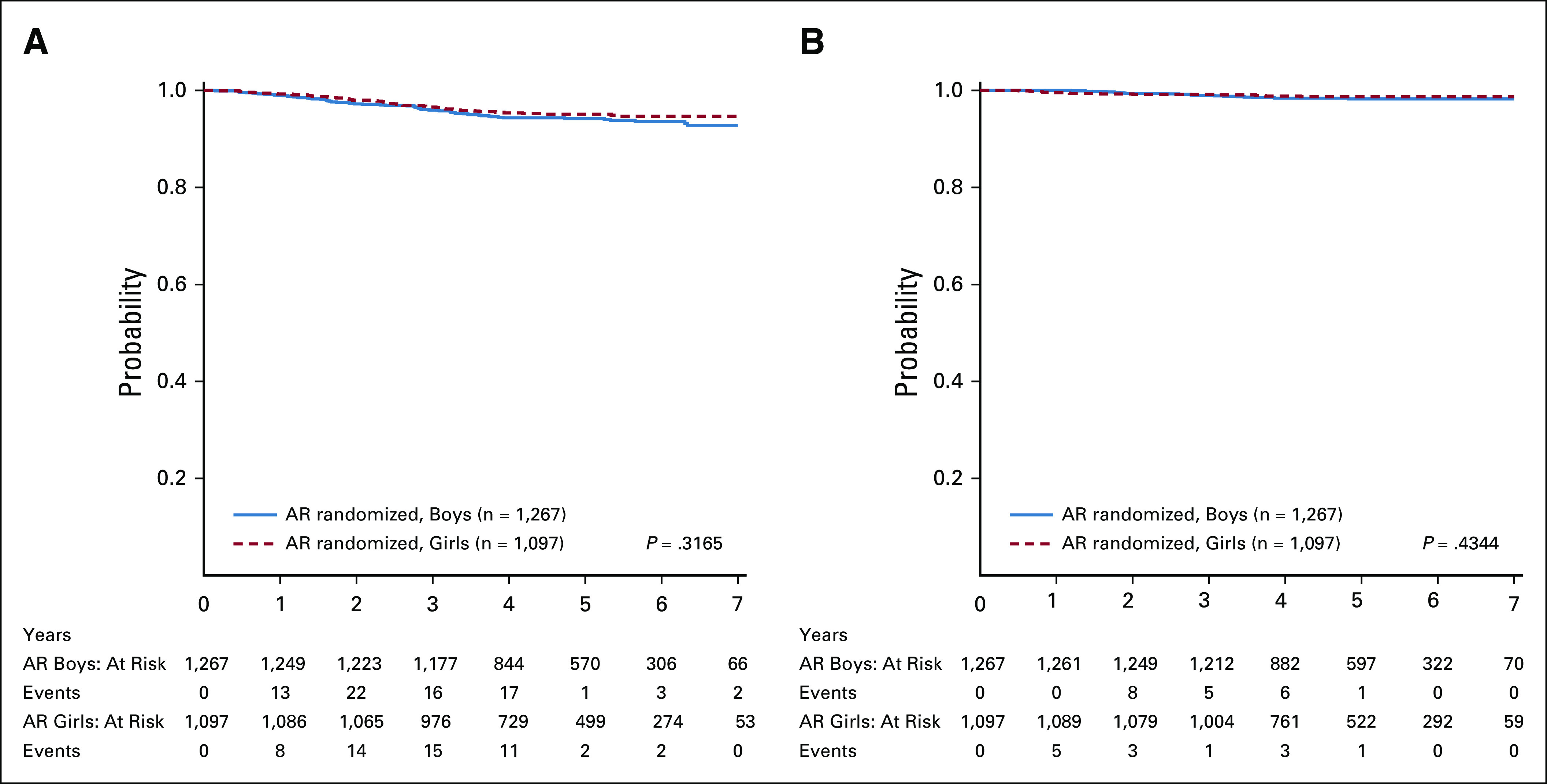
(A) DFS from the start of maintenance for the randomly assigned AR patients by sex. (B) OS from the start of maintenance for the randomly assigned AR patients by sex. AR, average risk; DFS, disease-free survival; OS, overall survival.
FIGURE A3.
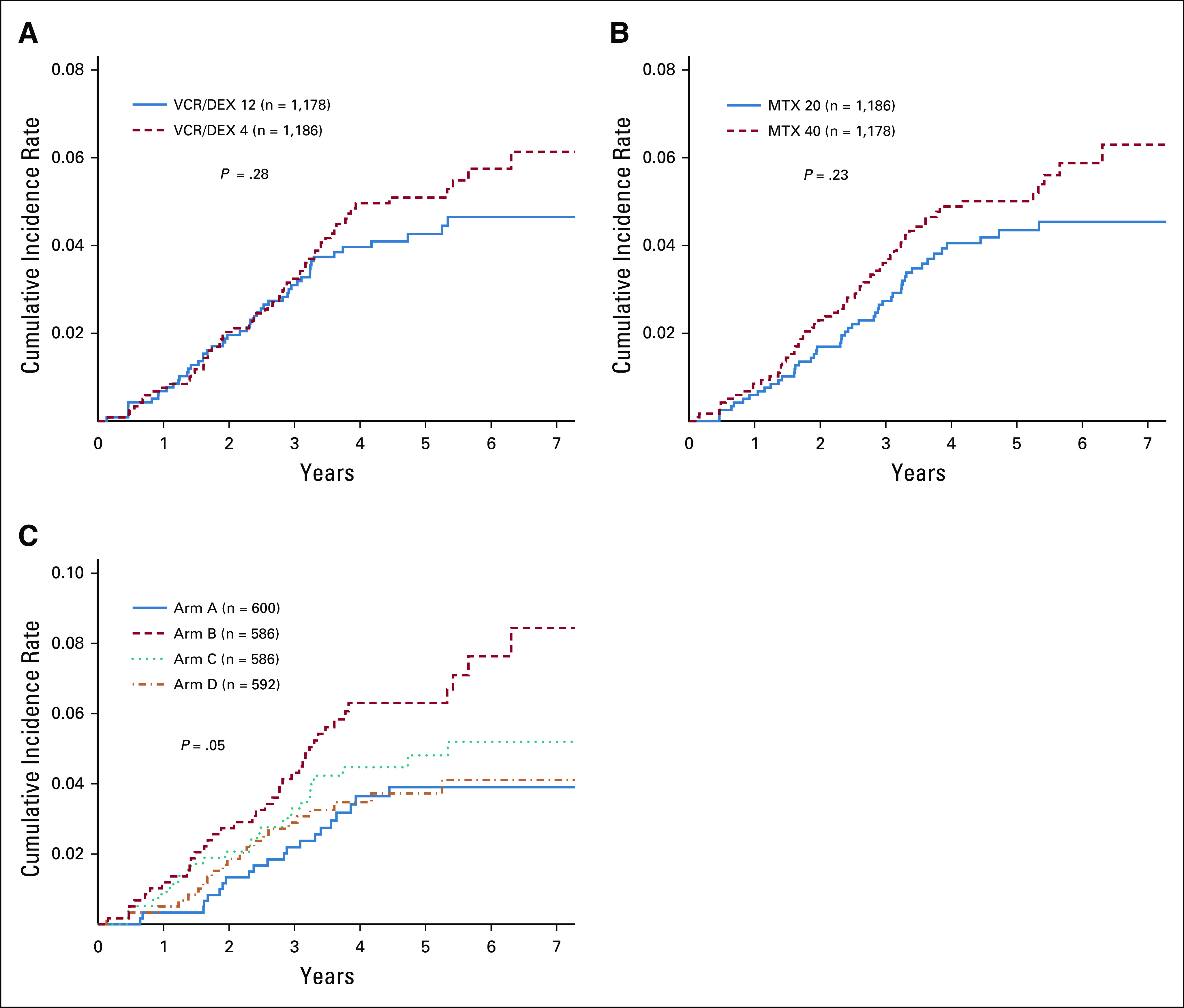
(A) Cumulative incidence of all relapses as first events by pulse frequency randomization. (B) Cumulative incidence of all relapses as first events by oral methotrexate dose random assignment. (C) Cumulative incidence of all relapses as first events by AR maintenance arm. AR, average risk.
Anne L. Angiolillo
Honoraria: Servier Pharmaceuticals
Travel, Accommodations, Expenses: Servier Pharmaceuticals
Reuven J. Schore
Research Funding: Janssen Research & Development, Amgen
John A. Kairalla
Stock and Other Ownership Interests: Johnson & Johnson
Meenakshi Devidas
Honoraria: PSI, Novartis
Patrick Zweidler-McKay
Stock and Other Ownership Interests: Immunogen
Michael J. Borowitz
Consulting or Advisory Role: Amgen
Research Funding: Becton Dickinson
Travel, Accommodations, Expenses: Beckman Coulter
Brent Wood
Honoraria: Amgen, Seattle Genetics, Abbvie, Janssen, Astellas Pharma
Consulting or Advisory Role: Sysmex
Research Funding: Amgen, Seattle Genetics, Pfizer, Juno Therapeutics, BiolineRx, Biosight, Stemline Therapeutics, Janssen Oncology, Novartis
Travel, Accommodations, Expenses: Amgen
Mary V. Relling
Research Funding: Servier Pharmaceutical
Elizabeth A. Raetz
Research Funding: Pfizer
Other Relationship: Celgene
Mignon L. Loh
Consulting or Advisory Role: MediSix Therapeutics
Stephen P. Hunger
Stock and Other Ownership Interests: Amgen, Merck
Honoraria: Amgen
Consulting or Advisory Role: Novartis
No other potential conflicts of interest were reported.
SUPPORT
Supported by grants from National Cancer Institute (NCI) grants to the Children's Oncology Group (U10 CA98543, U10 CA98413, U10 CA 180886, and U10 CA 180899) and by research funding from St. Baldrick's Foundation and BD Biosciences, and U24 CA114766 (COG Specimen Banking), and Human Specimen Banking in NCI-Sponsored Clinical Trials (1U24-CA196173).
PRIOR PRESENTATION
Presented in part at the 61st Annual Meeting of the American Society of Hematology, Orlando, FL, December 7-10, 2019.
CLINICAL TRIAL INFORMATION
NCT01190930 (AALL0932)
A.L.A. and R.J.S. contributed equally as first authors. E.A.R., M.L.L., and S.P.H. contributed equally as senior authors.
DISCLAIMERS
A.L.A. has been on an advisory board and received honoraria from Shire Pharmaceuticals and Servier Pharmaceuticals. R.J.S. has been on an advisory board and received honoraria from Baxalta Pharmaceuticals and Shire Pharmaceuticals. E.A.R. has received research funding from Pfizer. M.L.L. has received research funding from Pfizer and honoraria for MediSix Therapeutics. S.P.H. has received honoraria from Amgen, Jazz Pharmaceuticals, Sigma Tau Pharmaceuticals, Spectrum Pharmaceuticals, and Erytech, and consulting fees from Novartis. M.L.L. is the Benioff Chair of Children's Health and the Deborah and Arthur Ablin Professor of Pediatric Molecular Oncology in the Department of Pediatrics, Benioff Children's Hospital, San Francisco, CA. S.P.H. is the Jeffrey E. Perelman Distinguished Chair in the Department of Pediatrics, Children's Hospital of Philadelphia. E.A.R. is the KiDS of NYU Foundation Professor at NYU Langone Health.
AUTHOR CONTRIBUTIONS
Conception and design: Anne L. Angiolillo, Reuven J. Schore, Meenakshi Devidas, Johann Hitzler, Kelly W. Maloney, William L. Carroll, Naomi J. Winick, Elizabeth A. Raetz, Mignon L. Loh, Stephen P. Hunger
Administrative support: Stephen P. Hunger
Collection and assembly of data: Anne L. Angiolillo, Reuven J. Schore, John A. Kairalla, Meenakshi Devidas, Karen R. Rabin, Patrick Zweidler-McKay, Michael J. Borowitz, Brent Wood, Andrew J. Carroll, Nyla A. Heerema, Johann Hitzler, Ashley R. Lane, William L. Carroll, Stephen P. Hunger
Data analysis and interpretation: Anne L. Angiolillo, Reuven J. Schore, John A. Kairalla, Meenakshi Devidas, Michael J. Borowitz, Mary V. Relling, Johann Hitzler, Kelly W. Maloney, Cindy Wang, Mylène Bassal, William L. Carroll, Naomi J. Winick, Mignon L. Loh, Stephen P. Hunger
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Excellent Outcomes With Reduced Frequency of Vincristine and Dexamethasone Pulses in Standard-Risk B-Lymphoblastic Leukemia: Results From Children's Oncology Group (COG) AALL0932
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I =Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Anne L. Angiolillo
Honoraria: Servier Pharmaceuticals
Travel, Accommodations, Expenses: Servier Pharmaceuticals
Reuven J. Schore
Research Funding: Janssen Research & Development, Amgen
John A. Kairalla
Stock and Other Ownership Interests: Johnson & Johnson
Meenakshi Devidas
Honoraria: PSI, Novartis
Patrick Zweidler-McKay
Stock and Other Ownership Interests: Immunogen
Michael J. Borowitz
Consulting or Advisory Role: Amgen
Research Funding: Becton Dickinson
Travel, Accommodations, Expenses: Beckman Coulter
Brent Wood
Honoraria: Amgen, Seattle Genetics, Abbvie, Janssen, Astellas Pharma
Consulting or Advisory Role: Sysmex
Research Funding: Amgen, Seattle Genetics, Pfizer, Juno Therapeutics, BiolineRx, Biosight, Stemline Therapeutics, Janssen Oncology, Novartis
Travel, Accommodations, Expenses: Amgen
Mary V. Relling
Research Funding: Servier Pharmaceutical
Elizabeth A. Raetz
Research Funding: Pfizer
Other Relationship: Celgene
Mignon L. Loh
Consulting or Advisory Role: MediSix Therapeutics
Stephen P. Hunger
Stock and Other Ownership Interests: Amgen, Merck
Honoraria: Amgen
Consulting or Advisory Role: Novartis
No other potential conflicts of interest were reported.
REFERENCES
- 1.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the children's oncology group J Clin Oncol 301663–16692012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulrooney DA, Hyun G, Ness KK, et al. The changing burden of long-term health outcomes in survivors of childhood acute lymphoblastic leukaemia: A retrospective analysis of the St Jude Lifetime Cohort Study Lancet Haematol 6e306–e3162019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bleyer WA, Sather HN, Nickerson HJ, et al. Monthly pulses of vincristine and prednisone prevent bone marrow and testicular relapse in low-risk childhood acute lymphoblastic leukemia: A report of the CCG-161 study by the Childrens Cancer Study Group J Clin Oncol 91012–10211991 [DOI] [PubMed] [Google Scholar]
- 4.Borowitz MJ, Devidas M, Hunger SP, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: A Children's Oncology Group Study Blood 1115477–54852008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lange BJ, Bostrom BC, Cherlow JM, et al. Double-delayed intensification improves event-free survival for children with intermediate-risk acute lymphoblastic leukemia: A report from the Children's Cancer Group Blood 99825–8332002 [DOI] [PubMed] [Google Scholar]
- 6.Conter V, Valsecchi MG, Silvestri D, et al. Pulses of vincristine and dexamethasone in addition to intensive chemotherapy for children with intermediate-risk acute lymphoblastic leukaemia: A multicentre randomised trial Lancet 369123–1312007 [DOI] [PubMed] [Google Scholar]
- 7.De Moerloose B, Suciu S, Bertrand Y, et al. Improved outcome with pulses of vincristine and corticosteroids in continuation therapy of children with average risk acute lymphoblastic leukemia (ALL) and lymphoblastic non-Hodgkin lymphoma (NHL): Report of the EORTC randomized phase 3 trial 58951 Blood 11636–442010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eden T, Pieters R, Richards S, et al. Systematic review of the addition of vincristine plus steroid pulses in maintenance treatment for childhood acute lymphoblastic leukaemia—an individual patient data meta-analysis involving 5,659 children Br J Haematol 149722–7332010 [DOI] [PubMed] [Google Scholar]
- 9.Bostrom BC, Sensel MR, Sather HN, et al. Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: A report from the Children's Cancer Group Blood 1013809–38172003 [DOI] [PubMed] [Google Scholar]
- 10.Kato M, Ishimaru S, Seki M, et al. Long-term outcome of 6-month maintenance chemotherapy for acute lymphoblastic leukemia in children Leukemia 31580–5842017 [DOI] [PubMed] [Google Scholar]
- 11.Lonsdale D, Gehan EA, Fernbach DJ, et al. Interrupted vs. continued maintenance therapy in childhood acute leukemia Cancer 36341–3521975 [DOI] [PubMed] [Google Scholar]
- 12.Schmiegelow K, Nielsen SN, Frandsen TL, et al. Mercaptopurine/Methotrexate maintenance therapy of childhood acute lymphoblastic leukemia: Clinical facts and fiction J Pediatr Hematol Oncol 36503–5172014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation N Engl J Med 3602730–27412009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winick N, Shuster JJ, Bowman WP, et al. Intensive oral methotrexate protects against lymphoid marrow relapse in childhood B-precursor acute lymphoblastic leukemia J Clin Oncol 142803–28111996 [DOI] [PubMed] [Google Scholar]
- 15.Bleyer WA.The clinical pharmacology of methotrexate: New applications of an old drug Cancer 4136–511978 [DOI] [PubMed] [Google Scholar]
- 16.Teresi ME, Crom WR, Choi KE, et al. Methotrexate bioavailability after oral and intramuscular administration in children J Pediatr 110788–7921987 [DOI] [PubMed] [Google Scholar]
- 17.Balis FM, Holcenberg JS, Poplack DG, et al. Pharmacokinetics and pharmacodynamics of oral methotrexate and mercaptopurine in children with lower risk acute lymphoblastic leukemia: A joint children's cancer group and pediatric oncology branch study Blood 923569–35771998 [PubMed] [Google Scholar]
- 18.Balis FM, Murphy RF, Lester CM, et al. The influence of route of administration on the hepatic uptake of methotrexate Cancer Drug Deliv 3239–2421986 [DOI] [PubMed] [Google Scholar]
- 19.Balis FM, Savitch JL, Bleyer WA.Pharmacokinetics of oral methotrexate in children Cancer Res 432342–23451983 [PubMed] [Google Scholar]
- 20.Dervieux T, Hancock M, Evans W, et al. Effect of methotrexate polyglutamates on thioguanine nucleotide concentrations during continuation therapy of acute lymphoblastic leukemia with mercaptopurine Leukemia 16209–2122002 [DOI] [PubMed] [Google Scholar]
- 21.Balis FM, Lester CM, Chrousos GP, et al. Differences in cerebrospinal fluid penetration of corticosteroids: Possible relationship to the prevention of meningeal leukemia J Clin Oncol 5202–2071987 [DOI] [PubMed] [Google Scholar]
- 22.Pui CH, Relling MV, Sandlund JT, et al. Rationale and design of Total Therapy Study XV for newly diagnosed childhood acute lymphoblastic leukemia Ann Hematol 83S124–S1262004suppl 1 [DOI] [PubMed] [Google Scholar]
- 23.Relling MV, Hancock ML, Boyett JM, et al. Prognostic importance of 6-mercaptopurine dose intensity in acute lymphoblastic leukemia Blood 932817–28231999 [PubMed] [Google Scholar]
- 24.Koren G, Ferrazini G, Sulh H, et al. Systemic exposure to mercaptopurine as a prognostic factor in acute lymphocytic leukemia in children N Engl J Med 32317–211990 [DOI] [PubMed] [Google Scholar]
- 25.Nielsen SN, Grell K, Nersting J, et al. DNA-thioguanine nucleotide concentration and relapse-free survival during maintenance therapy of childhood acute lymphoblastic leukaemia (NOPHO ALL2008): A prospective substudy of a phase 3 trial Lancet Oncol 18515–5242017 [DOI] [PubMed] [Google Scholar]
- 26.Schmiegelow K, Heyman M, Gustafsson G, et al. The degree of myelosuppression during maintenance therapy of adolescents with B-lineage intermediate risk acute lymphoblastic leukemia predicts risk of relapse Leukemia 24715–7202010 [DOI] [PubMed] [Google Scholar]
- 27.Schmiegelow K, Schroder H, Gustafsson G, et al. Risk of relapse in childhood acute lymphoblastic leukemia is related to RBC methotrexate and mercaptopurine metabolites during maintenance chemotherapy. Nordic Society for Pediatric Hematology and Oncology J Clin Oncol 13345–3511995 [DOI] [PubMed] [Google Scholar]
- 28.Bhatia S, Landier W, Hageman L, et al. Systemic exposure to thiopurines and risk of relapse in children with acute lymphoblastic leukemia: A Children's Oncology Group study JAMA Oncol 1287–2952015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia J Clin Oncol 1418–241996 [DOI] [PubMed] [Google Scholar]
- 30.Matloub Y, Bostrom BC, Hunger SP, et al. Escalating intravenous methotrexate improves event-free survival in children with standard-risk acute lymphoblastic leukemia: A report from the Children's Oncology Group Blood 118243–2512011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schemper M, Smith TL.A note on quantifying follow-up in studies of failure time Control Clin Trials 17343–3461996 [DOI] [PubMed] [Google Scholar]
- 32.Kaplan EL, Meier P.Nonparametric estimation from incomplete observations J Am Stat Assoc 53457–4811958 [Google Scholar]
- 33.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples Br J Cancer 351–391977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fleming TR, Harrington DP, O'Brien PC.Designs for group sequential tests Control Clin Trials 5348–3611984 [DOI] [PubMed] [Google Scholar]
- 34a. http://www.r-project.org
- 35.McNeer JL, Nachman JB.The optimal use of steroids in paediatric acute lymphoblastic leukaemia: No easy answers Br J Haematol 149638–6522010 [DOI] [PubMed] [Google Scholar]
- 36.Reynolds CR, Kamphaus RW. BASC-2: Behavior Assessment System for Children. ed 2. Circle Pines, MN: AGS Publishing; 2004. [Google Scholar]
- 37.Sioka C, Kyritsis AP.Central and peripheral nervous system toxicity of common chemotherapeutic agents Cancer Chemother Pharmacol 63761–7672009 [DOI] [PubMed] [Google Scholar]
- 38.Walsh D, Avashia J.Glucocorticoids in clinical oncology Cleve Clin J Med 59505–5151992 [DOI] [PubMed] [Google Scholar]
- 39.Limburg H, Shaw AK, McBride ML.Impact of childhood cancer on parental employment and sources of income: A Canadian pilot study Pediatr Blood Cancer 5193–982008 [DOI] [PubMed] [Google Scholar]
- 40.Stevens B, Croxford R, McKeever P, et al. Hospital and home chemotherapy for children with leukemia: A randomized cross-over study Pediatr Blood Cancer 47285–2922006 [DOI] [PubMed] [Google Scholar]
- 41.Hockenberry M, Krull K, Moore K, et al. Longitudinal evaluation of fine motor skills in children with leukemia J Pediatr Hematol Oncol 29535–5392007 [DOI] [PubMed] [Google Scholar]
- 42.Postma TJ, Benard BA, Huijgens PC, et al. Long-term effects of vincristine on the peripheral nervous system J Neurooncol 1523–271993 [DOI] [PubMed] [Google Scholar]
- 43.Zheng DJ, Lu X, Schore RJ, et al. Longitudinal analysis of quality-of-life outcomes in children during treatment for acute lymphoblastic leukemia: A report from the Children's Oncology Group AALL0932 trial Cancer 124571–5792018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pui CH, Sandlund JT, Pei D, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude Children's Research Hospital Blood 1042690–26962004 [DOI] [PubMed] [Google Scholar]