PURPOSE
Children's Oncology Group (COG) AALL0331 tested whether pegaspargase intensification on a low-intensity chemotherapy backbone would improve the continuous complete remission (CCR) rate in a low-risk subset of children with standard-risk B-acute lymphoblastic leukemia (ALL).
METHODS
AALL0331 enrolled 5,377 patients with National Cancer Institute standard-risk B-ALL (age 1-9 years, WBC < 50,000/μL) between 2005 and 2010. Following a common three-drug induction, a cohort of 1,857 eligible patients participated in the low-risk ALL random assignment. Low-risk criteria included no extramedullary disease, < 5% marrow blasts by day 15, end-induction marrow minimal residual disease < 0.1%, and favorable cytogenetics (ETV6-RUNX1 fusion or simultaneous trisomies of chromosomes 4, 10, and 17). Random assignment was to standard COG low-intensity therapy (including two pegaspargase doses, one each during induction and delayed intensification) with or without four additional pegaspargase doses at 3-week intervals during consolidation and interim maintenance. The study was powered to detect a 4% improvement in 6-year CCR rate from 92% to 96%.
RESULTS
The 6-year CCR and overall survival (OS) rates for the entire low-risk cohort were 94.7% ± 0.6% and 98.7% ± 0.3%, respectively. The CCR rates were similar between arms (intensified pegaspargase 95.3% ± 0.8% v standard 94.0% ± 0.8%; P = .13) with no difference in OS (98.1% ± 0.5% v 99.2% ± 0.3%; P = .99). Compared to a subset of standard-risk study patients given identical therapy who had the same early response characteristics but did not have favorable or unfavorable cytogenetics, outcomes were significantly superior for low-risk patients (CCR hazard ratio 1.95; P = .0004; OS hazard ratio 5.42; P < .0001).
CONCLUSION
Standard COG therapy without intensified pegaspargase, which can easily be given as an outpatient with limited toxicity, cures nearly all children with B-ALL identified as low-risk by clinical, early response, and favorable cytogenetic criteria.
INTRODUCTION
Five-year survival for pediatric B-acute lymphoblastic leukemia (B-ALL) exceeds 90%.1,2 Although outcomes are even better among patients with National Cancer Institute (NCI) standard-risk (SR) B-ALL, only 50% of those experiencing relapse survive and 36% of all B-ALL deaths occur in this SR group.1,3,4
CONTEXT
Key Objective
Retrospective analysis of Children's Oncology Group trials identified a subset of patients with standard-risk B-acute lymphoblastic leukemia (B-ALL), defined by favorable clinical features, early response characteristics, and either ETV6-RUNX1 or trisomies of chromosomes 4, 10, and 17, who had < 10% chance of relapse. We hypothesized that post-induction pegaspargase intensification for such low-risk patients treated prospectively on a low-intensity treatment backbone would improve continuous complete remission.
Knowledge Generated
Outcomes were outstanding. Pegaspargase intensification added toxicity but did not improve 6-year continuous complete remission or overall survival rates.
Relevance
This Children's Oncology Group B-ALL treatment backbone, including a three-drug induction, a low-intensity 4-week consolidation with no Berlin-Frankfurt-Muenster Ib phase, single interim maintenance and delayed intensification phases, no high-dose methotrexate, and limited anthracycline or alkylator exposure, is easily delivered in an outpatient setting with very low risk of significant treatment-related morbidity or mortality and results in 6-year overall survival approaching 100% in low-risk B-ALL.
Risk-adapted therapy intensification has produced steady survival improvements while minimizing treatment-related toxicity.1,5 The Children's Oncology Group (COG) implemented a revised ALL risk classification system in 2004 based on retrospective analysis of data from earlier trials.6 Incorporating clinical, biologic, early response, and minimal residual disease (MRD) criteria, a subset of SR patients representing about 27% of the overall B-ALL population was identified as having approximately 91%-92% event-free survival (EFS) and 95% overall survival (OS) rates at 4 years. These SR-low patients had no extramedullary disease, rapid early response (RER) to induction therapy, end-induction bone marrow (BM) MRD < 0.1%, and one of two defined favorable sentinel cytogenetic features.
Several prognostic somatic genetic alterations have been identified in B-ALL.5,7 Two major favorable risk features are ETV6-RUNX1 fusion and hyperdiploidy with simultaneous trisomies of chromosomes 4, 10, and 17, which is related in part to enhanced asparaginase sensitivity.6,8-11 Pegaspargase intensification appears to be both efficacious and well tolerated in younger patients, providing a rationale to investigate this strategy in SR-low patients.12,13 Given their outstanding survival, it is imperative that strategies to further improve survival not add significant toxicity to standard therapy.14
COG AALL0331 used a three-drug induction for patients with newly diagnosed SR B-ALL, with refined risk assignment at end-induction.15 SR-low patients who consented to post-induction random assignment received standard therapy with or without four additional doses of pegaspargase given at 3-week intervals during consolidation and interim maintenance with the primary objective to determine if intensified pegaspargase improved the continuous complete remission (CCR) rate. A secondary objective was to compare outcomes of these patients with a subset of SR-average patients given identical therapy who met all SR-low eligibility criteria but did not have either favorable or unfavorable cytogenetics.
METHODS
Patients and Treatment
Children with newly diagnosed B-ALL of age 1-9 years with initial WBC count < 50,000/μL were eligible for AALL0331, following mandatory registration on the classification or biology study AALL03B1. All patients received a standard three-drug induction with dexamethasone, vincristine, and pegaspargase, plus intrathecal chemotherapy (Table 1). Risk assignment was refined at end-induction, based on presenting features, cytogenetic and molecular findings, and induction response, into SR-low, SR-average, and SR-high cohorts, as previously published (Fig 1).15
TABLE 1.
Treatment Summary
FIG 1.
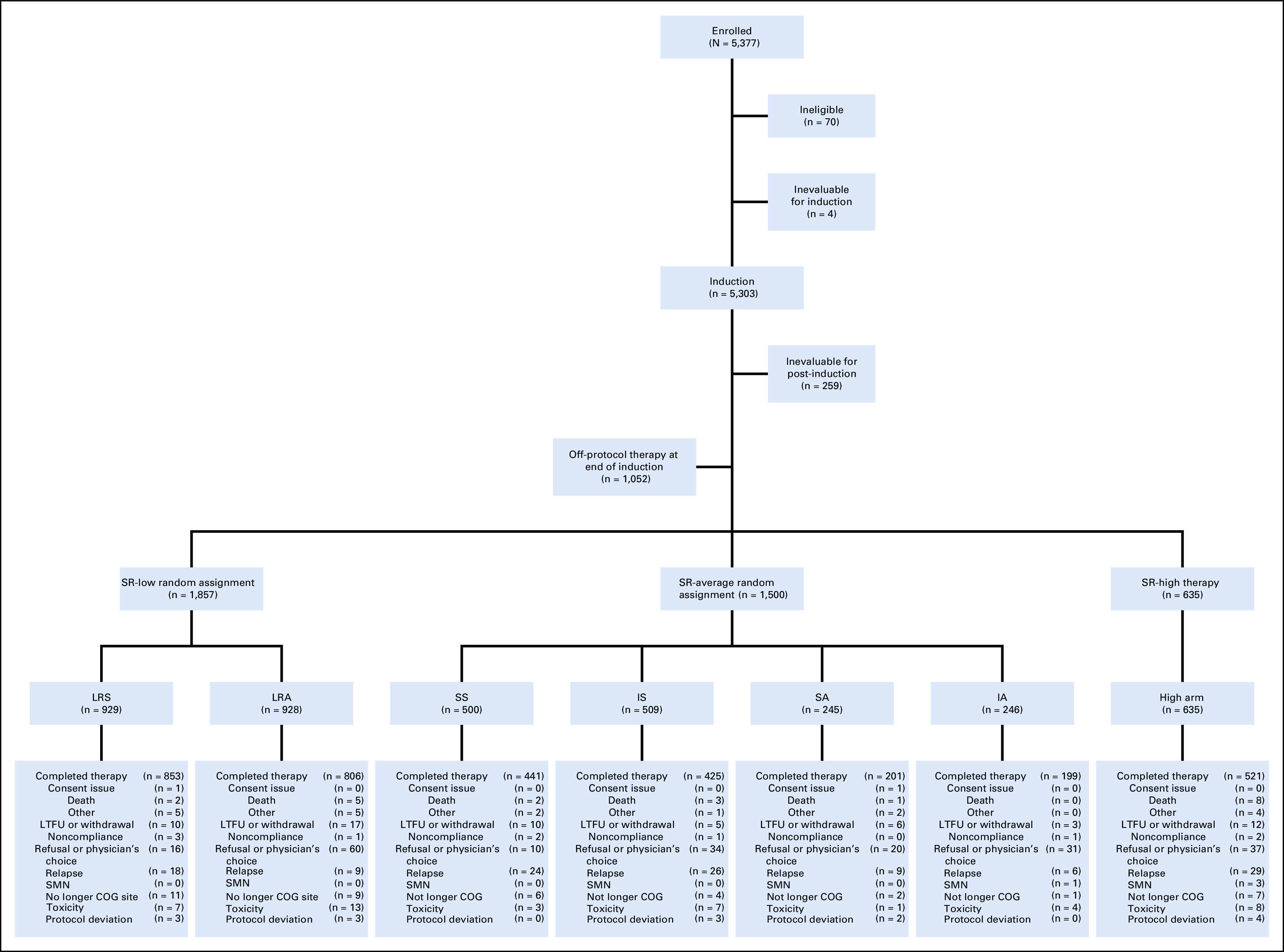
CONSORT diagram for risk-stratified therapy. Reasons for ineligibility (n = 70) included missing or incorrect consent (n = 12), incorrect diagnosis (n = 12), incorrect timing to start of therapy (n = 12), no or insufficient samples (11), no testicular exam before enrollment (n = 5), ineligible for classification study (n = 4), prior therapy before enrollment (n = 3), institutional review board record issues (n = 3), started therapy before enrollment on classification study (n = 2), samples not sent to an approved cytogenetics lab (n = 2), no CNS status determined at enrollment (n = 2), no repeat diagnostic marrow within 1 week before enrollment (n = 1), and no signature on the short consent in patient's native language (n = 1). Regimens LRS and LRA include post-amendment patient cohorts LRS with IV methotrexate and LRA with IV methotrexate, respectively. Regimens SS, IS, SA, and IA include standard versus intensified consolidation and standard versus augmented interim maintenance. Regimen SS includes post-amendment patient cohort SS with IV methotrexate. COG, Children's Oncology Group; IA, intensified consolidation and augmented interim maintenance; IS, intensified consolidation and standard interim maintenance; IV, intravenous; LRA, low risk with intensified pegaspargase; LRS, low risk standard; LTFU, lost to follow-up; SA, standard consolidation and augmented interim maintenance; SMN, second malignant neoplasm; SR, standard risk; SS, standard consolidation.
Criteria for participation in the SR-low post-induction random assignment included all of the following (Table 1 footnote): no corticosteroid pretreatment; no CNS, testicular, or other extramedullary leukemia (organomegaly not included); favorable cytogenetics defined as either (1) simultaneous trisomies of chromosomes 4, 10, and 17, or (2) ETV6-RUNX1 fusion (by centrally performed and reviewed fluorescence in situ hybridization); no high-risk features; and RER to induction therapy (by locally assessed BM morphology at day 8/15 and centrally performed and reviewed BM MRD by multicolor flow cytometry at day 29).16,17 SR-low patients, including those with Down syndrome, were randomly assigned to receive standard consolidation and interim maintenance phases with (regimen low risk with intensified pegaspargase [LRA]) or without (low risk standard [LRS]) four additional pegaspargase doses (2,500 IU/m2) administered every 3 weeks. After September 2008, based on matured Children's Cancer Group CCG-1991 results, all patients received escalating-dose intravenous (IV) methotrexate without leucovorin rescue (current COG standard of care) in place of oral methotrexate during interim maintenance (Table 1, regimens LRS with IV methotrexate [LRS-IV] and LRA with IV methotrexate [LRA-IV]).18 Concurrently, based on COG AALL0232 results, all patients received alternate-week rather than continuous dexamethasone during delayed intensification to limit osteonecrosis risk.19
AALL0331 was approved by the NCI and institutional review boards of participating institutions. Informed consent for study participation and induction therapy was obtained from a parent or guardian before initiation of protocol therapy in accordance with Department of Health and Human Services guidelines; a second consent was obtained for post-induction therapy.
Toxicity Monitoring
Adverse event data were collected using the NCI Common Terminology Criteria for Adverse Events (reported using version 4.0), supplemented with the NCI Adverse Event Expedited Reporting System. Targeted toxicities that required reporting included pancreatitis (grade ≥ 2), CNS hemorrhage (grade ≥ 2), cerebrovascular ischemia (grade ≥ 2), and osteonecrosis (any grade). Nontargeted toxicities (nonhematologic) were reported for grade ≥ 3.
Statistical Analysis
SR-low patients were randomly assigned (1:1 using method of permuted blocks) following induction to post-induction therapy with standard versus intensified pegaspargase. The primary end point was CCR, defined as time from random assignment to first event (relapse, remission death, or second malignant neoplasm) or last contact if event-free. OS was calculated as time from enrollment to death or last contact. Six-year CCR or OS values are provided throughout. The random assignment was designed to enroll 1,800 patients to detect a 4% improvement in 6-year CCR from 92% to 96% (relative hazard rate = 0.4896; 4-year minimum follow-up, one-sided α = 5%; expected event horizon, 95). The α × time2 spending function was used to determine the upper efficacy stopping boundaries; the lower futility boundaries were based on testing the alternative hypothesis at the .005 level.20 The cumulative power to detect differences by the last (fourth) interim analysis was 96.7%. The results of the random assignment were released by the data monitoring committee when the expected event horizon was achieved. Kaplan-Meier method with standard errors of Peto was used to estimate survival curves, with between-group comparisons using the log-rank test.21-23 Multivariable Cox regression analyses were used to examine the impact of various risk factors (age, WBC, treatment, risk group, cytogenetics, and day 8 and 29 MRD) on CCR. Cumulative incidence rates were computed using the cumulative incidence function for competing risks, with between-group comparisons using the K-sample test.21 Chi-square test or Fisher's exact test were used to compare proportions. Per protocol design, one-sided P values are presented for efficacy end point (CCR, OS) comparisons, with two-sided P-values for all other comparisons. All analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC). Graphics were generated using R version 3.0.1.24 Data are current as of June 30, 2017, with a median follow-up time of 7.97 years.
RESULTS
Participants
AALL0331 enrolled 5,377 patients at 185 COG institutions between April 2005 and May 2010, with 5,303 eligible and evaluable for induction therapy and 5,044 for post-induction therapy (Fig 1).15 Of the 3,992 patients consenting to post-induction therapy, 1,857 (46.5%) participated in the SR-low random assignment, with 929 on standard (LRS 617 and LRS-IV 312) and 928 on intensified pegaspargase regimens (LRA 620 and LRA-IV 308). Baseline and end-induction patient characteristics are shown in Table 2. ETV6-RUNX1 was present in 1,139 (61.3%), triple trisomy in 712 (38.3%), and both in 6 (0.3%) cases.
TABLE 2.
Patient Characteristics of the Standard-Risk-Low Population
Therapy-Related Outcomes
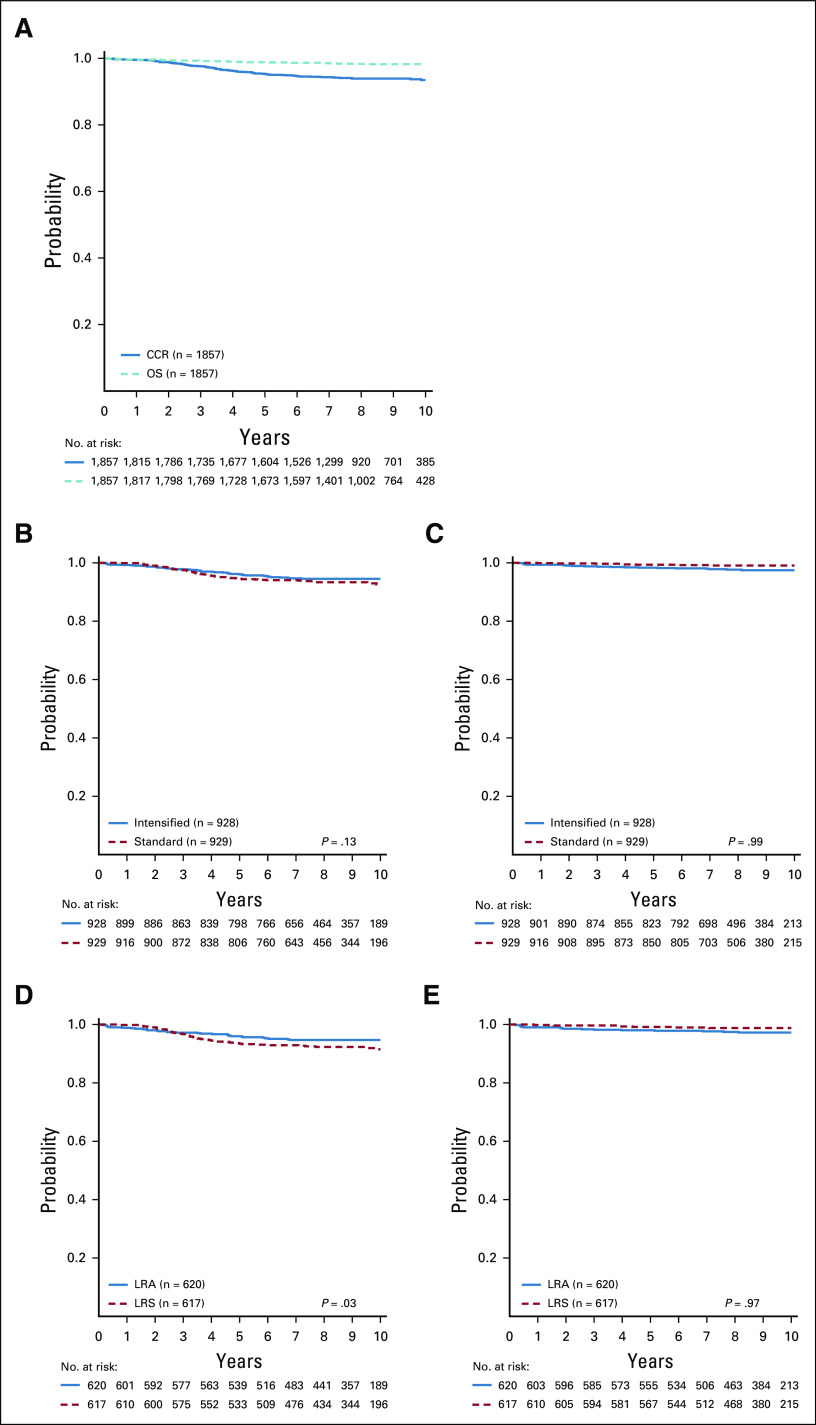
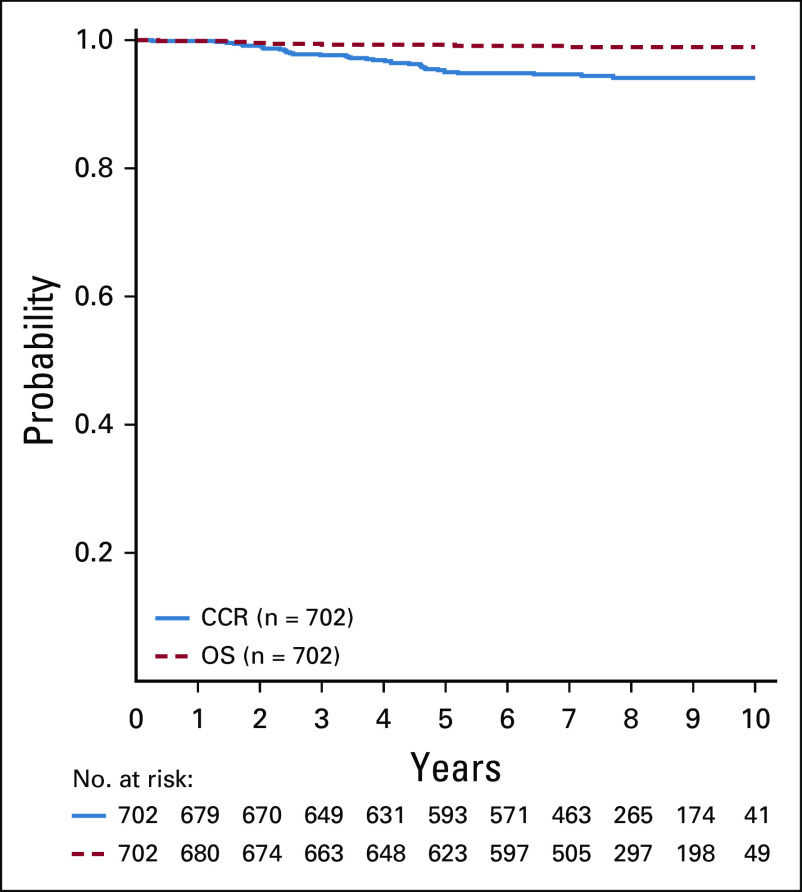
Among all SR-low patients, CCR and OS rates were 94.7% ± 0.6% and 98.7% ± 0.3%, respectively (Fig 2A). The CCR rates were not significantly different for intensified pegaspargase (LRA and LRA-IV) compared with standard therapy (LRS and LRS-IV) (95.3% ± 0.8% v 94.0% ± 0.8%; P = .13), with no difference in OS (98.1% ± 0.5% v 99.2% ± 0.3%; P = .99) (Figs 2B and 2C). Outcomes for patients randomly assigned to pre-amendment regimens (LRA and LRS) showed superior CCR favoring intensified pegaspargase (95.3% ± 0.9% v 92.9% ± 1.1%; P = .03) with no difference in OS (97.9% ± 0.6% v 99.0% ± 0.4%; P = .97) (Figs 2D and 2E). Outcomes for patients randomly assigned to post-amendment regimens (LRA-IV and LRS-IV) showed no difference in either CCR (95.4% ± 1.3% v 96.2% ± 1.2%; P = .79) or OS (98.6% ± 0.7% v 99.7% ± 0.4%; P = .95).
FIG 2.
CCR and OS curves. (A) CCR and OS curves for the entire standard-risk (SR)-low population. Six-year CCR and OS rates were 94.7% ± 0.6% and 98.7% ± 0.3%, respectively. (B) CCR curves for standard (LRS, LRS with IV methotrexate [LRS-IV]) versus intensified pegaspargase (LRA, LRA with IV methotrexate [LRA-IV]) randomly assigned cohorts. Six-year CCR rates were 94.0% ± 0.8% and 95.3% ± 0.8%, respectively. (C) OS curves for standard (LRS, LRS-IV) versus intensified pegaspargase (LRA, LRA-IV) randomly assigned cohorts. Six-year OS rates were 99.2% ± 0.3% and 98.1% ± 0.5%, respectively. (D) CCR curves for LRS versus LRA randomly assigned regimens. Six-year CCR rates were 92.9% ± 1.1% and 95.3% ± 0.9%, respectively. (E) OS curves for LRS versus LRA randomly assigned regimens. Six-year OS rates were 99.0% ± 0.4% and 97.9% ± 0.6%, respectively. CCR, continuous complete remission; IV, intravenous; LRA, low risk with intensified pegaspargase; LRS, low risk standard; OS, overall survival.
Based on the study amendment, we evaluated the anticipated benefit of escalating-dose IV methotrexate over oral methotrexate in relevant treatment cohorts. Among standard therapy patients, 6-year CCR and OS rates for regimens LRS-IV and LRS were 96.3% ± 1.2% versus 92.9% ± 1.1% (P = .06) and 99.7% ± 0.4% versus 99.0% ± 0.4% (P = .2), respectively. Importantly, no difference was observed among intensified pegaspargase therapy patients (LRA-IV v LRA) or the combined cohorts (LRS-IV/LRA-IV v LRS/LRA) in CCR or OS.
Cytogenetics-Related Outcomes
Among SR-low patients (excluding six with both features), triple trisomy was associated with superior CCR (96.3% ± 0.8% v 93.7% ± 0.8%; P = .01) and OS (99.4% ± 0.3% v 98.2% ± 0.4%; P = .02) compared with ETV6-RUNX1.
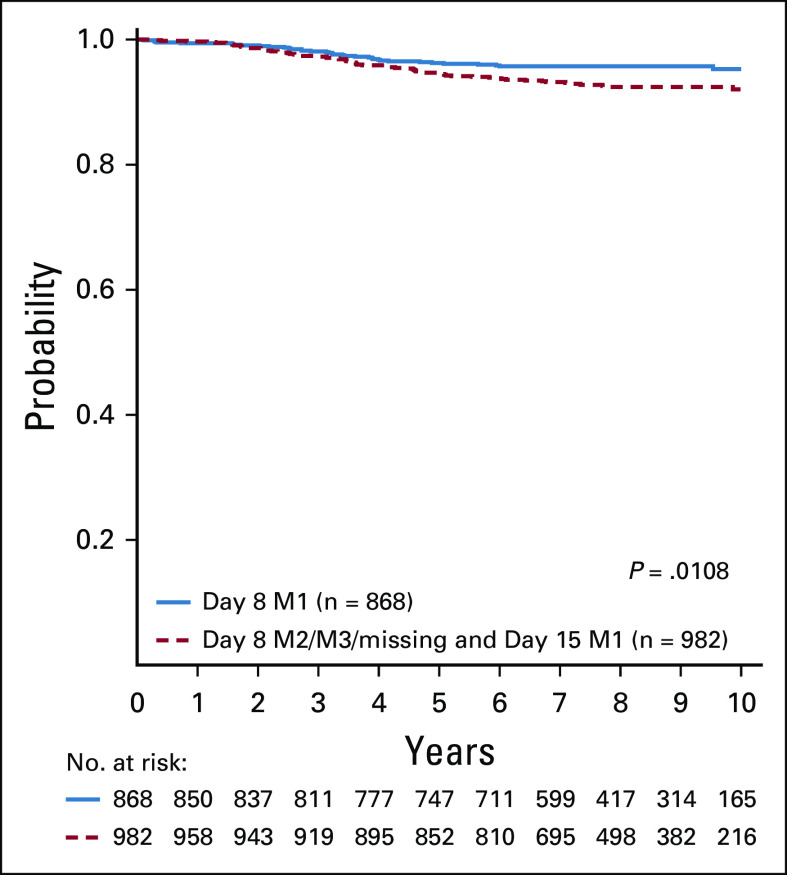
Overall, 47% (868/1,850) of patients achieved < 5% marrow blasts at day 8, and this response differed by cytogenetic category (ETV6-RUNX1 53% [591/1,125], triple trisomy 38% [271/710]). Among the entire SR-low cohort, the 6-year CCR rate was better for patients with < 5% BM blasts at day 8 compared with those who did not achieve < 5% BM blasts until day 15 (95.7% ± 0.7% v 93.8% ± 0.8%; P = .0108) (Appendix Fig A1, online only). A similar difference was observed for ETV6-RUNX1 (94.8% ± 1.0% v 92.6% ± 1.2%; P = .02) but not for triple trisomy (97.7% ± 1.0% v 95.4% ± 1.1%; P = .2). There was no difference in OS for any group.
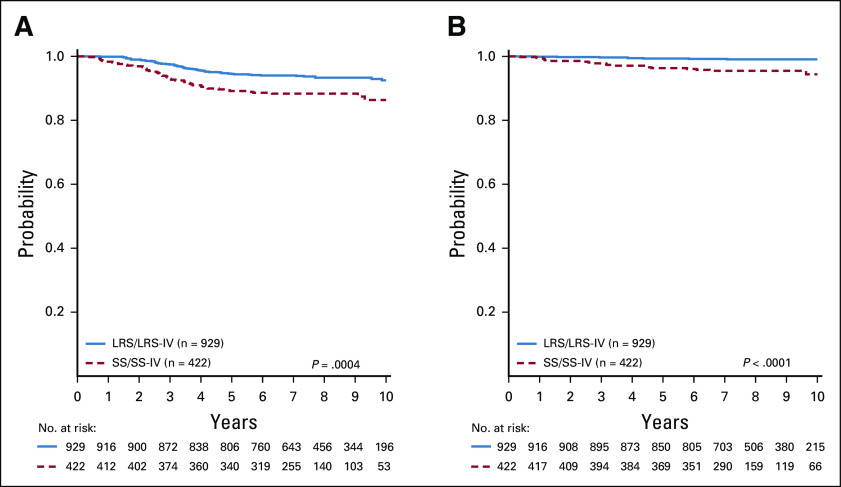
We identified a cohort of AALL0331 SR-average patients (n = 422) meeting all SR-low criteria but without favorable or unfavorable cytogenetics, and who were randomly assigned on the SR-average stratification to receive therapy identical to LRS or LRS-IV.15 Comparative analyses showed superior CCR (94.0% ± 0.8% v 88.6% ± 1.7%; hazard ratio [HR], 1.95 [95% CI, 1.34 to 2.85]; P = .0004) and OS (99.2% ± 0.3% v 96.1% ± 1.0%; HR, 5.42 [95% CI, 2.37 to 12.39]; P < .0001) in SR-low versus SR-average patients (Figs 3A and 3B). Multivariable Cox regression analyses were used to examine this comparison and found that age (≥ 6 v < 6 years: P = .0051), risk group (SR-average v SR-low: P = .0014), day 29 MRD (0.01%-0.1% v < 0.01%: P < .001), and cytogenetics (ETV6-RUNX1 v triple trisomies: P = .0498) were associated with inferior outcome, whereas there was no significant effect of day 8 peripheral blood (PB) MRD or WBC (Appendix Table A1, online only).
FIG 3.
Survival comparisons for SR-low and SR-average cohorts. (A) CCR curves for SR-low (LRS and LRS-IV) versus SR-average (SS and SS-IV) randomly assigned standard therapy cohorts. Analysis only includes patients in the SR-average population who met all SR-low criteria except for favorable cytogenetics. Six-year CCR rates were 94.0% ± 0.8% versus 88.6% ± 1.7%, respectively. (B) OS curves for SR-low (LRS and LRS-IV) versus SR-average (SS and SS-IV) randomly assigned standard therapy cohorts. Six-year OS rates were 99.2% ± 0.3% versus 96.1% ± 1.0%, respectively. CCR, continuous complete remission; IV, intravenous; LRS, low risk standard; LRS-IV, LRS with IV methotrexate; OR, overall survival; SR, standard risk; SS, standard consolidation and standard interim maintenance.
MRD-Related Outcomes
Induction day 29 BM MRD levels are shown in Table 2. Approximately 91% of study patients achieved MRD < 0.01%, the remainder having MRD ≥ 0.01% and < 0.1%. Additional analyses were performed to estimate outcomes among patients with available day 8 PB MRD data (n = 1,036). In patients with day 8 PB and day 29 BM MRD < 0.01%, 6-year CCR and OS were 96.3% ± 1.4% and 98.6% ± 0.8%, respectively (Appendix Fig A2, online only). In patients with day 8 PB MRD ≥ 0.01% and day 29 BM MRD < 0.01%, 6-year CCR and OS were 94.8% ± 0.9% and 99.1% ± 0.4%, respectively (Appendix Fig A3, online only). Among 812 patients meeting the current MRD low-risk criteria used in COG AALL1731 (ClinicalTrials.gov identifier: NCT03914625) (day 8 PB MRD < 1.0% and day 29 BM MRD < 0.01%), 6-year CCR and OS were 96.0% ± 0.7% and 99.0% ± 0.4%, respectively. In multivariable Cox regression analyses, age (≥ 6 v < 6 years: P = .0289), day 8 MRD (≥ 1% v < 1%: P = .0407), day 29 MRD (0.01%-0.1% v < 0.01%: P < .001), and cytogenetics (ETV6-RUNX1 v triple trisomies: P = .0058) were associated with inferior outcome (Appendix Table A2, online only).
Events
In the entire SR-low cohort (1,857 patients), 106 events occurred (Table 3). Compared to standard therapy, intensified pegaspargase therapy was associated with fewer relapses (36 v 53) but more remission deaths (10 v 2). Using the COG relapsed ALL risk classification system, 16 of the 89 (18%) relapses were high risk (any relapse within 18 months plus BM relapses 18-36 months) and 73 (82%) were standard risk (Appendix Table A3, online only). The combined number of second malignancies was low (n = 5). The 6-year cumulative incidence rates of isolated marrow, isolated CNS, and combined relapses among all patients were 2.1% ± 0.3%, 1.1% ± 0.3%, and 0.5% ± 0.2%, respectively, with a nonsignificant overall lower rate with intensified pegaspargase (3.6% ± 0.6% v 5.3% ± 0.8%; P = .07). The cumulative incidence of remission deaths among all patients was 0.6% ± 0.2%, and was significantly higher with intensified pegaspargase (1.0% ± 0.3% v 0.2% ± 0.2%; P = .02).
TABLE 3.
DFS Event Summary by Standard-Risk-Low Randomly Assigned Regimen
Toxicity
Toxicities are summarized in Table 4. Symptomatic pancreatitis was reported more frequently with intensified pegaspargase (3.7% v 0.8%; P < .0001) and was primarily moderate in severity (1 grade 2, 33 grade 3, and 7 grade 4). Osteonecrosis was nominally more common with intensified pegaspargase therapy (3.0% v 1.8%; P = .099) and occurred with equally low frequency post-amendment across both regimens (1.3% [n = 8]) using alternate-week dexamethasone. Cerebrovascular ischemia occurred in three standard and six intensified pegaspargase therapy patients (3 grade 2, 5 grade 4, and 1 grade unknown). CNS hemorrhage was also rare, occurring in four standard and three intensified pegaspargase therapy patients (three grade 2, one grade 4, one grade 5, and two grade unknown).
TABLE 4.
Toxicity Summary by Standard Risk-Low Randomly Assigned Regimen
As expected, anaphylaxis occurred more often with intensified pegaspargase (7.8% v 0.7%; P < .0001). Neutropenia and febrile neutropenia were more common with intensified pegaspargase during both consolidation (24.1% v 12.9%; P < .0001; 4.0% v 1.9%; P = .009) and interim maintenance (39.8% v 22.2%; P < .0001; 14.2% v 6.1%; P < .0001), and infection was more common during interim maintenance (16.0% v 9.7%; P < .0001).
Of the nine SR-low on-treatment deaths, seven occurred on intensified pegaspargase therapy (three in delayed intensification, two in interim maintenance, and two in maintenance), and two occurred on standard therapy during maintenance (Table 3). None of the deaths were attributed to pegaspargase, and none were in patients with Down syndrome.
DISCUSSION
COG AALL0331 prospectively identified and treated 1,857 patients with SR-low B-ALL with low-intensity post-induction therapy with or without intensified pegaspargase. Overall results were excellent, with 6-year CCR 94.7% and OS 98.7%. Patients receiving standard therapy including escalating-dose IV methotrexate (LRS-IV) had 6-year CCR 96.3% and OS 99.7%. These outstanding outcomes demonstrate the prognostic strength of specific variables and identify a cohort for which limiting therapeutic toxicity should be paramount.
Our primary objective was to determine if post-induction pegaspargase intensification improved the CCR rate from 92% to 96% and we found no difference between arms. Confirming the results of Children's Cancer Group CCG-1991, the change to IV methotrexate during interim maintenance improved CCR in standard therapy patients (LRS-IV v LRS) and was well tolerated.18
There were remarkably few remission deaths (12/1,857, 0.6%), with 10 on intensified pegaspargase regimens (three of these during follow-up). Six deaths were infection-related. Pre-maintenance infection deaths (n = 3) were all on regimen LRA, consistent with the nearly two-fold elevated risk of neutropenia with intensified pegaspargase. Maintenance infection deaths (n = 3) were equally rare across both cohorts. Similar findings were reported for EORTC-CLG trial 58951, with improved disease-free but not OS rates, and more infection-related remission deaths, in patients with SR-ALL receiving prolonged asparaginase therapy; overall infection rates during consolidation and late intensification were also higher when preceded by dexamethasone during induction.25 In another study, reduced pegaspargase exposure from individualized dosing on DCOG ALL-11 was associated with fewer infections during intensification compared with standard dosing used on ALL-10 among medium-risk patients.26
Pancreatitis (n = 41) and osteonecrosis (n = 45) were the most common targeted toxicities observed. Not surprisingly, pancreatitis occurred disproportionately with intensified pegaspargase (3.7% v 0.8%), as reported for NOPHO ALL08.27 Other targeted toxicities were rare, including osteonecrosis after institution of alternate-week dexamethasone during delayed intensification (n = 8). Notably, only a single death related to targeted toxicities was reported on study (CNS hemorrhage on regimen LRA-IV) and that event occurred during maintenance, well after completion of pegaspargase therapy. A detailed analysis of asparaginase discontinuation on AALL0331 demonstrated that patients with SR-ALL with RER who halted pegaspargase for reasons including hypersensitivity were not at elevated risk of relapse.28 This observation is in contrast to the results of JACLS ALL-02, in which asparaginase discontinuation among patients with ETV6-RUNX1–positive B-ALL was associated with inferior EFS on multivariate analysis.29 Regardless, the added burden of therapy associated with pegaspargase intensification is not justified, given the fact that standard therapy with escalating IV methotrexate during interim maintenance (LRS-IV) provides a nearly 100% chance of cure in this sizable low-risk cohort.
The parallel design of AALL0331 allowed us to directly evaluate the prognostic significance of favorable cytogenetics using two cohorts given identical standard therapy and whose presenting characteristics were the same except for the presence (LRS/LRS-IV) or absence (standard consolidation and standard interim maintenance [SS], SS with IV methotrexate [SS-IV]) of ETV6-RUNX1 or triple trisomy.15 Results of this secondary study objective showed that favorable genetics was associated with striking advantages in both CCR (HR, 1.95; P = .0004) and OS (HR, 5.42; P < .0001). To our knowledge, we are the first to report this important finding based on a prospective comparative analysis. The inherent benefit conferred by favorable cytogenetics has also been demonstrated separately in a subset of patients with NCI high-risk B-ALL treated on COG AALL0232 with 5-year EFS and OS rates of 94.9% and 98.1%, respectively.30 The prognostic impact of ETV6-RUNX1 is complex and likely influenced by clonal heterogeneity and cytogenetic context.31 Recent clinical data suggest that ETV6-RUNX1 confers sensitivity to asparaginase and methotrexate, compatible with our observations.32
Triple trisomy was associated with superior CCR and OS compared with ETV6-RUNX1 among SR-low patients, reflected in fewer relapse events of all types, although the underlying basis of this finding is unclear. Notably, a larger proportion of cases with ETV6-RUNX1 had < 5% BM blasts on day 8 (52%) than with triple trisomy (38%). Earlier response translated to improved CCR only in cases with ETV6-RUNX1, while OS was no different with either cytogenetic abnormality.
With regard to more recent RER criteria adopted in subsequent COG ALL trials, analyses on a subset of our SR-low patients validated the use of day 8 PB MRD in lieu of day 8/15 marrow assessment and a more stringent day 29 BM MRD cutoff of < 0.01%. Patients with day 8 PB MRD < 0.01% achieved a higher CCR rate compared with use of the AALL0331 criteria (96.3% v 94.7%), and those with day 8 PB MRD < 1.0% did nearly as well (95.3% ± 0.5%). These refinements reduce burden of care and focus attention on patients for whom intensified therapy is most appropriate.
Reducing treatment intensity to limit toxicity without compromising efficacy may be successful in select settings. Variably defined clinical and MRD-based favorable cohorts on UKALL 2003 and DCOG ALL10 experienced less toxicity and fewer remission deaths with preservation of leukemic outcomes by modifying or restricting delayed intensification.33,34 On AIEOP-Berlin-Frankfurt-Muenster ALL 2000, however, SR patients 1-6 years or with the ETV6-RUNX1 did well given lower-intensity delayed intensification, whereas other cohorts fared poorly, with no measurable reduction in toxicity across the trial.35 An alternative approach has been to eliminate the most-toxic elements of therapy.36,37
The outstanding outcomes achieved for SR-low patients on our study were obtained with a three-drug induction, a low-intensity 4-week consolidation without the Berlin-Frankfurt-Muenster Ib phase, no high-dose IV methotrexate, single interim maintenance and delayed intensification phases, limited anthracycline (75 mg/m2) and alkylator (1 g/m2) exposure, and only two doses of pegaspargase on the standard regimens. All post-induction therapy can be easily delivered in an outpatient setting with very low risk of significant treatment-related morbidity or mortality. These results have substantial implications for childhood ALL treatment in low- and middle-income countries and provide a compelling argument to develop fluorescence in situ hybridization and MRD testing to identify these low-risk patients in those settings. Moreover, our findings support efforts to reduce the burden of therapy in SR-low patients without compromising outcome.
Appendix
FIG A1.
CCR in SR-low patients with day 8 versus day 15 M1 marrow and day 29 MRD < 0.1%. Six-year CCR for SR-low patients with day 8 M1/day 29 MRD < 0.1% versus day 15 M1/day 29 MRD < 0.1% were 95.7% ± 0.7% versus 93.8% ± 0.8%, respectively. CCR, continuous complete remission; MRD, minimal residual disease; SR, standard risk.
FIG A2.
CCR and OS in SR-low patients with day 8 PB MRD < 0.01% and day 29 marrow MRD < 0.01%. Six-year CCR and OS for SR-low patients with day 8 PB MRD < 0.01% were 96.3% ± 1.4% and 98.6% ± 0.8%, respectively. CCR, continuous complete remission; MRD, minimal residual disease; OS, overall survival; PB, peripheral blood; SR, standard risk.
FIG A3.
CCR and OS in SR-low patients with day 8 PB MRD ≥ 0.01% and day 29 marrow MRD < 0.01%. Six-year CCR and OS for SR-low patients with day 8 PB MRD ≥ 0.01% and day 29 MRD < 0.01% were 94.8% ± 0.9% and 99.1% ± 0.4%, respectively. CCR, continuous complete remission; MRD, minimal residual disease; OS, overall survival; PB, peripheral blood; SR, standard risk.
TABLE A1.
Multivariable Cox Regression Analysis: SR-Averagea Versus SR-Lowb
TABLE A2.
Multivariable Cox Regression Analysis: Standard-Risk-Low Cohort
TABLE A3.
Breakdown of Relapses by Site and Duration of CCR
Leonard A. Mattano
Stock and Other Ownership Interests: Pfizer, Amgen, Monsanto
Consulting or Advisory Role: Pfizer, Novartis, Melinta Therapeutics
Meenakshi Devidas
Honoraria: PSI, Novartis
Michael J. Borowitz
Consulting or Advisory Role: Amgen
Research Funding: Becton Dickinson
Travel, Accommodations, Expenses: Beckman Coulter
Julie M. Gastier-Foster
Research Funding: Bristol-Myers Squibb, Incyte
Nina S. Kadan-Lottick
Honoraria: Medtronic, Boston Scientific
Consulting or Advisory Role: Medtronic, Boston Scientific
Speakers' Bureau: Medtronic, Boston Scientific
Yousif H. Matloub
Employment: Takeda
Stock and Other Ownership Interests: Amgen, AstraZeneca
David T. Marshall
Leadership: First String Research
Stock and Other Ownership Interests: First Choice Health
Consulting or Advisory Role: Isoray
Mignon L. Loh
Consulting or Advisory Role: MediSix Therapeutics
Elizabeth A. Raetz
Research Funding: Pfizer
Other Relationship: Celgene
Brent L. Wood
Honoraria: Amgen, Seattle Genetics, Abbvie, Janssen, Astellas Pharma, Roche Diagnostics
Consulting or Advisory Role: Sysmex
Research Funding: Amgen, Seattle Genetics, Pfizer, Juno Therapeutics, BiolineRx, Biosight, Stemline Therapeutics, Janssen Oncology, Novartis
Travel, Accommodations, Expenses: Amgen
Stephen P. Hunger
Stock and Other Ownership Interests: Amgen, Merck
Honoraria: Amgen
Consulting or Advisory Role: Novartis
William L. Carroll
Other Relationship: Amgen
No other potential conflicts of interest were reported.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
PRIOR PRESENTATION
Presented in part at the American Society of Hematology Annual Meeting, San Francisco, CA, December 2014 (abstract #793).
SUPPORT
Supported by grants U10 CA98543, U10 CA98413, U10 CA180886, 1U24-CA196173, and U10 CA180899 from the National Institutes of Health and by the St. Baldrick's Foundation.
S.P.H., W.L.C., and N.J.W. contributed equally as senior authors.
AUTHOR CONTRIBUTIONS
Conception and design: Leonard A. Mattano, Meenakshi Devidas, Kelly W. Maloney, Alison M. Friedmann, Michael J. Borowitz, Julie M. Gastier-Foster, Nina S. Kadan-Lottick, Yousif H. Matloub, Linda C. Stork, Mignon L. Loh, Elizabeth A. Raetz, Stephen P. Hunger, William L. Carroll, Naomi J. Winick
Administrative support: Stephen P. Hunger
Provision of study materials or patients: Michael J. Borowitz, Julie M. Gastier-Foster, Nyla A. Heerema, William L. Carroll
Collection and assembly of data: Leonard A. Mattano, Meenakshi Devidas, Kelly W. Maloney, Alison M. Friedmann, Michael J. Borowitz, Andrew J. Carroll, Julie M. Gastier-Foster, Nyla A. Heerema, Nina S. Kadan-Lottick, David T. Marshall, Brent L. Wood, Stephen P. Hunger
Data analysis and interpretation: Leonard A. Mattano, Meenakshi Devidas, Kelly W. Maloney, Cindy Wang, Patrick Buckley, Michael J. Borowitz, Nina S. Kadan-Lottick, Linda C. Stork, Mignon L. Loh, Stephen P. Hunger, William L. Carroll, Naomi J. Winick
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Favorable Trisomies and ETV6-RUNX1 Predict Cure in Low-Risk B-Cell Acute Lymphoblastic Leukemia: Results From Children's Oncology Group Trial AALL0331
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Leonard A. Mattano
Stock and Other Ownership Interests: Pfizer, Amgen, Monsanto
Consulting or Advisory Role: Pfizer, Novartis, Melinta Therapeutics
Meenakshi Devidas
Honoraria: PSI, Novartis
Michael J. Borowitz
Consulting or Advisory Role: Amgen
Research Funding: Becton Dickinson
Travel, Accommodations, Expenses: Beckman Coulter
Julie M. Gastier-Foster
Research Funding: Bristol-Myers Squibb, Incyte
Nina S. Kadan-Lottick
Honoraria: Medtronic, Boston Scientific
Consulting or Advisory Role: Medtronic, Boston Scientific
Speakers' Bureau: Medtronic, Boston Scientific
Yousif H. Matloub
Employment: Takeda
Stock and Other Ownership Interests: Amgen, AstraZeneca
David T. Marshall
Leadership: First String Research
Stock and Other Ownership Interests: First Choice Health
Consulting or Advisory Role: Isoray
Mignon L. Loh
Consulting or Advisory Role: MediSix Therapeutics
Elizabeth A. Raetz
Research Funding: Pfizer
Other Relationship: Celgene
Brent L. Wood
Honoraria: Amgen, Seattle Genetics, Abbvie, Janssen, Astellas Pharma, Roche Diagnostics
Consulting or Advisory Role: Sysmex
Research Funding: Amgen, Seattle Genetics, Pfizer, Juno Therapeutics, BiolineRx, Biosight, Stemline Therapeutics, Janssen Oncology, Novartis
Travel, Accommodations, Expenses: Amgen
Stephen P. Hunger
Stock and Other Ownership Interests: Amgen, Merck
Honoraria: Amgen
Consulting or Advisory Role: Novartis
William L. Carroll
Other Relationship: Amgen
No other potential conflicts of interest were reported.
REFERENCES
- 1.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the Children's Oncology Group J Clin Oncol 301663–16692012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pui CH, Yang JJ, Hunger SP, et al. Childhood acute lymphoblastic leukemia: Progress through collaboration J Clin Oncol 332938–29482015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen K, Devidas M, Cheng SC, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: A Children's Oncology Group study Leukemia 222142–21502008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia J Clin Oncol 1418–241996 [DOI] [PubMed] [Google Scholar]
- 5.Pui CH, Carroll WL, Meshinchi S, et al. Biology, risk stratification, and therapy of pediatric acute leukemias: An update J Clin Oncol 29551–5652011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schultz KR, Pullen DJ, Sather HN, et al. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: A combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children's Cancer Group (CCG) Blood 109926–9352007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunger SP, Mullighan CG.Acute lymphoblastic leukemia in children N Engl J Med 3731542–15522015 [DOI] [PubMed] [Google Scholar]
- 8.Kaspers GJL, Smets LA, Pieters R, et al. Favorable prognosis of hyperdiploid common acute lymphoblastic leukemia may be explained by sensitivity to antimetabolites and other drugs: Results of an in vitro study Blood 85751–7561995 [PubMed] [Google Scholar]
- 9.Ramakers-van Woerden NL, Pieters R, Loonen AH, et al. TEL/AML1 gene fusion is related to in vitro drug sensitivity for L-asparaginase in childhood acute lymphoblastic leukemia Blood 951094–10992000 [PubMed] [Google Scholar]
- 10.Shurtleff SA, Bujis A, Behm FG, et al. TEL/AML1 fusion resulting from a cryptic t(12;21) is the most common genetic lesion in pediatric ALL and defines a subgroup of patients with an excellent prognosis Leukemia 91985–19891995 [PubMed] [Google Scholar]
- 11.Sutcliffe MJ, Shuster JJ, Sather HN, et al. High concordance from independent studies by the Children's Cancer Group (CCG) and Pediatric Oncology Group (POG) associating favorable prognosis with combined trisomies of 4, 10, and 17 in children with NCI standard-risk B-precursor acute lymphoblastic leukemia: A Children's Oncology Group (COG) initiative Leukemia 19734–7402005 [DOI] [PubMed] [Google Scholar]
- 12.Abshire TC, Pollock BH, Billett AL, et al. Weekly polyethylene glycol conjugated L-asparaginase compared with biweekly dosing produces superior induction remission rates in childhood relapsed acute lymphoblastic leukemia: A Pediatric Oncology Group Study Blood 961709–17152000 [PubMed] [Google Scholar]
- 13.Silverman LB, Gelber RD, Kimball Dalton V, et al. Improved outcome for children with acute lymphoblastic leukemia: Results of Dana-Farber Consortium Protocol 91-01 Blood 971211–12182001 [DOI] [PubMed] [Google Scholar]
- 14.Hunger SP, Winick NJ, Sather HN, et al. Therapy of low-risk subsets of childhood acute lymphoblastic leukemia: When do we say enough? Pediatr Blood Cancer 45876–8802005 [DOI] [PubMed] [Google Scholar]
- 15.Maloney KW, Devidas M, Wang C, et al. Outcome in children with standard-risk B-cell acute lymphoblastic leukemia: Results of Children's Oncology Group trial AALL0331 J Clin Oncol 38602–6122020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borowitz MJ, Devidas M, Hunger SP, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: A Children's Oncology Group study Blood 1115477–54852008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borowitz MJ, Wood BL, Devidas M, et al. Prognostic significance of minimal residual disease in high risk B-ALL: A report from Children's Oncology Group study AALL0232 Blood 126964–9712015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matloub Y, Bostrom BC, Hunger SP, et al. Escalating intravenous methotrexate improves event-free survival in children with standard-risk acute lymphoblastic leukemia: A report from the Children's Oncology Group Blood 118243–2512011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsen E, Devidas M, Chen S, et al. Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B-acute lymphoblastic leukemia: A report from Children's Oncology Group study AALL0232 J Clin Oncol 342380–23882016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freidlin B, Korn EL.A comment on futility monitoring Controlled Clin Trials 23355–3662002 [DOI] [PubMed] [Google Scholar]
- 21.Gray RJ.A class of K-sample tests for comparing the cumulative incidence of a competing risk Ann Statist 161141–11541988 [Google Scholar]
- 22.Kaplan E, Meier P.Nonparametric estimation from incomplete observations J Am Stat Assoc 53457–4811958 [Google Scholar]
- 23.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples Br J Cancer 351–391977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.R version 3.0.1. http://www.R-project.org
- 25.Mondelaers V, Suciu S, De Moerloose B, et al. Prolonged versus standard native E. coli asparaginase therapy in childhood acute lymphoblastic leukemia and non-Hodgkin lymphoma: Final results of the EORTC-CLG randomized phase III trial 58951 Haematologica 1021727–17382017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kloos RQH, Pieters R, Jumelet FMV, et al. Individualized asparaginase dosing in childhood acute lymphoblastic leukemia J Clin Oncol 38715–7242020 [DOI] [PubMed] [Google Scholar]
- 27.Albertsen BK, Grell K, Abrahamsson J, et al. Intermittent versus continuous PEG-asparaginase to reduce asparaginase-associated toxicities: A NOPHO ALL2008 randomized study J Clin Oncol 371638–16462019 [DOI] [PubMed] [Google Scholar]
- 28.Gupta S, Wang C, Raetz EA, et al. Impact of asparaginase discontinuation on outcome in childhood acute lymphoblastic leukemia: A report from the Children's Oncology Group J Clin Oncol 381897–19052020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Usami I, Imamura T, Takahashi Y, et al. Discontinuation of L-asparaginase and poor response to prednisolone are associated with poor outcome of ETV6-RUNX1-positive pediatric B-cell precursor acute lymphoblastic leukemia Int J Hematol 109477–4822019 [DOI] [PubMed] [Google Scholar]
- 30.Raetz AE, Loh ML, Devidas M, et al. Genetic and response-based risk classification identifies a subgroup of NCI high risk childhood B-lymphoblastic leukemia (HR B-ALL) with outstanding outcomes: A report from the Children's Oncology Group (COG) Blood. 2015;126:807. (abstr 126) [Google Scholar]
- 31.Ampatzidou M, Papadhimitriou SI, Paterakis G, et al. ETV6/RUNX1-positive childhood acute lymphoblastic leukemia (ALL): The spectrum of clonal heterogeneity and its impact on prognosis Cancer Genet 2241–112018 [DOI] [PubMed] [Google Scholar]
- 32.Bhojwani D, Pei D, Sandlund JT, et al. ETV6-RUNX1-positive childhood acute lymphoblastic leukemia: Improved outcome with contemporary therapy Leukemia 26265–2702012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pieters R, de Groot-Kruseman H, Van der Velden V, et al. Successful therapy reduction and intensification for childhood acute lymphoblastic leukemia based on minimal residual disease monitoring: Study ALL10 from the Dutch Childhood Oncology Group J Clin Oncol 342591–26012016 [DOI] [PubMed] [Google Scholar]
- 34.Vora A, Goulden N, Wade R, et al. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): A randomised controlled trial Lancet Oncol 14199–2092013 [DOI] [PubMed] [Google Scholar]
- 35.Schrappe M, Bleckmann K, Zimmermann M, et al. Reduced-intensity delayed intensification in standard-risk pediatric acute lymphoblastic leukemia defined by undetectable minimal residual disease: Results of an international randomized trial (AIEOP-BFM ALL 2000) J Clin Oncol 36244–2532017 [DOI] [PubMed] [Google Scholar]
- 36.Chauvenet AR, Martin PL, Devidas M, et al. Antimetabolite therapy for lesser-risk B-lineage acute lymphoblastic leukemia of childhood: A report from Children's Oncology Group study P9201 Blood 1101105–11112007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pedrosa F, Coustan-Smith E, Zhou Y, et al. Reduced-dose intensity therapy for pediatric lymphoblastic leukemia: Long-term results of the Recife RELLA05 pilot study Blood 1351458–14662020 [DOI] [PMC free article] [PubMed] [Google Scholar]