Abstract.
Invasive Salmonella infection is a common cause of acute febrile illness (AFI) among children in sub-Saharan Africa; however, diagnosing Salmonella bacteremia is challenging in settings without blood culture. The Uganda AFI surveillance system includes blood culture-based surveillance for etiologies of bloodstream infection (BSIs) in hospitalized febrile children in Uganda. We analyzed demographic, clinical, blood culture, and antimicrobial resistance data from hospitalized children at six sentinel AFI sites from July 2016 to January 2019. A total of 47,261 children were hospitalized. Median age was 2 years (interquartile range, 1–4) and 26,695 (57%) were male. Of 7,203 blood cultures, 242 (3%) yielded bacterial pathogens including Salmonella (N = 67, 28%), Staphylococcus aureus (N = 40, 17%), Escherichia spp. (N = 25, 10%), Enterococcus spp. (N = 18, 7%), and Klebsiella pneumoniae (N = 17, 7%). Children with BSIs had longer median length of hospitalization (5 days versus 4 days), and a higher case-fatality ratio (13% versus 2%) than children without BSI (all P < 0.001). Children with Salmonella BSIs did not differ significantly in length of hospitalization or mortality from children with BSI resulting from other organisms. Serotype and antimicrobial susceptibility results were available for 49 Salmonella isolates, including 35 (71%) non-typhoidal serotypes and 14 Salmonella serotype Typhi (Typhi). Among Typhi isolates, 10 (71%) were multi-drug resistant and 13 (93%) had decreased ciprofloxacin susceptibility. Salmonella strains, particularly non-typhoidal serotypes and drug-resistant Typhi, were the most common cause of BSI. These data can inform regional Salmonella surveillance in East Africa and guide empiric therapy and prevention in Uganda.
INTRODUCTION
Invasive Salmonella disease, including typhoid and paratyphoid fever and invasive non-typhoidal salmonellosis (iNTS), is a significant cause of morbidity and mortality in sub-Saharan Africa.1–4 In 2017, an estimated 10.9 million cases of typhoid fever and 116,800 deaths were attributed to Salmonella enterica serotype Typhi (Typhi) globally.5 Sub-Saharan Africa accounted for 15.9% of global deaths resulting from typhoid and paratyphoid fever, and mortality rates were highest among young children.5 An estimated 535,000 cases of iNTS and 77,500 deaths occurred globally, with the highest disease incidence in sub-Saharan Africa (34.5 cases per 100,000 person-years) and in children younger than 5 years old (34.3 cases per 100,000 person-years).6 In resource-limited settings, it has traditionally been challenging to determine the true disease incidence because diagnosis was dependent on the availability of blood culture. However, recently, modeling and high-quality incidence studies have used multipliers or health-care utilization surveys to account for under-ascertainment.7–9
In Uganda, robust surveillance data on the common etiologies of acute febrile illness (AFI) in children are lacking. Several studies have evaluated the contribution of community-acquired bacterial bloodstream infections (BSIs) to non-malarial AFI in hospitalized children. These studies have primarily targeted specific sub-populations, including children younger than 5 years old or children with immunocompromising conditions (severe malnutrition and/or HIV infection).10–12 Additional blood culture-based surveillance data are needed in a broader population of children. The Uganda AFI surveillance system was developed to address these data gaps and expand diagnostic capacity for non-malarial causes of AFI.
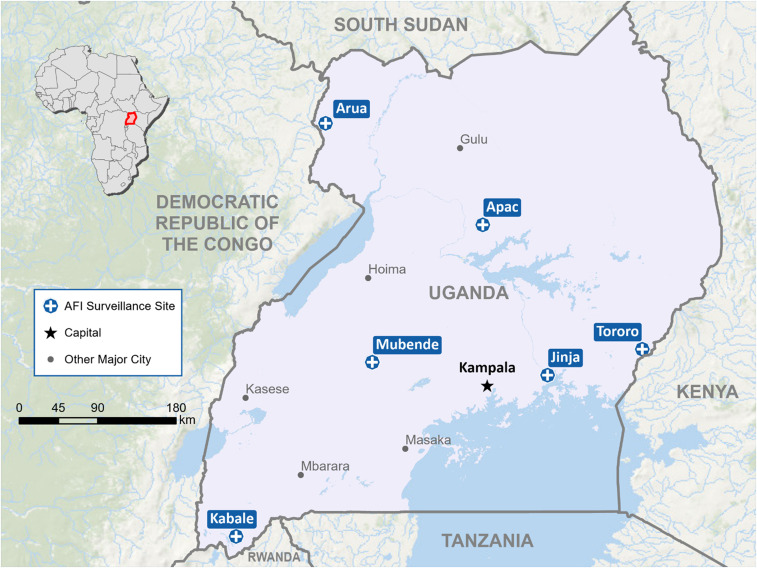
Since 2016, the Uganda AFI surveillance system has conducted sentinel surveillance for non-malarial causes of AFI in hospitalized children ≤ 14 years old across 6 sites in Uganda (Figure 1). The system was developed to build a sustainable laboratory and surveillance capacity for identifying (non-malarial) etiologies of AFI in children. The system was implemented through a consortium of public–private partnerships, including the Uganda Ministry of Health, the Uganda Virus Research Institute, the Infectious Diseases Institute, Makerere University Department of Medical Microbiology (DMM), the Infectious Diseases Research Collaboration, the Health Information Systems Program Uganda, and the U.S. CDC. The AFI surveillance system generated crucial data on the non-malarial etiologies of AFI in hospitalized Ugandan children, evaluating for BSIs, arboviruses (West Nile, yellow fever, dengue, chikungunya, and Zika), and zoonotic diseases (brucellosis, leptospirosis, and rickettsioses). As the preliminary data on Uganda AFI serological surveillance for arboviruses and zoonotic diseases have been presented previously,13 in this report we present a sub-analysis of Uganda AFI surveillance system data from 2016 to 2019 to characterize the incidence, clinical features, outcomes, and antimicrobial resistance (AMR) patterns for Salmonella BSI among febrile hospitalized children in Uganda.
Figure 1.
Acute febrile illness (AFI) surveillance sites— Uganda, 2016–2019. This figure appears in color at www.ajtmh.org.This figure appears in color at www.ajtmh.org.
MATERIALS AND METHODS
AFI surveillance.
The AFI surveillance proposal was approved by the Uganda Ministry of Health and the U.S. CDC, with a CDC non-research determination (NCEZID #031416) for public health surveillance.13 Inpatient surveillance sites included Apac and Tororo District hospitals, and Arua, Kabale, Jinja, and Mubende regional referral hospitals (RRHs) (Figure 1). The RRHs are referral locations for district hospitals, and each served a catchment area of more than 2 million people. Five inpatient sites (Apac, Jinja, Kabale, Mubende, and Tororo) were established previously for malaria surveillance. They were located strategically to monitor the impact of malaria interventions, including insecticidal nets and indoor residual spraying of insecticides, and to represent varying malaria transmission.14,15 An additional inpatient site, Arua, was added for geographic representation in northern Uganda. Site activation occurred between July 2016 and October 2017 (Supplemental Figure 1). Surveillance data collection ended at all sites in January 2019. The detailed development of this surveillance platform and site activations with phased introduction of on-site diagnostic capacity for blood culture and serology have been described previously.13 Briefly, the standard practice at each hospital was to test all children ≤ 14 years old with fever (defined as history of subjective fever or documented temperature ≥ 37.5°C) for malaria using rapid diagnostic testing (RDT) and/or microscopy. The AFI testing algorithm recommended that admitted children with fever and a negative malaria test should be considered for further testing with blood cultures. Testing decisions are ultimately driven by clinicians; therefore, blood cultures were collected outside of the algorithm based on individual clinical judgment. A standardized medical record form and a real-time web-based data management platform, the District Health Information System (DHIS2), were used to streamline epidemiological, clinical, and laboratory data collection across sites.
Antimicrobial treatment decisions were also at clinician discretion and in accordance with national guidelines. Per the 2016 Uganda Clinical Guidelines, the first-line agent for treatment of typhoid fever was ciprofloxacin, with chloramphenicol, ceftriaxone (for severe disease), and amoxicillin (in pregnancy) as alternatives. The recommended first-line agents for children with uncomplicated malaria were artemisinin-based combination therapies. For severe malaria, the first-line agent was IV artesunate, with quinine or artemether as alternatives.
We reviewed Uganda AFI surveillance system data for patients with culture-confirmed Salmonella BSIs from July 2016 through January 2019. Data obtained from the DHIS2 included demographic (age and gender), clinical (symptom history, admission diagnosis, antimicrobial therapy, length of hospital stay, discharge diagnosis, and disposition) and laboratory (malaria testing results and blood culture results) information.
Microbiological methods.
Blood samples were inoculated into BD BACTECTM Peds PlusTM medium bottles (Becton, Dickinson and Company, Franklin Lakes, NJ), with collection volume up to 3 mL/bottle dependent on patient age as follows: 1 mL for ≤ 1 year old, 2 mL for 2 to 3 years old, and 3 mL for ≥ 3 years old. Inoculated blood bottles were incubated on-site using the BACTEC 9050 System or transported to a central laboratory at DMM in Kampala. Before installation of on-site BACTEC 9050 Systems, or if on-site systems became non-functional, all blood cultures were processed centrally in Kampala. Positive bottles were transported to the central laboratory for identification and antimicrobial susceptibility testing (AST). Isolates were identified using standard microbiological methods, and, in the case of Salmonella species, sero-grouping and serotyping were performed. In addition, 49 Salmonella isolates were shipped to the CDC, in Atlanta, GA, for confirmatory identification, serotyping, and AST. The isolates were tested for susceptibility to 14 antimicrobial agents: amoxicillin/clavulanic acid, ampicillin, azithromycin, cefoxitin, chloramphenicol, ceftriaxone, ciprofloxacin, gentamicin, meropenem, nalidixic acid, streptomycin, sulfisoxazole, tetracycline, and trimethoprim-sulfamethoxazole using the broth microdilution method (SensititreTM; Thermo Fisher Scientific, Grand Island, NY) according to the manufacturer’s instructions. The criteria used to categorize minimum inhibitory concentration results are based on current breakpoints provided by the Clinical and Laboratory Standards Institute.16
Definition of terms.
Blood culture and AMR data for Salmonella isolates were obtained from a composite of three sources: the DHIS2, review of DMM laboratory records, and CDC serotyping and AST summary reports. Patients with evidence of bacterial pathogen growth from blood culture were considered to have BSI. Patients with no bacterial growth from blood culture were defined as patients without BSI. Growth of any of the following organisms was considered a contaminant: Bacillus spp., coagulase-negative Staphylococcus, Corynebacterium spp., Micrococcus spp., Rhodococcus spp., or viridans group streptococci. For Salmonella, multi-drug resistance (MDR) was defined as resistance to at least one antimicrobial in three or more drug classes.17 Nalidixic acid-resistant or ciprofloxacin intermediate isolates were considered to have decreased ciprofloxacin susceptibility (DCS). Malaria positivity was defined as a positive RDT or blood smear. For children with Salmonella BSIs, we used patient identifiers to link clinical and blood culture data manually from the DHIS2, DMM, and CDC identifiers included: specimen identification numbers, patient name, age, gender, and hospitalization and specimen collection dates.
Data analysis.
Analyses were conducted using SAS v9.4 software (SAS Institute, Cary, NC). Demographic and clinical characteristics of the children were presented and compared across age categories (< 1, 1–4, and 5–14 years old), BSI status (positive or negative), and BSI pathogen (Salmonella or non-Salmonella). We also determined the cumulative frequency of inpatient prescribed antimicrobials and antimalarials for children with BSIs. In all comparisons, we used χ2 or Fisher’s exact test as appropriate for categorical variables. For comparisons of continuous variables, we used the Wilcoxon rank-sum and Kruskal-Wallis tests as appropriate.
RESULTS
Baseline characteristics.
From July 1, 2016 to January 31, 2019, there were 47,261 pediatric admissions across the six surveillance hospitals (Table 1). The median age was 2 years old (interquartile range [IQR], 1–4), with 26,695 (57%) admissions among males, and median length of hospitalization was 3 days (range, 2–5 days). The most common presenting symptoms were fever (N = 40,876, 87%) and cough (N = 29,067, 62%). Fewer hospitalized patients presented with vomiting (N = 15,417, 33%), diarrhea (N = 12,354, 27%), or difficulty breathing (N = 10,719, 23%). Malaria positivity among tested patients was 44%, ranging between 2% and 66% across hospitals (Supplemental Table 1). The most common discharge diagnoses were malaria (N = 14,979, 39%), pneumonia (N = 7,410, 19%), and sepsis (N = 7,229, 19%). Use of antimicrobials or antimalarials in the week before admission was self-reported by 6,483 (15%) and 8,100 (18%) of patients, respectively (Table 1). Among 4,220 (8.9%) patients with data on HIV status, 203 (4.8%) were HIV seropositive. Death occurred in 1,691 (4%) of the admissions.
Table 1.
Demographic and clinical characteristics of pediatric admissions by age category
| Characteristic | All admissions | < 1 y old | 1–4 y old | 5–14 y old | P value |
|---|---|---|---|---|---|
| Total admission, N (%) | 47,261 | 11,675 (25) | 24,629 (52) | 10,957 (23) | < 0.001 |
| LOS, d; median (IQR) | 3 (2–5) | 3 (2–5) | 3 (2–5) | 3 (2–5) | |
| Gender, N (%) | – | – | – | – | 0.032 |
| Male | 26,695/47,043 (57) | 6,666 (57) | 13,951 (57) | 6,078 (56) | |
| Female | 20,348/47,043 (43) | 4,958 (43) | 10,556 (43) | 4,834 (44) | |
| Symptoms, N (%) | |||||
| Fever | 40,876/46,898 (87) | 9,679 (84) | 21,906 (89) | 9,291 (85) | < 0.001 |
| Vomiting | 15,417/46,430 (33) | 3,856 (34) | 7,976 (33) | 3,585 (33) | 0.131 |
| Diarrhea | 12,354/46,430 (27) | 4,558 (40) | 6,503 (27) | 1,293 (12) | < 0.001 |
| Bloody diarrhea | 1,510/46,238 (3) | 402 (4) | 805 (3) | 303 (3) | 0.006 |
| Cough | 29,067/46,528 (62) | 7,586 (67) | 15,806 (65) | 5,675 (53) | < 0.001 |
| Difficulty breathing | 10,719/46,421 (23) | 3,886 (34) | 5,250 (22) | 1,583 (15) | < 0.001 |
| Convulsions | 4,634/46,296 (10) | 924 (8) | 2,888 (12) | 822 (8) | < 0.001 |
| Altered consciousness | 814/45,798 (2) | 157 (1) | 456 (2) | 201 (2) | 0.003 |
| Admission diagnosis, N (%) | |||||
| Anemia | 8,013/44,137 (18) | 1,120 (10) | 4,209 (18) | 2,684 (27) | < 0.001 |
| Pneumonia | 9,077/44,137 (21) | 3,552 (33) | 4,543 (20) | 982 (10) | < 0.001 |
| Respiratory infection | 5,783/44,137 (13) | 1,359 (13) | 3,320 (14) | 1,104 (11) | < 0.001 |
| Sepsis | 8,881/44,137 (20) | 2,559 (24) | 4,448 (19) | 1,874 (19) | < 0.001 |
| Sickle cell | 2,328/44,137 (5) | 94 (1) | 887 (4) | 1,347 (13) | < 0.001 |
| Typhoid fever | 76/44,137 (0.17) | 2 (0) | 22 (0) | 52 (1) | < 0.001 |
| Diarrhea | 6,205/44,137 (14) | 2,671 (25) | 3,148 (14) | 386 (4) | < 0.001 |
| Malaria | 19,620/44,137 (44) | 2,840 (26) | 11,270 (48) | 5,510 (55) | < 0.001 |
| Malnutrition | 2,369/44,137 (5) | 601 (6) | 1,557 (7) | 211 (2) | < 0.001 |
| Discharge diagnosis, N (%) | |||||
| Anemia | 6,173/38,895 (16) | 832 (9) | 3,274 (16) | 2,067 (23) | < 0.001 |
| Pneumonia | 7,410/38,895 (19) | 2,839 (30) | 3,751 (18) | 820 (9) | < 0.001 |
| Respiratory infection | 3,976 /38,895 (10) | 932 (10) | 2,360 (11) | 684 (8) | < 0.001 |
| Sepsis | 7,229/38,895 (19) | 1,964 (21) | 3,668 (18) | 1,597 (18) | < 0.001 |
| Sickle cell | 2,416/38,895 (6) | 93 (1) | 904 (4) | 1,419 (16) | < 0.001 |
| Typhoid fever | 57/38,895 (0.15) | 5 (0.01) | 21 (0.05) | 31 (0.08) | < 0.001 |
| Diarrhea | 4,796/38,895 (12) | 2,202 (24) | 2,349 (11) | 245 (3) | < 0.001 |
| Malaria | 14,979/38,895 (39) | 1,956 (21) | 8,570 (42) | 4,453 (49) | < 0.001 |
| Malnutrition | 1,887/38,895 (5) | 497 (5) | 1,232 (6) | 158 (2) | < 0.001 |
| Malaria positivity, N (%) | – | – | – | – | < 0.001 |
| Positive | 19,128/43,892 (44) | 2,633 (25) | 11,132 (48) | 5,363 (53) | – |
| Negative | 23,455/43,892 (53) | 7,440 (71) | 11,619 (50) | 4,396 (44) | – |
| Not tested | 1,309/43,892 (3) | 387 (4) | 612 (3) | 310 (3) | – |
| Antimicrobial before admission, N (%) | 6,483/44,401 (15) | 1,892 (17) | 3,163 (14) | 1,428 (14) | < 0.001 |
| Antimalarial before admission, N (%) | 8,100/44,284 (18) | 1,249 (11) | 4,530 (20) | 2,321 (23) | < 0.001 |
| HIV status, N (%) | – | – | – | – | < 0.001 |
| HIV seropositive | 203/4,220 (5) | 32 (1) | 106 (1) | 65 (2) | – |
| HIV seronegative | 4,017/4,220 (24) | 986 (25) | 2,130 (23) | 901 (24) | – |
| Not tested | 12,840/17,060 (75) | 2,853 (74) | 7,217 (76) | 2,770 (74) | – |
| Died, N (%) | 1,691/43,805 (4) | 613 (6) | 741 (3) | 337 (3) | < 0.001 |
IQR = interquartile range; LOS = length of stay in the hospital.
Presentation by age categories.
Most hospital admissions were children 1 to 4 years old (24,629, 52%) compared with infants younger than 1 year (11,675, 25%), and children 5 to 14 years old (10,957, 23%; Table 1). Fever, cough, and vomiting were the most common presenting symptoms within each age group. The prevalence of clinical symptoms (except vomiting) was statistically different by the three age groups (Table 1). Malaria was the most common discharge diagnosis for 1- to 4-year-olds (N = 8,570, 42%) and 5- to 14-year-olds (N = 4,453, 49%), whereas pneumonia was the most common discharge diagnosis for infants (N = 2,839, 30%). A greater proportion of infants died (N = 613, 6%) compared with children in all other age groups (N = 741 [3%] and 337 [3%]; P < 0.001).
Bloodstream infections.
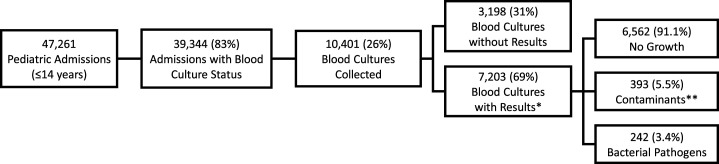
Of 47,261 pediatric admissions, 39,344 (83%) had available data on whether blood cultures were collected (Figure 2). Among these, 10,401 (26%) had blood cultures indicated as collected. Of 7,203 collected blood cultures for which results were available, 6,563 (91%) showed no growth, 393 (5.4%) yielded a contaminant, and 242 (3.4%) yielded one or more pathogens. The contamination rates across five surveillance hospitals ranged from 1% to 7%; however, in the sixth AFI site, with few blood culture results available, the contamination rate was 52% (42/81) (Supplemental Table 1). Among 242 positive blood cultures, the most frequently isolated pathogens were Salmonella (N = 67, 28%), Staphylococcus aureus (N = 40, 17%), Escherichia spp. (N = 25, 10%), Enterococcus spp. (N = 18, 7%), and Klebsiella pneumoniae (N = 17, 7%) (Supplemental Table 2).
Figure 2.
Flow diagram of blood culture results.
Children with BSIs had longer median length of hospitalization than children without BSIs (5 days versus 4 days, P < 0.001; Table 2). There were no statistically significant differences between the two groups by age or frequency of self-reported antimicrobial use during the week before admission. Presenting with difficulty breathing (N = 44, 20%) and discharge diagnosis of pneumonia (N = 31, 17%) were both less common among the 237 children with BSIs compared with among the 6,563 children without BSIs (N = 1,916, 30% and N = 1,572, 29%, respectively; both P < 0.001). Conversely, a discharge diagnosis of malnutrition (N = 22, 12%) was more common in children with BSIs, than in children without BSIs (N = 302, 6%, P < 0.001). Sepsis was the most common discharge diagnosis in both groups, reported in 64 (36%) children with BSIs and 1,619 (30%) of children without BSIs (P = 0.089). Thirty-two (14%) of the admissions with BSIs had a positive malaria test. None of the admissions with BSIs were reported to be HIV seropositive. In-hospital mortality was more common among children with BSIs than among children without BSIs (N = 28, 13% versus N = 145, 2%; P < 0.001). The most frequently prescribed antimicrobials for admissions with BSIs were gentamicin (N = 109, 46%), ceftriaxone (N = 31, 13%), and ampicillin (N = 9, 4%); artesunate, an antimalarial, was prescribed for 64 (27%) admissions with BSIs. No use of azithromycin, fluoroquinolones, or meropenem was reported.
Table 2.
Clinical characteristics of pediatric admissions based on bloodstream infection and Salmonella bloodstream infection status
| Characteristic | Without BSI (N = 6,563) | With BSI (N = 237) | P value | Non-Salmonella BSI (N = 170) | Salmonella BSI* (N = 44) | P value |
|---|---|---|---|---|---|---|
| Age, median (IQR) | 1 (0–4) | 1 (0–3) | 0.7791 | 1 (0–3) | 3 (1–5.5) | < 0.001 |
| LOS, d; median (IQR) | 4 (3–6) | 5 (4–9) | < 0.001 | 5 (3–9) | 5.5 (4–10) | 0.2541 |
| Age group, y; n (%) | – | – | 0.5190 | – | – | 0.0030 |
| < 1 | 1,872 (29) | 62 (26) | 55 (32) | 4 (9) | ||
| 1–4 | 3,352 (51) | 130 (55) | 90 (53) | 27 (61) | ||
| 5–14 | 1,339 (20) | 45 (19) | 25 (15) | 13 (30) | ||
| Gender, n (%) | – | – | 0.0305 | – | – | 0.2342 |
| Male | 3,694 (56) | 149 (63) | 102 (61) | 31 (70) | ||
| Female | 2,869 (44) | 86 (37) | 66 (39) | 13 (30) | ||
| Symptoms, n (%) | ||||||
| Fever | 6,264 (96) | 221 (94) | 0.1251 | 154 (92) | 44 (100) | 0.0456† |
| Vomiting | 2,213 (34) | 68 (30) | 0.1620 | 44 (27) | 16 (36) | 0.2431 |
| Diarrhea | 1,903 (30) | 71 (31) | 0.5549 | 45 (28) | 18 (41) | 0.1041 |
| Bloody diarrhea | 220 (3) | 6 (3) | 0.5348 | 6 (4) | 0 (0) | 0.3435† |
| Cough | 4,566 (71) | 148 (65) | 0.0509 | 105 (65) | 31 (70) | 0.4837 |
| Difficulty breathing | 1,916 (30) | 44 (20) | < 0.001 | 38 (23) | 6 (14) | 0.1639 |
| Convulsions | 474 (7) | 25 (11) | 0.0442 | 19 (12) | 5 (11) | 0.9467 |
| Discharge diagnosis, n (%) | ||||||
| Anemia | 550 (10) | 23 (13) | 0.2385 | 16 (13) | 7 (19) | 0.3299 |
| Pneumonia | 1,572 (29) | 31 (17) | < 0.001 | 26 (20) | 3 (8) | 0.0828 |
| Respiratory infection | 664 (12) | 19 (11) | 0.5137 | 13 (10) | 4 (11) | NA*† |
| Sepsis | 1,619 (30) | 64 (36) | 0.0893 | 46 (36) | 12 (32) | 0.6715 |
| Sickle cell | 510 (9) | 13 (7) | 0.3336 | 10 (8) | 1 (3) | 0.4587† |
| Typhoid fever | 9 (0) | 18 (10) | < 0.001† | 1 (1) | 11 (30) | < 0.001† |
| Diarrhea | 974 (18) | 20 (11) | 0.0195 | 14 (11) | 3 (8) | 0.7652† |
| Malaria | 363 (7) | 19 (11) | 0.0404 | 5 (4) | 11 (30) | < 0.001† |
| Malnutrition | 302 (6) | 22 (12) | < 0.001 | 20 (16) | 2 (5) | 0.1678† |
| Malaria co-infection, n (%) | – | – | < 0.001 | – | – | 0.001† |
| Positive | 331 (5) | 32 (14) | 13 (8) | 12 (27) | ||
| Negative | 6,077 (95) | 192 (85) | 147 (91) | 30 (68) | ||
| Not tested | 0 (0) | 3 (1) | 1 (1) | 2 (5) | ||
| HIV status, n (%) | – | – | < 0.001 | – | – | 0.1797 |
| HIV seropositive | 27 (2) | 0 (0) | 0 (0) | 0 (0) | ||
| HIV seronegative | 843 (72) | 29 (22) | 23 (26) | 4 (14) | ||
| Not tested | 305 (26) | 103 (78) | 66 (74) | 25 (86) | ||
| Antimicrobial before admission, n (%) | 1,042 (16) | 37 (17) | 0.9277 | 23 (15) | 8 (19) | 0.5255 |
| Antimalarial before admission, n (%) | 898 (14) | 51 (23) | < 0.001 | 22 (14) | 19 (44) | < 0.001 |
| Died, n (%) | 145 (2) | 28 (13) | < 0.001 | 24 (15) | 3 (7) | 0.1614 |
BSI = bloodstream infection; IQR = interquartile range; LOS = length of stay in the hospital; NA = not applicable.
Isolates with confirmatory serotype and antimicrobial susceptibility testing conducted at the CDC, and matched to clinical data. Excludes 23 Salmonella spp. isolates without confirmatory testing (N = 18) or linkage to clinical data (N = 5).
Fisher’s exact test.
Salmonella BSI.
Of 67 Salmonella isolates, 49 were submitted for confirmatory identification, serotyping, and minimum inhibitory concentration testing (Table 3). Thirty-five isolates were NTS serotypes (21 Enteritidis, 13 Typhimurium, and one I 4,5,12:i:-) and 14 isolates were serotype Typhi. Of the Enteritidis and Typhimurium isolates, 19 (56%) of 34 were MDR and one (3%) was MDR with DCS (Table 3). The most common AMR pattern was resistance to ampicillin, chloramphenicol, streptomycin, sulfisoxazole, tetracycline, and trimethoprim-sulfamethoxazole, which was seen for 17 of 21 (81%) of Enteritidis isolates. Most Typhimurium isolates (N = 11, 84%) were susceptible to all tested antimicrobials. The 14 Salmonella Typhi isolates were from five of the six sites. The most common resistance pattern observed for Typhi was resistance to ampicillin, chloramphenicol, streptomycin, sulfisoxazole, nalidixic acid, and trimethoprim-sulfamethoxazole (10 isolates). Nine (64%) of 14 Typhi isolates were MDR with DCS, one (7%) was MDR only, and four (29%) had DCS only (Table 3). All isolates were susceptible to ceftriaxone, azithromycin, or meropenem.
Table 3.
Antimicrobial resistance pattern by Salmonella serotype (N = 49)
| Serotype | N | MDR only* (N = 20), N (%) | DCS only† (N = 4), N (%) | Both MDR and DCS (N = 10), N (%) | No resistance (N = 15), N (%) |
|---|---|---|---|---|---|
| Non-typhoidal | 35 | 19 (54) | 0 (0) | 1 (3) | 15 (43) |
| Enteritidis | 21 | 17 (81) | 0 (0) | 0 (0) | 4 (19) |
| Typhimurium | 13 | 1 (8) | 0 (0) | 1 (8) | 11 (84) |
| I 4,[5],12:i:- | 1 | 1 (100) | 0 (0) | 0 (0) | 0 (0) |
| Typhi | 14 | 1 (7) | 4 (29) | 9 (64) | 0 (0) |
DCS = decreased ciprofloxacin susceptibility; MDR = multi-drug resistant.
MDR isolates exhibited resistance to at least one antibiotic in three or more drug classes. Nineteen non-typhoidal isolates were resistant to ampicillin, chloramphenicol, and trimethoprim-sulfamethoxazole. One Salmonella enteritidis isolate was resistant to ampicillin, streptomycin, sulfisoxazole, and tetracycline, but susceptible to chloramphenicol and trimethoprim-sulfamethoxazole.
Isolates with DCS exhibited resistance to nalidixic acid and were intermediate to ciprofloxacin.
Overall, 8 (19%) admissions with Salmonella BSIs were treated with ceftriaxone, which is considered appropriate antimicrobial therapy. However, more admissions were treated with gentamicin (N = 11, 26%) or ampicillin (N = 8, 18%), which were likely ineffective. Compared with children admitted with non-Salmonella BSIs, children admitted with Salmonella BSIs were older (median age, 3 years versus 1 year; P < 0.001), and higher proportions reported receiving antimalarials (N = 19, 44% versus N = 22, 14%; P < 0.001) and antimicrobials (N = 8, 19% versus N = 23, 15%) in the week before admission. Malaria co-infection occurred in 13 (8%) patients with non-Salmonella BSIs compared with 12 (27%) patients with Salmonella BSIs (P = 0.001). The two groups did not differ by gender, clinical symptoms (apart from fever), length of hospitalization, or mortality.
Of 49 confirmed Salmonella isolates, 44 (90%) were linked with patient clinical data (Supplemental Table 3). Median age was 3 years (IQR, 1–5.5 years) and 70% were male. Patients with iNTS were younger than those with typhoid fever (median age, 2 years; IQR, 1–3 years versus median age, 5 years; IQR, 2–8 years) (Supplemental Table 3). All 44 patients with Salmonella BSIs presented with fever. Cough (N = 31, 70%), followed by diarrhea (N = 18, 41%) and vomiting (N = 16, 36%), were other frequently reported symptoms (Table 2). None had bloody diarrhea. Median length of hospitalization was 5.5 days (IQR, 4–10 days).
Three (10%) of 31 patients with NTS BSIs and available clinical data died; two of the three had malaria co-infection and required blood transfusion (Supplemental Table 3). All three were male and presented with fever, cough, and diarrhea, and received antimalarials preceding admission. The youngest decedent, a 1-year-old, was hospitalized for 6 days in 2016 and had also presented with convulsions. He had MDR Salmonella Enteritidis BSI and malaria co-infection and was treated with intravenous artesunate and paracetamol; no antimicrobials were recorded as given. Cause of death was listed as septicemia and severe malaria. A 7-year-old decedent, also with malaria co-infection, was hospitalized for 16 days in 2017. He had MDR Salmonella Enteritidis BSI and was treated with artesunate, penicillin, and amoxicillin. Causes of death were listed as severe malaria, anemia, sickle cell disease, and respiratory infection. Last, a 3-year-old decedent was hospitalized for 1 day in 2017 with a diagnosis of pneumonia. His blood culture yielded Salmonella Typhimurium that was susceptible to all tested antimicrobials. He was treated with artesunate, gentamicin, and ampicillin; no cause of death was listed. All 14 admissions with Salmonella serotype Typhi BSI recovered.
DISCUSSION
Bacterial BSIs are a leading cause of severe febrile illness in children in sub-Saharan Africa.3,18 Our study found bacterial pathogens in 3% of 7,203 blood cultures from children hospitalized at six sentinel hospitals. The proportion of BSIs attributable to recovered pathogens was similar to findings in other hospital-based surveillance studies among febrile pediatric patients in East Africa.19–26 Median prevalence of BSIs of 14.6% (range, 3.4–38.2%) was calculated in a recent systematic review and meta-analysis of community-acquired blood stream infection (Co-BSI) studies among 19,838 febrile hospitalized patients in nine countries in Africa.4 Children ≤ 15 years old accounted for 11,078 (55.8%) of patients analyzed.
Adult and pediatric Co-BSI studies in Uganda found a prevalence of 24% and 18%, respectively, among 305 adult (15–65 years old) and 250 pediatric (< 5 years old) febrile patients admitted to an urban tertiary hospital.11,27 In the pediatric study, eligibility was limited to children with negative malaria smears and no history of recent antimicrobial use. The lower detection of BSIs in our study may have been the result of prior antimicrobial use (reported for 16% of children with BSIs) and challenges with blood volume collection, particularly in malnourished patients, and clinician-directed collection of blood culture samples.
Co-BSI has been associated with high mortality among hospitalized African children.28–30 In our study, children with BSIs had higher proportions of malaria co-infection and worse outcomes than children without BSIs, including a longer median duration of hospitalization and a higher mortality ratio. The higher proportions of malaria co-infection should be interpreted with caution as the decision to obtain a blood culture was clinician directed, and testing practices varied by site. In addition, given the suggested testing algorithm, few patients with positive malaria test had blood cultures performed, which may have selected for testing in patients with more clinically severe disease. In the algorithm, we recommended only testing those patients without a known diagnosis of malaria based on resource allocation and the limited number of blood culture bottles available per site. None of the children with BSIs were confirmed or known to be HIV seropositive, but only 12% had documented test results.
Salmonella was the most commonly isolated pathogen (28%) in our study, with predominance of NTS serotypes Enteritidis and Typhimurium among the subset of strains that were serotyped. Among non-Salmonella pathogens, Staphylococcus aureus and Escherichia spp. were isolated most frequently. These results are consistent with findings from the Typhoid Fever Surveillance in Africa Program (TSAP) study; Salmonella were the primary pathogens isolated from blood culture of 13,431 febrile hospitalized patients from across 13 sub-Saharan African sentinel sites in 10 countries from 2010 to 2014.9 In contrast to our findings, a higher proportion of Typhi (24%) were identified compared with NTS (17%) in the TSAP study, which may be reflective of the non-random selection of TSAP sites, with priority given to locations with reported typhoid fever occurrence.31 Staphylococcus aureus (12%), E. coli (8%), and S. pneumoniae (8%) were the other frequently isolated pathogens in the TSAP study. Similarly, in the meta-analysis of Co-BSIs in 24 African studies, NTS (29.5%) was the most common pathogen isolated, followed by S. pneumoniae and E. coli.4
In African children, NTS infections have been associated with several risk factors, including young age, malnutrition, sickle cell disease, severe anemia, HIV, and malaria infection.32–40 In our study, children with and without Salmonella BSIs did not differ by age group or frequency of diagnoses of malnutrition, sickle cell disease, anemia, and HIV infection. However, the median age of children with NTS BSI was younger than that of children with Typhi BSI. Multiple observational studies have shown a positive correlation between the malaria transmission intensity in an area and the incidence of NTS, and conversely, a negative correlation with the incidence of typhoid fever.41–44 Notably, in our study, 81% of NTS isolates were detected from one site with high malaria transmission intensity. Arua, in northwest Uganda, has an estimated malaria prevalence of more than 60% and an entomological inoculation rate of more than 100 infective mosquito bites per person per year.45,46 We found high proportions of AMR among Salmonella isolates; 57% of NTS and 71% of Typhi were MDR. A previous AMR study from Uganda also found MDR in 50% of NTS and 60% of Typhi.26
In addition, we detected DCS in 3% of NTS and in 93% of Typhi isolates. Typhi strains with DCS have been reported sporadically in sub-Saharan Africa, including from Kenya, the Democratic Republic of the Congo, South Africa, and Zimbabwe.47–50 In the TSAP study, 48% of NTS and 47% of Typhi strains were MDR, whereas DCS was uncommon (3% of NTS and 9% of Typhi).9 Notably, there was a greater prevalence of MDR Typhi in East Africa (Kenya and Tanzania), as we found in our study, than in many parts of West Africa.9,47
Regional heterogeneity in Typhi AMR patterns has been attributed to the emergence and expansion of the MDR H58 haplotype (genotype 4.3.1) from South Asia into East Africa.51–54 Recent genomic and phylogenetic analyses have further shown that some MDR 4.3.1 Typhi strains in Kenya and Uganda now share mutations that confer reduced susceptibility to fluoroquinolones, raising concern for the rapid spread of ciprofloxacin-resistant Typhi across East Africa.51,55 In our study, Enteritidis strains exhibited MDR phenotypes, but none demonstrated DCS. The sequencing data from the isolates in our study will be published separately. In NTS strains, genomic analyses have also detected emergence of MDR Salmonella Typhimurium strains (sequence type ST313), including strains that have acquired extended spectrum beta lactamase resistance. These Typhimurium strains, with conferred ceftriaxone resistance, have been reported sporadically from Kenya, Malawi, and the Democratic Republic of the Congo.56–59 Globally, Salmonella strains continue increasingly to acquire antimicrobial resistance, highlighting the urgent need for global AMR surveillance to inform antimicrobial treatment. MDR has been widespread in Asia and Africa since the 1990s.51 As empiric treatment guidelines changed correspondingly from the former first-line agents to ciprofloxacin, widespread fluoroquinolone resistance emerged. Alarmingly, in 2016, the first outbreak of an extensively drug-resistant Typhi strain (with MDR, ciprofloxacin and ceftriaxone resistance) emerged in Pakistan.60–65 In 2018, another outbreak of highly drug-resistant Typhi (with ciprofloxacin and ceftriaxone resistance) emerged in Iraq.66
Our findings had several limitations. First, admission practices varied by site, and blood culture testing was clinician directed, which may have introduced selection bias. Venipunctures were difficult to obtain in some pediatric patients, and we did not measure blood culture volumes systematically to ensure adequate samples. These factors likely contributed to the high proportion of blood cultures without results or with no growth (Figure 2). Not all sites were activated for surveillance at the same time, and stockouts in blood culture collection materials meant blood culture testing was available inconsistently across the sites during the study period (Supplemental Figure 2). At the time of this analysis, for non-Salmonella isolates, identification to the level of species, subspecies, or serotype was dependent on the laboratory capacity in-country. Because of the unavailability of reagents or the difficulty in performing phenotypic identification, species-level identification was not available for many organisms.
For some patients, surveillance data were incomplete, of variable quality, or unavailable. History of prior antimicrobial or antimalarial use was self-reported, and HIV serological (antibody) testing was not performed routinely. Apart from admission and discharge diagnoses, we did not capture data on underlying chronic medical conditions associated with NTS infections. Although we did not evaluate the timing and selection of antimicrobials in relation to blood culture results, it is likely that many treatment decisions were empiric and not based on targeted therapy. Anecdotally, delays in specimen transport and processing led to slow turnaround in reporting of blood culture results. As 32% of children with Salmonella bacteremia had discharge diagnoses of sepsis, this could explain the preponderance of (likely empiric) treatment with ampicillin and gentamicin in this group. Given the high rates of MDR at our sites, ampicillin was likely ineffective therapy for children with Salmonella bacteremia. Gentamicin, even if susceptible in vitro, should not be used to treat invasive Salmonella given its poor penetration of intracellular sites of infection.67
Despite these limitations, our findings provide valuable data on the clinical features and AMR patterns among hospitalized Ugandan children with Salmonella BSIs, including the detection of MDR Salmonella strains. In strengthening blood culture-based diagnostic capacity, the AFI surveillance system addresses a critical gap in the detection of non-malarial causes of fever in hospitalized Ugandan children.
Invasive Salmonella, predominantly NTS serotypes, were the leading cause of bacterial BSIs among Ugandan children hospitalized with AFI. High proportions of antimicrobial resistance were detected in both NTS and Typhi strains, consistent with regional MDR phenotypes reported in East Africa. The increasing global threat of AMR limits treatment options for potentially fatal Salmonella BSIs in children, and empiric treatment guidelines should be informed by local AMR data. This highlights the urgent need for improved capacity for blood culture and AST in sub-Saharan Africa and other lower resource settings. These data can inform regional Salmonella AMR surveillance and more targeted use of antimicrobials and prevention measures, including typhoid vaccines, in Uganda.
Supplemental tables
Acknowledgments:
We thank the following individuals and institutions for their contributions to project management, coordination, and/or surveillance: hospital staff and surveillance officers at Arua, Jinja, Kabale, and Mubende RRHs and Tororo and Apac District hospitals; Vance Brown and Jaco Homsy from CDC Uganda; Hannington Tasimwa from the Department of Medical Microbiology at Makerere University; Ray Ransom and George Odongo from the CDC Division of Global Health Protection; Moses Kamaya from Makerere University; Richard Walwema from the Infectious Disease Institute; Ruth Kigozi from the Infectious Disease Research Collaboration; Prosper Behumbiize from Health Information Systems Program Uganda; the Uganda Ministry of Health; and the Uganda National Health Laboratories. We also thank Graeme Prentice-Mott from the CDC Division of Foodborne, Waterborne, and Environmental Diseases for assistance in data replication, and Jeff Higgins from the CDC Geospatial Research, Analysis, and Services Program for mapping support.
Note: Supplemental tables and figures appear at www.ajtmh.org.
REFERENCES
- 1.Crump JA, Youssef FG, Luby SP, Wasfy MO, Rangel JM, Taalat M, Oun SA, Mahoney FJ, 2003. Estimating the incidence of typhoid fever and other febrile illnesses in developing countries. Emerg Infect Dis 9: 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crump JA, 2012. Typhoid fever and the challenge of nonmalaria febrile illness in sub-Saharan Africa. Clin Infect Dis 54: 1107–1109. [DOI] [PubMed] [Google Scholar]
- 3.Prasad N, Murdoch DR, Reyburn H, Crump JA, 2015. Etiology of severe febrile illness in low-and middle-income countries: a systematic review. PLoS One 10: e0127962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marchello CS, Dale AP, Pisharody S, Rubach MP, Crump JA, 2019. Prevalence of community-acquired bloodstream infections among hospitalized patients in Africa and Asia: a systematic review and meta-analysis. Antimicrob Agents Chemother 64: e01974–e019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanaway JD, Reiner RC, Blacker BF, Goldberg EM, Khalil IA, Troeger CE, Andrews JR, Bhutta ZA, Crump JA, Im J, 2019. The global burden of typhoid and paratyphoid fevers: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis 19: 369–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanaway JD, Parisi A, Sarkar K, Blacker BF, Reiner RC, Hay SI, Nixon MR, Dolecek C, James SL, Mokdad AH, 2019. The global burden of non-typhoidal Salmonella invasive disease: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis 19: 1312–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crump JA, 2019. Progress in typhoid fever epidemiology. Clin Infect Dis 68: S4–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrews JR, Barkume C, Yu AT, Saha SK, Qamar FN, Garrett D, Luby SP, 2018. Integrating facility-based surveillance with healthcare utilization surveys to estimate enteric fever incidence: methods and challenges. J Infect Dis 218: S268–S276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marks F, Von Kalckreuth V, Aaby P, Adu-Sarkodie Y, El Tayeb MA, Ali M, Aseffa A, Baker S, Biggs HM, Bjerregaard-Andersen M, 2017. Incidence of invasive Salmonella disease in sub-Saharan Africa: a multicentre population-based surveillance study. Lancet Glob Health 5: e310–e323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachou H, Tylleskär T, Kaddu-Mulindwa DH, Tumwine JK, 2006. Bacteraemia among severely malnourished children infected and uninfected with the human immunodeficiency virus-1 in Kampala, Uganda. BMC Infect Dis 6: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kibuuka A, Byakika-Kibwika P, Achan J, Yeka A, Nalyazi JN, Mpimbaza A, Rosenthal PJ, Kamya MR, 2015. Bacteremia among febrile Ugandan children treated with antimalarials despite a negative malaria test. Am J Trop Med Hyg 93: 276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musiime V, Cook A, Bakeera-Kitaka S, Vhembo T, Lutakome J, Keishanyu R, Prendergast AJ, Lubwama S, Robertson V, Hughes P, 2013. Bacteremia, causative agents and antimicrobial susceptibility among HIV-1-infected children on antiretroviral therapy in Uganda and Zimbabwe. Pediatr Infect Dis J 32: 856–862. [DOI] [PubMed] [Google Scholar]
- 13.Lamorde M, Mpimbaza A, Walwema R, Kamya M, Kapisi J, Kajumbula H, Sserwanga A, Namuganga JF, Kusemererwa A, Tasimwa H, 2018. A cross-cutting approach to surveillance and laboratory capacity as a platform to improve health security in Uganda. Health Secur 16: S76–S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nankabirwa JI, Arinaitwe E, Rek J, Kilama M, Kizza T, Staedke SG, Rosenthal PJ, Rodriguez-Barraquer I, Briggs J, Greenhouse B, 2020. Malaria transmission, infection, and disease following sustained indoor residual spraying of insecticide in Tororo, Uganda. Am J Trop Med Hyg 103: 1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raouf S, Mpimbaza A, Kigozi R, Sserwanga A, Rubahika D, Katamba H, Lindsay SW, Kapella BK, Belay KA, Kamya MR, 2017. Resurgence of malaria following discontinuation of indoor residual spraying of insecticide in an area of Uganda with previously high-transmission intensity. Clin Infect Dis 65: 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute , 2020. Performance Standards for Antimicrobial Susceptibility Testing. Supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute. [Google Scholar]
- 17.National Antimicrobial Resistance Monitoring System , 2019. Glossary of Terms Related to Antibiotic Resistance. Atlanta, GA:Centers for Disease Control. Available at: https://www.cdc.gov/narms/resources/glossary.html. Accessed May 12, 2021.
- 18.Reddy EA, Shaw AV, Crump JA, 2010. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis 10: 417–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crump JA, Morrissey AB, Nicholson WL, Massung RF, Stoddard RA, Galloway RL, Ooi EE, Maro VP, Saganda W, Kinabo GD, 2013. Etiology of severe non-malaria febrile illness in northern Tanzania: a prospective cohort study. PLoS Negl Trop Dis 7: e2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blomberg B, Manji KP, Urassa WK, Tamim BS, Mwakagile DS, Jureen R, Msangi V, Tellevik MG, Holberg-Petersen M, Harthug S, 2007. Antimicrobial resistance predicts death in Tanzanian children with bloodstream infections: a prospective cohort study. BMC Infect Dis 7: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christopher A, Mshana SE, Kidenya BR, Hokororo A, Morona D, 2013. Bacteremia and resistant Gram-negative pathogens among under-fives in Tanzania. Ital J Pediatr 39: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dougle M, Hendriks E, Sanders E, Dorigo-Zetsma J, 1997. Laboratory investigations in the diagnosis of septicaemia and malaria. East Afr Med J 74: 353–356. [PubMed] [Google Scholar]
- 23.Okwara F, Obimbo E, Wafula E, Murila F, 2004. Bacteraemia, urinary tract infection and malaria in hospitalised febrile children in Nairobi: is there an association? East Afr Med J 81: 47–51. [DOI] [PubMed] [Google Scholar]
- 24.Petit P, Haarlem J, Poelman M, Haverkamp M, Wamola I, 1995. Bacteraemia in patients presenting with fever. East Afr Med J 72: 116–120. [PubMed] [Google Scholar]
- 25.Walsh AL, Phiri AJ, Graham SM, Molyneux EM, Molyneux ME, 2000. Bacteremia in febrile Malawian children: clinical and microbiologic features. Pediatr Infect Dis J 19: 312–318. [DOI] [PubMed] [Google Scholar]
- 26.Kajumbula H, Fujita AW, Mbabazi O, Najjuka C, Izale C, Akampurira A, Aisu S, Lamorde M, Walwema R, Bahr NC, 2018. Antimicrobial drug resistance in blood culture isolates at a tertiary hospital, Uganda. Emerg Infect Dis 24: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ssali FN, Kamya MR, Wabwire-Mangen F, Kasasa S, Joloba M, Williams D, Mugerwa RD, Ellner JJ, Johnson JL, 1998. A prospective study of community-acquired bloodstream infections among febrile adults admitted to Mulago Hospital in Kampala, Uganda. J Acquir Immune Defic Syndr Hum Retrovirol 19: 484–489. [DOI] [PubMed] [Google Scholar]
- 28.Berkley JA, Lowe BS, Mwangi I, Williams T, Bauni E, Mwarumba S, Ngetsa C, Slack MP, Njenga S, Hart CA, 2005. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med 352: 39–47. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen MV, Sarpong N, Krumkamp R, Dekker D, Loag W, Amemasor S, Agyekum A, Marks F, Huenger F, Krefis AC, 2012. Incidence and characteristics of bacteremia among children in rural Ghana. PLoS One 7: e44063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sigaúque B, Roca A, Mandomando I, Morais L, Quintó L, Sacarlal J, Macete E, Nhamposa T, Machevo S, Aide P, 2009. Community-acquired bacteremia among children admitted to a rural hospital in Mozambique. Pediatr Infect Dis J 28: 108–113. [DOI] [PubMed] [Google Scholar]
- 31.Von Kalckreuth V, Konings F, Aaby P, Adu-Sarkodie Y, Ali M, Aseffa A, Baker S, Breiman RF, Bjerregaard-Andersen M, Clemens JD, 2016. The Typhoid Fever Surveillance in Africa Program (TSAP): clinical, diagnostic, and epidemiological methodologies. Clin Infect Dis 62: S9–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crump JA, Heyderman RS, 2014. Invasive Salmonella infections in Africa. Trans R Soc Trop Med Hyg 108: 673–675. [DOI] [PubMed] [Google Scholar]
- 33.Faragher E, Haan J, van Lent PL, Rockett KA, Teo Y, Richardson A, Khoka M, Molyneux M, Malange P, Bates I, 2008. Severe anemia in Malawian children. N Engl J Med 358: 2291. [DOI] [PubMed] [Google Scholar]
- 34.Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA, 2012. Invasive non-typhoidal Salmonella disease: an emerging and neglected tropical disease in Africa. Lancet Glob Health 379: 2489–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feasey NA, Everett D, Faragher EB, Roca-Feltrer A, Kang’ombe A, Denis B, Kerac M, Molyneux E, Molyneux M, Jahn A, 2015. Modelling the contributions of malaria, HIV, malnutrition and rainfall to the decline in paediatric invasive non-typhoidal Salmonella disease in Malawi. PLoS Negl Trop Dis 9: e0003979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacLennan CA, Levine MM, 2013. Invasive nontyphoidal Salmonella disease in Africa: current status. Expert Rev Anti Infect Ther 11: 443–446. [DOI] [PubMed] [Google Scholar]
- 37.Maltha J, Guiraud I, Kaboré B, Lompo P, Ley B, Bottieau E, Van Geet C, Tinto H, Jacobs J, 2014. Frequency of severe malaria and invasive bacterial infections among children admitted to a rural hospital in Burkina Faso. PLoS One 9: e89103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morpeth SC, Ramadhani HO, Crump JA, 2009. Invasive non-Typhi Salmonella disease in Africa. Clin Infect Dis 49: 606–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park SE, Pak GD, Aaby P, Adu-Sarkodie Y, Ali M, Aseffa A, Biggs HM, Bjerregaard-Andersen M, Breiman RF, Crump JA, 2016. The relationship between invasive nontyphoidal Salmonella disease, other bacterial bloodstream infections, and malaria in sub-Saharan Africa. Clin Infect Dis 62: S23–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takem EN, Roca A, Cunnington A, 2014. The association between malaria and non-typhoid Salmonella bacteraemia in children in sub-Saharan Africa: a literature review. Malar J 13: 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biggs HM, Lester R, Nadjm B, Mtove G, Todd JE, Kinabo GD, Philemon R, Amos B, Morrissey AB, Reyburn H, 2014. Invasive Salmonella infections in areas of high and low malaria transmission intensity in Tanzania. Clin Infect Dis 58: 638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mackenzie G, Ceesay SJ, Hill PC, Walther M, Bojang KA, Satoguina J, Enwere G, d’Alessandro U, Saha D, Ikumapayi UN, 2010. A decline in the incidence of invasive non-typhoidal Salmonella infection in The Gambia temporally associated with a decline in malaria infection. PLoS One 5: e10568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mtove G, Amos B, Nadjm B, Hendriksen IC, Dondorp AM, Mwambuli A, Kim DR, Ochiai RL, Clemens JD, Von Seidlein L, 2011. Decreasing incidence of severe malaria and community-acquired bacteraemia among hospitalized children in Muheza, north-eastern Tanzania, 2006–2010. Malar J 10: 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scott JAG, Berkley JA, Mwangi I, Ochola L, Uyoga S, Macharia A, Ndila C, Lowe BS, Mwarumba S, Bauni E, 2011. Relation between falciparum malaria and bacteraemia in Kenyan children: a population-based, case-control study and a longitudinal study. Lancet Glob Health 378: 1316–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeka A, Gasasira A, Mpimbaza A, Achan J, Nankabirwa J, Nsobya S, Staedke SG, Donnelly MJ, Wabwire-Mangen F, Talisuna A, 2012. Malaria in Uganda: challenges to control on the long road to elimination: I. Epidemiology and current control efforts. Acta Trop 121: 184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maziarz M, Kinyera T, Otim I, Kagwa P, Nabalende H, Legason ID, Ogwang MD, Kirimunda S, Emmanuel B, Reynolds S, 2017. Age and geographic patterns of Plasmodium falciparum malaria infection in a representative sample of children living in Burkitt lymphoma-endemic areas of northern Uganda. Malar J 16: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al-Emran HM, Eibach D, Krumkamp R, Ali M, Baker S, Biggs HM, Bjerregaard-Andersen M, Breiman RF, Clemens JD, Crump JA, 2016. A multicountry molecular analysis of Salmonella enterica serovar Typhi with reduced susceptibility to ciprofloxacin in sub-Saharan Africa. Clin Infect Dis 62: S42–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lunguya O, Lejon V, Phoba M-F, Bertrand S, Vanhoof R, Verhaegen J, Smith AM, Keddy KH, Muyembe-Tamfum J-J, Jacobs J, 2012. Salmonella typhi in the Democratic Republic of the Congo: fluoroquinolone decreased susceptibility on the rise. PLoS Negl Trop Dis 6: e1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith A, Govender N, Keddy K, 2010. Quinolone-resistant Salmonella typhi in South Africa, 2003–2007. Epidemiol Infect 138: 86–90. [DOI] [PubMed] [Google Scholar]
- 50.Mashe T, Gudza-Mugabe M, Tarupiwa A, Munemo E, Mtapuri-Zinyowera S, Smouse SL, Sooka A, Stray-Pedersen B, Smith AM, Mbanga J, 2019. Laboratory characterisation of Salmonella enterica serotype Typhi isolates from Zimbabwe, 2009–2017. BMC Infect Dis 19: 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Britto CD, Wong VK, Dougan G, Pollard A, 2018. A systematic review of antimicrobial resistance in Salmonella enterica serovar Typhi, the etiological agent of typhoid. PLoS Negl Trop Dis 12: e0006779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dyson ZA, Klemm EJ, Palmer S, Dougan G, 2019. Antibiotic resistance and typhoid. Clin Infect Dis 68: S165–S170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong VK, Baker S, Pickard DJ, Parkhill J, Page AJ, Feasey NA, Kingsley RA, Thomson NR, Keane JA, Weill F-X, 2015. Phylogeographical analysis of the dominant multidrug-resistant H58 clade of Salmonella typhi identifies inter- and intracontinental transmission events. Nat Genet 47: 632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kariuki S, Revathi G, Kiiru J, Mengo DM, Mwituria J, Muyodi J, Munyalo A, Teo YY, Holt KE, Kingsley RA, 2010. Typhoid in Kenya is associated with a dominant multidrug-resistant Salmonella enterica serovar Typhi haplotype that is also widespread in Southeast Asia. J Clin Microbiol 48: 2171–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park SE, Pham DT, Boinett C, Wong VK, Pak GD, Panzner U, Espinoza LMC, von Kalckreuth V, Im J, Schütt-Gerowitt H, 2018. The phylogeography and incidence of multi-drug resistant typhoid fever in sub-Saharan Africa. Nat Commun 9: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kariuki S, Okoro C, Kiiru J, Njoroge S, Omuse G, Langridge G, Kingsley RA, Dougan G, Revathi G, 2015. Ceftriaxone-resistant Salmonella enterica serotype Typhimurium sequence type 313 from Kenyan patients is associated with the blaCTX-M-15 gene on a novel IncHI2 plasmid. Antimicrob Agents Chemother 59: 3133–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luvsansharav U-O, Wakhungu J, Grass J, Oneko M, Nguyen V, Bigogo G, Ogola E, Audi A, Onyango D, Hamel M, 2020. Exploration of risk factors for ceftriaxone resistance in invasive non-typhoidal Salmonella infections in western Kenya. PLoS One 15: e0229581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Msefula CL, Kingsley RA, Gordon MA, Molyneux E, Molyneux ME, MacLennan CA, Dougan G, Heyderman RS, 2012. Genotypic homogeneity of multidrug resistant S. typhimurium infecting distinct adult and childhood susceptibility groups in Blantyre, Malawi. PLoS One 7: e42085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Puyvelde S, Pickard D, Vandelannoote K, Heinz E, Barbé B, de Block T, Clare S, Coomber EL, Harcourt K, Sridhar S, 2019. An African Salmonella Typhimurium ST313 sublineage with extensive drug-resistance and signatures of host adaptation. Nat Commun 10: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chatham-Stephens K, Medalla F, Hughes M, Appiah GD, Aubert RD, Caidi H, Angelo KM, Walker AT, Hatley N, Masani S, 2019. Emergence of extensively drug-resistant Salmonella typhi infections among travelers to or from Pakistan—United States, 2016–2018. MMWR Morb Mortal Wkly Rep 68: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klemm EJ, Shakoor S, Page AJ, Qamar FN, Judge K, Saeed DK, Wong VK, Dallman TJ, Nair S, Baker S, 2018. Emergence of an extensively drug-resistant Salmonella enterica serovar Typhi clone harboring a promiscuous plasmid encoding resistance to fluoroquinolones and third-generation cephalosporins. MBio 9: e00105–e00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Levine MM, Simon R, 2018. The gathering storm: is untreatable typhoid fever on the way? MBio 9: e00482–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qamar FN, Yousafzai MT, Khalid M, Kazi AM, Lohana H, Karim S, Khan A, Hotwani A, Qureshi S, Kabir F, 2018. Outbreak investigation of ceftriaxone-resistant Salmonella enterica serotype Typhi and its risk factors among the general population in Hyderabad, Pakistan: a matched case-control study. Lancet Infect Dis 18: 1368–1376. [DOI] [PubMed] [Google Scholar]
- 64.Andrews JR, Qamar FN, Charles RC, Ryan ET, 2018. Extensively drug-resistant typhoid: are conjugate vaccines arriving just in time? N Engl J Med 379: 1493–1495. [DOI] [PubMed] [Google Scholar]
- 65.Andrews JR, Baker S, Marks F, Alsan M, Garrett D, Gellin BG, Saha SK, Qamar FN, Yousafzai MT, Bogoch I, 2019. Typhoid conjugate vaccines: a new tool in the fight against antimicrobial resistance. Lancet Infect Dis 19: e26–e30. [DOI] [PubMed] [Google Scholar]
- 66.François Watkins L, et al. 2020. Update on extensively drug-resistant Salmonella serotype Typhi infections among travelers to or from Pakistan and report of ceftriaxone-resistant Salmonella serotype Typhi infections among travelers to Iraq–United States, 2018–2019. MMWR Morb Mortal Wkly Rep 69: 618–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crump JA, Sjölund-Karlsson M, Gordon MA, Parry CM, 2015. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clin Microbiol Rev 28: 901–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.