Abstract.
Granulomatous amebic encephalitis (GAE) caused by Acanthamoeba is a rare infection with central nervous system (CNS) involvement usually with fatal consequences. Currently, information regarding GAE in children is scarce and is limited only to case reports and case series. A 13-year-old immunocompetent male patient with a 6-month history of progressive and intermittent headaches presented to our institution. One week before hospital admission, the patient showed signs of CNS involvement. Magnetic resonance imaging revealed multiple lesions with supra- and infratentorial cerebral abscesses. An empiric treatment with combined antibiotics was given, but the patient died after 20 days of hospital stay. A postmortem diagnosis confirmed GAE. Although it is a rare disease in pediatric patients, GAE should be considered in children with a chronic history of fever, headache, and vomiting with CNS involvement.
INTRODUCTION
Free-living amoebas such as Naegleria fowleri, Acanthamoeba spp., and Balamuthia madrillaris are currently considered as emerging organisms, with high pathogenicity that may affect the central nervous system (CNS). Infection with these organisms represents a mortality rate greater than 95%. Acanthamoeba sp. was discovered in 1930, and since then at least 24 species have been described. It is ubiquitous in nature and has been isolated from soil, brackish water, and heating and air conditioning units.1,2
Although Acanthamoeba spp. commonly cause granulomatous amebic encephalitis (GAE) in immunocompromised adults, disseminated infection has been rarely described in children.3–20 This article presents a rare case of an immunocompetent adolescent with a postmortem diagnosis of GAE.
Case report.
A previously healthy 13-year-old boy from northeastern Mexico was admitted to our hospital in November 2017, and relevant history in his medical record showed that the patient indulged in recreational activities in a river.
He reported a 6-month history of progressive and intermittent headaches in the left hemisphere with dizziness during the past 5 months. Later he showed symptoms of altered mental status, drowsiness, altered personality, visual hallucinations, lower extremities paresis, and swallowing problems.
According to the child neurologist, the patient was somnolence with stupor periods, was disoriented, and had slurred speech, difficulty in calculating simple math, irregular and dyskinetic movements, left predominance dysmetria, and cranial nerve palsy (including III, VI, VII, IX, X, XI, and XII).
The hemogram at admission revealed a mild lymphopenia (white blood cell count, 10.7 K/μL; neutrophils, 9.11 K/μL; lymphocytes, 0.918; hemoglobin, 15.2 g/dL; platelets, 277,000/mm3). Magnetic resonance imaging (MRI) showed multiple lesions, supra- and infratentorial cerebral abscesses with enhancement, and perilesional edema located in basal ganglia and in the internal capsule (Figure 1). The evaluation of the cerebrospinal fluid (CSF) analysis showed white blood cell count 98/mm3, mononuclear predominance, glucose 48 mg/dL, and 42.5 mg/dL proteins. The CSF cultures were negative for bacterial and fungal species. The following tests were found negative: CSF culture, KOH, IgM and IgG toxoplasma, ELISA for HIV, IgM and IgG for cytomegalovirus, Ziehl-Neelsen, galactomannan test, and cytology for malignant cells.
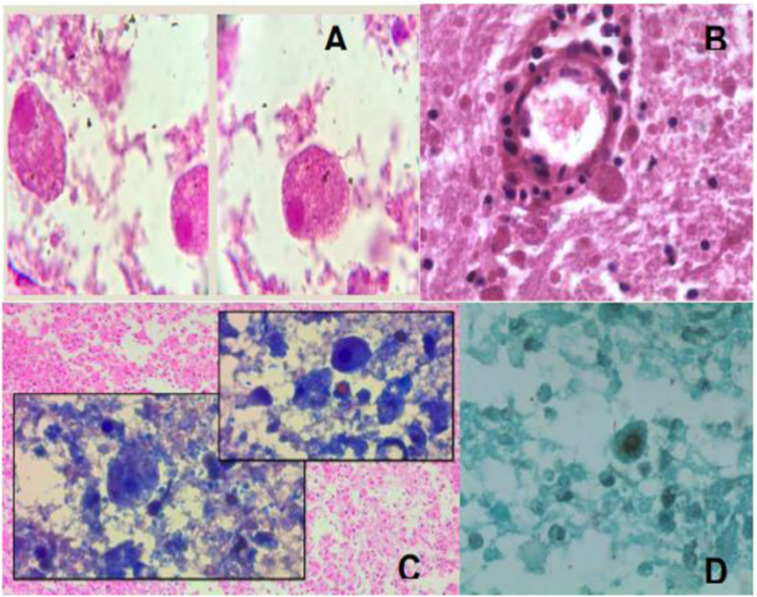
Figure 1.
Histopathology from the patient´s brain tissue. (A and B) Multiple trophic forms are observed scattered in different sizes and different projections (some tending to be lateral), some of them with a small translucent halo (due to the lysis of the tissues). The rounded shapes suggest precyst with two layers. Trophozoites are observed with a single nucleolus (hematoxylin and eosin). (C and D) Some trophozoites have fine thorny projections around them. In some smears in the perivascular areas, trophozoites of different shapes and sizes can be seen, giving the appearance of leaving the blood vessels. The presence of precyst and cystic forms, the single nucleolus, and the thorny fine projections around the trophozoites are related to Acanthamoeba sp. This figure appears in color at www.ajtmh.org.
Empirical antibiotic treatment with vancomycin (60 mg/kg), ceftriaxone (100 mg/kg), and metronidazole (40 mg/kg) was started, with no adverse events. Neurologic deterioration required intensive care unit (ICU) support, but the patient´s condition deteriorated progressively with intracranial hypertension secondary to hydrocephalus and cerebral edema. The patient died 20 days after admission to our ICU.
The parents agreed to an autopsy. Gross examination of the brain revealed a cerebral abscess on the basal ganglia with signs of hypoxia, edema, and extensive necrosis. Hematoxylin and eosin, periodic acid-Schiff stain, and Grocott stain revealed the presence of cyst forms that varied in size from 12 to 14.7 μm. Trophozoites evaluation showed a single nucleolus and the typical fine thorny projections around them, which confirmed the presence of Acanthamoeba sp. (Figure 2).
Figure 2.
Magnetic resonance image showing multiple round and heterogeneous lesions at level of the caudate nucleous head. This figure appears in color at www.ajtmh.org.
DISCUSSION
Acanthamoeba spp. cause GAE, an insidious and chronic granulomatous CNS infection that can last a few weeks to 2 years.1,2 Acanthamoeba spp. encephalitis affecting immunocompetent patients is extremely rare. The exact prevalence worldwide is unclear.
Clinical cases of GAE due to Acanthamoeba spp. have been rarely reported in children (Table 1). We performed a literature search of core databases including Medline (US National Library of Medicine), SciELO (Scientific Electronic Library Online), LILACS (Latin American and Caribbean Literature in Health Science), African Index Medicus (WHO), the Excerpta Medica Database (Embase; Elsevier, Amsterdam, Netherlands]), Scopus (Elsevier), and Europe PMC (European Bioinformatics Institute) between 1960 and 2020 using a combination of the keywords (“Acanthamoeba sp.” or “acanthamoebiasis” and “granulomatous amoebic encephalitis”), with a narrow search specific to pediatric patients (0–18 years) to identify case reports and case series. References were reviewed to identify additional cases. Cases were included if the article was published between January 1960 and April 2020; if the age of the patient was between 0 and 18 years; and if Acanthamoeba spp. infection was confirmed via histologic analysis, polymerase chain reaction, cytology of cerebrospinal fluid smear, or growth culture in agar with a stock of Escherichia coli. For the statistical analysis, a χ2 test was used to analyze categorical variables. Statistical significance was assumed at P < 0.05. Statistical analysis was performed using SPSS v.21 (IBM Corp., Armonk, NY).
Table 1.
Characteristics and clinical presentation of patients with granulomatous amebic encephalitis (N = 30) during the period 1960–2020.
| Variable | Value | P value (P < 0.05) |
|---|---|---|
| Median age | 9 y (9 mo to 17 y) | |
| Sex | ||
| Male | 20 (67%) | |
| Female | 10 (33%) | |
| Median (range) time from onset of symptoms to diagnosis of GAE (d) | ||
| Survivor group (N = 7) | 26 (10–42) | 0.165 |
| Nonsurvivor group (N = 15) | 120 (8–540) | |
| Median (range) duration of hospital length stay (d) | ||
| Survivor group (N = 5) | 51 (28–90) | 0.026 |
| Nonsurvivor group (N = 11) | 18 (2–120) | |
| Clinical manifestations (N = 30) | ||
| Early symptoms | 24 (80%) | |
| Fever | 18 (7%) | |
| Headache | 15 (50%) | |
| Vomiting | 10 (33%) | |
| Nausea | 14 (47%) | |
| CNS involvement | 12 (40%) | |
| Neck stiffness | 9 (30%) | |
| Seizures | 3 (10%) | |
| Hemiparesis | 2 (7%) | |
| Cranial nerve palsy | 1 (3%) | |
| Drowsiness | 1 (3%) | |
| Hallucinations | 1 (3%) | |
| Ataxia | ||
| Ophthalmitis |
CNS = central nervous system; GAE = granulomatous amebic encephalitis.
From 1960 to 2020, 30 pediatric cases with GAE were reported. Fourteen (46%) cases were reported from India; three (10%) cases from the USA and Peru; two (6.6%) cases from Austria; and one (3.3%) case each from South Korea, Indonesia, Iran, Malaysia, Mexico, Sweden, Turkey, and Venezuela. Median age was 9 years (range, 9 months to 17 years); 67% of cases were male. Twenty-six (87%) patients were healthy before the Acanthamoeba spp. infection; among those who were not healthy, two patients had received an allogeneic hematopoietic stem cell transplantation due to acute myeloid leukemia and dyskeratosis congenital, respectively; one patient had severe acute malnutrition; and one patient had acute lymphoblastic leukemia. Previous medical history of recreational activities in rivers was documented in six (20%) cases, and cutaneous lesions were documented in five (17%) cases. The median incubation period, based on patients with a known single exposure (N = 5), was 34 days (range, 8–180 days). Sixteen (53%) cases had available information regarding hospital length stay. Median hospital length stay among survivors (N = 5) was 51 days (range, 28–90 days), compared with 18 days (range, 2–120 days) among nonsurvivors (N = 11), with a statistical significance (P = 0.026). There were 12 (40%) survivors: three patients fully recovered, and three patients had residual neurologic deficits. Information regarding outcomes was not available for six patients.
Seven (23%) of 30 patients with GAE were sent home after the first presentation to the hospital. Three cases were initially misdiagnosed as tuberculosis, two cases as sinusitis, two case as pharyngitis, and one case as cysticercosis. Eleven (36%) cases had information available regarding initial diagnosis: six cases were diagnosed as bacterial meningitis, two cases as meningoencephalitis, one case as brain abscess, one case as tuberculosis, and one case as herpes simplex encephalitis. Granulomatous amebic encephalitis was diagnosed postmortem in seven (23%) cases. In these seven cases, the trophozoites of Acanthamoeba spp. were seen in CSF, four of which grew in agar culture with an E. coli stock. Two patients (6.6%) required polymerase chain reaction only to confirm the diagnosis.
Twenty (67%) cases had a documented computed tomography (CT) or MRI. Results from 19 (95%) cases were read as abnormal. Interpretations of the MRI included multiple hypointense lesions with ring enhancement in 7 of 11 (64%) patients. Interpretations of the CT were more varied, with pyocephalus, hyperdensity indicative of bleed, intraparenchymal hemorrhagic, and cerebral edema.
Treatment data for 28 of 30 (93%) patients were available. Of these patients, 17 (63%) were treated for GAE, 11 (64%) of whom survived (Supplemental Table). All cases received intravenous therapy. All cases received antifungal therapy; amphotericin B was administered in nine (53%) cases, fluconazole in five (29%) cases, ketoconazole in three (18%) cases, and voriconazole in one (6%) case. Trimethoprim-sulfamethoxazole and rifampin were used in 14 (82%) cases. Sixteen (94%) patients received combination therapy. Three (18%) cases presented a therapeutic failure with the use of trimethoprim-sulfamethoxazole monotherapy before the combination therapy. Of 16 patients, 13 (81%) received a combination of three drugs, two (31%) received two drugs, and one (6%) received one drug. Among the patients who received a three-drug regimen, 76% survived.
In conclusion, GAE should be suspected in children with subacute to chronic constitutional symptoms, CNS involve-ment, and multiple lesions with ring enhancement on brain imaging. Direct microscopy of skin lesions or CSF samples looking for trophozoites might be added to the panel diagnosis. Although no treatment has provided a successful outcome, a combination approach with three drugs might be considered.
This current review of the literature highlights the importance of including GAE in the differential diagnosis of children with chronic and subacute CNS syndrome with no apparent cause. This review contributes to the knowledge of the epidemiology of this disease to decrease fatal sequences.
Supplemental tables
Acknowledgments:
We thank Jose Luis Romero, MD, and his team members of the Hospital Infantil de Mexico Federico Gomez for a special contribution to examining the pathological stains of this case.
Note: Supplemental table appears at www.ajtmh.org.
REFERENCES
- 1.Trabelsi H, et al. 2012. Pathogenic free-living amoebae: epidemiology and clinical review. Pathol Biol (Paris) 60: 399–405. [DOI] [PubMed] [Google Scholar]
- 2.Visvesvara GS, Moura H, Schuster FL, 2007. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol 50: 1–26. [DOI] [PubMed] [Google Scholar]
- 3.Kernohan JW, Magath TB, Schloss GT, 1960. Granuloma of brain probably due to Endolimax williamsi. Arch Pathol 70: 576–580. [PubMed] [Google Scholar]
- 4.Martinez AJ, et al. 1977. Meningoencephalitis due to Acantamoeba sp. pathogenesis and clinico-pathological study. Acta Neuropathol 37: 183–191. [DOI] [PubMed] [Google Scholar]
- 5.Martinez AJ, Garcia AC, Halks-Miller M, Arce-Vela R, 1980. Granulomatous amebic encephalitis presenting as a cerebral mass lesion. Acta Neuropathol 51: 85–91. [DOI] [PubMed] [Google Scholar]
- 6.Martinez AJ, et al. 1994. Granulomatous amebic encephalitis: a review report of a spontaneous case from Venezuela. Acta Neuropathol 87: 430–434. [DOI] [PubMed] [Google Scholar]
- 7.Im K, Kim DS, 1998. Acantamoebiasis in Korea: two new cases with clinical cases review. Yonsei Med J 39: 478–484. [DOI] [PubMed] [Google Scholar]
- 8.Singhal T, et al. 2001. Successful treatment of Acanthamoeba meningitis with combination oral antimicrobials. Pediatr Infect Dis J 20: 623–627. [DOI] [PubMed] [Google Scholar]
- 9.Saxena A, Mittal S, Burman P, Garg P, 2009. Acanthamoeba meningitis with successful outcome. Int J Pediatr 76: 1063–1064. [DOI] [PubMed] [Google Scholar]
- 10.Abd H, et al. 2009. Ante mortem diagnosis of amoebic encephalitis in a hematopoietic stem cell transplanted patient. Scand J Infect Dis 41: 619–622. 10.1080/00365540903015117. [DOI] [PubMed] [Google Scholar]
- 11.Lackner P, et al. 2010. Acute granulomatous acanthamoeba encephalitis in a immunocompetent patient. Neurocrit Care 12: 91–94. [DOI] [PubMed] [Google Scholar]
- 12.Maritschnegg P, et al. 2011. Granulomatous amebic encephalitis in a child with acute lymphoblastic leukemia successfully treatment with multimodal antimicrobial therapy and hyperbaric oxygen. J Clin Microbiol 49: 446–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandra SR, Adwani S, Mahadevan A, 2014. Acanthamoeba meningoencephalitis. Ann Indian Acad Neurol 17: 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das S, et al. 2016. Central nervous system infection due to acanthamoeba: a case series. Trop Parasitol 6: 88–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunawan PI, Idarto A, Saharso D, 2016. Acanthamoeba infection in a drowning child. Ethiop J Health Sci 26: 289–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coven SL, et al. 2017. Acanthamoeba granulomatous amoebic encephalitis after pediatric hematopoietic stem cell transplant. Pediatr Transplant 21: 1–6. [DOI] [PubMed] [Google Scholar]
- 17.Megha K, Shegal R, Khurana S, 2018. Genotyping of Acanthamoeba spp. isolated from patients with granulomatous amoebic encephalitis. Indian J Med Res 456–459. 10.4103/ijmr.IJMR_1564_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanafiah M, et al. 2018. Acanthamoeba encephalitis in an immunocompetent child and review of the imaging features of intracranial acanthamoebic infections in immunocompetent patients. Neurol Asia 23: 179–184. [Google Scholar]
- 19.Sütcu M, et al. 2018. Granulomatous Amebic encephalitis caused by Acanthamoeba in an immunocompetent child. Turk J Pediatr 60: 340–343. [DOI] [PubMed] [Google Scholar]
- 20.Cabello-Vilchez AM, et al. 2020. Fatal granulomatous amoebic encephalitis due to free-living amoebae in two different hospitals in Lima, Perú. Neuropathology 40: 180–184. 10.1111/neup.12617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.