Abstract.
Rhinosporidiosis is a chronic mucosal infection caused by Rhinosporidium seeberi, an aquatic protistan parasite. It presents as nasal or ocular polypoidal or vascularized masses. It is endemic in tropical and subtropical areas, especially in South Asia; R. seeberi´s endemicity in the Americas is often overlooked. The objective of this study was to describe the demographic and clinical characteristics of patients with rhinosporidiosis in the Americas, its management, and patient outcomes. This study is a systematic review of cases of human rhinosporidiosis in the Americas reported in the literature from 1896 to February 28, 2019. This review screened 1,994 reports, of which 115 were eligible for further analysis. The selected reports described 286 cases of human rhinosporidiosis between 1896 and 2019. Cases were diagnosed in Brazil (32.2%), Colombia (24.4%), Paraguay (12.6%), and the United States (11.9%). The majority of the cases (91%) occurred in geographic areas with altitudes < 1,000 m above sea level and in areas with median temperatures ≥ 25°C (67.3%). Most of the patients presented nasal (65%) and ocular involvement (35%). Surgical treatment was provided for 99.6% of patients, but 19.8% of them recurred. This review describes the under-recognized geographic distribution and clinical presentation of rhinosporidiosis in the Americas and highlights clinical differences to cases in Asia, specifically in reference to a higher prevalence of ocular disease and higher relapse rates.
INTRODUCTION
Rhinosporidiosis is a chronic mucosal infection caused by Rhinosporidium seeberi. It is a pathogen with debated taxonomy because neither culture methods nor animal models are available. It had been classified as a sporozoan and as a fungus; however, it has been reclassified under a new clade of aquatic protistan parasites, Ichthyosporea (Mesomycetozoea), through molecular methods.1–6 The presumed natural habitat of R. seeberi is stagnant water, and infection probably occurs through transepithelial penetration. The most common clinical presentation is that of polypoidal or vascularized masses, especially in the nasal cavity (70–75%) and in the eye (10–18%).7–9 Rare locations include the genitourinary tract, anal canal, lung, liver, spleen, bone, and brain.7,10–13 Documentation of mature sporangia and endospores in tissue biopsy is the gold standard for diagnosis. Surgical resection is the treatment of choice; anecdotally, dapsone has been used to prevent recurrences.1,11,12,14
The disease has an almost universal distribution; cases have been reported in all continents except Australia. It is endemic in tropical and subtropical areas. Most cases are reported in South Asia, especially India and Sri Lanka (88%), followed by South America and Africa.2,7,9–11 Although the disease was first described in Argentina more than a century ago, adequate environmental conditions for transmission are present throughout the Americas, and endemic areas in Paraguay and Brazil have been reported, the endemicity of R. seeberi in the Americas is still underrecognized.10,15–18 Characterization of this disease in the continent has been limited due to a paucity of reports, often published in low-impact journals.
The objective of this review was to describe the demographic and clinical characteristics of patients with rhinosporidiosis in the Americas, its management, and patient outcomes.
MATERIALS AND METHODS
Eligibility criteria.
All the studies that described human cases of rhinosporidiosis in the Americas were included. Studies describing cases of rhinosporidiosis in animals or continents other than America were excluded.
Search strategy.
A search of the Lilacs, PubMed, and Google Scholar databases was performed from 1896 to February 28, 2019, with the following search terms: (“Rhinosporidium” [MeSH Terms] OR “rhinosporidiosis” [MeSH Terms] OR “rhinosporidium” [All Fields] OR “Rhinosporidium seeberi” [All Fields] OR “rhinosporidiosis” [All Fields] OR “rinosporidiosis” [DeCS]). The search limit was species (“Humans”). The search was performed without language restrictions. A manual search for references cited in the reviewed articles was performed. Authors of articles with incomplete information were contacted.
Criteria for case selection.
The first investigators (PS, ZN, ACA) performed the literature search, eliminated duplicate articles, and reviewed the titles and abstracts against the predefined eligibility criteria. Full-text articles were obtained from the databases, main authors, universities, and scientific organizations. Full-text articles were reviewed by four investigators (PS, ZN, CJJ, and ACA); any discrepancy was resolved by consensus between the authors.
Data extraction and analysis.
Information about sex, age, lesion location, type of lesion, type of treatment, outcome, risk factors, and place of residence of each patient was registered in a Microsoft Office Excel database. Geolocation with the ArcGIS Online program was performed for cases that described the place of residence.
Ethics.
This was a retrospective study, based on previously published articles. Approval by the investigation ethics committee was not required.
RESULTS
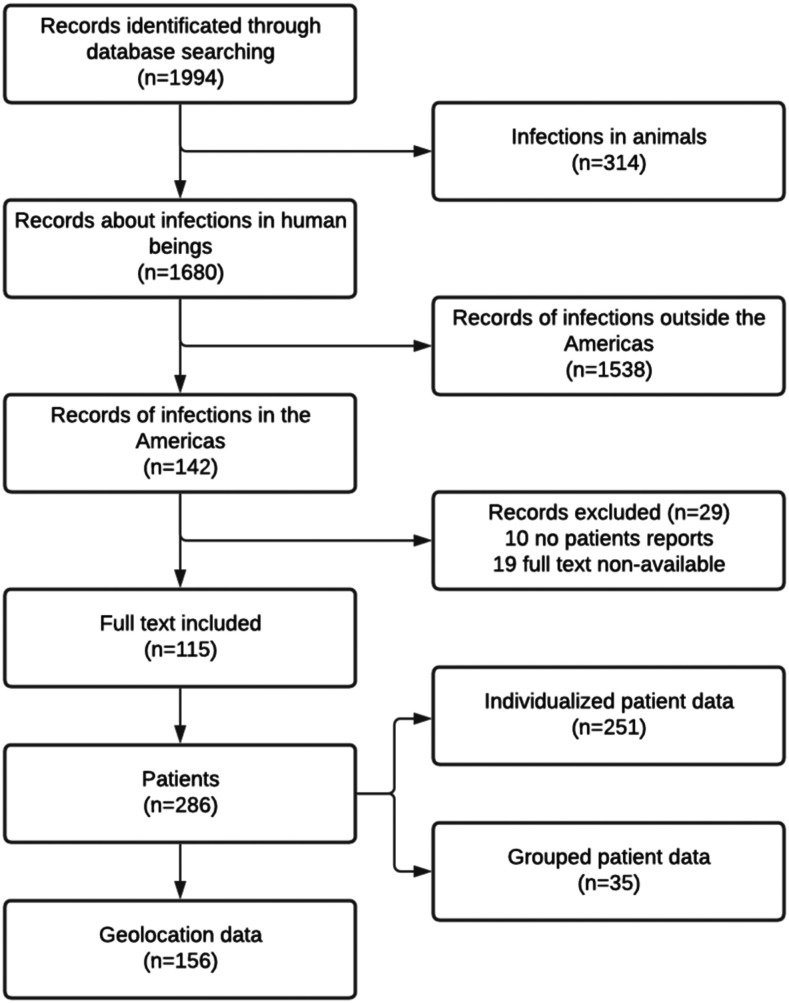
A total of 1,994 articles were screened; these included 874 articles in the Google Scholar database, 504 in the PubMed database, and 613 in the Lilacs database. Screening by title and abstract resulted in the exclusion of 314 articles referring to animals, 1,538 articles of cases from continents other than America, and 10 articles that were not case reports. The full-text versions of 19 eligible articles were not available and were not included in the final analysis. Ultimately, 115 articles were selected for final analysis, describing 286 cases of rhinosporidiosis (Figure 1; Supplemental materials). Of these 286 cases, 156 had reported residence data for geolocation. One of the reports was a case series that presented compiled data rather than individual patient information.
Figure 1.
Flow diagram of database search.
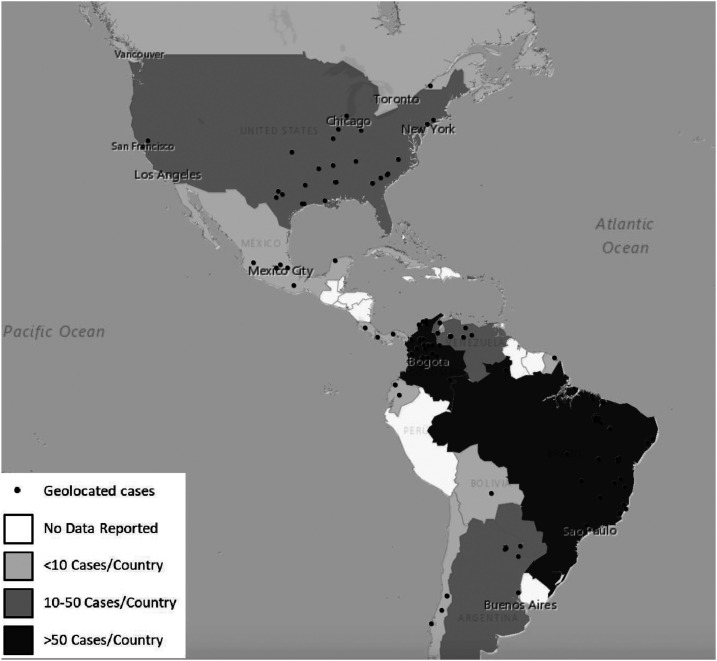
The selected reports described 286 rhinosporidiosis human cases between 1896 and 2019 in the Americas. Cases were diagnosed in Brazil (92 cases, 32.2%), Colombia (64 cases, 24.4%), Paraguay (36 cases, 12.6%), United States (34 cases, 11.9%), Venezuela (18 cases, 6.3%), and Argentina (13 cases, 4.5%) (Table 1). Geolocation for these cases is depicted in Figure 2. For the geographic areas involved, the median altitude above sea level was 134 m (interquartile range [IQR]: 30–357.5), the median annual precipitation was 1,356 mm (IQR: 1,011–1,891), and the median average annual temperature was 25.9°C (IQR: 20.2–27.4). City of origin was available for 156 patients; 91% (142) of cases occurred in geographic areas with altitudes < 1,000 m above sea level and 67.3% (105) in regions with median temperatures ≥ 25°C.
Table 1.
Demographic characteristics of patients with rhinosporidiosis in the Americas
| Characteristic | Patients (N = 286), n (%) |
|---|---|
| Country | |
| Brazil | 92 (32.2) |
| Colombia | 64 (22.4) |
| Paraguay | 36 (12.6) |
| United States | 34 (11.9) |
| Venezuela | 18 (6.3) |
| Argentina | 13 (4.5) |
| Mexico | 9 (3.1) |
| Chile | 5 (1.7) |
| Ecuador | 4 (1.4) |
| Cuba | 4 (1.4) |
| Canada | 2 (0.7) |
| Panama | 2 (0.7) |
| Bolivia | 1 (0.3) |
| Costa Rica | 1 (0.3) |
| French Guyana | 1 (0.3) |
| Average temperature (°C), median (IQR) | 25.9 (20.2–27.4) |
| Temperature, °C (N = 105) | |
| < 15 | 10 (9.5) |
| 15–17.9 | 8 (7.6) |
| 18–23.9 | 20 (19) |
| ≥ 24 | 67 (63.8) |
| Altitude (a.a.s.l.), median (IQR) | 134 (30–357.5) |
| Altitude (a.a.s.l.) | |
| 0–900 | 93 (88.6) |
| 901–1,700 | 5 (4.8) |
| 1,701–2,500 | 2 (1.9) |
| > 2,500 | 5 (4.8) |
| Annual precipitation (mm), median (IQR) | 1,356 (1,011–1,891) |
| < 750 | 11 (10.5) |
| 750–1,249 | 30 (28.6) |
| 150–1,749 | 36 (34.3) |
| ≥ 1,750 | 28 (26.7) |
a.a.s.l. = altitude above sea level; IQR = interquartile range.
Figure 2.
Geolocation of the reported cases of rhinosporidiosis in the Americas. This figure appears in color at www.ajtmh.org.
Most patients were male (N = 220; 77.7%), with a median age of 15 years (IQR: 11–24.8) (Table 2). Risk factors were described for 33 patients; the most common one was exposure to free-flowing or stagnant water. Lesions were located in the nasal area in 65% (N = 186) of patients and in the ocular area in 35% (N = 100) of patients. These lesions were described as polypoid in 156 patients (68.7%), tumoral in 39 patients (17.2%), and papillomatous in 21 patients (9.3%). Only two patients with ocular rhinosporidiosis presented with staphyloma.19,20 Surgical treatment alone was provided for 236 patients (99.6%), 24 (10.3%) patients underwent cauterization in addition to surgical treatment, and seven (3.1%) patients received medications in addition to surgical treatment. Disease recurrence was documented in 26 of 131 cases (19.8%) for whom follow-up information was available. Of these 26 patients, one had HIV infection, and four had two or more documented episodes of recurrence. Of the 16 patients treated with surgical resection and cauterization for whom follow-up information was available, three (18.8%) had recurrent disease.
Table 2.
Clinical characteristics, treatment, and outcome of patients with rhinosporidiosis in the Americas
| Variable | Patients (N = 286) n (%) |
|---|---|
| Sex (N = 283) | |
| Male | 220 (77.7) |
| Age (years), median (IQR) | 15 (11–24.8) |
| < 10 | 35 (17.2) |
| 10–19 | 97 (47.5) |
| 20–30 | 37 18.1) |
| > 30 | 35 (17.2) |
| Risk factor (N = 33) | |
| Free flowing or stagnant water (wells, rivers, and dams) | 24 (72.7) |
| Horses, cows, and dogs | 10 (30.3) |
| Goats | 1 (3) |
| Cotton picking | 1 (3) |
| Lesion localization | |
| Nasal | 186 (65) |
| Nasal | 184 (64.3) |
| Nasopharynx | 2 (0.7) |
| Ocular | 100 (35) |
| Conjunctival | 90 (31.5) |
| Palpebral | 6 (2.1) |
| Ocular | 3 (1.0) |
| Lacrimal | 1 (0.3) |
| Type of lesion (N = 227) | |
| Polypoid | 156 (68.7) |
| Tumoral | 39 (17.2) |
| Papillomatous | 21 (9.3) |
| Granulomatous | 4 (1.8) |
| Flat | 4 (1.8) |
| Hemangiomatous like | 1 (0.4) |
| Carcinomatous like | 1 (0.4) |
| Verrucous like | 1 (0.4) |
| Medical treatment (N = 234) | |
| No | 228 (97.4) |
| Dapsone | 2 (0.9) |
| Antibiotic | 2 (0.9) |
| Diamino diphenyl sulfone | 2 (0.9) |
| Itraconazole | 1 (0.4) |
| Surgical treatment (N = 237) | 236 (99.6) |
| Cauterization (N = 234) | 24 (10.3) |
| Cryotherapy (N = 234) | 2 (0.9) |
| Outcome (N = 131) | |
| No relapse | 105 (80.2) |
| Relapse | 26 (19.8) |
DISCUSSION
Although R. seeberi is a pathogen with an almost universal distribution, its associated disease, rhinosporidiosis, has been greatly overlooked in endemic areas of the Americas.11,16,17 It is renowned as an Asiatic disease and is characterized as endemic in Paraguay and Brazil only by a few authors.10,15–18 In this systematic review, we identified 115 articles describing 286 cases of rhinosporidiosis in the Americas.
The countries with most of the reported cases were Brazil (32.2%), Colombia (22.4%), Paraguay (12.6%), and the United States (11.9%). The relative frequency of cases in these geographic areas is not surprising because most of the reported cases occur in ecosystems found throughout these countries: arid areas (precipitation < 200 mm) or tropical areas (average temperature > 24°C, altitude 0–1,000 m above sea level, and annual precipitation of 1,750–2,000 mm).3,11,18,20 However, although 91% of cases did occur at low altitudes (< 1,000 m above sea level, median altitude of 134 m above sea level), only 67.3% of the cases occurred in geographic areas with median temperatures ≥ 25°C.
Based on circumstantial data, the presumed natural habitat of R. seeberi is stagnant or ground waters.3,11,12 More convincing evidence of this association comes from an outbreak in Serbia, where 17 patients bathed during a holiday in the same lake and developed rhinosporidiosis.21 In this review, 72.7% of the cases for whom this information was available reported exposure to free-flowing or stagnant water. Reports of rhinosporidiosis in dry areas of Sri Lanka suggest R. seeberi can survive in these types of environments and be infective.9,11,22 The presumed mode of infection is the contact of a disrupted epithelial layer with water or dust containing R. seeberi. Similar to what is reported in India, most of the cases in this review lived in cities with rivers or lakes in the vicinity, where people usually bathe.8,9,11
Similar patients with rhinosporidiosis from other geographic areas, patients in the Americas are young (median age 15 years) and predominantly male.8,9,23 Nasal compromise is the most frequent clinical presentation reported in both Asian cohorts and in the Americas.1,3,9,12 Patients with nasal involvement usually present with a slow disease course of nasal obstruction, tumor-like masses, or bleeding.3,12,14 With the exception of predominant ocular involvement reported in an outbreak in Serbia and in Sri Lanka where the main exposure was to water bodies,9,21 ocular involvement is reported only for 10–18% of the cases in geographic areas outside of the Americas; in contrast, 35% of patients in the Americas present with ocular involvement.7–9,12 The relatively high incidence of ocular disease in the Americas could be related to the transmission mechanism with direct ocular inoculation (in dusty areas or direct contact with river water), as proposed by some authors, or to the pathogenicity of R. seeberi species in the Americas.9,24 Patients with ocular involvement usually present with tumor-like masses or polyps affecting the conjunctiva and lacrimal sac. Patients complain of a foreign body sensation, ocular irritation, and tearing. Rare complications are visual impairment and staphyloma, which occurs due to scleral thinning and herniation of the intraocular content.7,8,20 In this series only two cases of staphyloma were reported.19,20
In the Americas, 99.6% of cases were treated by surgical resection, which is the gold standard for treatment.9,20 Despite surgical management, recurrence was reported for 19.8% of the cases with available follow-up information. This number is in sharp contrast with a 2–5.8% relapse rate reported for patients in Asia, with the exception of one case series in Sri Lanka that reported a recurrence rate of 37% and up to 100% for patients with disseminated disease.1,7,9–11,13,23 Recurrences may be related to incomplete surgical excision of the lesion by its base; thus, some authors have proposed adding cauterization of the wound to surgical resection to prevent relapse.20 However, our study showed that cauterization after surgery did not improve relapse rates (18.8%).10,25 Other possible causes for the high relapse rate are re-exposure to the pathogen, underlying host factors, or higher virulence of the pathogen specific to the Americas species. In the Americas, most patients had short follow-up periods of 6 months to a year; only in a few cases the follow-up period extended for up to 8 years.10,19,20,26–34 The follow-up period in Asian cases is usually only 6–18 months. The Sri Lanka cohort reports a high recurrence rate but does not specify the follow-up period, which may have been longer.8,9,13,23,25,35–38
This study has some limitations. The main limitation of this study was its reliance on published data, which probably represent just a fraction of the total number of cases in an area due to under-reporting. Moreover, the reported cases in the literature probably depict unusual cases, which may represent a bias toward a higher incidence of ocular compromise and high relapse rates. Finally, some articles were not available for review or had incomplete information.
In conclusion, this review highlights the neglected endemicity of R. seeberi in the Americas, especially in low-altitude areas in South America (< 1,000 m above sea level). The clinical presentation is similar to the one reported in Asia, with a higher prevalence of ocular involvement and higher relapse rates. The generalized unawareness of this disease´s local endemicity may translate to a lack of recognition of its clinical presentation and missed opportunities for diagnosis and treatment. Thus, educating the medical community about where this disease presents, as well as on its early recognition and treatment, is of critical importance.
Supplemental tables
Note: Supplemental table appears at www.ajtmh.org.
REFERENCES
- 1.Arseculeratne SN, 2005. Rhinosporidiosis: what is the cause? Curr Opin Infect Dis 18: 113–118. [DOI] [PubMed] [Google Scholar]
- 2.Vilela R, Mendoza L, 2012. The taxonomy and phylogenetics of the human and animal pathogen Rhinosporidium seeberi: a critical review. Rev Iberoam Micol 29: 185–199. [DOI] [PubMed] [Google Scholar]
- 3.Vélez A, Jiménez G, Hidrón A, Talero S, Agudelo CA, 2018. Rhinosporidiosis in Colombia: case series and literature review. Trop Doct 48: 289–293. [DOI] [PubMed] [Google Scholar]
- 4.Silva V, Pereira CN, Ajello L, Mendoza L, 2005. Molecular evidence for multiple host-specific strains in the genus Rhinosporidium. J Clin Microbiol 43: 1865–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fredricks DN, Jolley JA, Lepp PW, Kosek JC, Relman DA, 2000. Rhinosporidium seeberi: a human pathogen from a novel group of aquatic protistan parasites. Emerg Infect Dis 6: 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herr RA, Ajello L, Taylor JW, Arseculeratne SN, Mendoza L, 1999. Phylogenetic analysis of Rhinosporidium seeberi’s 18S small-subunit ribosomal DNA groups this pathogen among members of the protoctistan Mesomycetozoa clade. J Clin Microbiol 37: 2750–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pal DK, Mallick AA, Majhi TK, Biswas BK, Chowdhury MK, 2012. Rhinosporidiosis in southwest Bengal. Trop Doct 42: 150–153. [DOI] [PubMed] [Google Scholar]
- 8.Chowdhury RK, Behera S, Bhuyan D, Das G, 2007. Oculosporidiosis in a tertiary care hospital of western Orissa, India: a case series. Indian J Ophthalmol 55: 299–301. [DOI] [PubMed] [Google Scholar]
- 9.Arseculeratne SN, Sumathipala S, Eriyagama NB, 2010. Patterns of rhinosporidiosis in Sri Lanka: comparison with international data. Southeast Asian J Trop Med Public Health 41: 175–191. [PubMed] [Google Scholar]
- 10.Almeida FA, Feitoza L de M, Pinho JD, de Mello GCF, Lages JS, Silva FF, da Silva RR, Silva GEB, 2016. Rhinosporidiosis: the largest case series in Brazil. Rev Soc Bras Med Trop 49: 473–476. [DOI] [PubMed] [Google Scholar]
- 11.Arseculeratne SN, 2002. Recent advances in rhinosporidiosis and Rhinosporidium seeberi. Indian J Med Microbiol 20: 119–131. [PubMed] [Google Scholar]
- 12.Sudarshan V, Goel NK, Gahine R, Krishnani C, 2007. Rhinosporidiosis in Raipur, Chhattisgarh: a report of 462 cases. Indian J Pathol Microbiol 50: 718–721. [PubMed] [Google Scholar]
- 13.Mithal C, Agarwal P, Mithal N, 2012. Ocular and adnexal rhinosporidiosis: the clinical profile and treatment outcomes in a tertiary eye care centre. Nepal J Ophthalmol 4: 45–48. [DOI] [PubMed] [Google Scholar]
- 14.Das S, Kashyap B, Barua M, Gupta N, Saha R, Vaid L, Banka A, 2011. Nasal rhinosporidiosis in humans: new interpretations and a review of the literature of this enigmatic disease. Med Mycol 49: 311–315. [DOI] [PubMed] [Google Scholar]
- 15.Seeber G, 1900. Un nuevo esporozuario parasito del hombre: dos casos encontrados en polipos nasales. MsD Thesis, Universidad Nacional de Buenos Aires. [Google Scholar]
- 16.Rolon A, 1974. Rinosporidiosis Epidemiologia en la Republica del Paraguay. Mycopathol Mycol Appl 52: 155–171. [DOI] [PubMed] [Google Scholar]
- 17.Vallarelli AFA, Rosa SP, de Souza EM, 2011. Rhinosporidiosis: cutaneous manifestation. An Bras Dermatol 86: 795–796. [DOI] [PubMed] [Google Scholar]
- 18.Lupi O, Tyring SK, McGinnis MR, 2005. Tropical dermatology: fungal tropical diseases. J Am Acad Dermatol 53: 931–951, quiz 952–954. [DOI] [PubMed] [Google Scholar]
- 19.Tzelikis PF de M, Martins HC, Vilela G, Akaishi L., 2007. Afilamento escleral em paciente com rinosporidiose conjuntival. Rev Bras Oftalmol 66: 337–340. [Google Scholar]
- 20.Almeida FA, Teixeira-Junior AAL, Pinho JD, Costa EF, Silva GEB, 2019. Evaluation of diagnosed cases of eye rhinosporidiosis in a public hospital of Maranhão, Northeast Brazil. BMC Ophthalmol 19: 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vukovic Z, Bobic-Radovanovic A, Latkovic Z, Radovanovic Z, 1995. An epidemiological investigation of the first outbreak of rhinosporidiosis in Europe. J Trop Med Hyg 98: 333–337. [PubMed] [Google Scholar]
- 22.Mendoza L, Vilela R, 2013. Presumptive synchronized nuclear divisions without cytokinesis in the Rhinosporidium seeberi parasitic life cycle. Microbiology 159: 1545–1551. [DOI] [PubMed] [Google Scholar]
- 23.Shrestha SP, Hennig A, Parija SC, 1998. Prevalence of rhinosporidiosis of the eye and its adnexa in Nepal. Am J Trop Med Hyg 59: 231–234. [DOI] [PubMed] [Google Scholar]
- 24.Senaratne T, Edussuriya K, Dhanapala M, Bandara A, Arseculeratne S, 2011. Ocular Rhinosporidiosis with Staphyloma Formation: a Case with Unusual Features. Available at: https://www.dovepress.com/ocular-rhinosporidiosis-with-staphyloma-formation-a-case-with-unusual–peer-reviewed-article-EB. Accessed January 12, 2011. [DOI] [PMC free article] [PubMed]
- 25.Khan I, Gogia S, Agarwal A, Swaroop A, 2014. Recurrent rhinosporidiosis: coblation assisted surgical resection-a novel approach in management. Case Rep Otolaryngol 2014: 609784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lasser A, Smith HW, 1976. Rhinosporidiosis. Arch Otolaryngol 102: 308–310. [DOI] [PubMed] [Google Scholar]
- 27.Prevost E, Kreutner A, Vallotton WW, Walker EM, 1980. Conjunctival lesion caused by Rhinosporidium seeberi. South Med J 73: 1077–1079. [DOI] [PubMed] [Google Scholar]
- 28.Anon. 2009, Nasal Rhinosporidiosis: Differential Diagnosis of Fungal Sinusitis and Inverted Papilloma. Available at: http://www.arquivosdeorl.org.br/conteudo/acervo_eng.asp?Id=595. Accessed January 19, 2019.
- 29.França GV, Gomes CC, Sakano E, Altemani AM, Shimizu LT, 1994. Nasal rhinosporidiosis in children. J Pediatr (Rio J) 70: 299–301. [DOI] [PubMed] [Google Scholar]
- 30.Jimenez JF, Young DE, Hough AJ, 1984. Rhinosporidiosis: a report of two cases from Arkansas. Am J Clin Pathol 82: 611–615. [DOI] [PubMed] [Google Scholar]
- 31.Anon., 2007. Nasal Rhinosporidiosis - Four Cases Relate and Literature Review. Available at: http://arquivosdeorl.org.br/additional/acervo_port.asp?id=428. Accessed January 11, 2019.
- 32.Mora JA, Salazar JA, Calderón C, García R, 1985. Rinosporidiosis en Costa Rica: revisión del tema e informe dell primer caso en nuestro país. Acta Med Costarric 28: 122–125. [Google Scholar]
- 33.Krauss MK, Bahamonde SH, Karle PM, 2016. Rinosporidiosis nasal: reporte de un nuevo caso y revisión de la literatura. Rev Otorrinolaringol Cir Cabeza Cuello 76: 320–324. [Google Scholar]
- 34.Zoroquiain P, Moreno A, Oddó D, 2014. Rinosporidiosis conjuntival diagnosticada mediante estudio histopatológico. Rev Chilena Infectol 31: 213–215. [DOI] [PubMed] [Google Scholar]
- 35.Capoor MR, Khanna G, Rajni, Batra K, Nair D, Venkatchalam VP, Aggarwal P, 2009. Rhinosporidiosis in Delhi, north India: case series from a non-endemic area and mini-review. Mycopathologia 168: 89–94. [DOI] [PubMed] [Google Scholar]
- 36.Ganekal S, Dorairaj S, Hegde S, Jhanji V, 2013. Conjunctival rhinosporidiosis. J Am Assoc Pediatr Ophthalmol Strabismus 17: 432–433. [DOI] [PubMed] [Google Scholar]
- 37.Senaratne T, Edussuriya K, Dhanapala M, Bandara A, Arseculeratne S, 2011. Ocular rhinosporidiosis with staphyloma formation: a case with unusual features. Eye Brain 3: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castelino AM, Rao SK, Biswas J, Gopal L, Madhavan HN, Kumar SK, 2000. Conjunctival rhinosporidiosis associated with scleral melting and staphyloma formation: diagnosis and management. Cornea 19: 30–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.