Abstract.
Neurocysticercosis (NCC) is endemic in many parts of the world, carrying significant neurological morbidity that varies according to whether lesions are located inside the cerebral parenchyma or in extraparenchymal spaces. The latter, in particular subarachnoid NCC, is assumed to be more severe, but no controlled studies comparing mortality between types of NCC exist. The aim of this study was to compare all-cause mortality between patients with intraparenchymal NCC and those with subarachnoid NCC. Vital status and sociodemographic characteristics were evaluated in patients with intraparenchymal viable, intraparenchymal calcified, and subarachnoid NCC attending a neurological referral hospital in Lima, Perú. Survival analyses using Kaplan–Meier curves and Cox proportional regression models were carried out to compare mortality rates between groups. From 840 NCC patients followed by a median time of 82.3 months, 42 (5.0%) died, six (1.8%) in the intraparenchymal viable group, four (1.3%) in the calcified group, and 32 (16.6%) in the subarachnoid group (P < 0.001). Older age and lower education were significantly associated with mortality. The age-adjusted hazard ratio for death in the subarachnoid group was 13.6 (95% CI: 5.6–33.0, P < 0.001) compared with the intraparenchymal viable group and 10.7 (95% CI: 3.7–30.8, P < 0.001) when compared with the calcified group. We concluded that subarachnoid disease is associated with a much higher mortality in NCC.
INTRODUCTION
Neurocysticercosis (NCC) is one of the most frequent parasitic infections of the central nervous system (CNS) globally and the most common cause of adult-onset epilepsy in endemic regions.1–3 Clinical manifestations of NCC vary according to the location and number of parasitic lesions in the CNS.1,4,5 The most common presentation of NCC involves viable cysts in the cerebral parenchyma (intraparenchymal NCC) and is commonly associated with seizures. Following the natural parasite involution or in response to antiparasitic therapy, some cysts resolve completely, although others result in a calcified scar.6 Seizures can continue regardless of parasite viability. Compared with the general population, patients with seizures have an increased risk of death because of trauma and sudden death associated with epilepsy.1,7,8
In some patients, the parasitic lesions are located in the cerebral ventricles or in the subarachnoid spaces (extraparenchymal NCC). Subarachnoid NCC is considered a particularly severe form of the disease, presenting with chronic inflammation and infiltration into neighboring spaces that lead to hydrocephalus, mass effects, intracranial hypertension, vasculitis, stroke, and cranial nerve involvement, among others and may be lethal if not treated promptly.1,4,5,9 Patients with subarachnoid NCC frequently require neurosurgical interventions, with additional risks for severe complications including even death.
Neurocysticercosis as an underlying or associated cause of death has been described.8,10,11 The literature refers to a extraparenchymal cyst including subarachnoid NCC as being associated with increased morbidity and mortality4,8; however, no comparative data are available. A better understanding of these differences would lead to improved and likely more intensive approaches to medical and surgical management in the group with the highest risk. We assessed life status in three large cohorts of patients with viable parenchymal NCC, calcified parenchymal NCC, and subarachnoid NCC to determine differences in all-cause mortality between these types of disease.
METHODS
Study subjects.
We included patients with NCC confirmed by neuroimaging who attended the Cysticercosis Unit at the Instituto Nacional de Ciencias Neurologicas, Lima, Peru, with baseline brain images (computed tomography [CT], or magnetic resonance imaging [MRI]) and serological results on enzyme-linked immunoelectrotransfer blot assay using lentil lectin purified glycoprotein antigens (LLGP-EITB). These subjects were been screened for participation or enrolled in prior clinical studies.12–18 We also included patients attending our unit for clinical advice. Patients with intraventricular NCC or those with degenerating brain cysts were excluded. Eligible patients were categorized to one of the three following groups:
(1) Calcified NCC: Patients with only intracranial calcifications compatible with NCC on brain CT scan with no documented history of previous viable lesions
(2) Intraparenchymal viable NCC: Patients with/viable NCC cysts inside the brain parenchyma observed on MRI and positive serology (specific antibodies to Taenia solium on EITB assay). They could have NCC calcifications.
(3) Subarachnoid NCC: Patients with viable NCC cysts in the subarachnoid space observed on brain MRI and positive EITB assay for cysticercosis. Patients with subarachnoid NCC could have additional parenchymal cysts (either viable or calcified).
This work expands a prior study which was conducted in a smaller group of 118 patients with parenchymal NCC and 50 patients with subarachnoid NCC that met the same definition.9
Procedures.
Demographic, serological, and radiological information were collected from the original databases including the date of the initial diagnosis of NCC. Data on age, gender, level of education, years of education, birth region, place of residence (Lima or outside Lima), and vital status as of October 24, 2018, as well as date of death when applicable, were collected from the National Registry of Identification and Marital Status (Registro Nacional de Identificación y Estado Civil - RENIEC in Spanish), which contains demographic information from all Peruvian citizens.
Statistical analysis.
Frequencies, median, interquartile ranges (IQR), and mortality rates (per 1,000 inhabitants) are reported for descriptive purposes. Direct and indirect standardization were used to adjust mortality rates by age. For direct standardization, the sum of the totals in each NCC group was used as reference. Age-adjusted mortality rates and standardized mortality ratios (SMRs) were calculated by indirect standardization using general mortality in the Peruvian population in the mid-year (2009) of the study period as reference. We used chi-squared and Wilcoxon rank-sum tests to assess differences in characteristics by NCC groups and by vital status. For survival analysis, we considered the time in years from the date of diagnosis by imaging to the date of death or date of vital status survey. The log-rank test was used to assess difference in the Kaplan–Meier groups according to NCC type and the Cox proportional regression model to report the hazard ratio (HR) between the groups using the intraparenchymal viable group as a reference.
Ethical considerations.
The study was reviewed and approved by the institutional review boards from the Universidad Peruana Cayetano Heredia and the Instituto Nacional de Ciencias Neurologicas, both in Lima, Peru. We used information stored in the hospital and prior study registries. To protect the subjects’ personal information, all personal identifiers were excluded from the study databases once the clinical and demographic data were linked to their respective follow-up life status.
RESULTS
We included 840 patients with NCC, including 335 with viable intraparenchymal lesions, 312 with calcified NCC, and 193 with subarachnoid NCC. Overall, 60% of the participants (n = 504/840) were male. There were significantly more male subjects in the viable intraparenchymal and in the subarachnoid NCC groups (n = 127/193, 65.8% and n = 257/335, 76.7% respectively), than in the calcified NCC group (n = 120/312, 38.5%) (P < 0.001, chi-squared test). The median age for all participants was 41.8 (IQR: 33.2–54.1) years. Patients with subarachnoid NCC were significantly older than those with parenchymal NCC, viable, or calcified (median 46.0 years, IQR: 37.4–58.8 versus 40.3, IQR: 33.0–53.0 and 39.1 years, IQR: 31.2–52.9, respectively, P < 0.001, Kruskal–Wallis test). Most of the participants were single in all groups (250/333, 75.1% in the intraparenchymal group 234/311, 75.2% in the calcified group; and 131/184, 71.2% in the subarachnoid group; P = 0.555). Also, the subarachnoid group had the highest proportion of participants without school education with 10.3% (19/184) compared with the calcified NCC group (14/311, 4.5%) and intraparenchymal viable NCC group (10/333, 3.0%) (P = 0.001) (Table 1).
Table 1.
Characteristics of the study subjects (n = 840)
| Characteristic | Total (n = 840) | Intraparenchymal viable (n = 335) | Calcified (n = 312) | Subarachnoid (n = 193) | P-value |
|---|---|---|---|---|---|
| Gender | < 0.001 | ||||
| Female | 336 (40%) | 78 (23.3%) | 192 (61.5%) | 66 (34.2%) | |
| Male | 505 (60%) | 257 (76.8%) | 120 (38.5%) | 127 (65.8%) | |
| Age (years) | 41.8 (33.2–54.1) | 40.3 (33.0–53.0) | 39.1 (31.2–52.9) | 46.0 (37.4–58.8) | < 0.001 |
| Marital status* | 0.555 | ||||
| Single | 615 (74.3%) | 250 (75.1%) | 234 (75.2%) | 131 (71.2%) | |
| Others | 213 (25.7%) | 83 (24.9%) | 77 (24.8%) | 53 (28.8%) | |
| Level of education* | 0.001 | ||||
| No studies | 43 (5.2%) | 10 (3%) | 14 (4.5%) | 19 (10.3%) | |
| Any studies | 785 (94.8%) | 323 (97%) | 297 (95.5) | 165 (89.7%) | |
| Years of education* | 11 (6–11) | 11 (6–11) | 11 (9–11) | 11 (6–11) | < 0.001 |
We could not gather data on marital status and education from 12 patients.
The median follow-up time for the entire study population was 6.8 (IQR: 2.9–9.4) years. As the enrolled patients came from original studies that were conducted in different years, there was a differential follow-up time, higher in the intraparenchymal viable (median 9.7 years, IQR: 8.0–11.2 years) than in the calcified NCC group (median 4.3 years, IQR: 2.0–5.6 years) or the subarachnoid NCC group (median 66.9 months, IQR: 14.9–98.4 months) (P < 0.001).
A total of 42 subjects died during the follow-up period, six (1.8%) in the intraparenchymal viable group, four (1.3%) in the calcified NCC group, and 32 (16.6%) in the subarachnoid NCC group (P < 0.001, chi-squared test). In comparison to survivors, the subjects who died were significantly older (median age 53.5 years, IQR: 41.5–60.3 years versus 41.1 years, IQR: 33.0–53.2 years, P < 0.001, Wilcoxon rank-sum test), and had lower education levels (19.4% with no studies versus 4.6%, P < 0.001, chi-squared test), with a low proportion of them being single (63.9% versus 74.8%, not statistically significant P = 0.145, chi-squared test) and a slightly higher proportion being male (73.8% versus 59.3%, not statistically significant P = 0.061, chi-squared test) (Table 2). Within the subarachnoid NCC group, the presence of concomitant intraparenchymal NCC lesions did not affect the likelihood of dying (23/32, 71.9% in those who died, compared with 117/161, 72.7%, P = 0.754, chi-squared test).
Table 2.
Characteristics according to vital status in all subjects
| Characteristic | Survived (n = 798) | Died (n = 42) | P-value |
|---|---|---|---|
| Gender | 0.061 | ||
| Female | 325 (40.7%) | 11 (26.2%) | |
| Male | 473 (59.3%) | 31 (73.8%) | |
| Age (years) | 41.1 (33.0–53.2) | 53.5 (41.5–60.3) | < 0.001 |
| Marital status* | |||
| Single | 592 (74.8%) | 23 (63.9%) | 0.145 |
| Others | 200 (25.2%) | 13 (36.1%) | |
| Level of education* | < 0.001 | ||
| No studies | 36 (4.6%) | 7 (19.4%) | |
| Any studies | 756 (95.4%) | 29 (80.6%) | |
| Years of education* | 11 (6–11) | 7 (4–11) | 0.003 |
We could not gather the marital status and education from 12 patients.
The crude mortality rate (deaths/1,000 inhabitants) was 165.80 in the subarachnoid group, 17.91 in the intraparenchymal group, and 12.82 in the calcified group. The respective age-adjusted mortality rates using direct standardization were 144.13, 17.83, and 12.35. Using indirect standardization with the 2009 Peruvian population as reference, the SMR was 1,603.9% in the subarachnoid group, 186.2% in the intraparenchymal group, and 117.4% in the calcified group. The respective age-adjusted mortality rates were 107.30, 12.45 and 7.85.
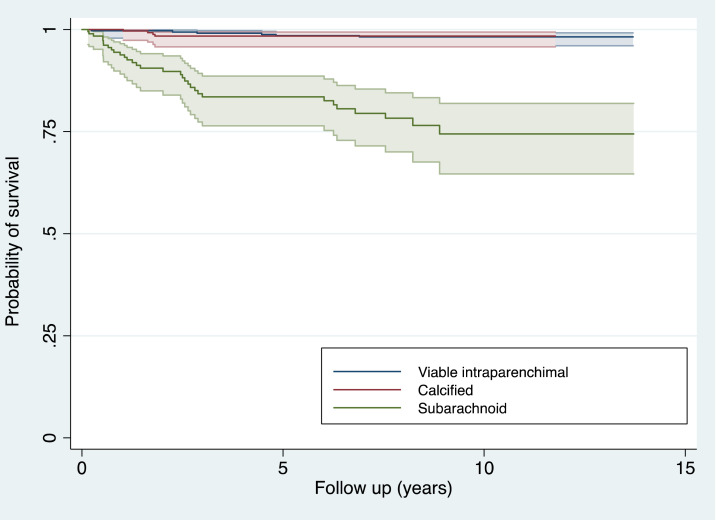
Figure 1 shows the impact of the type of NCC on mortality as Kaplan–Meier survival curves. The 5-year survival probability for the intraparenchymal group was 98.2 (95% CI: 96.1–99.2), similar to the calcified group that had a survival of 98.4 (95% CI: 95.8–99.4), but it was much lower in the subarachnoid group with 83 (95% CI: 76.4–88.6). At 10 years, the survival probabilities were still the same for the viable intraparenchymal and calcified groups and even lower for the subarachnoid group with 74.4 (95% CI: 64.6–81.9) (P < 0.001, log-rank test).
Figure 1.
Probability of survival in patients with intraparenchymal viable, calcified, and subarachnoid Neurocysticercosis. This figure appears in color at www.ajtmh.org.
In a crude Cox proportional hazard regression model, the subarachnoid NCC group had an HR of dying of any cause of 15.08 (95% CI: 6.27–36.29, P < 0.001) compared with the intraparenchymal viable group, and 11.70 (95% CI: 4.10–33.43, P < 0.001) compared with the calcified group. Age adjustment affected only slightly the HR values to 13.57 (95% CI: 5.58–32.99, P < 0.001) and 10.70 (95% CI: 3.72–30.78, P < 0.001).
DISCUSSION
Our study demonstrated a much higher mortality rate in subarachnoid NCC than either viable or calcified NCC. Although this could be apparent to clinicians in endemic regions, controlled studies are absent from the literature. In addition, quantification of the markedly higher mortality risk of subarachnoid NCC should help focus in improving the management and perhaps early diagnosis of this type of disease to decrease this excessive mortality.
Neurocysticercosis is an endemic zoonosis in most developing countries, where it constitutes the most common cause of adult-onset epilepsy. Cases are also increasing in developed countries because of migration.1–3,7 The spectrum of clinical presentation is wide and not limited to epilepsy. It results from several factors including the number, size, stage, and location of the parasites, as well as the immune response of the host.4,5,19 From all variables influencing the presentation and prognosis of NCC, perhaps the one with the stronger impact is the localization of the parasites inside the CNS. Intraparenchymal NCC follows a more benign course and usually presents with epilepsy.1,5 Extraparenchymal NCC tends to cause intracranial hypertension and hydrocephalus, symptoms associated with cranial nerve involvement, and even stroke related to vasculitis in the most severe cases. Its more aggressive form, subarachnoid NCC, involves lesions in spaces such as the Sylvian fissures and basal cisterns,4 is frequently associated with lesion growth, and may require surgical management to remove the NCC lesions and/or treat hydrocephalus.1,4,5
Because of the nature of their neurological symptoms, both types of NCC can be associated with increased mortality. Epilepsy patients are more prone to accidents and trauma because of seizures.20 Also, antiepileptic drug use and concomitant psychiatric conditions may be associated to increased suicide risk in epilepsy patients.21,22 Patients with generalized seizures may present with sudden unexpected death in epilepsy.23,24 In the subarachnoid NCC group, intracranial hypertension may lead to death if not treated promptly. Sudden death in a patient with hydrocephalus with no history of epilepsy has been reported.25 Neurosurgery in NCC includes cyst extraction and shunt placing, and new techniques have reduced the perioperative complications. However, shunt failure and late infection are not uncommon26 and may also lead to death. Antiparasitic treatment also increases the risk of death because of inflammation that can lead to seizures, edema, and hydrocephalus. Brain stem compression due to mass effect is another cause of death.1,4,5,8 In a recent series, mortality in a treated group of subarachnoid NCC patients with a close follow-up was null.27 In Peru, poor access to specialized health services such as MRI is common in regions where this disease is endemic, which may also contribute to mortality.
The nature of our case-based study design does not allow us to extrapolate our findings toward city- or countrywide numbers. In Mexico, deaths due to NCC were 0.5% of the annual incidence cases of NCC with a loss of 7,062-disability adjusted life years in 2012,28 accounting for a total economic loss of over 289 million USD due to both morbidity and premature mortality.29 In the United States, most NCC-related deaths occurred in immigrant Latin American patients and were related to hydrocephalus, brain compression, and epilepsy, for an annual mortality rate of 0.06 per million population over a 13-year period.30 Neurocysticercosis mortality in Sao Paulo, Brazil, decreased from high 2.51 deaths in 1987 to 0.88 deaths per 1,000,000 inhabitants in 2003. A nationwide study in Brazil showed a similar trend but also showed higher mortality rates in highly endemic locations. Most deaths were also associated to intracranial hypertension, followed by cerebral edema and hydrocephalus.10,11
The overall mortality was 5.0%. As expected, mortality was much higher in the subarachnoid group (16.6%) than in the other two groups (1.8 in intraparenchymal viable and 1.3% in calcified NCC). Subarachnoid NCC had the highest crude and age-adjusted mortality rates, followed by intraparenchymal viable and calcified groups. Similar differences were found when standardizing to the Peruvian population, with a marked increase in mortality for subarachnoid cases, almost twice the risk for individuals with viable parenchymal cysts, and only a slight increase for those with calcified lesions only. Factors associated with increased mortality in bivariate analysis were older age and lower education, and there was a marginal association with higher risk for males. In the survival analysis, we found hazard risks more than 10 even after age adjustment.
Our study has limitations. Our study population was seen at a neurological referral facility where patients have access to appropriate neurological diagnosis and care. In endemic regions, it is likely that some patients with NCC die before being diagnosed or receiving specialized care, and as such, our numbers may underestimate the true mortality of the disease. This study describes any-cause mortality that may include causes unrelated to NCC. We did not have data on the specific cause of death. However, this should not differentially affect the mortality by type of NCC, as suggested by the concordance in mortality rates for both groups of parenchymal NCC. We also included follow-up data from two different periods, and secular trends may have influenced our analyses. Finally, we included patients with intraparenchymal lesions in the subarachnoid group because these mixed forms are treated as subarachnoid NCC, prioritizing the most severe form. Although it is possible that the additional impact of parenchymal cysts could have worsened the prognosis of the underlying subarachnoid disease, we did not find differences in the distribution of these mixed forms in both the surviving and dead groups.
Subarachnoid NCC is already a challenge. Its diagnosis requires advanced imaging tools, and its management requires specialized and prolonged surgical and medical care. Factors that we believe may have contributed to mortality in this group and need to be explored in future studies are the delay in diagnosis, the presence of hydrocephalus, and intracranial hypertension. Other factors related to treatment that could increase the risk of death are surgery complications and long-term use of corticosteroids. The markedly high mortality described here for patients with subarachnoid NCC should prompt clinicians and policy makers to impulse the development of interventions aimed to decrease mortality in this higher risk group.
Acknowledgments:
J. A., I. G., and J. B. were partially supported by an FIC NIH training grant (TW001140).
REFERENCES
- 1.Garcia HH, Nash TE, Del Brutto OH, 2014. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol 13: 1202–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fabiani S, Bruschi F, 2013. Neurocysticercosis in Europe: still a public health concern not only for imported cases. Acta Trop 128: 18–26. [DOI] [PubMed] [Google Scholar]
- 3.Ong S, Talan DA, Moran GJ, Mower W, Newdow M, Tsang VCW, Pinner RW; EMERGEncy ID NET Study Group , 2002. Neurocysticercosis in radiographically imaged seizure patients in U.S. emergency departments. Emerg Infect Dis 8: 608–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleury A, Carrillo-Mezo R, Flisser A, Sciutto E, Corona T, 2011. Subarachnoid basal neurocysticercosis: a focus on the most severe form of the disease. Expert Rev Anti Infect Ther 9: 123–133. [DOI] [PubMed] [Google Scholar]
- 5.Garcia HH, Gonzalez AE, Gilman RH, 2020. Taenia solium cysticercosis and its impact in neurological disease. Clin Microbiol Rev 33: e00085-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bustos JA, et al. For the Cysticercosis Working Group in Peru , 2020. Frequency and determinant factors for calcification in neurocysticercosis. Clin Infect Dis ciaa784. Available at: https://academic.oup.com/cid/advance-article-abstract/doi/10.1093/cid/ciaa784/5859152?redirectedFrom=fulltext. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruno E, Bartoloni A, Zammarchi L, Strohmeyer M, Bartalesi F, Bustos JA, Santivañez S, García HH, Nicoletti A; COHEMI Project Study Group , 2013. Epilepsy and neurocysticercosis in Latin America: a systematic review and meta-analysis. PLoS Negl Trop Dis 7: e2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeGiorgio CM, Houston I, Oviedo S, Sorvillo F, 2002. Deaths associated with cysticercosis. Report of three cases and review of the literature. Neurosurg Focus 12: e2. [DOI] [PubMed] [Google Scholar]
- 9.Siu-Chang D, 2015. Neurocisticercosis Subaracnoidea Basal, Mortalidad Y Factores Asociados. Lima, Peru: Universidad Peruana Cayetano Heredia. Available at: http://repositorio. upch.edu.pe/handle/upch/4434. [Google Scholar]
- 10.Martins-Melo FR, Ramos AN, Cavalcanti MG, Alencar CH, Heukelbach J, 2016. Neurocysticercosis-related mortality in Brazil, 2000–2011: epidemiology of a neglected neurologic cause of death. Acta Trop 153: 128–136. [DOI] [PubMed] [Google Scholar]
- 11.Santo AH, 2007. Cysticercosis-related mortality in the State of São Paulo, Brazil, 1985–2004: a study using multiple causes of death. Cad Saude Publica 23: 2917–2927. [DOI] [PubMed] [Google Scholar]
- 12.Garcia HH, Pretell EJ, Gilman RH, Martinez SM, Moulton LH, Del Brutto OH, Herrera G, Evans CAW, Gonzalez AE, 2004. A trial of antiparasitic treatment to reduce the rate of seizures due to cerebral cysticercosis. N Engl J Med 350: 249–258. [DOI] [PubMed] [Google Scholar]
- 13.Nash TE, Pretell EJ, Lescano AG, Bustos JA, Gilman RH, Gonzalez AE, Garcia HH; Cysticercosis Working Group in Peru , 2008. Perilesional brain oedema and seizure activity in patients with calcified neurocysticercosis: a prospective cohort and nested case-control study. Lancet Neurol 7: 1099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia HH, et al. Cysticercosis Working Group in Peru , 2011. Pharmacokinetics of combined treatment with praziquantel and albendazole in neurocysticercosis. Br J Clin Pharmacol 72: 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Callacondo D, Garcia HH, Gonzales I, Escalante D, Nash TE; Cysticercosis Working Group in Peru , 2012. High frequency of spinal involvement in patients with basal subarachnoid neurocysticercosis. Neurology 78: 1394–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia HH, et al. Cysticercosis Working Group in Peru , 2014. Efficacy of combined antiparasitic therapy with praziquantel and albendazole for neurocysticercosis: a double-blind, randomised controlled trial. Lancet Infect Dis 14: 687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia HH, Gonzales I, Lescano AG, Bustos JA, Pretell EJ, Saavedra H, Nash TE; Cysticercosis Working Group in Peru , 2014. Enhanced steroid dosing reduces seizures during antiparasitic treatment for cysticercosis and early after. Epilepsia 55: 1452–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia HH, Lescano AG, Gonzales I, Bustos JA, Pretell EJ, Horton J, Saavedra H, Gonzalez AE, Gilman RH; Cysticercosis Working Group in Peru , 2016. Cysticidal efficacy of combined treatment with praziquantel and albendazole for parenchymal brain cysticercosis. Clin Infect Dis 62: 1375–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arroyo G, et al. Cysticercosis Working Group in Peru , 2018. Antibody banding patterns of the enzyme-linked immunoelectrotransfer blot and brain imaging findings in patients with neurocysticercosis. Clin Infect Dis 66: 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watila MM, Balarabe SA, Ojo O, Keezer MR, Sander JW, 2018. Overall and cause-specific premature mortality in epilepsy: a systematic review. Epilepsy Behav 87: 213–225. [DOI] [PubMed] [Google Scholar]
- 21.Dreier JW, Pedersen CB, Gasse C, Christensen J, 2019. Antiepileptic drugs and suicide: role of prior suicidal behavior and parental psychiatric disorder. Ann Neurol 86: 951–961. [DOI] [PubMed] [Google Scholar]
- 22.Mula M, Sander JW, 2015. Suicide and epilepsy: do antiepileptic drugs increase the risk? Expert Opin Drug Saf 14: 553–558. [DOI] [PubMed] [Google Scholar]
- 23.Scorza FA, Arida RM, de Mendonça PRF, Cavalheiro EA, Leite JP, 2012. Neurocysticercosis: a new trend in SUDEP research? Rev Soc Bras Med Trop 45: 280. [DOI] [PubMed] [Google Scholar]
- 24.Holmes NE, Iles LE, Danks RA, Korman TM, 2010. Neurocysticercosis causing sudden death. Am J Forensic Med Pathol 31: 117–119. [DOI] [PubMed] [Google Scholar]
- 25.Llompart Pou JA, Gené A, Ayestarán JI, Saus C, 2005. Neurocysticercosis presenting as sudden death. Acta Neurochir (Wien) 147: 785–786. [DOI] [PubMed] [Google Scholar]
- 26.Reddy GK, Bollam P, Caldito G, 2012. Ventriculoperitoneal shunt surgery and the risk of shunt infection in patients with hydrocephalus: long-term single institution experience. World Neurosurg 78: 155–163. [DOI] [PubMed] [Google Scholar]
- 27.Nash TE, O’Connell EM, Hammoud DA, Wetzler L, Ware JM, Mahanty S, 2020. Natural history of treated subarachnoid neurocysticercosis. Am J Trop Med Hyg 102: 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhattarai R, Budke CM, Carabin H, Proaño JV, Flores-Rivera J, Corona T, Ivanek R, Snowden KF, Flisser A, 2012. Estimating the non-monetary burden of neurocysticercosis in Mexico. PLoS Negl Trop Dis 6: e1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhattarai R, Carabin H, Proaño JV, Flores-Rivera J, Corona T, Flisser A, León-Maldonado L, Budke CM, 2019. The monetary burden of cysticercosis in Mexico. PLoS Negl Trop Dis 13: e0007501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorvillo FJ, DeGiorgio C, Waterman SH, 2007. Deaths from cysticercosis, United States. Emerg Infect Dis 13: 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]