Abstract
Mantle cell lymphoma (MCL) accounts for 5%–10% of all lymphomas. The disease’s genetic hallmark is the t(11; 14)(q13; q32) translocation. In younger patients, the first-line treatment is chemoimmunotherapy followed by autologous stem cell transplantation. Upon disease progression, novel and targeted agents such as the BTK inhibitor ibrutinib, the BCL-2 inhibitor venetoclax, or the combination of both are increasingly used, but even after allogeneic stem cell transplantation or CAR T-cell therapy, MCL remains incurable for most patients. Chronic antigenic stimulation of the B-cell receptor (BCR) is thought to be essential for the pathogenesis of many B-cell lymphomas. LRPAP1 has been identified as the autoantigenic BCR target in about 1/3 of all MCLs. Thus, LRPAP1 could be used to target MCL cells, however, there is currently no optimal therapeutic format to integrate LRPAP1. We have therefore integrated LRPAP1 into a concept termed BAR, for B-cell receptor antigens for reverse targeting. A bispecific BAR body was synthesized consisting of the lymphoma-BCR binding epitope of LRPAP1 and a single chain fragment targeting CD3 or CD16 to recruit/engage T or NK cells. In addition, a BAR body consisting of an IgG1 antibody and the lymphoma-BCR binding epitope of LRPAP1 replacing the variable regions was synthesized. Both BAR bodies mediated highly specific cytotoxic effects against MCL cells in a dose-dependent manner at 1–20 µg/mL. In conclusion, LRPAP1 can substitute variable antibody regions in different formats to function in a new therapeutic approach to treat MCL.
Introduction
Mantle cell lymphoma (MCL) accounts for 5%–10% of all lymphomas with an annual incidence rate of 0.86 per 100 000 population.1,2 MCL shows a variable clinical course ranging from indolent to aggressive. The disease’s genetic hallmark is the t(11; 14)(q13; q32) translocation that transposes CCND1 to the immunoglobulin heavy chain locus leading to overexpression of the cell cycle regulator cyclin D1.1 Standard first-line treatment for younger patients with good performance status has been established within the “MCL younger trial of the European Mantle Cell Lymphoma Network.” It consists of chemoimmunotherapy (6 alternating cycles of R-CHOP and R-DHAP) followed by autologous stem cell transplantation (ASCT).3 Rituximab maintenance therapy after ASCT was shown to further prolong event-free survival.4 Upon disease progression, novel and targeted therapies such as the BTK inhibitor ibrutinib, the BCL-2 inhibitor venetoclax or the combination of both are increasingly used but even after allogeneic stem cell transplantation or CAR T-cell therapy, MCL remains incurable for most patients.5–9 The need for new therapeutic options is further emphasized by the fact that the median age of patients with MCL is close to 70 years, an age excluding ASCT as therapeutic option for many patients.2 For this patient population, the European MCL Elderly trial reported an OS time of 6.4 years when treated with 6 cycles of R-CHOP followed by rituximab maintenance.10
B-cell receptor (BCR) signaling has been identified as an important pathway in B-cell lymphomagenesis, and there is increasing evidence for antigenic BCR stimulation as a proliferation trigger.11 Autoantigens have been proposed as stimulating ligands of the BCR and its pathway in different types of lymphoma.12
Over the last years, our group has identified several autoantigens as a specific target of the BCR/paraprotein from different B cell malignancies. These include the following: Hyperphosphorylated Paratarg-7 and sumoylated HSP90 were each described as the antigenic targets for approximately 15% of the monoclonal immunoglobulin from monoclonal gammopathy of unknown significance and multiple myeloma (MM) patients.13,14 ARS2 has been identified as the autoantigenic target of the BCR from approximately 25% of diffuse large B-cell lymphomas (DLBCLs) of the ABC type and neurabin-I/SAMD14 as BCR target of more than half of primary CNS lymphomas (PCNSL).15,16 Similarly, LRPAP1 was found to be the antigenic target of the BCR in 1/3 of MCL cases.17 The mechanism underlying the autoimmunogenicity of the respective antigens has not been fully elucidated, but for some BCR targets a posttranslational modification (hyperphosphorylation of paratarg-7, sumoylation of HSP90, hypophosphorylation of ARS2 and hyperglycosylation of neurabin-I/SAMD14) might be the reason for the breakdown of self-tolerance.13–16
In the context of MCL, no posttranslational modifications were found for LRPAP1, leaving the origin of the immune reaction against LRPAP1 unexplained.17 LRPAP1 consists of 357 amino acids (uniprot accession number: P30533) resulting in a molecular weight of 39 kDa.18 It is described to play a role in the megalin/cubilin endocytosis pathway as an antagonist of the LDL receptor family.19 It was shown that the epitope region of LRPAP1, which is reactive to the BCR of some MCLs, spans from amino acids 264 to 318.17
The BCR of a patient´s lymphoma cells is unique and differs from the BCRs of all other patient’s B cells in its variable, antigen-binding region, making it an attractive target for specific therapeutic approaches. Several formats have been used to pursue BCR targeting, among others anti-idiotypes, that is, antibodies directed against the variable region of lymphoma BCRs. Their intended mechanism of action is to elicit an immune response against the lymphoma cells expressing the respective BCR.20,21 These anti-idiotype antibodies were either isolated from lymphoma patients following vaccination with the BCR obtained from lymphoma cells, or they were obtained from sera of animals, immunized with lymphoma BCRs.22–25
Another approach to target the BCR of a malignant B-cell clone are “peptibodies” obtained by phage display selection as described by Levy et al.26 The main problem of these approaches is, however, their applicability, because they are directed against a unique BCR of a given malignant B-cell clone, necessitating a specific reagent for each individual patient.
In contrast, BCR antigens like LRPAP1 are predominant for a given lymphoma subtype and cover a considerable proportion of patients within each lymphoma entity. Thus, LRPAP1-based treatment strategies have the potential to function effectively in a significant percentage of patients with MCL. We have recently shown that BCR antigens can be used to target B-cell lymphoma cells in vitro in an approach designated as BARs (BCR antigens for reverse targeting).15–17,27 The aim of this study was to identify and test different therapeutic LRPAP1-based BAR bodies to target MCL cells.
Materials and methods
Bacteria, cell lines, and cell culture
The DLBCL cell line U2932 was kindly provided by M.-L. Hansmann from the Dr. Senckenberg Institute of Pathology of the Goethe University Hospital Frankfurt. HEK 293T cells and MCL cell lines MAVER1, Mino and Granta-519 were purchased from the DSMZ (Braunschweig, Germany). The cell line Z138 was bought from ATCC (American Type Culture Collection, Manassas, VA). For authentication, identity of the VH gene sequences with published sequences was demonstrated by PCR analysis and sequencing. Cells were cultured in RPMI 1640 medium (Pan Biotech, Aidenbach, Germany), supplemented to a concentration of 4 mM glutamine and 10% FCS.
Peripheral blood mononuclear cells (PBMCs) were prepared by Ficoll density centrifugation (1500 rpm for 30 min) and used on day 2 after preparation without stimulation.
NK cells were isolated from PBMCs by magnetic depletion of all non-NK cells using the CD56+/CD16+ human NK-Cell Isolation Kit (Miltenyi Biotech GmbH, Bergisch Gladbach, Germany) according to the manufacturer`s instructions. NK cells were isolated immediately before the ADCC assay without additional activation. The viability of NK cells after isolation was 95% and the percentage of the CD16+ fraction was between 90 and 98% as assessed by flow cytometry.
T cells were isolated from PBMCs using the Pan T Cell Isolation Kit, human by Miltenyi Biotech GmbH and cultured for 24 hours at 37°C on αCD3/αCD28 coated plates.
DH5α competent Escherichia coli was obtained from Thermo Scientific (168 Third Avenue, Waltham, MA) and used for general cloning and subcloning. TG1 E. coli were used for expression of the Fab-format LRPAP1 BAR bodies.
Cloning strategy for bispecific LRPAP1 constructs
Variable light-chain (VL) and variable heavy-chain (VH) domains of the anti-CD3 OKT 3 hybridoma and the anti-CD16 3G8 hybridoma were cloned into a pcDNA 3.1 vector (Invitrogen, Carlsbad, CA) by standard cloning techniques, followed by the DNA sequence of the LRPAP1 epitope, respectively (Supplemental Digital Figure 1, http://links.lww.com/HS/A179). The single-chain fragment (scFv) containing the variable heavy and light chain of the anti-CD16 hybridoma, linked by a glycine-serine linker, was bought from GenScript (GenScript USA Inc., Piscataway, NJ).
The primers used for PCR amplification of anti-CD3 scFv and the LRPAP1 epitope are shown in Table 1.
Table 1.
Primers Used for Bispecific LRPAP1 BAR-bodies
| Bispecific Constructs | Name | Sequence (5′-3′) |
|---|---|---|
| LRPAP1 epitope Aa 263–317 |
LRPAP1 Bispecific AA 263-EcoRV-sense |
GATATCATGCTGGCGCAGTCCGCCAAC |
| LRPAP1 Bispecific AA 317-EcoRV-antisense |
GATATCCACACGCTCGCCGTCGCC | |
| VH Anti CD3 scFv | Anti CD3-VH-HindIII-sense | AAGCTTGCCACCATGCAGGTCCAGCTGCAGCAG |
| Anti CD3-VH-BamHI-antisense | GGATCCACCACCACCGGAGCCGCCGCCGCCAGAACCACC ACCACCAGAACCACCACCACCTGTTGTTTTGGCTGAGGA |
|
| VL Anti CD3 scFv | Anti CD3-VK-BamHI-sense | GGATCCCAAATTGTTCTCACCCAGTC |
| Anti CD3-VK-EcoRI-antisense | GAATTCGATCCGCCACCGCCAGAGCCACCTCCGCC TGAACCGCCTCCACCAGTTGGTGCAGTATCAGCC |
BAR=B-cell receptor antigens for reverse targeting.
VH and VL were linked by a glycine-serine linker, as was the LRPAP1 epitope to VL, resulting in a VH-(GlySer)4-VL-(GlySer)3-LRPAP1 peptide chain. The LRPAP1 epitope was tagged with a histidine tail for subsequent detection and purification.
PCR products, amplified with these primers, were subcloned into a PCR 2.1 vector using the TOPO TA Cloning Kit (ThermoFisher Scientific, Waltham, MA) and then assembled in the pcDNA 3.1 vector. The final cloning product was used to transfect HEK 293T cells for production of bispecific constructs using X-tremeGENE HP DNA Transfection Reagent (Sigma-Aldrich Chemie GmbH, Munich, Germany).
Expression, purification and detection of bispecific LRPAP1 constructs
Expressed proteins were purified using cobalt-based “Immobilized Metal Affinity Chromatography.” In short, transfected cells were harvested and lysed in 10mM TRIS pH 8 buffer for 30 minutes at 4°C. Following this, 50 µL of Talon beads (ThermoFisher Scientific) were added and incubated for 30 minutes on ice. After 3 washing steps, proteins were eluted with 150 mM imidazole and rebuffered in PBS. Both constructs (anti-CD3/LRPAP1, anti-CD16/LRPAP1) were detected via an N-terminal polyhistidine His6-tag. Lysates of HEK 293T cells, transfected with pcDNA 3.1 vectors containing anti-CD3/LRPAP1 and anti-CD16/LRPAP1 were loaded onto and separated in a 10% SDS-PAGE gel and transferred to a PVDF membrane using a transblot semidry transfer cell (Bio-Rad Laboratories GmbH, Hercules, CA). After blocking overnight at 4°C in PBS/10% nonfat dry milk, transferred proteins were incubated for 1 hour at room temperature with murine anti-His antibody at 1:2000 (Qiagen, Hilden, Germany) followed by HRP-labeled anti-mouse IgG antibody (Bio-Rad Laboratories GmbH) diluted 1:3000. A chemiluminescence reagent (New England Biolabs, Ipswich, MA, USA) was used for immunoblot detection.
Cloning strategy for the Fab-format LRPAP1 BAR body
A modified pCES1 vector was used to assemble the Fab-format LRPAP1 BAR bodies,28 comprising the MCL binding epitope of LRPAP1 in substitution for the heavy and light chain variable domains (VH + VL) and 2 constant domains, CH1 + CL (Supplemental Digital Figure 3, http://links.lww.com/HS/A179). The former variable region that is replaced by a fragment of LRPAP1 is called BAR-region.
Three versions (A, B, and C) of the Fab-format LRPAP1 BAR bodies were cloned. These 3 versions show similar lengths of their BAR region, that is, approximately 120 amino acids to mimic the length of natural variable regions. The Fab-format BAR body versions differ in their amino acid sequence selected from the different regions of the LRPAP1 epitope/affinity region: version A (amino acids 263–350 of LRPAP1), version B (amino acids 230–350 of LRPAP1), and version C (amino acids 198–317 of LRPAP1). Primers and restriction sites used are listed in Table 2. All versions contained the MCL binding LRPAP1 epitope (amino acids 264 – 318)17 but at different positions from 5′ to 3′. The LRPAP1 sequence integrated in version A is only 87 amino acids long, since the MCL-reactive epitope is located at the 3′ end of LRPAP1.
Table 2.
Primers Used for Fab-format LRPAP1 BAR-bodies
| Fab-formatBAR-Body | Name | Sequence (5′-3′) |
|---|---|---|
| Version AHeavy chain | LRPAP1 Fab AA 263-Ncol-sense |
CCATGGCCCTGGCGCACTCCGCCAAC |
| LRPAP1 Fab AA 350-BstE2-antisense |
GGTGACCCCGGAGATCCTGCCGGACAC | |
| Version ALight chain | LRPAP1 Fab AA 263-ApaLl-sense |
GTGCACAGCTGGCGCAGTCCGCCAAC |
| LRPAP1 Fab AA 350-Xho1-antisense |
CTCCAGGGAGATCCTGCCCGACAG | |
| Version BHeavy chain | LRPAP1 Fab AA 230-Ncol-sense |
CCATGGCCATCAACCAGGGCCTGGAC |
| LRPAP1 Fab AA 350-BstE2-antisense |
GGTGACCCCGGAGATCCTGCCGGACAC | |
| Version BLight chain | LRPAP1 Fab AA 230-ApaL1-sense |
GTGCACAGATCAACCAGGGCCTGGAG |
| LRPAP1 Fab AA 350-Xho1-antisense |
CTCCAGGGAGATCCTGCCCGACAG | |
| Version CHeavy chain | LRPAP1 Fab AA 198-Ncol-sense |
CCATGGCCGAAATCCACGAGAACGTC |
| LRPAP1 FAb AA 317-BstE2-antisense |
GGTGACCACACGCTCGCCGTCGCC | |
| Version CLight chain | LRPAP1 Fab AA 198-ApaL1-sense |
GTCCACAGGAAATCCACGAGAACGTC |
| LRPAP1 Fab AA 317-Xho1-antisense |
CTCGAGCACACGCTCGCCGTCGCC |
BAR=B-cell receptor antigens for reverse targeting.
Expression, purification, and detection of Fab-format LRPAP1 BAR bodies
E. coli bacteria of the TG1 strain were transformed with the modified pCES1 vector comprising the Fab-format LRPAP1 BAR body. Recombinant soluble Fab-format BAR bodies were expressed and purified as described previously.28,29 In short, 50 µL of IPTG were added to 50 mL of TY medium and TG1 bacteria (cell density of 0.6–0.8 measured at 600 nm) for 4 hours at 30°C. Then, the medium was centrifuged at 4000 rpm for 15 minutes. After lysing, His-tagged Fab-format LRPAP1 BAR bodies were purified by Immobilized Metal Affinity Chromatography as described for the bispecific constructs. Proteins were eluted with 150 mM imidazole for 5 minutes at room temperature and detected by western blot analysis using recombinant mouse anti-His6 antibody (Qiagen) and goat anti-mouse IgG (H + L)-HRP conjugate (Bio-Rad, Munich, Germany), followed by chemiluminescence (New England Biolabs).
Cloning strategy for the IgG1-format LRPAP1 BAR bodies
A pSfi FLAG-Tag expression vector was used to assemble the sequence of the IgG1-format LRPAP1 BAR body (Supplemental Digital Figure 5, http://links.lww.com/HS/A179). The pSfi FLAG-Tag vector was generated from the pEGFP-C1 vector of Clontech (Mountain View, CA) by removing the eGFP ORF and replacing it with a FLAG-Tag. An IgG1 sequence comprising a heavy chain variable region, the heavy chain constant regions CH1-CH3, a furin cleavage site, an autoproteolytic 2A peptide sequence, a light chain variable and a constant region30 was used as template. VH and VL were exchanged with the sequence of LRPAP1 that had been tested as Fab-format BAR body and selected for further experiments (Version A, amino acids 263–350) containing the MCL reactive epitope (amino acids 264–318). Primers and restriction sites used are listed in Table 3. Restriction enzymes MunI and BstEII (ThermoFisher Scientific) were used to replace the VH region with the LRPAP1 fragment (amino acids 263–350) and AgeI and SmaI were used to integrate amino acids 263–350 of LRPAP1 into the VL region.
Table 3.
Primers Used for the IgG1-format LRPAP1 BAR-body
| IgG1-formatBAR-Body | Name | Sequence (5′-3′) |
|---|---|---|
| Heavy chain | LRPAP1 AA 263-MunI-sense | CAATTGCTGGCGCAGTCCGCCAA |
| LRPAP1 AA 350-BstEII-antisense | GGTACCGGAGATCCTGCCGGACAG | |
| Light chain | LRPAP1 AA 263-AgeI-sense | ACCGGTCTGGCGCAGTCCGCCAA |
| LRPAP1 AA 350-SmaI-antisense | CCCGGGGGAGATCCTGCCGGACAG |
BAR=B-cell receptor antigens for reverse targeting.
Expression, purification and detection of the IgG1-format LRPAP1 BAR bodies
HEK 293T cells were used for production of IgG1-format LRPAP1 BAR bodies. 1 µg plasmid DNA diluted in 100 µL RPMI1640 was mixed with 3 µL of X-tremeGENE HP DNA Transfection Reagent (Sigma-Aldrich Chemie GmbH, Munich, Germany). The mixture was incubated at room temperature for 10 minutes and then added to HEK 293 T cell cultures drop by drop.
Fifty microliters of supernatant of the transfected HEK 293T cells was incubated with 200 µL ANTI-FLAG M2 Affinity Gel (Sigma-Aldrich, St. Louis, MO, USA) overnight at 4°C. After 2 rounds of centrifugation (10 min at 2500 rpm) 3 washing steps followed with 1 mL PBS. Centrifugation during the washing steps was performed at 10 000 rpm for 30 seconds. Elution of IgG1 LRPAP1 BAR bodies from the matrix was done by adding 250 µL glycine pH 3 for 2 minutes with subsequent centrifugation at 10 000 rpm for 30 seconds. Supernatant containing the isolated IgG1 LRPAP1 BAR bodies was supplemented with 25 µL Na2HPO4. Overnight dialysis against PBS was performed to transfer purified IgG1 LRPAP1 BAR bodies into PBS.
For detection, IgG1 LRPAP1 BAR bodies were loaded on to a 10% SDS-PAGE gel and transferred to a PVDF membrane using a transblot semidry transfer cell (Bio Rad). The PVDF membrane was then incubated for 45 minutes at room temperature with murine monoclonal ANTI-FLAG M2 antibody (Sigma-Aldrich, St. Louis, MO, USA) at 1:2,500. Thereafter, the membrane was rinsed with TBS 5 times for 2 minutes and incubated for 45 minutes with goat anti-mouse IgG (H+L)-HRP conjugate (Bio-Rad, Feldkirchen) at 1:3000. After another washing step, to rinse the membrane with TBS 5 times for 2 minutes each, the chemiluminescence reagent LumiGLO (Cell Signaling Technology, Frankfurt, Germany) was used for immunoblotting.
Flowcytometric binding assays
Binding of all constructs to lymphoma cell lines (MAVER1, Z138, Granta-519, Mino, U2932), PBMCs and isolated T/NK cells was assessed by flow cytometry. All flow cytometry analyses were performed using a BD FACS Canto Flow Cytometer and data was analyzed with FCSalyzer 0.9.18-alpha.
NK cells isolated from PBMCs (CD56+/CD16+ human NK-Cell Isolation Kit, Miltenyi Biotech GmbH) were detected using 2-color flow cytometry with FITC-coupled αCD16 antibodies and PE-coupled αCD56 antibodies.
Bispecific LRPAP1 BAR bodies:
For staining of lymphoma cells, NK cells or PBMCs, single-color flow cytometry was used. For this, approximately 5 × 10E6 cells (1 × 10E6/mL) were incubated for 45 minutes at room temperature with anti-CD3/LRPAP1 or anti-CD16/LRPAP1 at a concentration of 3 µg/mL. Thereafter, cells were incubated with PE-labeled anti-His antibody (ThermoFisher Scientific) for 30 minutes at room temperature. Isolated T-cells (Pan T Cell Isolation Kit, human by Miltenyi Biotech GmbH) were stained using 2-color flow cytometry with bispecific BAR bodies (secondary system with PE as in single-color experiments) and FITC-coupled anti-CD4 or anti-CD8 antibodies.
Fab-format LRPAP1 BAR bodies
Binding to MCL cell lines was tested using 5 × 10E6 MAVER1 or Granta-519 cells that were incubated with 5 μg/mL of Fab-format LRPAP1 BAR bodies (versions A, B, and C) for 30 minutes at 4°C, washed with PBS and stained with 5 µL of Penta-His-APC-labeled antibody (Qiagen) for 30 minutes at 4°C.
IgG1-format LRPAP1 BAR bodies
To determine binding properties to lymphoma cells (MAVER1, Z138, Mino, Granta-519, U2932) or isolated NK cells, 5 × 10E6 cells were incubated with 10 µg/mL IgG1-format LRPAP1 BAR body for 30 minutes, washed in PBS and stained with murine monoclonal ANTI-FLAG M2 antibody for 30 minutes at 4°C (Sigma-Aldrich, St. Louis, MO, USA). After another washing step in PBS, the mixture was incubated with goat anti-mouse IgG (H+L), APC for 30 minutes at 4°C (ThermoFisher Scientific). IgG1 format BAR bodies incorporating neurabin-I, a BCR antigen of primary central nervous system lymphomas, served as controls.16 BAR bodies were tested for binding on U2932 cells with or without a SAMD14/neurabin-I-reactive BCR.
Cytotoxicity assays
Cell-mediated cytotoxicity of all constructs was determined in lactate dehydrogenase (LDH) release assays (“Cytotoxicity Detection Kit” by Roche). For bispecific constructs, 2 × 10E3 lymphoma cells (MAVER1, Z138, Mino, Granta-519) per well were incubated with anti-CD3/LRPAP1 (20/10/5.0/1.0 μg/mL) or anti-CD16/LRPAP1 (20/10/5.0/1.0 μg/mL) or PBS as control. Isolated T cells were added at an effector:target (E:T) ratio of 5:1 and isolated NK cell were used at an E:T ratio of 2.5:1.
IgG1-format LRPAP1 BAR bodies were applied at concentrations of 20, 10, 5.0, 1.0 μg/mL or 10, 5, 2.5, and 1.25 µg/mL. IgG1-format BAR bodies incorporating the BCR antigen neurabin-I16 and PBS served as controls. The cell lines MAVER1, Z138, Mino, Granta-519 and U2932 were used as target cells. For NK cell experiments 2 × 10E3 target cells were used while 5 × 10E3 target cells were used in PBMC experiments. Isolated NK cells or PBMCs were added to target cells and BAR bodies at an E/T ratio of 2.5:1 for NK cells and 10:1 for PBMCs, corresponding to 5 × 10E3 NK cells and 5 × 10E4 PBMCs per well.
Percent of specific lysis was determined as (experimental lysis minus spontaneous lysis)/(maximum lysis − spontaneous lysis) × 100. The maximum lysis was determined by adding 10% Triton X-100. LDH was measured according to the protocol of the LDH assay kit (Roche, Mannheim, Germany). Read-out was done using a Victor II microplate reader (PerkinElmer, Rodgau, Germany). All experiments were performed in triplicate.
Results
To determine the optimal therapeutic format for LRPAP1, we designed and tested 2 different approaches. First, bispecific BAR bodies (anti-CD3/LRPAP1 and anti-CD16/LRPAP1), consisting of a recombinant single-chain fragment (scFv) against CD3 or CD16 and the MCL binding epitope of LRPAP1, were produced. The scFv arm should bind to the T-cell co-receptor CD3 or to CD16 and engage T or NK cells, while LRPAP1 should bind to MCL cells with a BCR specific for LRPAP1, thus redirecting and activating T or NK cells against MCL cells while sparing normal B-cells. Second, LRPAP1 was integrated into an IgG antibody format in exchange for the variable regions to generate LRPAP1 BAR bodies. Both formats were assessed for binding properties and therapeutic potential.
Cloning, expression, and detection of bispecific constructs
We used standard cloning techniques to create the two bispecific constructs. scFvs targeting CD3 (OKT 3 hybridoma) on T cells or CD16 (3G8 hybridoma) on NK cells represent the effector arms of the constructs. To favor specific contact between effector cells and MCL cells, we linked both the anti-CD3 and anti-CD16 scFv to the BCR-binding epitope of LRPAP1 (Supplemental Digital Figure 1, http://links.lww.com/HS/A179). αCD3/LRPAP1 and αCD16/LRPAP1 consist of 1044 and 1002 base pairs corresponding to 348 and 334 amino acids, respectively. Western Blot analysis reveals bands of the respected sizes for both constructs just over 35 kDa, which confirms the estimated size of 38.6 and 37.1 kDa (Supplemental Digital Figure 2, http://links.lww.com/HS/A179).
Bispecific LRPAP1 BAR bodies bind highly selective to effector cells and LRPAP1-reactive MCL cells
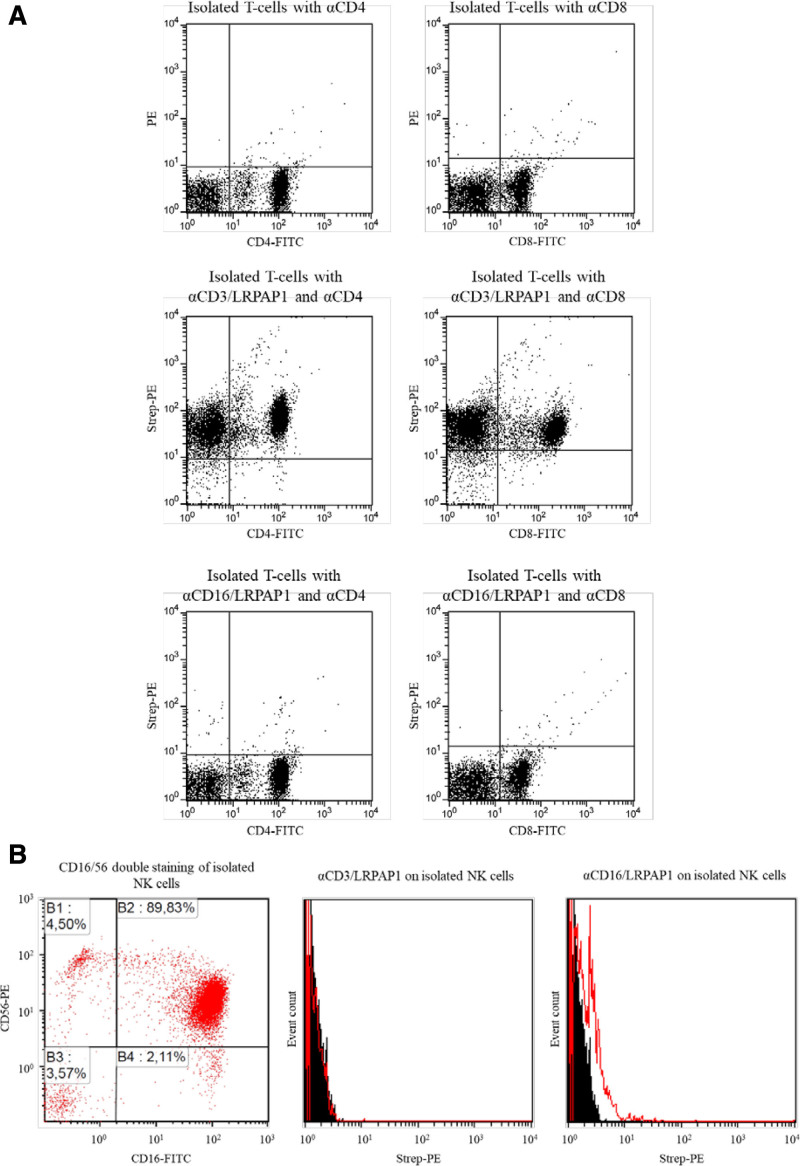
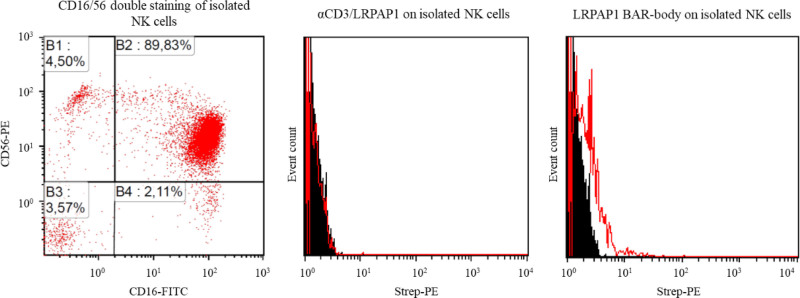
The specific binding of the constructs to effector and target cells was tested by flow cytometry. Figure 1A (upper panel) shows CD4+ and CD8+ cells in the absence of any BAR bodies. As indicated by the increase in PE fluorescence, anti-CD3/LRPAP1 (Figure 1A, middle panel) but not anti-CD16/LRPAP1 (Figure 1B, lower panel) showed binding to isolated CD4+ and CD8+ T cells. Likewise, anti-CD16/LRPAP1 but not anti-CD3/LRPAP1 demonstrated binding capacity to isolated NK cells. When isolated NK cells are incubated anti-CD16/LRPAP1 and secondary system, a small but clear and significant shift in PE fluorescence can be observed (Figure 1B).
Figure 1.
Binding of bispecific BAR-bodies to effector cells. (A) T cells were isolated from PBMCs using the Pan T Cell Isolation Kit, human by Miltenyi Biotech GmbH. Isolated T cells were stained with FITC-coupled anti-CD4 and anti-CD8 antibodies and with the secondary system of LRPAP1 bispecific BAR-bodies (anti-His AB → biotinylated anti-mouse AB → Strep-PE) only (upper dot plots), αCD3/LRPAP1 bispecific BAR-bodies (middle dot plots) and αCD16/LRPAP1 bispecific BAR-bodies (lower dot plots). αCD3/LRPAP1 bispecific BAR-bodies but not αCD16/LRPAP1 bispecific BAR-bodies show binding to CD4+ and CD8+ T cells. (B) NK cells were isolated from PBMCs by magnetic depletion of all non-NK cells using the CD56+/CD16+ human NK-Cell Isolation Kit (Miltenyi Biotech GmbH). αCD16 antibodies were FITC-coupled and αCD56 antibodies were PE-coupled. The viability of the NK cells after isolation averaged 95% and the CD16+ fraction was between 90 and 98% (dot plot on the left). αCD16/LRPAP1 bispecific BAR-bodies (histogram on the right) but not αCD3/LRPAP1 bispecific BAR-bodies (histogram in the middle) showed binding to isolated NK cells. The secondary system for detection was used as in (A). BAR=B-cell receptor antigens for reverse targeting; PBMC=peripheral blood mononuclear cell.
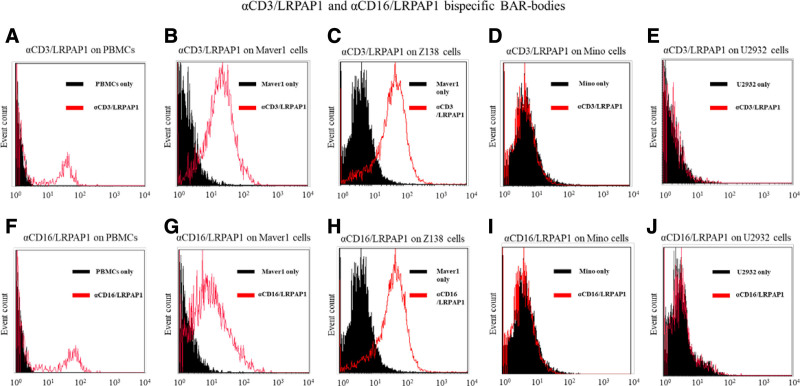
Next, we confirmed that anti-CD3/LRPAP1 and anti-CD16/LRPAP1 bind to the LRPAP1-reactive MCL cell lines MAVER1 (Figure 2B and G) and Z138 (Figure 2C and H) but not to cells of the MCL cell line Mino expressing BCRs not reactive to LRPAP1 (Figure 2D and I). In addition, no unspecific binding of the bispecific BAR bodies to DLBCL cells of the cell line U2932 was observed (Figure 2E and J).
Figure 2.
Binding of bispecific BAR-bodies to lymphoma cells. Flow cytometric assessment of the binding properties of bispecific BAR-bodies. αCD3/LRPAP1 and αCD16/LRPAP1 show binding capacity to PBMCs (A and F). Both constructs bind to cells of the LRPAP1-reactive MCL cell lines MAVER1 (B and G) and Z138 (C and H), which express a BCR that is reactive to LRPAP1. αCD3/LRPAP1 and αCD16/LRPAP1 did not bind to cells of the MCL cell line Mino that express a BCR of different specificity (D and I). Also, both bispecific constructs did not bind to cells of the DLBCL cell line U2932 that were used as further controls (E and J). For experiments, 5 × 10E6 lymphoma cells or PBMCs were incubated with αCD3/LRPAP1 and αCD16/LRPAP1 (1 µg/mL). For detection, PE-labeled anti-His antibody or APC-labeled anti-His antibody was used. BAR=B-cell receptor antigens for reverse targeting; BCR=B-cell receptor; DLBCL=diffuse large B-cell lymphoma; MCL=mantle cell lymphoma; PBMC=peripheral blood mononuclear cell.
Bispecific LRPAP1 BAR bodies mediate highly selective cytotoxic effects to MCL cells
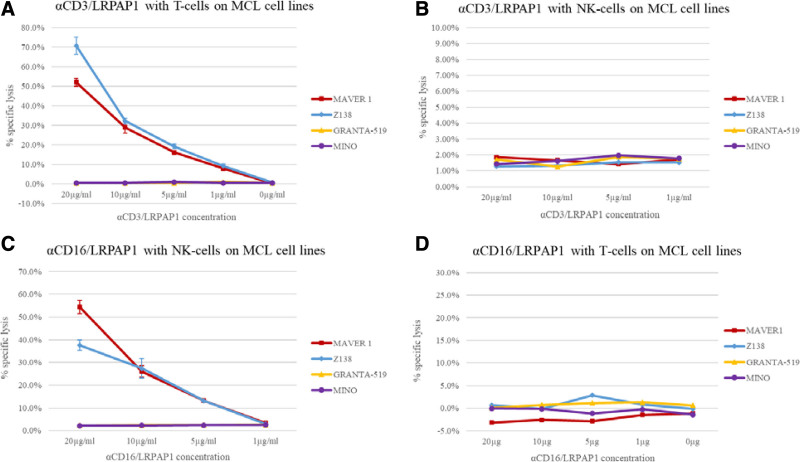
The cytotoxic effects of bispecific BAR bodies were determined by LDH release assays over 4 hours. In the presence of isolated T cells, anti-CD3/LRPAP1 induced dose-dependent lysis of the LRPAP1-reactive MCL cell lines MAVER1 and Z138, up to 70% or 50%, respectively (Figure 3 A). Granta-519 and Mino cells, which express BCRs with no reactivity toward LRPAP1, were not affected by anti-CD3/LRPAP1.
Figure 3.
Bispecific LRPAP1 BAR-bodies—LDH release assays. Isolated T cells and NK cells were used as effector cells. T cells were isolated from PBMCs using the Pan T Cell Isolation Kit, human by Miltenyi Biotech GmbH and cultured for 24 h at 37°C on αCD3/αCD28 coated plates. NK cells were isolated from PBMCs by magnetic depletion of all non-NK cells using the CD56+/CD16+ human NK-Cell Isolation Kit (Miltenyi Biotech GmbH). NK cells were isolated immediately before the ADCC assay without additional activation (eg, by IL-2). Cytotoxic effects of bispecific LRPAP1 BAR-bodies were determined by LDH release. αCD3/LRPAP1 induced specific lysis in LRPAP1-reactive cell lines MAVER1 and Z138 at concentrations of 1.0, 5.0, 10, and 20 µg/mL, respectively. With increasing doses of αCD3/LRPAP1-specific lysis intensified and reached 71% in Z138 and 52% in MAVER1 cells. Granta-519 and Mino cells (not LRPAP1-reactive) were not affected by αCD3/LRPAP1 (A). When the same experiments were repeated using αCD3/LRPAP1 and isolated NK cells, no cytotoxic effects were observed (B). αCD16/LRPAP1 induced cytotoxicity in LRPAP1-reactive MAVER1 and Z138 cells when isolated NK cells were used as effector cells. At 1.0, 5.0, 10, and 20 µg/mL specific lysis reached 3%, 13%, 27%, and 38% in Z138 cells and 3%, 13%, 26%, and 54% in MAVER1 cells. αCD16/LRPAP1 had no effect on Granta-519 and Mino cells (C). When the same experiments were repeated using αCD16/LRPAP1 and isolated T cells, no cytotoxic effects were observed (D). For experiments, 2 × 10E3 lymphoma cells (MAVER1, Z138, Granta-519, Mino) were incubated with αCD3/LRPAP1 and αCD16/LRPAP1 (20/10/5.0/1.0 μg/mL) or PBS and isolated T cells at an E:T ratio of 5:1 (10,000 T cells) or isolated NK cell at an E:T ratio of 2.5:1 (5000 NK cells). Specific lysis was calculated as compared to Triton X-100 controls and is shown in percentage on the y-axis. All experiments were performed in triplicate. BAR=B-cell receptor antigens for reverse targeting; BCR=B-cell receptor.
Anti-CD3/LRPAP1 exerts its cytotoxicity specifically in the presence of T cells, as it had no effect on any MCL cell line in the presence of isolated NK-cells but no T cells (Figure 3B). Anti-CD16/LRPAP1 induced dose-dependent cytotoxicity in MAVER1 and Z138 cells and again had no effect on the LRPAP1 nonreactive Granta-519 and Mino cells when incubated with isolated NK cells (Figure 3C). This cytotoxicity was specific for NK cells as no cytotoxic effects were observed in the presence of T cells (Figure 3D).
Identification of a Fab-format BAR body version with binding properties to MCL cells expressing LRPAP1-reactive BCRs
To integrate LRPAP1 into an antibody format (LRPAP1 BAR body), we initially used the Fab-format to identify the optimal position of the epitope in the variable region. For this, we cloned and expressed 3 different Fab-format BAR bodies and replaced the variable regions with the BCR-binding epitope of LRPAP1 (amino acids 264–318). The MCL-reactive epitope of LRPAP1 was complemented by adjacent LRPAP1 sequences at either the 3´ end (version A), the 5´ end (version C) or both ends (version B) as shown in Supplemental Digital Figure 3, http://links.lww.com/HS/A179. LRPAP1 Fab-format BAR bodies were produced in TG1 E. coli bacteria and western blot analysis confirmed the estimated molecular mass of just under 55 kDa (Supplemental Digital Figure 4, http://links.lww.com/HS/A179).
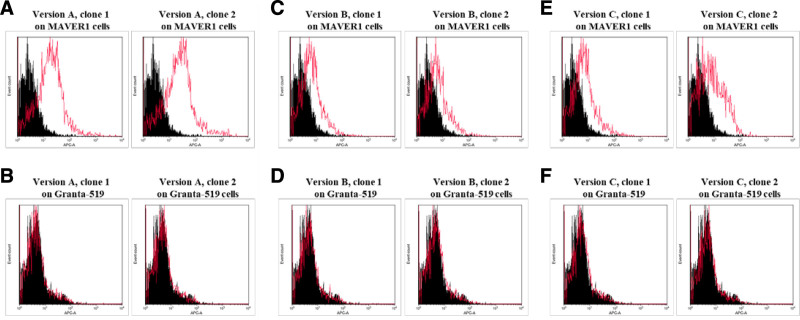
To identify the optimal orientation for the LRPAP1 epitope in the former variable region, all 3 Fab-format BAR body versions were tested by flow cytometry. MAVER1 cells were stained with the three versions (A, B, and C) of the LRPAP1 Fab-format BAR bodies (Figure 4A, C, and E). We produced and tested 2 clones of each version. Granta-519 MCL cells expressing a BCR with no reactivity towards LRPAP1 served as control cells (Figure 4B, D, and F). All versions showed binding to MAVER1 cells and no detectable binding to Granta-519 cells. Both clones of version A (MCL-binding epitope of LRPAP1 at the 5´ end of the former variable region) showed the most intensive reaction with the LRPAP1-reactive BCR of MAVER1 cells (Figure 4A), and their topical orientation of the LRPAP1 epitope in the variable region was chosen for the development of IgG1-format LRPAP1 BAR bodies.
Figure 4.
Fab-format LRPAP1 BAR-bodies—binding assays. Flow cytometric binding assay to identify the optimal conformation for Fab-format LRPAP1 BAR-bodies. MAVER1 cells were stained with 2 clones of Fab-format BAR bodies versions A, B, and C (A, C, and E). Granta-519 mantle cell lymphoma cells were used as controls (B, D, and F). Fab-format BAR-bodies of version A showed the most intensive staining reaction of MAVER1 cells and no detectable binding to Granta-519 cells (A and B). The conformation of the Fab-format BAR-body, version A, was therefore chosen for the further development of IgG1-format LRPAP1 BAR-bodies. 5 × 10E6 lymphoma cells (MAVER1 or Granta-519) were incubated with Fab-format BAR-bodies (5 µg/mL). APC labeled anti-His antibody was used for detection. BAR=B-cell receptor antigens for reverse targeting.
Expression and detection of an IgG1-format LRPAP1 BAR body
Version A of the Fab-format BAR bodies was used as template for the generation of a full length IgG1-format BAR body by integrating LRPAP1 instead of variable regions (Supplemental Digital Figure 5, http://links.lww.com/HS/A179) to target the BCR of MCLs reactive to LRPAP1. Three clones of LRPAP1 IgG1-format BAR bodies were produced eukaryotically in HEK293 cells and western blot analysis confirmed detection of a single band of the right size (Supplemental Digital Figure 6, http://links.lww.com/HS/A179). The estimated molecular mass for the LRPAP1 IgG1-format BAR body is 150 kDa, resembling the molecular mass of full-length IgG antibodies.
IgG1-format LRPAP1 BAR bodies show binding to NK cells and to lymphoma cells expressing LRPAP1-reactive BCRs
First, IgG1-format LRPAP1 BAR bodies were tested for specific affinity to isolated NK cells (Figure 5, left panel).
Figure 5.
Binding of IgG1-format LRPAP1 BAR-bodies to NK cells. NK cells were isolated from PBMCs by magnetic depletion of all non-NK cells using the CD56+/CD16+ human NK-Cell Isolation Kit (Miltenyi Biotech GmbH). αCD16 antibodies were FITC-coupled and αCD56 antibodies were PE-coupled. The viability of the NK cells after isolation averaged 95% and the CD16+ fraction was between 90 and 98% (dot plot on the left). IgG1-format LRPAP1 BAR-bodies (histogram on the right) but not αCD3/LRPAP1 bispecific BAR-bodies (histogram in the middle) showed binding to isolated NK cells. Mouse Anti-Flag and APC-coupled anti-mouse antibodies were used as secondary system. BAR=B-cell receptor antigens for reverse targeting; PBMC=peripheral blood mononuclear cell.
We confirmed that IgG1-format LRPAP1 BAR bodies bind to isolated NK cells (Figure 5, right panel). This binding is specific since αCD3/LRPAP1 BAR bodies did not bind to NK cells (Figure 5).
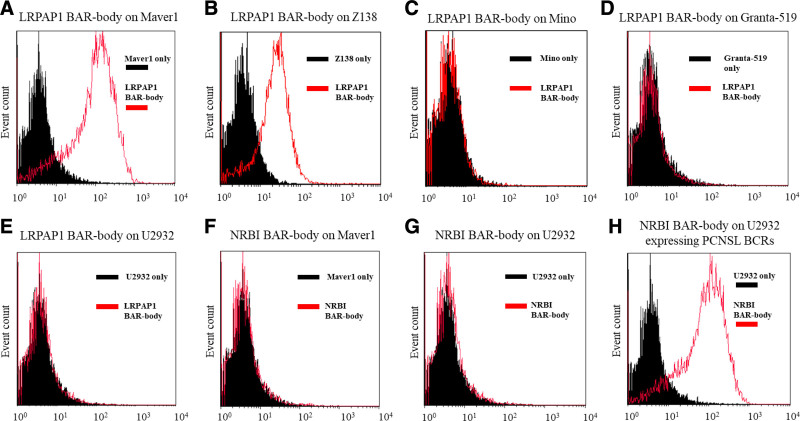
Next, we tested if IgG1-format LRPAP1 BAR bodies bind to lymphoma cells with or without LRPAP1 reactive BCRs. IgG1-format BAR bodies integrating the PCNSL autoantigen neurabin-I/SAMD14 were used as additional controls. The IgG1 LRPAP1 BAR body recognizes selectively MAVER1 and Z138 cells (Figure 6A and B), but not MCL cell lines expressing not LRPAP1-reactive BCRs like Mino and Granta-519 (Figure 6 C and D). Accordingly, the DLBCL cell line U2932 is also recognized by LRPAP1 BAR bodies (Figure 6 E). IgG1 BAR bodies comprising the BCR antigen SAMD14/neurabin-I as binding domain did also not bind to the MCL cell line MAVER1 (Figure 6 F) nor to the DLBCL cell line U2932 (Figure 6 G) but showed strong binding to U2932 cells that were manipulated to express a SAMD14/neurabin-I-reactive BCR (Figure 6 H). Together this proves that the IgG1-format LRPAP1 BAR bodies bind specifically to NK cells and to MCL cells expressing an LRPAP1-reavtive BCR.
Figure 6.
IgG1-format BAR-bodies—binding assays. IgG1 LRPAP1 BAR-bodies were tested for binding to different lymphoma cell lines: MCL cell lines MAVER1, Z138, Mino and Granta-519 (A–D) and the DLBCL cell line U2932 (E). MAVER1 and Z138 cells express a BCR that is reactive to LRPAP1. For further controls, we used U2932 cells that were engineered to express a BCR derived from PCNSL with known reactivity toward neurabin-I/SAMD14 and the IgG1-format BAR-body integrating the PCNSL autoantigen neurabin-I/SAMD14. The IgG1 LRPAP1 BAR-body showed highly specific binding to MAVER1 and Z138 cells (A and B) but not to the MCL cell lines Mino and Granta-519 (C and D). In addition, IgG1-format LRPAP1 BAR-bodies did not bind to cells of the DLBCL cell line U2932 (E). To emphasize the specificity of our approach, an IgG1 BAR-body integrating neurabin-I/SAMD14 was tested for binding to MAVER1 and U2932 cells. Neurabin-I/SAMD14 BAR-bodies did not bind to the MCL cell line MAVER1 (F) or to the DLBCL cell line U2932 (G) but showed strong binding to U2932 cells expressing a recombinant, neurabin-I/SAMD14-reactive, BCR (H). 5 × 10E6 lymphoma cells and 10 µg/mL of IgG1 BAR-bodies were used for each experiment. Murine monoclonal ANTI-FLAG M2 antibody (Sigma-Aldrich, St. Louis, MO, USA) and goat anti-mouse IgG (H+L), APC (Thermo Scientific) were used for detection. BAR=B-cell receptor antigens for reverse targeting; BCR=B-cell receptor; DLBCL=diffuse large B-cell lymphoma.
IgG1-format LRPAP1 BAR bodies mediate cytotoxic effects to MCL cells expressing corresponding BCRs
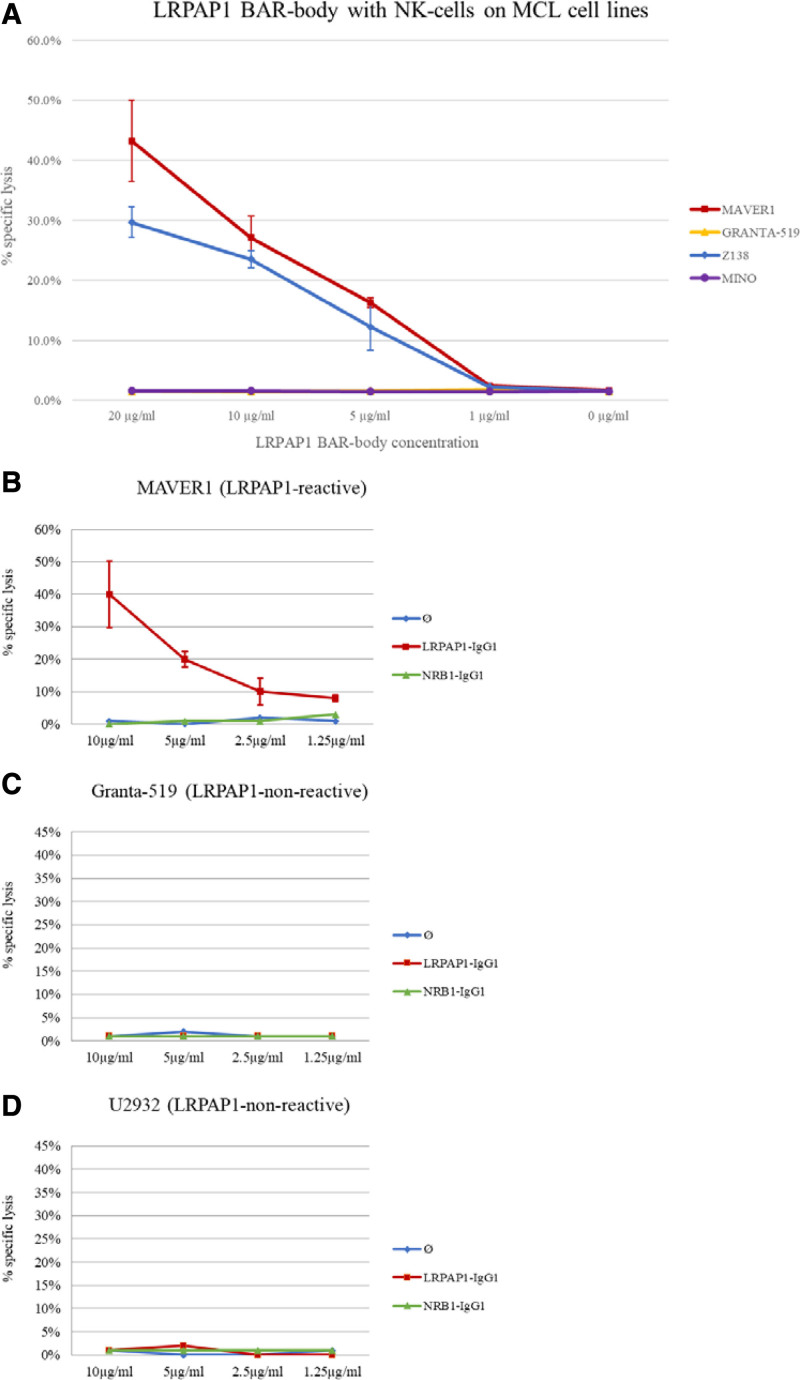
IgG1-LRPAP1 BAR bodies were tested for NK cell- and PBMC-mediated specific cytotoxicity against MAVER1 and Z138 lymphoma cells expressing a BCR with LRPAP1 reactivity and against control cells (Granta-519, Mino, U2932). IgG1 BAR bodies integrating SAMD14/neurabin-I served as control.
IgG1 LRPAP1 BAR bodies induced dose-dependent, specific, lysis of 2.5%, 16.3%, 27.2%, and 43.3% at 1.0, 5.0, 10, and 20 µg/mL in MAVER1 cells and of 2.3%, 12.3%, 23.5%, and 29.7% in Z138 cells in the presence of NK cells at an E:T ratio of 2.5:1. (Figure 7 A). In contrast, IgG1 LRPAP1 BAR bodies had no effect on Granta-519 and Mino cells. As additional control experiment, LRPAP1 BAR bodies were tested for PBMC-mediated specific lysis on the cell lines MAVER1, Granta-519, and U2932, with IgG1 BAR bodies incorporating SAMD14/neurabin-I as BCR-binding domain as controls (Figure 7B–D). As expected, IgG1-format LRPAP1 BAR bodies showed specific lysis of MAVER1 cells (Figure 7B) but had no effect on Granta-519 cells (Figure 7C) or U2932 cells (Figure 7D). Incubation of lymphoma cells with the neurabin-I/SAMD14 control BAR body had no effect on LDH release (Figure 7B–D).
Figure 7.
IgG1-format BAR-body—LDH release assays. Cytotoxic effects of IgG1-format LRPAP1 BAR-bodies as determined by LDH release. BAR-bodies were tested for NK cell- and PBMC-mediated specific cytotoxicity against different MCL and DLBCL cell lines. MAVER1 and Z138 cells express an LRPAP1-reactive BCR; Granta-519, Mino, and U2932 cells express BCRs of different specificity. IgG1 LRPAP1 BAR-bodies and isolated NK cells induced specific cell lysis of 2.5%, 16.3%, 27.2%, and 43.3% at 1.0, 5.0, 10, and 20 µg/mL in MAVER1 cells and of 2.3%, 12.3%, 23.5%, and 29.7% in Z138 cells. In control experiments, IgG1 LRPAP1 BAR-bodies and isolated NK cells had no effect on Granta-519 and Mino cells (A). 2 × 10E3 lymphoma cells (MAVER1, Z138, Granta-519, and Mino) were incubated with IgG1 LRPAP1 BAR-bodies at 1.0, 5.0, 10, or 20 µg/mL and NK cell at an E:T ratio of 2.5:1 (5000 NK cells). To underline the specificity of IgG1 LRPAP1 BAR-bodies, they were also tested on cells of the DLBCL cell line U2932 and simultaneously with a control-BAR-body incorporating the irrelevant autoantigen neurabin-I/SAMD14 (B–D). PBMCs were used as effector cells used at an E:T ratio of 10:1. As expected, IgG1-format LRPAP1 BAR-bodies showed specific lysis of MAVER1 cells (B) but had no effect on Granta-519 cells (C) or U2932 cells (D). Incubation of lymphoma cells with the neurabin-I/SAMD14 control-BAR-body had no effect on LDH release (B–D). All experiments were performed in triplicate. BAR=B-cell receptor antigens for reverse targeting; BCR=B-cell receptor; DLBCL=diffuse large B-cell lymphoma; LDH=lactate dehydrogenase; PBMC=peripheral blood mononuclear cell.
Discussion
In this article, we describe the generation of 2 new strategies for the treatment of MCL, both integrating LRPAP1, the protein that has been identified as autoantigenic target of approximately 45% of MCL cases. The first format, realized as two bispecific constructs (bispecific BAR body), recruits either CD3-positive T or CD16-positive NK effector cells via single chain fragments and connects these scFv to LRPAP1 to target MCL cells with LRPAP1-reactive BCRs. The second format mimics the conformation of an IgG1 antibody with the MCL-reactive epitopes of LRPAP1 replacing the variable regions. In this second format, LRPAP1 is (like in the bispecific BAR bodies) used to target MCL cells with LRPAP1-reactive BCRs but effector cells and complement factor are recruited and activated by the Fc portion of the BAR body.
In 1961, Nisonoff et al generated the first bispecific antibody by pepsin digestion and re-oxidation of two different antibodies.31 Since then a multitude of bispecific molecules of various formats have been reported that were produced using different techniques32 making bispecific antibodies one of the fastest growing fields of drug research with currently more than 50 bispecific antibodies in clinical development.33 However, only one bispecific antibody is currently approved by the European Medicines Agency, that is, the anti-CD19/anti-CD3 single-chain construct Blinatumomab for relapsed, refractory or minimal residual disease (MRD) positive B-cell acute lymphatic leukemia (B-ALL).
Both bispecific BAR constructs described here were able to induce specific lysis of MCL cells with the CD16 directed, NK-cell recruiting construct requiring slightly higher concentrations to reach similar cytotoxic effects as the CD3 directed bispecific BAR construct in case PBMCs were used as effector cells (Supplemental Digital Figure 7, http://links.lww.com/HS/A179). The most important characteristic of this new format is its high specificity with restriction to the malignant clone. Whereas conventional antibody-based therapies target common B-cell antigens like CD19, CD20 or CD79b, but do not discriminate between normal and neoplastic B cells,34–36 the bispecific BAR bodies are designed to distinguish malignant and normal B cells by their BCR. This unique quality could help to prevent some of the most-feared side effects of Blinatumomab, that is, neurological symptoms and cytokine release syndromes.34
In addition, side effects due to long-lasting depletion of the normal healthy B-cell repertoire, usually associated with CD19 or CD20 directed therapies with the risk of infections, would not occur with constructs that exclusively target the BCR of the malignant clone.3,9,34 Another important advantage is, that, compared to previous approaches that aim to target the BCR of lymphoma cells, that is, anti-idiotype antibodies and peptibodies,20,26 bispecific LRPAP1 constructs would represent an “off-the-shelf” approach, applicable to a large proportion of patients with MCL.
The relatively small size of bispecific constructs like Blinatumomab of roughly over 54 kDa has both, advantages and disadvantages. Tissue penetration and passing physiological barriers like the blood-brain barrier is facilitated by small size. On the other hand, renal clearance is increased and the loss of a Fc-portion that can be recognized by the neonatal Fc receptor (FcRn) prohibits FcRn-mediated recycling.37,38 Both effects lead to fast elimination and necessitate continuous infusion to reach therapeutic levels of plasma concentration.37
Our bispecific BAR constructs have an approximate size of 37–38 kDa, which is even less than Blinatumomab, and thus, the above-discussed advantages and disadvantages should be amplified. When compared in a cross-study, in vitro setting to Blinatumomab,39 the concentration needed to mediate similar cell lysis is 1000-fold higher with the LRPAP1 incorporating bispecific BAR bodies (100 ng/mL) as compared to Blinatumomab (100 pg/mL). The reason for this is unknown. Since the interaction between LRPAP1 and the BCR of MCL cells has not been quantified yet, for example by plasmon resonance imaging, it can be speculated that the affinity of this interaction is lower than the affinity of the CD19 hybridoma used in Blinatumomab towards CD19. A possibly reduced affinity of LRPAP1 to its cognate MCL BCR may contribute to the high concentrations needed as compared to the anti-CD19/anti-CD3 bispecific antibody Blinatumomab.
In a second step, we designed a construct mimicking the conformation of an IgG1 antibody (IgG1-format BAR body) replacing the variable regions with the MCL-binding epitope of the autoantigen LRPAP1. The resulting construct incorporates the constant regions of Ig light and heavy chains and 4 fragments of LRPAP1 molecules per construct (see Supplemental Digital Figure 5, http://links.lww.com/HS/A179). The IgG1-format BAR body mediates highly specific lysis to cells expressing a BCR with LRPAP1-reactivity. Similar to the bispecific constructs, the concentration needed to reach 50% specific lysis is significantly higher than the concentration that has been reported for rituximab.35 In comparison to bispecific BAR bodies, IgG1-format BAR bodies will have different pharmacokinetic properties. Tissue penetration and passing physiological barriers will be hampered by their size of approximately 150 kDa but evidence from clinical trials studying antibodies of similar-size suggests meaningful benefit in MCL.40 In analogy to normal antibodies, we estimate IgG1-format BAR bodies to avoid renal filtration and to participate in the FcRn-mediated recycling pathway, which will result in an elimination half-life time of up to 20 days.41
The optimal format to integrate the MCL BCR antigen LRPAP1 has yet to be determined and further antibody-based formats such as immunotoxins and CAR-T cells have to be considered. For conclusive results as to the pharmacokinetic properties, in vivo experiments have to be performed. Both formats tested in this study are associated with the advantages of BCR-directed therapies and have the potential to be new treatment options for MCL with minimal side effects due to their ultimate specificity for the malignant clone.
Acknowledgments
We dedicate this work to Michael Pfreundschuh, the intellectual father of the BAR approach. We are grateful to the entire team of the José-Carreras-Centre for Immuno- and Gene Therapy and the Department of Internal Medicine I of Saarland University Medical School for their continuous logistic and intellectual support.
Disclosures
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Sources of funding
This work was supported by a grant from Wilhelm-Sander-Stiftung (a charity foundation in Munich, Germany).
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
References
- 1.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016; 127:2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith A, Crouch S, Lax S, et al. Lymphoma incidence, survival and prevalence 2004-2014: sub-type analyses from the UK’s Haematological Malignancy Research Network. Br J Cancer. 2015; 112:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hermine O, Hoster E, Walewski J, et al. Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet. 2016; 388:565–575. [DOI] [PubMed] [Google Scholar]
- 4.Le Gouill S, Thieblemont C, Oberic L, et al. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N Engl J Med. 2017; 377:1250–1260. [DOI] [PubMed] [Google Scholar]
- 5.Dreyling M, Jurczak W, Jerkeman M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet. 2016; 387:770–778. [DOI] [PubMed] [Google Scholar]
- 6.Davids MS, Roberts AW, Seymour JF, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-hodgkin lymphoma. J Clin Oncol. 2017; 35:826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tam CS, Anderson MA, Pott C, et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med. 2018; 378:1211–1223. [DOI] [PubMed] [Google Scholar]
- 8.Tessoulin B, Ceballos P, Chevallier P, et al. Allogeneic stem cell transplantation for patients with mantle cell lymphoma who failed autologous stem cell transplantation: a national survey of the SFGM-TC. Bone Marrow Transplant. 2016; 51:1184–1190. [DOI] [PubMed] [Google Scholar]
- 9.Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020; 382:1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle cell lymphoma (MCL): long-term follow-up of the randomized European MCL elderly trial. J Clin Oncol. 2020; 38:248–256. [DOI] [PubMed] [Google Scholar]
- 11.Young RM, Staudt LM. Targeting pathological B cell receptor signalling in lymphoid malignancies. Nat Rev Drug Discov. 2013; 12:229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young RM, Wu T, Schmitz R, et al. Survival of human lymphoma cells requires B-cell receptor engagement by self-antigens. Proc Natl Acad Sci U S A. 2015; 112:13447–13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Preuss KD, Pfreundschuh M, Weigert M, et al. Sumoylated HSP90 is a dominantly inherited plasma cell dyscrasias risk factor. J Clin Invest. 2015; 125:2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Preuss KD, Pfreundschuh M, Ahlgrimm M, et al. A frequent target of paraproteins in the sera of patients with multiple myeloma and MGUS. Int J Cancer. 2009; 125:656–661. [DOI] [PubMed] [Google Scholar]
- 15.Thurner L, Hartmann S, Bewarder M, et al. Identification of the atypically modified autoantigen Ars2 as the target of B-cell receptors from activated B cell-type diffuse large B-cell lymphoma. Haematologica. 2020; 105:xxx–xxx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thurner L, Preuss KD, Bewarder M, et al. Hyper-N-glycosylated SAMD14 and neurabin-I as driver autoantigens of primary central nervous system lymphoma. Blood. 2018; 132:2744–2753. [DOI] [PubMed] [Google Scholar]
- 17.Thurner L, Hartmann S, Fadle N, et al. LRPAP1 is a frequent proliferation-inducing antigen of BCRs of mantle cell lymphomas and can be used for specific therapeutic targeting. Leukemia. 2019; 33:148–158. [DOI] [PubMed] [Google Scholar]
- 18.Korenberg JR, Argraves KM, Chen XN, et al. Chromosomal localization of human genes for the LDL receptor family member glycoprotein 330 (LRP2) and its associated protein RAP (LRPAP1). Genomics. 1994; 22:88–93. [DOI] [PubMed] [Google Scholar]
- 19.Herz J, Goldstein JL, Strickland DK, et al. 39-kDa protein modulates binding of ligands to low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. J Biol Chem. 1991; 266:21232–21238. [PubMed] [Google Scholar]
- 20.Kwak LW, Campbell MJ, Czerwinski DK, et al. Induction of immune responses in patients with B-cell lymphoma against the surface-immunoglobulin idiotype expressed by their tumors. N Engl J Med. 1992; 327:1209–1215. [DOI] [PubMed] [Google Scholar]
- 21.Levy R, Ganjoo KN, Leonard JP, et al. Active idiotypic vaccination versus control immunotherapy for follicular lymphoma. J Clin Oncol. 2014; 32:1797–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hough DW, Eady RP, Hamblin TJ, et al. Anti-idiotype sera raised against surface immunoglobulin of human neoplastic lymphocytes. J Exp Med. 1976; 144:960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamblin TJ, Abdul-Ahad AK, Gordon J, et al. Preliminary experience in treating lymphocytic leukaemia with antibody to immunoglobulin idiotypes on the cell surfaces. Br J Cancer. 1980; 42:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meeker TC, Lowder J, Maloney DG, et al. A clinical trial of anti-idiotype therapy for B cell malignancy. Blood. 1985; 65:1349–1363. [PubMed] [Google Scholar]
- 25.Miller RA, Maloney DG, Warnke R, et al. Treatment of B-cell lymphoma with monoclonal anti-idiotype antibody. N Engl J Med. 1982; 306:517–522. [DOI] [PubMed] [Google Scholar]
- 26.Torchia J, Weiskopf K, Levy R. Targeting lymphoma with precision using semisynthetic anti-idiotype peptibodies. Proc Natl Acad Sci U S A. 2016; 113:5376–5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bewarder M, Kiefer M, Moelle C, et al. Integration of the B-cell receptor antigen Neurabin-I/SAMD14 into an antibody format as new therapeutic approach for the treatment of primary CNS lymphoma. Front Oncol. 2020; 10:580364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Haard HJ, van Neer N, Reurs A, et al. A large non-immunized human Fab fragment phage library that permits rapid isolation and kinetic analysis of high affinity antibodies. J Biol Chem. 1999; 274:18218–18230. [DOI] [PubMed] [Google Scholar]
- 29.Hoogenboom HR, Griffiths AD, Johnson KS, et al. Multi-subunit proteins on the surface of filamentous phage: methodologies for displaying antibody (Fab) heavy and light chains. Nucleic Acids Res. 1991; 19:4133–4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freitag J, Heink S, Roth E, et al. Towards the generation of B-cell receptor retrogenic mice. PLoS One. 2014; 9:e109199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NISONOFF A, RIVERS MM. Recombination of a mixture of univalent antibody fragments of different specificity. Arch Biochem Biophys. 1961; 93:460–462. [DOI] [PubMed] [Google Scholar]
- 32.Riethmüller G. Symmetry breaking: bispecific antibodies, the beginnings, and 50 years on. Cancer Immun. 2012; 12:12. [PMC free article] [PubMed] [Google Scholar]
- 33.Trivedi A, Stienen S, Zhu M, et al. Clinical pharmacology and translational aspects of bispecific antibodies. Clin Transl Sci. 2017; 10:147–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017; 376:836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herter S, Herting F, Mundigl O, et al. Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol Cancer Ther. 2013; 12:2031–2042. [DOI] [PubMed] [Google Scholar]
- 36.Palanca-Wessels MC, Czuczman M, Salles G, et al. Safety and activity of the anti-CD79B antibody-drug conjugate polatuzumab vedotin in relapsed or refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukaemia: a phase 1 study. Lancet Oncol. 2015; 16:704–715. [DOI] [PubMed] [Google Scholar]
- 37.Zhu M, Wu B, Brandl C, et al. Blinatumomab, a bispecific T-cell engager (BiTE(®)) for CD-19 targeted cancer immunotherapy: clinical pharmacology and its implications. Clin Pharmacokinet. 2016; 55:1271–1288. [DOI] [PubMed] [Google Scholar]
- 38.Wu B, Sun YN. Pharmacokinetics of Peptide-Fc fusion proteins. J Pharm Sci. 2014; 103:53–64. [DOI] [PubMed] [Google Scholar]
- 39.Löffler A, Kufer P, Lutterbüse R, et al. A recombinant bispecific single-chain antibody, CD19 x CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood. 2000; 95:2098–2103. [PubMed] [Google Scholar]
- 40.Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). J Clin Oncol. 2005; 23:1984–1992. [DOI] [PubMed] [Google Scholar]
- 41.Ryman JT, Meibohm B. Pharmacokinetics of Monoclonal Antibodies. CPT Pharmacometrics Syst Pharmacol. 2017; 6:576–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.