Abstract
PURPOSE
Prostate cancer (PCa) becomes resistant to androgen ablation through adaptive upregulation of the androgen receptor in response to the low-testosterone microenvironment. Bipolar androgen therapy (BAT), defined as rapid cycling between high and low serum testosterone, disrupts this adaptive regulation in castration-resistant PCa (CRPC).
METHODS
The TRANSFORMER (Testosterone Revival Abolishes Negative Symptoms, Fosters Objective Response and Modulates Enzalutamide Resistance) study is a randomized study comparing monthly BAT (n = 94) with enzalutamide (n = 101). The primary end point was clinical or radiographic progression-free survival (PFS); crossover was permitted at progression. Secondary end points included overall survival (OS), prostate-specific antigen (PSA) and objective response rates, PFS from randomization through crossover (PFS2), safety, and quality of life (QoL).
RESULTS
The PFS was 5.7 months for both arms (hazard ratio [HR], 1.14; 95% CI, 0.83 to 1.55; P = .42). For BAT, 50% decline in PSA (PSA50) was 28.2% of patients versus 25.3% for enzalutamide. At crossover, PSA50 response occurred in 77.8% of patients crossing to enzalutamide and 23.4% to BAT. The PSA-PFS for enzalutamide increased from 3.8 months after abiraterone to 10.9 months after BAT. The PFS2 for BAT→enzalutamide was 28.2 versus 19.6 months for enzalutamide→BAT (HR, 0.44; 95% CI, 0.22 to 0.88; P = .02). OS was 32.9 months for BAT versus 29.0 months for enzalutamide (HR, 0.95; 95% CI, 0.66 to 1.39; P = .80). OS was 37.1 months for patients crossing from BAT to enzalutamide versus 30.2 months for the opposite sequence (HR, 0.68; 95% CI, 0.36 to 1.28; P = .225). BAT adverse events were primarily grade 1-2. Patient-reported QoL consistently favored BAT.
CONCLUSION
This randomized trial establishes meaningful clinical activity and safety of BAT and supports additional study to determine its optimal clinical integration. BAT can sensitize CRPC to subsequent antiandrogen therapy. Further study is required to confirm whether sequential therapy with BAT and enzalutamide can improve survival in men with CRPC.
INTRODUCTION
Since the discovery by Charles Huggins of remarkable palliative benefit from castration in men with symptomatic prostate cancer (PCa), the mainstay of treatment has been inhibition of androgen receptor (AR) function through primary androgen deprivation (ADT).1 Although highly effective, therapeutic resistance is almost universal. Second-generation therapies that potently inhibit AR have become standard therapy based on modest improvements in survival versus placebo,2,3 but resistance increases with each subsequent line of AR-directed therapy.4-6 Importantly, PCa cells can develop resistance to androgen ablation through an adaptive marked upregulation of AR over time in response to low-androgen conditions (Data Supplement, online only).7-9 Preclinical studies document that adaptive AR upregulation produces therapeutic vulnerability allowing PCa cells to be killed by exposure to supraphysiologic testosterone.9-12 Episodic exposure to supraphysiologic testosterone can produce downregulation of AR levels leading to potential resensitization to androgen-ablative therapies (Data Supplement).13 Initial clinical studies documented the safety of rapid cycling between polar extremes of supraphysiologic and near-castrate serum testosterone, a concept termed bipolar androgen therapy (BAT), in asymptomatic men with metastatic castration-resistant PCa (CRPC).14,15 The key findings have been that BAT was safe, did not accelerate disease progression, produced sustained prostate-specific antigen (PSA) and objective responses (ORs), and resensitized response to subsequent antiandrogens.14,15
CONTEXT
Key Objective
Is bipolar androgen therapy (BAT) superior to enzalutamide and does BAT overcome antiandrogen resistance in patients with metastatic prostate cancer progressing on abiraterone?
Knowledge Generated
BAT was not superior to enzalutamide but demonstrated similar time to progression and prostate-specific antigen response following treatment with abiraterone. BAT is safe, has meaningful clinical activity, can enhance quality of life, and markedly improve the magnitude and duration of response to enzalutamide.
Relevance
Sequential BAT→enzalutamide could be a safe and effective single third-line therapy for men with metastatic castration-resistant prostate cancer progressing on abiraterone. Further study is warranted to define the potential for this sequential treatment to produce significant survival improvement in men with castration-resistant prostate cancer.
Here, we hypothesized that BAT would have superior efficacy against PCa made resistant as a result of chronic exposure to low androgen and adaptively sensitize these cells to antiandrogens. We conducted the TRANSFORMER (Testosterone Revival Abolishes Negative Symptoms, Fosters Objective Response and Modulates Enzalutamide Resistance) trial to compare the effects of BAT versus the antiandrogen enzalutamide in asymptomatic men with CRPC progressing on abiraterone. Additionally, we explored the effect of sequential exposure to AR agonists or antagonists by allowing crossover to the opposite treatment upon progression.
METHODS
Trial Design
TRANSFORMER (ClinicalTrials.gov identifier: NCT02286921) was a multicenter, open-label, randomized, phase II trial whose objective was to determine the effectiveness of BAT versus enzalutamide on clinical or radiographic progression-free survival (PFS) in men with metastatic CRPC (mCRPC) progressing on abiraterone. Secondary objectives were to determine the effects on overall survival (OS), PSA-PFS, adverse events (AEs), and quality of life (QoL). Although crossover was not mandated, patients with radiographic progression on either arm who continued to meet eligibility requirements had the option to cross over to the opposite treatment. The objectives for this crossover were to evaluate time to PSA progression and time to second PSA progression from randomization through crossover treatment (termed PFS2). PSA50 response was an end point for both study phases.
Patients and Treatment
Eligible patients were asymptomatic with mCRPC documented by computed tomography (CT), technetium-99 bone scan, or both and Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of ≤ 2. Patients had evidence of PSA or radiographic progression after treatment with abiraterone acetate and prednisone. Patients were ineligible if they had pain because of mCRPC requiring treatment intervention or opioids or prior treatment with docetaxel or cabazitaxel for mCRPC. The Clinical Protocol and Data Supplement are available with the full text of this article (online only).
Patients were randomly assigned (1:1) to receive testosterone cypionate (at US Food and Drug Administration [FDA]–approved dose of 400 mg intramuscularly once every 28 days) or enzalutamide (160 mg by mouth daily) until clinical or radiographic progression or prohibitive toxicity. Patients were concurrently maintained on continuous testosterone suppression via surgical castration or luteinizing hormone–releasing hormone agonists or antagonists. At progression, asymptomatic patients who continued to meet eligibility requirements were allowed to cross over to alternate therapy. Clinical status and PSA were assessed each cycle during initial phase and crossover. CT and bone scan were obtained every 12 weeks during initial phase but not at crossover. Patients on either study arm with clinical progression because of pain from PCa were not permitted to cross over. QoL was assessed at baseline and 1, 3, 6, and 12 months postrandomization using RAND-SF36 Quality of Life Survey, FACIT-F Version 4, I-PANAS-SF, International Index of Erectile Function (IIEF), and the Brief Pain Inventory, respectively.
Randomization was performed centrally using a minimization approach, with stratification by length of prior abiraterone exposure (< or ≥ 6 months) and clinical center.
Trial Oversight
The trial was designed and led by the principal investigator (S.R.D.) and co-investigators at Johns Hopkins (M.A.E. and E.S.A.). The trial was conducted at 17 US academic centers. The authors were solely responsible for writing the manuscript.
A Transformative Impact Award from the Department of Defense (DoD) provided financial support for trial conduct. DoD representatives reviewed and approved the protocol and consent documents at each participating site but were not otherwise involved in any study aspect. ADT, enzalutamide, testosterone cypionate, and all subsequent treatments were accessed and administered according to local standard practice. The authors vouch for the accuracy and completeness of the reported data and for fidelity to the protocol.
An independent data and safety monitoring committee reviewed the progress and results of the trial. The trial was conducted in accordance with the principles of Good Clinical Practice guidelines and Declaration of Helsinki. The protocol was independently reviewed and approved as required at each participating institution. All patients provided written informed consent.
End Points
The primary end point of clinical or radiographic PFS was measured as the interval from randomization to the earliest sign of radiographic progression according to the criteria of the PCa Working Group 2 (PCWG2) for bone lesions and the RECIST version 1.1 for soft-tissue lesions, the development of symptoms or complications attributable to cancer progression, or the initiation of another anticancer treatment for PCa16 and censored at the date of last scan or clinical visit for those who did not have the event at the time of data cutoff. The secondary end point of OS was the interval from randomization to death and censored at the date of last known alive. PSA-PFS was measured as the interval from randomization to the time of PSA progression according to the PCWG2 criteria (a confirmed relative increase in the PSA level from the nadir value by ≥ 25% and by ≥ 2 ng/mL) or censored at the last date of PSA assessment for patients without PSA progression. The secondary end point PFS2 was defined as the interval from randomization to second PSA progression following crossover therapy. For patients who did not cross over, PFS2 was censored at the time of PFS or last follow-up with no progression on initial treatment. OR was defined as complete response or partial response per RECIST and PCWG2 among those with measurable baseline disease. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.02. AE data were collected during the treatment period, with a final safety assessment performed 30-42 days after the cessation of the trial regimen.
Statistical Analysis
Assuming a median PFS of 6 months in the enzalutamide group on the basis of two previous studies of enzalutamide in patients with mCRPC progressing on abiraterone, we determined that enrollment of 194 patients (with 156 PFS events) would provide a power of 80% to detect a hazard ratio (HR) of 0.667 in the BAT group versus the enzalutamide group, with a one-sided type I error of 0.05. Two interim analyses of efficacy and futility for PFS were conducted as planned, the first after approximately 45% of the information and the second after 70% of the information. An independent data and safety monitoring committee reviewed interim data and recommended to continue to full accrual.
The primary efficacy end point PFS and the secondary efficacy end points PSA-PFS, OS, and PFS2 were based on the intention-to-treat principle and included all patients who had undergone randomization. Patients who had undergone randomization and received a dose of any trial drug were included in safety analyses.
PFS and other time-to-event end points were estimated using Kaplan-Meier method, and each was compared between the arms using a stratified log-rank test, with stratification factor of duration of prior abiraterone treatment (< or ≥ 6 months). The Cox regression model, stratified for the same baseline stratification factor, was used to estimate HRs between the two arms and corresponding 95% CIs. For each QoL module, summary statistics of scores was reported at baseline and 1, 3, 6, and 12 months postrandomization. Scores at each follow-up time, as well as change pre- and post-treatment, were compared between the arms using Mann-Whitney tests.
RESULTS
From April 2015 to April 2018, we randomly assigned 195 men to receive either BAT (94 patients) or enzalutamide (101 patients) across 17 sites in the United States (Fig 1). The data cutoff date for this report was November 2019; median follow-up time among patients who are alive is 31.9 months. Baseline characteristics of all the patients are summarized in Table 1.
FIG 1.
TRANSFORMER CONSORT diagram. BAT, bipolar androgen therapy.
TABLE 1.
Characteristics of the Patients at Baseline
Primary End Point
The primary analysis of PFS was performed in November 2018, after progression had occurred in 156 patients. The median PFS was 5.6 months in the BAT arm versus 5.7 months in the enzalutamide arm (HR, 1.13; 95% CI, 0.82 to 1.57; P = .45) (Fig 2A). With additional follow-up at data cutoff in November 2019, results remained unchanged (5.7 months for both arms; HR, 1.14; 95% CI, 0.83 to 1.55; P = .42). In a prespecified analysis, PFS in men with short prior response to abiraterone (< 6 months) favored BAT (HR, 0.60; 95% CI, 0.29 to 1.25), whereas PFS in those with longer prior response to abiraterone (≥ 6 months) favored enzalutamide (HR, 1.31; 95% CI, 0.93 to 1.84; Pinteraction = .10) (Table 2 and Data Supplement).
FIG 2.
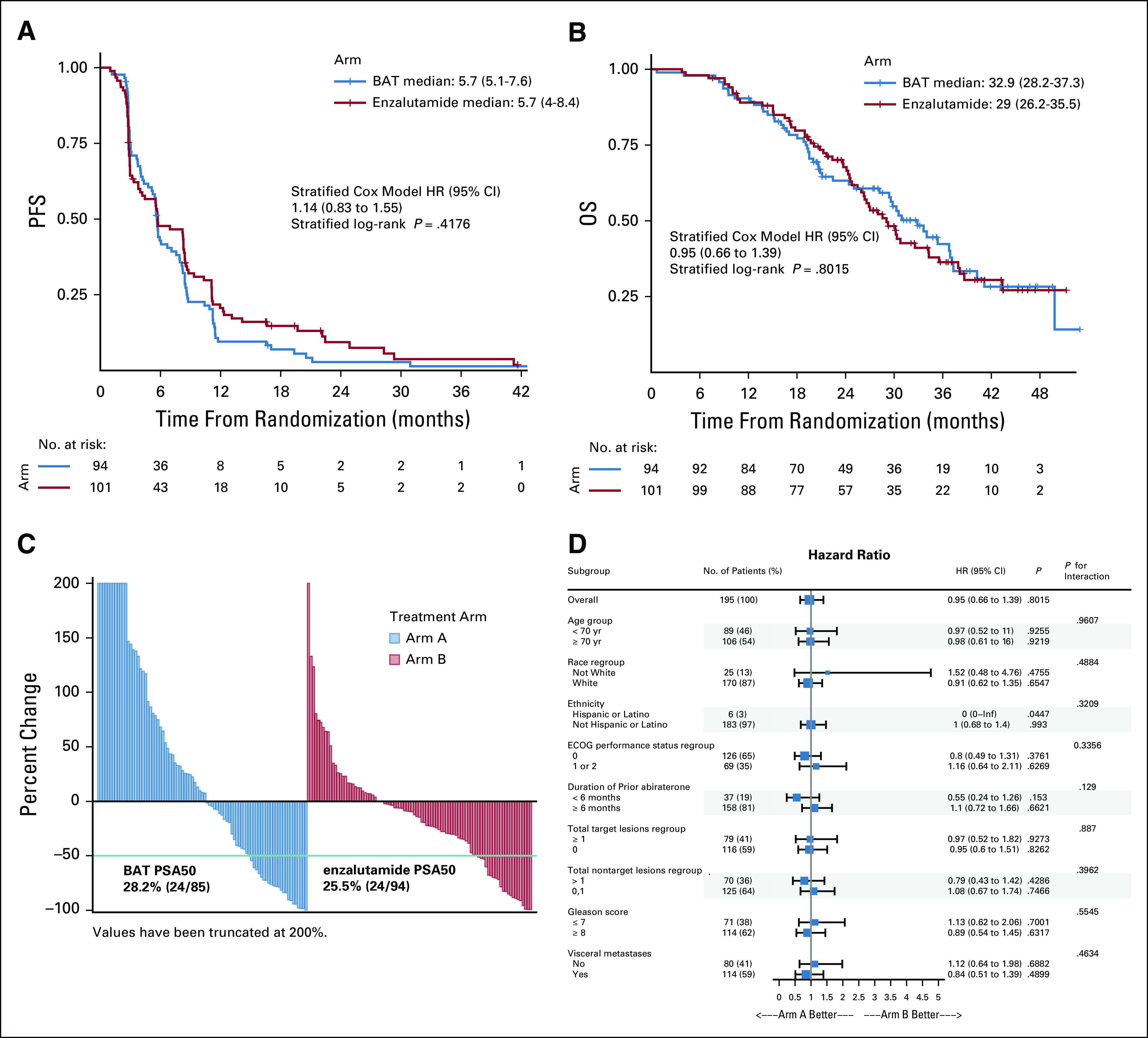
Kaplan-Meier estimates of (A) PFS and (B) OS, (C) waterfall plot of PSA response to initial therapy, (D) subgroup analysis of OS. BAT, bipolar androgen therapy; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; INF, infinity; OS, overall survival; PFS, progression-free survival; PSA, prostate-specific antigen.
TABLE 2.
Prespecified Secondary Efficacy End Points (ITT Population)
Secondary End Points
Median OS was not statistically different, but hypothesis-generating, for the BAT arm compared with the enzalutamide arm (32.9 v 29.0 months; HR, 0.95; 95% CI, 0.66 to 1.39; P = .80) (Fig 2B and Table 2). In a subset analysis, OS in men with short prior response to abiraterone (< 6 months) favored BAT (HR, 0.55; 95% CI, 0.24 to 1.26), whereas OS in those with longer prior response to abiraterone (≥ 6 months) favored enzalutamide (HR, 1.08; 95% CI, 0.71 to 1.64; Pinteraction = .14) (Fig 2D). The percentage of patients who achieved a PSA50 response during the initial phase of treatment was similar between the two groups (28.2% [24/85] for BAT versus 25.5% [24/94] for enzalutamide) (Fig 2C and Table 2). Time to first PSA progression was short for both the groups but favored the enzalutamide arm (2.8 months for BAT v 3.8 months for enzalutamide; HR, 1.51; 95% CI, 1.06 to 2.16; P = .02) (Table 2). Conversely, the OR rate favored the BAT group over enzalutamide (24.2% [8/33] v 4.2% [1/24], respectively; P = .07) (Table 2).
Crossover Treatment
Patients who remained asymptomatic and continued to meet eligibility requirements were provided the opportunity to cross over, after a 28-day washout period, to the alternate treatment at time of progression. Crossover was not permitted in patients in either arm with clinical progression because of pain from PCa. Overall, 37 (39.3%) patients initially on BAT crossed over to receive enzalutamide, whereas 48 (47.6%) patients crossed from enzalutamide to BAT (Table 1). For patients who did not cross over, approximately equal numbers (14% on BAT and 18% on enzalutamide) had clinical progression. Overall, 37% of patients receiving BAT and 43% receiving enzalutamide crossed over as a result of radiographic progression (Data Supplement).
The majority of the patients who crossed over did so as a result of radiographic progression (95% of the BAT group and 90% of the enzalutamide group) (Data Supplement). There was no significant difference in characteristics (age, ECOG PS, race, ethnicity, target lesions, nontarget lesions, and duration of prior abiraterone therapy) of the crossover population compared with the noncrossover population (Data Supplement). Characteristics of each crossover arm were similar (Data Supplement). Crossover to enzalutamide following BAT was associated with greater benefits than crossover to BAT following enzalutamide, for all secondary end points (Table 2). Median OS for those crossing over to enzalutamide post-BAT was 37.1 months versus 30.2 months for those crossing to BAT postenzalutamide (HR, 0.68; 95% CI, 0.36 to 1.28; P = .23) (Fig 3A and Table 1) versus 28.6 months for those who received enzalutamide-only without crossover (HR, 0.52; 95% CI, 0.29 to 0.96; P = .03) versus 25 months (HR, 0.46; 95% CI, 0.25 to 0.84; P = .009) for those who received BAT-only without crossover (Fig 3B and Table 1). The OR of 28.6% (10/35) for enzalutamide post-BAT was higher than the response of 7.3% (3/41) with BAT postenzalutamide (Table 2). The PSA50 response was 77.8% (28/36) for those who crossed to enzalutamide compared with 21.3% (10/47) for those who crossed to BAT (Fig 4A and Table 1). Patients receiving enzalutamide immediately after abiraterone had significantly shorter median PSA-PFS with enzalutamide (3.8 months) compared with those who received enzalutamide following BAT (10.9 months) (HR, 0.45; 95% CI, 0.24 to 0.86; P = .008) (Table 2).
FIG 3.
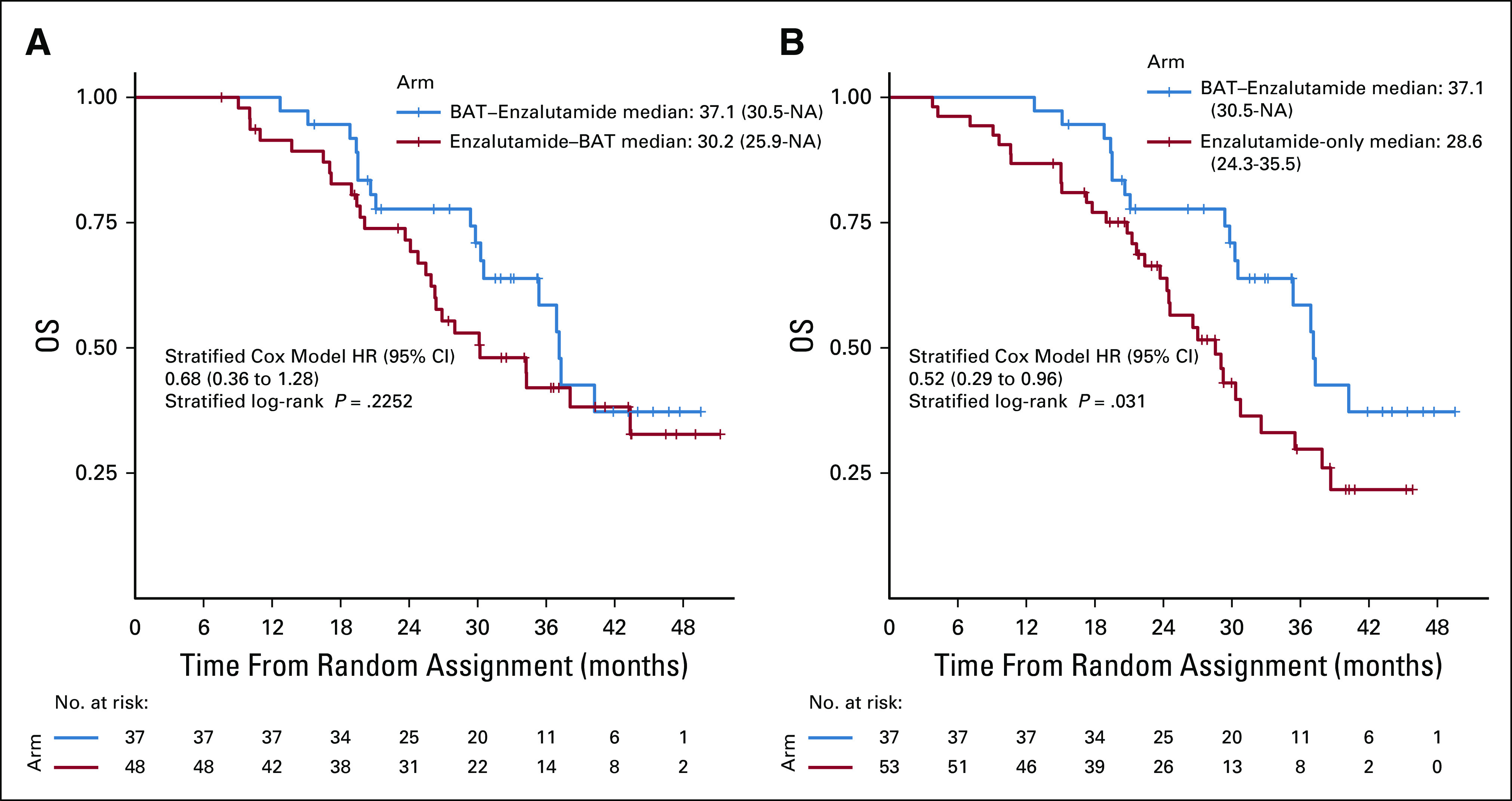
Kaplan-Meier estimates of OS in the crossover population. (A) Comparison of OS in the subset of patients receiving BAT→enzalutamide versus enzalutamide→BAT, after eliminating those who came off study without crossing over. (B) Comparison of OS in the subset of patients receiving BAT→enzalutamide (after eliminating patients who did not cross over) versus enzalutamide-only patients who did not cross over to receive BAT. BAT, bipolar androgen therapy; HR, hazard ratio; NA, not accessible; OS, overall survival.
FIG 4.
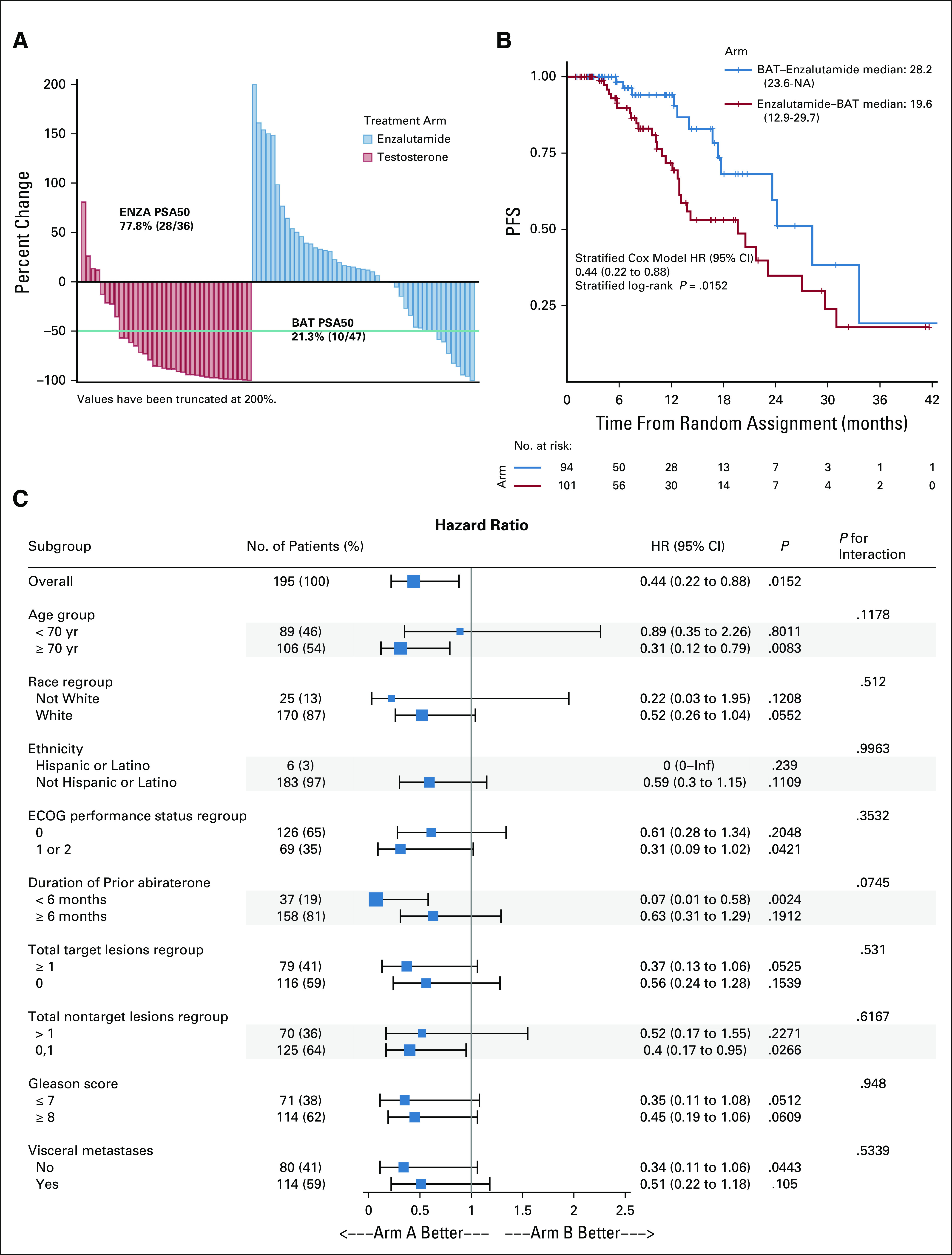
(A) Waterfall plot of PSA response to crossover therapy, (B) Kaplan-Meier estimates of PFS2, (C) subgroup analysis of PFS2. BAT, bipolar androgen therapy; ECOG, Eastern Cooperative Oncology Group; PFS, progression-free survival; PSA, prostate-specific antigen.
Considering the sequencing of BAT and enzalutamide, patients who received the treatment sequence of BAT→enzalutamide had significantly longer PFS2 compared with the opposite sequence (28.2 v 19.6 months; HR, 0.44; 95% CI, 0.22 to 0.88; P = .02) (Fig 4B and Data Supplement). Subgroup analysis of PFS2 favored the BAT→enzalutamide sequence (arm A) across all subgroups (Fig 4C).
Androgen Receptor Expression
Baseline blood samples (n = 187) were analyzed for transcript expression of full-length AR (AR-FL) and the truncated ligand-independent AR variant (AR-V7) in circulating tumor cells (CTCs), according to previously published methods.17 Overall, 41% of patients on BAT and 37% on enzalutamide tested positive for AR-FL, consistent with increased AR expression in CTCs following abiraterone pretreatment (Table 3). AR-V7 transcript was detected at baseline in 12% of patients on BAT and 7% on enzalutamide (Table 3). Detection of AR-FL and AR-V7 transcripts were both generally associated with shorter PFS and OS on BAT and enzalutamide (although not all differences were statistically significant), consistent with the broad negative prognostic impact in patients with mCRPC (Table 3 and Data Supplement).17 However, neither AR-FL nor AR-V7 status was predictive of better or worse clinical outcomes using BAT or enzalutamide, suggesting that neither factor can be used as a treatment selection biomarker in this context.
TABLE 3.
Effect of AR-FL and AR-V7 Expression on PFS and OS
Safety and QoL
The majority of AEs were grade 1-2 (BAT, 68.5%; enzalutamide, 62.8%); grade 3-4 AEs occurred in 28.1% of patients on BAT and 35.1% on enzalutamide (Table 4). Only one grade 5 AE of death not otherwise specified was observed in a patient on enzalutamide. Serious AEs occurred in 19.1% of patients on BAT and 20.6% on enzalutamide. The percentage discontinuing therapy as a result of AEs was slightly higher for BAT (9.0%) than enzalutamide (5.2%) (Table 4).
TABLE 4.
Summary of AEs During Initial Treatment (Safety Analysis Population)
The incidence of AEs was generally similar in the two groups. Notable exceptions included fatigue with 48.5% of patients on enzalutamide experiencing grade 1-2 and 7.2% of patients grade 3-4 fatigue, compared with 31.5% of BAT patients experiencing only grade 1-2 fatigue. Enzalutamide was associated with a higher percentage of constitutional symptoms such as anorexia, depression, anxiety, insomnia, headache, and generalized muscle weakness as well as GI complaints (diarrhea, constipation, abdominal pain, and flatulence). BAT was associated with increased sexual side effects (hot flashes, breast tenderness, and gynecomastia) and musculoskeletal complaints (peripheral edema and generalized musculoskeletal pain).
Patient-reported QoL consistently favored BAT at 1, 3, and 6 months after initiation of treatment (Data Supplement).
DISCUSSION
The TRANSFORMER trial is unique in that it compares two treatments with diametrically opposite effects on the AR therapeutic target. In this trial, BAT was not superior to enzalutamide with respect to the primary end point clinical or radiographic PFS in asymptomatic men with mCRPC progressing on abiraterone. Although not powered to show equivalency, the treatments were similar in terms of median PFS (5.7 months in both the arms), time to PSA progression (2.8 v 3.8 months), and PSA50 responses (28.2% v 25.5%). The similarity of response, despite the opposing nature of the treatments, may relate to PCa cells’ ability to adaptively regulate AR levels in response to androgen levels. Interestingly, the greatest benefit from BAT was in patients experiencing progression on prior abiraterone within 6 months, suggesting that BAT may partially reverse lineage plasticity in PCa cells losing AR addiction.18 Unfortunately, neither baseline AR-FL nor AR-V7 expression was identified as a potential treatment selection biomarker. However, consistent with the hypothesis that increased AR-FL can make PCa resistant to androgen ablation but vulnerable to high-dose testosterone,9 PFS was significantly increased for BAT and decreased for enzalutamide in AR-FL–positive patients (Table 3).
BAT also maintained or improved QoL, particularly in domains of fatigue and physical and sexual function compared with enzalutamide. The incidence of AEs was similar between treatments and primarily low-grade. BAT was associated with less fatigue and GI and constitutional symptoms but increased edema, generalized pain, and sexual side effects compared with enzalutamide.
Approximately 40% of patients crossed over to the opposite treatment at progression. There were no significant differences between noncrossover versus crossover patient characteristics. Patients who crossed to enzalutamide post-BAT showed significantly enhanced response compared with those who received enzalutamide immediately after progression on abiraterone. Median time to PSA progression increased to 10.9 months compared with 3.8 months, PSA50 response improved to 78% versus 25%, and OR improved to 29% versus 4%. Overall, our results support our hypothesis that BAT may reverse antiandrogen resistance via adaptive downregulation of AR expression (Data Supplement).
The use of PSA progression is nuanced because PSA expression is directly stimulated by testosterone, which could likely shorten time to PSA progression on BAT. However, as an exploratory end point we measured PFS2, which was significantly increased for patients treated with BAT→enzalutamide compared with the opposite sequence (28.2 v 19.6 months, respectively). Although our PFS2 results do not include the duration of treatment with prior abiraterone, they compare favorably with Khalaf et al19 who reported median PFS2 of 19.3 months in 73 patients treated with abiraterone followed by enzalutamide. Median survival of 25-28 months has been reported in small studies of patients with mCRPC receiving abiraterone followed by enzalutamide.19-21 In contrast, in our study, median postabiraterone survival for BAT→enzalutamide was 37.1 months.
In conclusion, TRANSFORMER establishes meaningful clinical activity of BAT and supports additional study to determine its optimal clinical integration. Although the trial failed to demonstrate superior PFS with BAT over enzalutamide in postabiraterone CRPC, it demonstrated that BAT is safe, enhances QoL, and has efficacy comparable to enzalutamide in this patient population. However, the most important finding is that postabiraterone, BAT can markedly improve the magnitude and duration of response to enzalutamide when used as an intervening therapy. These results support further evaluation of sequential BAT→enzalutamide as a single therapy. Further study is warranted to define the potential for sequential treatment to produce significant survival improvement in men with CRPC.
ACKNOWLEDGMENT
We thank the many patients who participated in this trial; the nurses, study coordinators, and research administrators at the participating hospitals; the administrative support of Nrushinga Mishra, Joshua McKean, and Melissa Cunningham at the DoD PCRP; and the members of the independent data monitoring committee: Dr Charles Ryan, Dr George Wilding, and Dr Christine Hann. In memory of Mr Bruce Hunsicker, founder of the One-in-Six Foundation, Akron, OH.
Samuel R. Denmeade
Stock and Other Ownership Interests: Sophiris Bio
Consulting or Advisory Role: Sophiris Bio
Travel, Accommodations, Expenses: Sophiris Bio
Neeraj Agarwal
Consulting or Advisory Role: Pfizer, Medivation/Astellas, Bristol-Myers Squibb, AstraZeneca, Nektar, Lilly, Bayer, Foundation One Inc, Pharmacyclics, Foundation Medicine, Astellas Pharma, Exelixis, AstraZeneca, Pfizer, Merck, Novartis, Eisai, Seattle Genetics, EMD Serono, Janssen Oncology, AVEO, Calithera Biosciences, MEI Pharma
Research Funding: Bayer, Bristol-Myers Squibb, GlaxoSmithKline, Takeda, Novartis, Pfizer, BN ImmunoTherapeutics, Exelixis, TRACON Pharma, Rexahn Pharmaceuticals, Amgen, AstraZeneca, Active Biotech, Bavarian Nordic, Calithera Biosciences, Celldex, Eisai, Genentech, Immunomedics, Janssen, Merck, Newlink Genetics, Prometheus, Sanofi
David C. Smith
Research Funding: Agensys, Incyte, Lilly, Novartis, Seattle Genetics, Bristol-Myers Squibb/Medarex, Genentech, Astellas Pharma, Bayer, ESSA, Roche, MedImmune
Michael T. Schweizer
Consulting or Advisory Role: Resverlogix
Research Funding: Janssen, AstraZeneca, Roche, Pfizer, Zenith Epigenetics, Madison Vaccines, Inc., Immunomedics, Bristol-Myers Squibb, Merck, Tmunity Therapeutics, Inc.
Mark N. Stein
Consulting or Advisory Role: Merck Sharp & Dohme, Exelixis, Exelixis, Xencor
Research Funding: Oncoceutics, Merck Sharp & Dohme, Janssen Oncology, Medivation/Astellas, Advaxis, Suzhou Kintor Pharmaceuticals, Harpoon, Bristol-Myers Squibb, Genocea Biosciences, Lilly, Nektar, Seattle Genetics, Xencor, Tmunity Therapeutics, Inc., Exelixis
Vasileios Assikis
Consulting or Advisory Role: Bayer
Speakers' Bureau: AstraZeneca
Przemyslaw W. Twardowski
Honoraria: Astellas Medivation, Bayer, Janssen, Genentech/Roche, Sanofi/Aventis
Consulting or Advisory Role: Sanofi/Aventis, Janssen
Speakers' Bureau: Astellas Pharma, Bayer, Janssen, Genentech, Sanofi
Thomas W. Flaig
Leadership: Aurora Oncology
Stock and Other Ownership Interests: Aurora Oncology
Consulting or Advisory Role: Seattle Genetics, Janssen Oncology
Research Funding: Novartis, Bavarian Nordic, Dendreon, GTx, Janssen Oncology, Medivation, Sanofi, Pfizer, Bristol-Myers Squibb, Roche/Genentech, Exelixis, Aragon Pharmaceuticals, Sotio, Tokai Pharmaceuticals, Astrazeneca/MedImmune, Lilly, Astellas Pharma, Agensys, Seattle Genetics, La Roche-Posay, Merck
Patents, Royalties, Other Intellectual Property: The University of Colorado has filed 2 patents related in which I am an inventor. These are related to early-stage bladder cancer treatment and detection. Neither is commercialized or licensed at this time.
Russell Z. Szmulewitz
Honoraria: Astellas Pharma
Consulting or Advisory Role: AstraZeneca, AbbVie, Exelixis, Merck, Amgen, Janssen Oncology, Sanofi, Astellas Pharma, Pfizer
Research Funding: AbbVie, Astellas Pharma, Incyte, Macrogenics, Janssen Oncology
Patents, Royalties, Other Intellectual Property: Patent licensed by The University of Chicago of which I am a co-inventor to Corcept Therapeutics for combination AR/GR inhibition in prostate cancer
Travel, Accommodations, Expenses: Corcept Therapeutics
Jeffrey M. Holzbeierlein
Consulting or Advisory Role: Basilea
Research Funding: MDxHealth
(OPTIONAL) Uncompensated Relationships: Astellas Medivation
Ralph J. Hauke
Stock and Other Ownership Interests: Aethlon
Research Funding: US Oncology, Bristol-Myers Squibb, Merck, Amgen, Novartis, Exelixis
Guru Sonpavde
Honoraria: UpToDate
Consulting or Advisory Role: Genentech, Merck, Eisai, AstraZenecam, Janssen, Bristol-Myers Squibb, Exelixis, EMD Serono, Astellas Pharma, Bicycle Therapeutics, Pfizer, Seattle Genetics
Speakers' Bureau: Physicans' Education Resource, Onclive, Research to Practice, Medscape
Research Funding: Janssen, Sanofi, AstraZeneca
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Other Relationship: AstraZeneca, Bristol-Myers Squibb, Astellas Pharma, Bavarian Nordic, Debiopharm GroupQED Therapeutics, Elsevier
Jorge A. Garcia
Honoraria: UpToDate, MerckJanssen, Amgen, Bayer, MJH Associates
Consulting or Advisory Role: Sanofi, Bayer, Eisai, Clovis Oncology, Targeted Oncology
Speakers' Bureau: Bayer, Sanofi, MerckSanofi/Aventis
Research Funding: Pfizer, Orion Pharma GmbH, Janssen Oncology, Genentech/Roche, Lilly, Merck
Travel, Accommodations, Expenses: Bayer, Sanofi, Eisai, Medivation/Astellas, Merck, Clovis Oncology, Janssen
Arif Hussain
Consulting or Advisory Role: AstraZeneca, Bayer, Exelixis, Janssen Oncology
Research Funding: Sotio, Merck, Bayer, Clovis Oncology, Pfizer, Constellation Pharmaceuticals, Nektar, Roche/Genentech, Infinity Pharmaceuticals
Oliver Sartor
Stock and Other Ownership Interests: Lilly, GlaxoSmithKline, AbbVie, Cardinal Health, United Health Group, PSMA Therapeutics, Clarity Pharmaceuticals, Noria Therapeutics, Clovis Oncology
Consulting or Advisory Role: Bayer, Sanofi, AstraZeneca, Dendreon, Constellation Pharmaceuticals, Advanced Accelerator Applications, Pfizer, Bristol-Myers Squibb, Bavarian Nordic, EMD Serono, Astellas Pharma, Progenics, Blue Earth Diagnostics, Myovant Sciences, Myriad Genetics, Novartis, Clarity Pharmaceuticals, Fusion Pharmaceuticals, Isotopen Technologien, Janssen, Noxopharm, Clovis Oncology, Noria Therapeutics, Point Biopharma, TeneoBio, Telix Pharmaceuticals, Theragnostics
Research Funding: Sanofi, Endocyte, Merck, InVitae, Constellation Pharmaceuticals, Advanced Accelerator Applications, AstraZeneca, Dendreon, Sotio, Janssen, Progenics
Expert Testimony: Sanofi
Travel, Accommodations, Expenses: Bayer, Johnson & Johnson, Sanofi, AstraZeneca, Progenics
Shifeng Mao
Consulting or Advisory Role: Sanofi, Cardinal Health
Speakers' Bureau: Bristol-Myers Squibb
Michael A. Carducci
Consulting or Advisory Role: Roche/Genentech, Pfizer, Foundation Medicine, Exelixis
Research Funding: Bristol-Myers Squibb, Pfizer, AstraZeneca, EMD Serono, Arcus Biosciences
Mark C. Markowski
Honoraria: Clovis Oncology, Exelixis
Mario A. Eisenberger
Leadership: Veru
Stock and Other Ownership Interests: Veru
Honoraria: Merck Sharp & Dohme, Bristol-Myers Squibb, Seattle Genetics
Emmanuel S. Antonarakis
Honoraria: Sanofi, Dendreon, Medivation, Janssen Biotech, ESSA, Astellas Pharma, Merck, AstraZeneca, Clovis Oncology
Consulting or Advisory Role: Sanofi, Dendreon, Janssen Biotech, ESSA, Merck, AstraZeneca, Clovis Oncology, Lilly, Bayer
Research Funding: Janssen Biotech, Johnson & Johnson, Sanofi, Dendreon, Aragon Pharmaceuticals, Exelixis, Millennium, Genentech, Novartis, Astellas Pharma, Tokai Pharmaceuticals, Merck, AstraZeneca, Clovis Oncology, Constellation Pharmaceuticals
Travel, Accommodations, Expenses: Sanofi, Dendreon, Medivation
No other potential conflicts of interest were reported.
SUPPORT
Supported by a Transformative Grant (W81XWH-14-2-0189) from the Department of Defense PCa Research Program.
CLINICAL TRIAL INFORMATION
ClinicalTrials.gov identifier: NCT02286921
AUTHOR CONTRIBUTIONS
Conception and design: Samuel R. Denmeade, Hao Wang, Przemyslaw W. Twardowski, Michael A. Carducci, Mario A. Eisenberger, Emmanuel S. Antonarakis
Administrative support: Neeraj Agarwal, Guru Sonpavde, Harry Cao, Ting Wang, Rehab Abdallah
Provision of study materials or patients: Neeraj Agarwal, David C. Smith, Vasileios Assikis, Russell Z. Szmulewitz, Ralph J. Hauke, Guru Sonpavde, Jorge A. Garcia, Oliver Sartor, Michael A. Carducci, Mark C. Markowski, Emmanuel S. Antonarakis
Collection and assembly of data: Samuel R. Denmeade, Neeraj Agarwal, David C. Smith, Michael T. Schweizer, Mark N. Stein, Vasileios Assikis, Russell Z. Szmulewitz, Jeffrey M. Holzbeierlein, Ralph J. Hauke, Guru Sonpavde, Arif Hussain, Oliver Sartor, Shifeng Mao, Harry Cao, Ting Wang, Rehab Abdallah, Vanessa Bolejack, Michael A. Carducci, Mark C. Markowski
Data analysis and interpretation: Samuel R. Denmeade, Hao Wang, Neeraj Agarwal, David C. Smith, Michael T. Schweizer, Mark N. Stein, Vasileios Assikis, Thomas W. Flaig, Russell Z. Szmulewitz, Ralph J. Hauke, Jorge A. Garcia, Arif Hussain, Oliver Sartor, Wei Fu, Su Jin Lim, Vanessa Bolejack, Channing J. Paller, Michael A. Carducci, Mark C. Markowski, Mario A. Eisenberger, Emmanuel S. Antonarakis
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
TRANSFORMER: A Randomized Phase II Study Comparing Bipolar Androgen Therapy Versus Enzalutamide in Asymptomatic Men With Castration-Resistant Metastatic Prostate Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Samuel R. Denmeade
Stock and Other Ownership Interests: Sophiris Bio
Consulting or Advisory Role: Sophiris Bio
Travel, Accommodations, Expenses: Sophiris Bio
Neeraj Agarwal
Consulting or Advisory Role: Pfizer, Medivation/Astellas, Bristol-Myers Squibb, AstraZeneca, Nektar, Lilly, Bayer, Foundation One Inc, Pharmacyclics, Foundation Medicine, Astellas Pharma, Exelixis, AstraZeneca, Pfizer, Merck, Novartis, Eisai, Seattle Genetics, EMD Serono, Janssen Oncology, AVEO, Calithera Biosciences, MEI Pharma
Research Funding: Bayer, Bristol-Myers Squibb, GlaxoSmithKline, Takeda, Novartis, Pfizer, BN ImmunoTherapeutics, Exelixis, TRACON Pharma, Rexahn Pharmaceuticals, Amgen, AstraZeneca, Active Biotech, Bavarian Nordic, Calithera Biosciences, Celldex, Eisai, Genentech, Immunomedics, Janssen, Merck, Newlink Genetics, Prometheus, Sanofi
David C. Smith
Research Funding: Agensys, Incyte, Lilly, Novartis, Seattle Genetics, Bristol-Myers Squibb/Medarex, Genentech, Astellas Pharma, Bayer, ESSA, Roche, MedImmune
Michael T. Schweizer
Consulting or Advisory Role: Resverlogix
Research Funding: Janssen, AstraZeneca, Roche, Pfizer, Zenith Epigenetics, Madison Vaccines, Inc., Immunomedics, Bristol-Myers Squibb, Merck, Tmunity Therapeutics, Inc.
Mark N. Stein
Consulting or Advisory Role: Merck Sharp & Dohme, Exelixis, Exelixis, Xencor
Research Funding: Oncoceutics, Merck Sharp & Dohme, Janssen Oncology, Medivation/Astellas, Advaxis, Suzhou Kintor Pharmaceuticals, Harpoon, Bristol-Myers Squibb, Genocea Biosciences, Lilly, Nektar, Seattle Genetics, Xencor, Tmunity Therapeutics, Inc., Exelixis
Vasileios Assikis
Consulting or Advisory Role: Bayer
Speakers' Bureau: AstraZeneca
Przemyslaw W. Twardowski
Honoraria: Astellas Medivation, Bayer, Janssen, Genentech/Roche, Sanofi/Aventis
Consulting or Advisory Role: Sanofi/Aventis, Janssen
Speakers' Bureau: Astellas Pharma, Bayer, Janssen, Genentech, Sanofi
Thomas W. Flaig
Leadership: Aurora Oncology
Stock and Other Ownership Interests: Aurora Oncology
Consulting or Advisory Role: Seattle Genetics, Janssen Oncology
Research Funding: Novartis, Bavarian Nordic, Dendreon, GTx, Janssen Oncology, Medivation, Sanofi, Pfizer, Bristol-Myers Squibb, Roche/Genentech, Exelixis, Aragon Pharmaceuticals, Sotio, Tokai Pharmaceuticals, Astrazeneca/MedImmune, Lilly, Astellas Pharma, Agensys, Seattle Genetics, La Roche-Posay, Merck
Patents, Royalties, Other Intellectual Property: The University of Colorado has filed 2 patents related in which I am an inventor. These are related to early-stage bladder cancer treatment and detection. Neither is commercialized or licensed at this time.
Russell Z. Szmulewitz
Honoraria: Astellas Pharma
Consulting or Advisory Role: AstraZeneca, AbbVie, Exelixis, Merck, Amgen, Janssen Oncology, Sanofi, Astellas Pharma, Pfizer
Research Funding: AbbVie, Astellas Pharma, Incyte, Macrogenics, Janssen Oncology
Patents, Royalties, Other Intellectual Property: Patent licensed by The University of Chicago of which I am a co-inventor to Corcept Therapeutics for combination AR/GR inhibition in prostate cancer
Travel, Accommodations, Expenses: Corcept Therapeutics
Jeffrey M. Holzbeierlein
Consulting or Advisory Role: Basilea
Research Funding: MDxHealth
(OPTIONAL) Uncompensated Relationships: Astellas Medivation
Ralph J. Hauke
Stock and Other Ownership Interests: Aethlon
Research Funding: US Oncology, Bristol-Myers Squibb, Merck, Amgen, Novartis, Exelixis
Guru Sonpavde
Honoraria: UpToDate
Consulting or Advisory Role: Genentech, Merck, Eisai, AstraZenecam, Janssen, Bristol-Myers Squibb, Exelixis, EMD Serono, Astellas Pharma, Bicycle Therapeutics, Pfizer, Seattle Genetics
Speakers' Bureau: Physicans' Education Resource, Onclive, Research to Practice, Medscape
Research Funding: Janssen, Sanofi, AstraZeneca
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Other Relationship: AstraZeneca, Bristol-Myers Squibb, Astellas Pharma, Bavarian Nordic, Debiopharm GroupQED Therapeutics, Elsevier
Jorge A. Garcia
Honoraria: UpToDate, MerckJanssen, Amgen, Bayer, MJH Associates
Consulting or Advisory Role: Sanofi, Bayer, Eisai, Clovis Oncology, Targeted Oncology
Speakers' Bureau: Bayer, Sanofi, MerckSanofi/Aventis
Research Funding: Pfizer, Orion Pharma GmbH, Janssen Oncology, Genentech/Roche, Lilly, Merck
Travel, Accommodations, Expenses: Bayer, Sanofi, Eisai, Medivation/Astellas, Merck, Clovis Oncology, Janssen
Arif Hussain
Consulting or Advisory Role: AstraZeneca, Bayer, Exelixis, Janssen Oncology
Research Funding: Sotio, Merck, Bayer, Clovis Oncology, Pfizer, Constellation Pharmaceuticals, Nektar, Roche/Genentech, Infinity Pharmaceuticals
Oliver Sartor
Stock and Other Ownership Interests: Lilly, GlaxoSmithKline, AbbVie, Cardinal Health, United Health Group, PSMA Therapeutics, Clarity Pharmaceuticals, Noria Therapeutics, Clovis Oncology
Consulting or Advisory Role: Bayer, Sanofi, AstraZeneca, Dendreon, Constellation Pharmaceuticals, Advanced Accelerator Applications, Pfizer, Bristol-Myers Squibb, Bavarian Nordic, EMD Serono, Astellas Pharma, Progenics, Blue Earth Diagnostics, Myovant Sciences, Myriad Genetics, Novartis, Clarity Pharmaceuticals, Fusion Pharmaceuticals, Isotopen Technologien, Janssen, Noxopharm, Clovis Oncology, Noria Therapeutics, Point Biopharma, TeneoBio, Telix Pharmaceuticals, Theragnostics
Research Funding: Sanofi, Endocyte, Merck, InVitae, Constellation Pharmaceuticals, Advanced Accelerator Applications, AstraZeneca, Dendreon, Sotio, Janssen, Progenics
Expert Testimony: Sanofi
Travel, Accommodations, Expenses: Bayer, Johnson & Johnson, Sanofi, AstraZeneca, Progenics
Shifeng Mao
Consulting or Advisory Role: Sanofi, Cardinal Health
Speakers' Bureau: Bristol-Myers Squibb
Michael A. Carducci
Consulting or Advisory Role: Roche/Genentech, Pfizer, Foundation Medicine, Exelixis
Research Funding: Bristol-Myers Squibb, Pfizer, AstraZeneca, EMD Serono, Arcus Biosciences
Mark C. Markowski
Honoraria: Clovis Oncology, Exelixis
Mario A. Eisenberger
Leadership: Veru
Stock and Other Ownership Interests: Veru
Honoraria: Merck Sharp & Dohme, Bristol-Myers Squibb, Seattle Genetics
Emmanuel S. Antonarakis
Honoraria: Sanofi, Dendreon, Medivation, Janssen Biotech, ESSA, Astellas Pharma, Merck, AstraZeneca, Clovis Oncology
Consulting or Advisory Role: Sanofi, Dendreon, Janssen Biotech, ESSA, Merck, AstraZeneca, Clovis Oncology, Lilly, Bayer
Research Funding: Janssen Biotech, Johnson & Johnson, Sanofi, Dendreon, Aragon Pharmaceuticals, Exelixis, Millennium, Genentech, Novartis, Astellas Pharma, Tokai Pharmaceuticals, Merck, AstraZeneca, Clovis Oncology, Constellation Pharmaceuticals
Travel, Accommodations, Expenses: Sanofi, Dendreon, Medivation
No other potential conflicts of interest were reported.
REFERENCES
- 1.Huggins C, Hodges CV.Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate Cancer Res 1293–2971941 [DOI] [PubMed] [Google Scholar]
- 2.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic PCa before chemotherapy N Engl J Med 371424–4332014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic PCa without previous chemotherapy N Engl J Med 368138–1482013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emamekhoo H, Barata PC, Edwin NC, et al. Evaluation of response to enzalutamide consecutively after abiraterone acetate/prednisone failure in patients with metastatic castration-resistant PCa Clin Genitourin Cancer 16429–4362018 [DOI] [PubMed] [Google Scholar]
- 5.Smith MR, Saad F, Rathkopf DE, et al. Clinical outcomes from androgen signaling-directed therapy after treatment with abiraterone acetate and prednisone in patients with metastatic castration-resistant PCa: Post hoc analysis of COU-AA-302 Eur Urol 7210–132017 [DOI] [PubMed] [Google Scholar]
- 6.de Bono JS, Chowdhury S, Feyerabend S, et al. Antitumour activity and safety of enzalutamide in patients with metastatic castration-resistant PCa previously treated with abiraterone acetate plus prednisone for >24 weeks in Europe Eur Urol 7437–452018 [DOI] [PubMed] [Google Scholar]
- 7.Linja MJ, Savinainen KJ, Saramaki OR, et al. Amplification and overexpression of androgen receptor gene in hormone-refractory PCa Cancer Res 613550–35552001 [PubMed] [Google Scholar]
- 8.Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy Nat Med 1033–392004 [DOI] [PubMed] [Google Scholar]
- 9.Isaacs JT, D'Antonio JM, Chen S, et al. Adaptive auto-regulation of androgen receptor provides a paradigm shifting rationale for bipolar androgen therapy (BAT) for castrate resistant human PCa Prostate 721491–15052012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Umekita Y, Hiipakka RA, Kokontis JM, et al. Human prostate tumor growth in athymic mice: Inhibition by androgens and stimulation by finasteride Proc Natl Acad Sci U S A 9311802–118071996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Litvinov IV, Vander Griend DJ, Antony L, et al. Androgen receptor as a licensing factor for DNA replication in androgen-sensitive PCa cells Proc Natl Acad Sci U S A 10315085–150902006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vander Griend DJ, Litvinov IV, Isaacs JT.Stabilizing androgen receptor in mitosis inhibits PCa proliferation Cell Cycle 6647–6512007 [DOI] [PubMed] [Google Scholar]
- 13.Denmeade SR, Isaacs JT.Bipolar androgen therapy: The rationale for rapid cycling of supraphysiologic androgen/ablation in men with castration resistant PCa Prostate 701600–16072010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schweizer MT, Antonarakis ES, Wang H, et al. Effect of bipolar androgen therapy for asymptomatic men with castration-resistant PCa: Results from a pilot clinical study. Sci Transl Med. 2015;7:269ra2. doi: 10.1126/scitranslmed.3010563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teply BA, Wang H, Luber B, et al. Bipolar androgen therapy in men with metastatic castration-resistant PCa after progression on enzalutamide: An open-label, phase 2, multicohort study Lancet Oncol 1976–862018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive PCa and castrate levels of testosterone: Recommendations of the PCa Clinical Trials Working Group J Clin Oncol 261148–11592008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in PCa N Engl J Med 3711028–10382014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beltran H, Hruszkewycz A, Scher HI, et al. The role of lineage plasticity in PCa therapy resistance Clin Cancer Res 256916–69242019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khalaf DJ, Annala M, Taavitsainen S, et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant PCa: A multicentre, randomised, open-label, phase 2, crossover trial Lancet Oncol 201730–17392019 [DOI] [PubMed] [Google Scholar]
- 20.Matsubara N, Yamada Y, Tabata KI, et al. Abiraterone followed by enzalutamide versus enzalutamide followed by abiraterone in chemotherapy-naive patients with metastatic castration-resistant PCa Clin Genitourin Cancer 16142–1482018 [DOI] [PubMed] [Google Scholar]
- 21.Terada N, Maughan BL, Akamatsu S, et al. Exploring the optimal sequence of abiraterone and enzalutamide in patients with chemotherapy-naïve castration-resistant PCa: The Kyoto-Baltimore Collaboration Int J Urol 24441–4482017 [DOI] [PubMed] [Google Scholar]