Introduction
Pharmacovigilance related to heart damage is of paramount importance at this early stage of massive coronavirus disease 2019 (COVID-19) campaigns to identify the profile of patients at risk.
Systemic reactogenicity, which leads to systemic adverse events, often occur after the second dose.
A possible explanation could be the molecular mimicry between SARS-CoV-2 viral proteins and cardiac structures or a nonspecific systemic inflammatory response secondary to vaccination (1).
Case Report
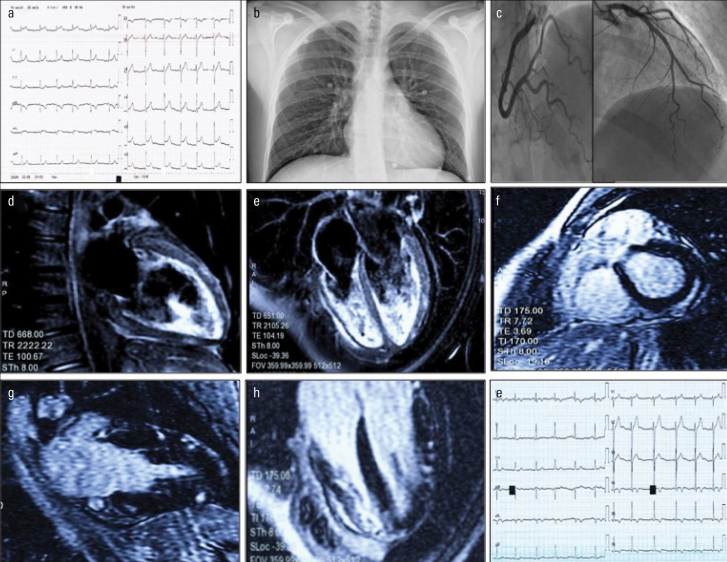
A 21-year-old male health worker with no previous medical or cardiac history was admitted to the emergency room with fever and cardiac-sounding chest pain. Electrocardiogram showed diffuse concave-upward ST-segment elevation and troponin elevation (troponin I: 6.53 ng/mL, C-reactive protein: 2.4 mg/dL) (Fig. 1a and 1b). He had been administered the second dose of the Pfizer mRNABNT162b2 Comirnaty® SARS-CoV-2 vaccine (the EU Brand Name for Pfizer & BioNTech’s COVID-19 Vaccine, Developed by Brand Institute) 30 hours earlier.
Figure 1.
a: Diffuse concave ST elevation with slightly widened QRS (90 ms, Axis + 60°)
b: Normal pulmonary texture on chest X-ray
c: Coronary arteries free from stenosis, TIMI 3 flow on all vessels
d and e: Patchy myocardial edema (water-sensitive T2-weighted sequence) in the anterior, inferior, and lateral walls. Mild pericardial effusion
f, g and h: Patchy epicardial hyperenhancement (late gadolinium enhancement sequence) in the posterior, anterior, inferior, and lateral walls
i: Electrocardiogram after one week with ST/T changes (note the negative T in the inferolateral site consensual to the signal changes reported on cardiac magnetic resonance)
The SARS-CoV-2 antigen rapid diagnostic test and the nasopharyngeal reverse transcriptase–polymerase chain reaction swab test were negative.
A transthoracic echocardiogram showed normal ejection fraction with hypokinesis of the left ventricular inferior and posterior walls. A workup of serological analysis for viral and bacterial infection was negative, except for a doubtful IgM positivity to Mycoplasma pneumoniae; therefore, prophylaxis with doxycycline was carried out (2).
Coronary angiography, which was performed to rule out coronary artery disease, showed no significant stenosis or flow abnormalities (Fig. 1c).
Bisoprolol and ramipril were started after a brief episode of nonsustained ventricular tachycardia.
Cardiac magnetic resonance imaging confirmed the diagnosis of myocarditis with evidence of edema and nonischemic delayed enhancement with a patchy pattern, predominantly epicardial (Fig. 1d–1h).
During hospitalization, the patient responded well to the treatment with regression of thoracic discomfort and partial ST/T resolution (Fig. 1i). The patient was discharged after 1 week without clinical events in the follow-up.
The alleged adverse event was reported to the Italian National Pharmacovigilance Network.
Discussion
Data regarding cardiac events after vaccination, particularly after vaccination with a newly developed vaccine, are limited.
However, whether myocarditis is causally related to the vaccination or is merely a chance occurrence remains unknown (3).
Establishing a causal link between administering a vaccine and a case of myocarditis exclusively on the logical fallacy of the “post hoc ergo propter hoc” is limited by many biasing factors and has limited scientific value, especially without a cardiac biopsy (4).
However, we can hypothesize 2 possible causal mechanisms. The first mechanism is that the vaccine triggered and exacerbated an autoimmune response in the context of a subacute Mycoplasma pneumoniae infection.
The second is that the vaccine triggered immunologically mediated heart damage induced by the autoimmune/inflammatory response to vaccine compounds in a patient with an unknown underlying disease or a predisposing immunological profile (5).
Postvaccination myocarditis is an infrequent event; this has been observed by comparing the millions of vaccinated individuals.
The potential benefit of a worldwide vaccination campaign against SARS-CoV-2 infection is far greater than the rare, self-limited, and benign course of myocarditis with preserved systolic function.
The risk/benefit profile is clearly in favor of the vaccine, especially considering hundreds of thousands of worldwide cases of myocarditis (about 1.4% of severe COVID-19 infections) (6) and millions of cases of SARS-CoV-2 pneumonia.
Conclusion
Vaccination is critically important in managing the current outbreak of COVID-19. Postvaccination adverse events are usually benign and self-limited. However, given the unusually rapid development of these vaccines and limited experience, it is essential to report any potential effects that may not be related to the vaccination.
In this case, the myocarditis episode was transient, was self-limiting, and had no consequent long-term impact on ventricular function.
Footnotes
Informed consent: Written informed consent was obtained from patient.
References
- 1.Ammirati E, Cavalotti C, Milazzo A, Pedrotti P, Soriano F, Schroeder JW, et al. Temporal Relation Between Second Dose BNT162b2 mRNA Covid-19 Vaccine and Cardiac involvement in a Patient with Previous SARS-COV-2 Infection. Int J Cardiol Heart Vasc. 2021:100778. doi: 10.1016/j.ijcha.2021.100778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.el-Khatib M, Lerner AM. Myocarditis in Mycoplasma pneumoniae pneumonia. Occurrence with hemolytic anemia and extraordinary titers of cold isohemagglutinins. JAMA. 1975;231:493–4. doi: 10.1001/jama.231.5.493. [DOI] [PubMed] [Google Scholar]
- 3.Su JR, McNeil MM, Welsh KJ, Marquez PL, Ng C, Yan M, et al. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990–2018. Vaccine. 2021;39:839–45. doi: 10.1016/j.vaccine.2020.12.046. [DOI] [PubMed] [Google Scholar]
- 4.Nagano N, Yano T, Fujita Y, Koyama M, Hasegawa R, Nakata J, et al. Hemodynamic Collapse After Influenza Vaccination: A Vaccine-Induced Fulminant Myocarditis? Can J Cardiol. 2020;36:1554.e5–7. doi: 10.1016/j.cjca.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Mele D, Flamigni F, Rapezzi C, Ferrari R. Myocarditis in COVID-19 patients: current problems. Intern Emerg Med. 2021:1–7. doi: 10.1007/s11739-021-02635-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halushka MK, Vander Heide RS. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021;50:107300. doi: 10.1016/j.carpath.2020.107300. [DOI] [PMC free article] [PubMed] [Google Scholar]