Abstract
Chronic kidney disease (CKD) is a significant health challenge associated with high cardiovascular mortality risk. Historically, cardiovascular mortality risk has been found to higher in men than women in the general population. However, recent research has highlighted that this risk may be similar or even higher in women than men in the CKD population. To address the inconclusive and inconsistent evidence regarding this relationship between sex and cardiovascular mortality within CKD patients, a systematic review and meta-analysis of articles published between January 2004 and October 2020 using PubMed/Medline, EMBASE, Scopus and Cochrane databases was performed. Forty-eight studies were included that reported cardiovascular mortality among adult men relative to women with 95% confidence intervals (CI) or provided sufficient data to calculate risk estimates (RE). Random effects meta-analysis of reported and calculated estimates revealed that male sex was associated with elevated cardiovascular mortality in CKD patients (RE 1.13, CI 1.03–1.25). Subsequent subgroup analyses indicated higher risk in men in studies based in the USA and in men receiving haemodialysis or with non-dialysis-dependent CKD. Though men showed overall higher cardiovascular mortality risk than women, the increased risk was marginal, and appropriate risk awareness is necessary for both sexes with CKD. Further research is needed to understand the impact of treatment modality and geographical distribution on sex differences in cardiovascular mortality in CKD.
Introduction
Chronic kidney disease (CKD) is a significant global health issue, resulting in premature death, reduced quality of life and substantial financial burden for patients [1]. In 2016, CKD was the 16th leading cause of death worldwide [2] and has been projected to be the 5th leading cause of death by 2040 [3]. The primary cause of mortality in CKD patients is cardiovascular disease, namely heart failure, myocardial infarction, sudden cardiac arrest and stroke [4].
Cardiovascular mortality is associated with several risk factors, including biological sex, age, smoking and obesity [5]. The effect of biological sex on cardiovascular mortality in CKD patients is unclear. Historically, the consensus has been that the life-long risk for cardiovascular mortality is higher in men than women within the general population [6]. However, few studies have focussed on sex differences in cardiovascular outcomes in the CKD population. A meta-analysis of renal function and the association of sex with cardiovascular mortality found that men had a higher mortality risk across all levels of renal function compared with women [7]. These findings are consistent with analyses of the European Renal Association-European Dialysis and Transplant Association Registry [8]. However, other population studies show that the hazard ratio (HR) for cardiovascular-specific (e.g. myocardial infarction; MI) and all-cause cardiovascular mortality is higher in women than men [9]. Moreover, more recent data from 2018 suggests that women with CKD have a higher annual risk of cardiovascular hospitalisations and death than men [10]. Thus the collective evidence regarding the role of sex on cardiovascular mortality among CKD patients is inconsistent and inconclusive.
To address this, we conducted a systematic review and meta-analysis of the literature published between 2004 and 2020. We sought to determine the effect of sex on cardiovascular mortality among CKD patients and identify if a need exists for risk stratification and sex-specific guidelines regarding cardiovascular mortality risk among CKD patients.
Materials and methods
Search strategies
This review was conducted per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines ([11]; see S1 Checklist). A systematic search of the literature published between January 2004 and October 2020 was conducted using PubMed/Medline, EMBASE, Scopus and the Cochrane library. A date limit was introduced based on the guidelines for Kidney Disease Outcomes Quality Initiative [12], which aimed to set a standard for the definition of CKD in the early 2000s, utilising eGFR as a measure of kidney disease progression. Combinations of appropriate Medical Subject Headings and keywords were used to comprehensively search the selected databases, with syntax amended for each database: (chronic renal insufficiency OR chronic kidney failure OR (chronic kidney adj (disease or insufficiency)) OR (chronic renal adj (disease or insufficiency)) OR end-stage renal disease OR uremia OR uraemia) AND (cardiovascular disease mortality OR (cardiovascular adj (mortality or death or event* or complication* or outcome*)) OR heart disease mortality) AND (sex factors OR male or female OR sex distribution OR sex characteristics OR sex ratio OR men OR women OR men or women or male* or female* or sex or gender or “sex difference*”). The search was limited to English. Additional details are provided in S1 Data Reference lists from relevant reviews were also screened for pertinent studies.
Study selection and eligibility criteria
Studies were included if they met the following criteria:
non-interventional cohort study (prospective/retrospective) of adult patients (aged > 18 years) with any stage of CKD, including dialysis patients, and CKD defined as an eGFR < 60 mL/min per 1·73 m2 (Stages 3–5 CKD).
reported rate/risk estimates of cardiovascular mortality of men relative to women as HRs or RRs with 95% confidence intervals or contained enough data to calculate risk estimates.
contained patient population data collected in or after 2004.
had a follow-up of at least one year.
If a group of studies drew participants from the same cohort, the study with more comprehensive data and longer follow-up duration was selected.
To reduce any confounding variables that may affect the association between CKD and cardiovascular mortality, we excluded studies if:
the patient population exclusively contained type 1 and 2 diabetes mellitus patients.
study participants had any infection, carcinoma, acute kidney injury, surgical interventions (e.g., kidney transplant or coronary artery bypass surgery).
study participants received any non-conventional drug treatments (e.g., chemotherapy).
Reviews, comments, letters to editors or unpublished studies were also excluded.
After removing all duplicates, records identified through database searches and other sources were screened by SS for eligibility by screening titles and abstracts. The full texts of the screened records were then independently reviewed against eligibility criteria by three reviewers, SS (100%), CMH (2%) and JKP (2%), where the percentage denotes the proportion of the studies screened by each reviewer. Disagreement regarding any study was resolved by discussion between the three reviewers.
Quality assessment
The Newcastle-Ottawa Scale (NOS) was used to assess the risk of bias for cohort studies (S2 Data) [13]. Since the primary objectives of the included studies differed from the research question of this systematic review, we adapted the NOS to assess the quality of data relevant to the research question. We therefore used three items to assess study quality: (i) selection of participants (including three domains); (ii) comparability of study results (including three domains); and (iii) outcome (including four domains). Each domain had a rating of “yes,” “no”, or “unclear.” If there was adequate data against a domain in the included study and met the criteria, it was classified as low risk of bias (yes). Conversely, a domain was classified as high risk of bias if adequate information was not available (no) or not enough data was available to make an assessment (unclear). “Yes” was scored as “1”, and “no” or “unclear” was scored as “0.” Scores were tallied up to calculate the final cumulative score. A study was considered high quality if the cumulative score was ≥ 4, and low quality if <4. Three reviewers assessed quality (SS (100%), JA (6.6%) and CMH (4.44%) and any disagreement was resolved through discussion.
Data extraction and data items
Data were extracted using an adapted form of the Data Extraction Template for systematic reviews (Cochrane Public Health Group; S3 Data). SS performed all data extraction with a proportion of duplicate data extraction performed by JA (6.6%) and CMH (4.44%), and any disagreements between reviewers resolved by discussion.
Data were collected for the number of men and women participants with CKD, CKD stage, mode of treatment, comorbidities, the average age of patients, length of follow-up and number of patients lost to follow-up. Outcome measures included rate/risk estimates of cardiovascular mortality of men relative to women as HRs and RRs with 95% CI and p-values, where cardiovascular death was defined as death due to any cardiovascular disease, such as myocardial infarction, heart failure, stroke, sudden cardiac arrest, and atrial fibrillation. Where rate estimates were not available, the absolute number of cardiovascular deaths stratified by sex were collected to calculate relative rates with 95% CI and p-values. Information was also collected for any adjustments for confounders, such as age, diabetes, hypertension, and obesity.
Additional data, where available, were also collected for: (i) sex-stratified cardiovascular mortality risk relative to a non-CKD population and (ii) cause-specific cardiovascular mortality, namely heart failure, myocardial infarction, and stroke. An effort was made to contact study authors in the case of missing data.
Statistical analysis
Extracted and calculated effect estimates hazard ratios (HR) and rate ratios (RR) were combined in the meta-analysis to calculate the overall risk estimate. A random-effects model was used for analysis to account for the variation of real effects across studies [14]. The overall estimate was denoted to be a risk estimate (RE). Heterogeneity among risk estimates was measured using the I2 statistic. The degree of heterogeneity was assessed from the I2 statistic using the following thresholds for interpretation: (1) 0% to 30%: marginal heterogeneity; (2) 30% to 50%: moderate heterogeneity; (3) 50% to 75%: substantial heterogeneity; and (4) 75% to 100%: represents considerable heterogeneity [14].
Exploratory subgroup meta-analyses were conducted on several variables that may have contributed to heterogeneity: quality of studies (high vs. low); geographical distribution (China vs. Taiwan vs. Korea vs. Japan vs. Europe vs. United States vs. Australia and New Zealand vs. Africa); sample size (<100 vs. 100–999 vs. >1000); length of follow-up in years (<2 vs. 2–5 vs. >5); male to female ratio in the study sample (0.4–0.8 vs. 0.9–1.1 vs. 1.2–2.8); and dialysis modality (haemodialysis (HD), peritoneal dialysis (PD), no dialysis). An additional subgroup analysis was conducted based on adjustment for confounders (adjusted vs. unadjusted) for reported HR from included studies.
A 95% CI with no overlap with the null effect value (1) were interpreted to demonstrate a statistically significant difference between sexes in risk of cardiovascular mortality. Publication bias was assessed via a funnel plot with pseudo-95% CI and the log of the RR as θ. All analyses were performed using the ‘meta’ package in STATA version 16 and Revman 5.
Results
Search result
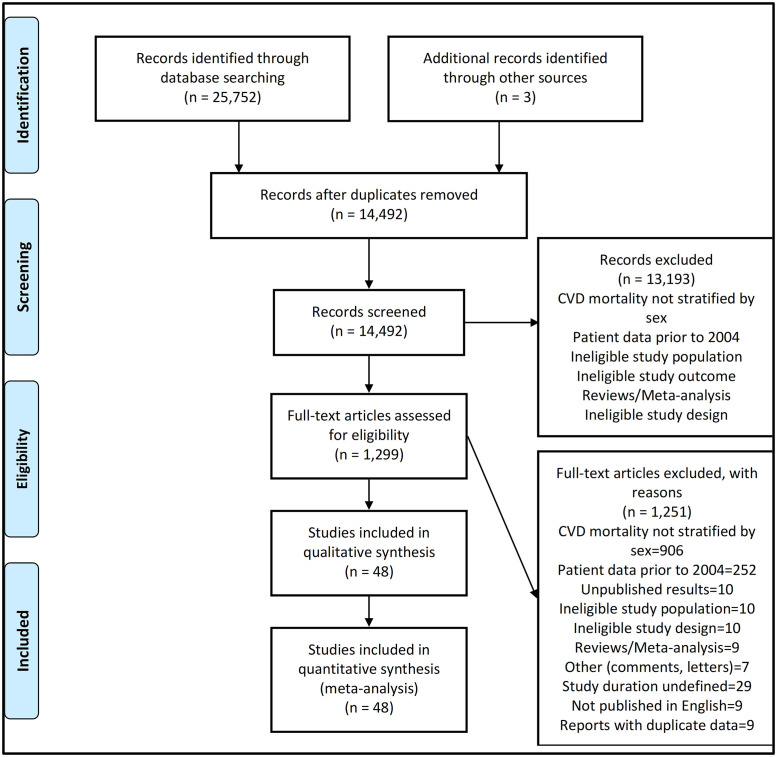
A systematic search of the literature identified a total of 14,492 studies (25,752 studies before removal of duplicate studies) with title and abstract screening identifying a total of 1,299 eligible studies (see Fig 1). A full-text review of eligible studies was evaluated against the eligibility criteria, yielding 57 studies. S1 Table details the excluded studies and reasons for exclusion. Of the 57 studies, nine identified studies contained data from the same study cohort. After careful evaluation, the studies with the most comprehensive data and longer follow-up time were included. The final review included a total of 48 studies.
Fig 1. Flow diagram of study selection and search results.
n, number of studies. CVD, Cardiovascular disease.
Characteristics of included studies
Characteristics of the included studies are detailed in Table 1. The total number of subjects across all the studies were 99,822, which comprised 51,069 men and 48,753 women. There was a higher proportion of men than women across all the studies (average ratio: 1.3; range: 0.4 [15] to 2.8 [16]), with the age of subjects typically ranging from 52–73 years, except for one study [17] which had a comparatively younger study population (average age: 36 years). All studies were published between 2010 and 2020, had an average duration of follow-up of 3.75 years (range: 1.28 [18] to 11 [19] years) and variable sample size (62 [20] to 38,377 [21] subjects).
Table 1. Summary of study characteristics.
| Author/country | Stage of CKD | Dialysis Modality | Sample size (ratio men: women) | Mean age | Follow-up yr. | Baseline comorbidities of study sample (%) | Study Factor | Outcome Factor | NOS score |
|---|---|---|---|---|---|---|---|---|---|
| Wang 2020 [45]/China | ESRD | HD | 205 (1.6) | 59.0 | 3.0 | HTN (90); DM (29.3) | Biomarkers soluble suppression of tumorigenicity 2 (sST2) and N-terminal pro-brain natriuretic peptide (NT-proBNP) | all-cause and cardiovascular mortality | 3 |
| Zhang 2020 [44]/China | ESRD | HD | 252 (1.3) | 57.1 | 6.1 | HTN (31.3), Coronary Heart Disease (6.7), DM (12.3), Cerebral infarction (14.3), Cerebral haemorrhage (3.2) | Plasma Trimethylamine-N-Oxide (TMAO) | cardiovascular and all-cause mortality | 2 |
| Yu 2020 [19]/China | ESRD | HD | 358 (1.1) | 74.0 | 11 | DM (50.3), HTN (95.8), CHF (13.7), Stroke (12), Arrhythmia (8.1), CHD (3.9), Peripheral vascular disease (0.8), Cancer (4.2) | Impact of vascular access | cardiovascular and all-cause mortality | 6 |
| Lee 2020 [59]/Taiwan | 3–5 | No | 472 (1.1) | 66.8 | 2.0 | DM (36.70); HTN (75.80); Coronary artery disease (19.6); Cerebrovascular disease (8.1); CHF (12) | Upstroke time | Cardiovascular and all-cause mortality | 4 |
| Tsai 2020 [55]/Taiwan | ESRD | PD | 133 (0.8) | 56.0 | 6.4 | DM (21.05), HTN (84.96) | Heart rhythm complexity | cardiovascular mortality | 3 |
| Simsek 2020 [60]/Turkey | 3,4 | No | 191 (0.7) | 66.3 | 6.3 | DM (19.40), HTN (57.10), Previous coronary heart disease (7.30), Dyslipidaemia (66.00) | N-terminal pro-brain natriuretic peptide (NT-proBNP) level | Mortality | 5 |
| Toyama 2019 [16]/Japan | ESRD | HD | 286 (2.8) | 70.5 | 2.0 | DM (34.62); HTN (77.98); Dyslipidaemia (49.30); Prior myocardial infarction (11.89) | stress myocardial perfusion single-photon emission computed tomography (SPECT) | cardiovascular/cerebrovascular events | 3 |
| Mizuiri 2019 [47]/Japan | ESRD | HD | 353 (2.0) | 68.0 | 3.0 | DM (40.2) | hypomagnesemia | All-cause and cardiovascular mortality | 3 |
| Chen 2019 [58]/Taiwan | 3–5 | No | 568 (1.5) | 66.0 | 2.7 | DM (57.57); HTN (85.56); CAD (79); Cerebrovascular disease (59) | aortic arch calcification (AoAC) and cardio-thoracic ratio (CTR) | all-cause and cardiovascular mortality | 5 |
| Yadav 2019 [46]/The Netherlands | ESRD | HD | 199 (1.5) | 70.0 | 4.0 | DM (39); HTN (79); CVD (55) | digital brachial index | Survival rates | 3 |
| Cano-Megias 2019 [57]/Spain | 4,5 | HD | 137 (1.2) | 61.5 | 10.0 | DM (27.7); HTN (89); CAD (20.1); Cerebrovascular disease (11.3); Previous cardiologic event (26.3) | coronary artery calcification (CaC) | Overall and cardiovascular mortality | 3 |
| Wu 2019 [28]/China | ESRD* | HD | 169 (1.20) | 60.2 | 7.0 | NA | baseline serum magnesium level | mortality | 4 |
| Saglimbene 2019 [29]/Europe and South America | ESRD | HD | 8110 (1.40) | 63.1 | 2.7 | HTN (85), DM (32), HF (19.1), MI (11.60), stroke (8.8), pulmonary disease (11.6), gastrointestinal disease (21.7) | n-3 polyunsaturated fatty acid dietary intake | mortality | 6 |
| Gong 2018 [48]/China | ESRD* | PD | 98 (0.96) | 52.5 | 6.0 | DM (21.4), History of CVD (7.1) | elevated serum sclerostin levels | mortality | 3 |
| Yayar 2018 [30]/Turkey | ESRD* | HD | 82 (0.78) | 57.9 | 4.0 | DM (26.8), History of CVD (35.4) | serum hepcidin-25 & sub-clinic atherosclerosis | mortality | 5 |
| Kawagoe 2018 [31]/Japan | ESRD* | HD | 1310 (1.40) | 67.9 | 2.0 | Not Reported | N-terminal-pro-B-type-natriuretic peptide | mortality | 5 |
| Kon 2018 [62]/Japan | 3–5 | Not clear | 27,362 (1.20) | 70.2 | 5.0 | Stroke (19.6), Heart disease (29.2) | baseline eGFR | 5-year all-cause & cardiovascular mortality | 3 |
| Navaneethan 2018 [21]/US | 3–5 | NA | 38,377 (0.80) | 71.7 | 4.5 | HTN (87.2), DM (29.8), CAD (24.2), HF (7.8), Cerebrovascular disease (10.4), PVC (3.7) | high-density lipoprotein cholesterol | mortality | 4 |
| Zhang 2017 [25]/China | ESRD* | HD | 414 (1.60) | 61.8 | 1.9 | DM (22.9), HTN (94), CVD (9.2) | soluble suppression of tumorigenicity 2 | mortality | 4 |
| Wu 2017 [49]/Taiwan | ESRD* | PD | 190 (0.80) | 52.6 | 4.6 | DM (15.3), CVD (21.1) | chest X-ray-detected aortic arch calcification | mortality | 6 |
| Peng 2017 [50]/China | ESRD* | PD | 345 (1.40) | 52.8 | 2.1 | DM (23.5), HTN (43.2), CVD (20) | prognostic nutritional index | cardiovascular disease mortality | 3 |
| Jeng 2017 [32]/Taiwan | ESRD* | HD | 136 (1.20) | 60.3 | 5.6 | DM (50), HTN (72.1), CVD (53.4) | proinflammatory monocytes levels | all-cause & cardiovascular mortality | 4 |
| Antunovic 2017 [20]/ Montenegro | ESRD | HD | 62 (0.90) | 57.8 | 2.0 | DM (8.1), HTN (32.3) | high-sensitive troponin I | mortality | 3 |
| Isla 2016 [17]/South Africa | ESRD | HD & PD | 340 (1.10) | 36.1 | 3.1 | DM (10.3), HTN (25.9) HIV positive (3.1) | causes and predictors | mortality | 4 |
| Lu 2016 [33]/Taiwan | ESRD* | HD | 154 (0.90) | 69.1 | 4.2 | DM (55.9), HTN (68.8) | number of endothelial progenitor cells | cardiovascular and all-cause mortality | 4 |
| Merle 2016 [22]/France | ESRD* | HD | 1983 (1.60) | 67.9 | 2.0 | DM (37.7), HTN (79.1), CVD (54.6) | low parathyroid hormone (PTH) status | mortality | 4 |
| Chen 2015 [37]/China | ESRD* | HD | 110 (1.40) | 55.2 | 3.5 | DM (57.3) | aortic artery calcification, cardiac valve calcification | mortality | 3 |
| Flythe 2015 [36]/US | ESRD* | HD | 10,758 (1.20) | 61.0 | 3.0 | DM (59.1), Heart Failure (43.1), CAD (13.4) | post-dialysis weights above and below the prescribed target weight | mortality | 5 |
| Tsai 2015 [35]/Taiwan | ESRD* | HD | 444 (0.87) | 61.6 | 4.3 | DM (32.7), CVD (20.9) | site of peripheral artery occlusive disease | all-cause & cardiovascular mortality | 6 |
| Oh 2015 [51]/Korea | ESRD* | PD | 335 (1.60) | 53.5 | 1.8 | DM (41.8), HTN (48.1), Coronary arterial disease (11.3), Peripheral arterial disease (7.5) | 3 biomarkers (N-terminal-pro-B-type-natriuretic peptide, Cardiac troponin T and high-sensitivity C-reactive protein) | mortality | 4 |
| Ulusoy 2015 [34]/Turkey | ESRD | HD | 238 (1.40) | 60.3 | 2.0 | DM (36.3), HTN (30.9), Peripheral vascular disease (6.3) | tenascin-C levels | cardiac mortality | 4 |
| Yoshitomi 2014 [61]/Japan | 3–5 | NA | 320 (2.10) | 70.0 | 2.5 | HTN (94), DM (51), History of CVD (19), Dyslipidaemia (73), History of IHD (19) | ankle-brachial blood pressure index | cardiovascular events and mortality | 1 |
| Okamoto 2014 [56]/Japan | ESRD* | HD, PD | 126 (1.30) | 67.0 | 5.0 | DM (52.4) | visceral fat area | mortality | 2 |
| Li 2014 [38]/China | ESRD* | HD | 278 (1.20) | 1.8 | DM (33.8), HTN (91.1), History of CVD (30.6) | pulmonary hypertension | cardiovascular mortality and events | 5 | |
| Honneger Bloch 2014 [39]/New Zealand | ESRD* | HD | 238 (1.10) | 63.0 | 2.0 | DM (64), History of MI (67) | high sensitivity troponin T | mortality | 4 |
| Oh 2014 [24]/Korea | ESRD | HD | 864 (1.50) | 59.7 | 1.5 | DM (56.3), HTN (48) | 3 biomarkers (N-terminal-pro-B-type-natriuretic peptide, Cardiac troponin T and high-sensitivity C-reactive protein) | mortality | 3 |
| Avramovski 2014 [26]/Macedonia | ESRD* | HD | 80 (2.00) | 59.3 | 2.6 | DM (20), HTN (46.2) | aortic pulse wave velocity | all-cause and cardiovascular mortality | 3 |
| Arsov 2014 [42]/ Macedonia, Germany, Sweden | ESRD* | HD | 169 (1.60) | 56.0 | 3.0 | DM (24), HTN (18), CVD (18) | skin autofluorescence and release of heart-type fatty acid binding protein in plasma | overall & CVD mortality | 3 |
| Lim 2013 [40]/Taiwan | ESRD* | HD | 248 (1.00) | 65.0 | 4.9 | DM (51.2), HTN (75.4) | serum oxidized albumin | all-cause & cardiovascular mortality | 4 |
| Li 2013 [52]/China | ESRD* | PD | 66 (0.89) | 62.1 | 3.5 | Non-diabetic, History of CVD (9.1) | insulin resistance | cardiovascular morbidity and mortality | 4 |
| Genovesi 2013 [27]/Italy | ESRD* | HD | 122 (1.80) | 69.8 | 3.9 | Ischaemic heart disease (37.7), DM (27.1), HTN (84.4), Dilated cardiomyopathy (41.8), Valvular heart disease (23.8), Dyslipidaemia (18), Ischaemic cerebral disease (14.8), Atrial fibrillation (41.8) | various risk factors | total mortality & sudden cardiac death | 3 |
| den Hoedt 2013 [41]/ Netherlands, Canada, Norway | ESRD | HD | 714 (1.70) | 64.1 | 3.0 | DM (24), History of CVD (44) | online hemodiafiltration versus low-flux haemodialysis | all-cause & CV morbidity and mortality | 4 |
| Murthy 2012 [18]/US | CKD Stages 3–5 | Not clear | 866 (1.00) | 71.1 | 1.3 | DM (44.8), HTN (91.2), Dyslipidaemia (70), Recent MI < = 30 days (18.9), Remote MI >30 days (22.6), Cerebrovascular disease (8), Peripheral vascular disease (9.1) | vasodilator function | mortality | 3 |
| An 2012 [54]/China | ESRD* | PD | 138 (1.40) | 53.0 | 3.2 | DM (23.9), CAD (29.1), Cerebrovascular disease (7.7), PVD (2.6) | neutrophil to lymphocyte ratio | cardiovascular & all-cause mortality | 6 |
| Wu 2012 [15]/Taiwan | ESRD* | HD | 112 (0.44) | 72.6 | 2.8 | DM (62.5), HTN (56.3) | Serum free p-cresyl sulphate levels | all-cause and CV mortality | 2 |
| Lee 2012 [53]/Taiwan | ESRD* | PD | 415 (1.30) | 55.8 | 2.9 | DM (47.2), CVD (34.9) | prevalence of aortic arch calcification | mortality | 6 |
| Kakiya 2012 [43]/Japan | ESRD* | HD | 494 (1.70) | 60.9 | 4.2 | Diabetic Nephropathy (22.3), HTN (86.2), Pre-existing CVD (33.6) | serum adrenal androgen dehydroepiandrosterone sulphate levels | mortality | 4 |
| Ogawa 2010 [23]/Japan | ESRD* | HD | 401 (2.10) | 61.5 | 4 | DM (33.2) | aortic arch calcification score | all-cause & cardiovascular mortality | 3 |
HD = Haemodialysis, PD = Peritoneal dialysis, ESRD = End-Stage Renal Disease, DM = Diabetes mellitus, HTN = Hypertension, CVD = Cardiovascular disease, PVD = Peripheral vascular disease, HF = Heart failure, MI = Myocardial infarction, CAD = Coronary artery disease. NOS = Newcastle-Ottawa Scale.
*Patients received dialysis therapy and thus assumed to have ESRD.
The majority of the studies reported results for end-stage renal disease (ESRD) patients on dialysis: 30 studies included subjects exclusively on HD [15, 16, 19, 20, 22–47], eight studies included subjects on PD [48–55], three studies included both HD and PD [17, 18, 56], and six studies reported their subjects were not on dialysis [21, 57–61]. Six studies defined their subjects as having CKD stages 3 to 5, [18, 21, 58, 59, 61, 62], while only one study described their subjects as CKD stages 3 and 4 [60]. In addition to this, most studies (33/48) reported comorbidity with diabetes mellitus and hypertension. Of the remaining studies, 15 studies did not report any comorbidity [23, 28, 30, 31, 35–37, 39, 41, 48, 49, 53, 54, 56, 62] and only one excluded diabetes as part of their patient selection criteria [52].
The majority of included studies were conducted in Asia (Japan, China, Taiwan; 30 in total). Among the remaining 18 studies: ten were undertaken in Europe (France, Italy, Macedonia, Sweden, Germany, Montenegro, The Netherlands, Spain and Turkey) [20, 22, 26, 27, 30, 34, 42, 46, 57, 60], three in the United States [18, 21, 36], one in Australia and New Zealand [39], one in South Africa [17], and two included more than one geographical region [29, 41].
Forty-four of the included studies were hospital/clinic-based, two were registry-based studies [21, 62], and one study was based on a previous clinical trial [60]. Thirty of the 48 studies had accounted for all study participants during follow-up [16, 19, 23, 25, 28–33, 35, 38–43, 45–47, 49–55, 58–60]; however, the remaining 18 studies did not clearly describe follow up method or status for all patients.
Risk of bias
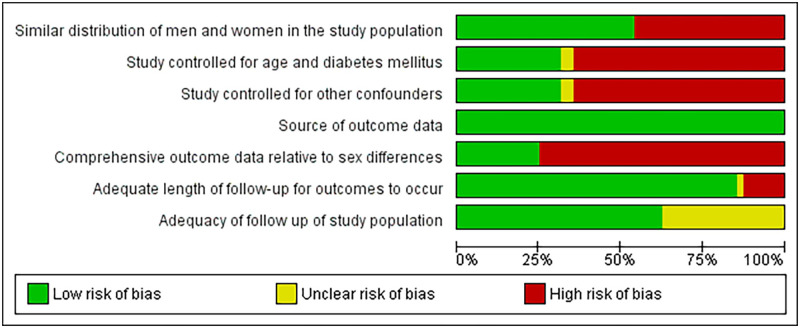
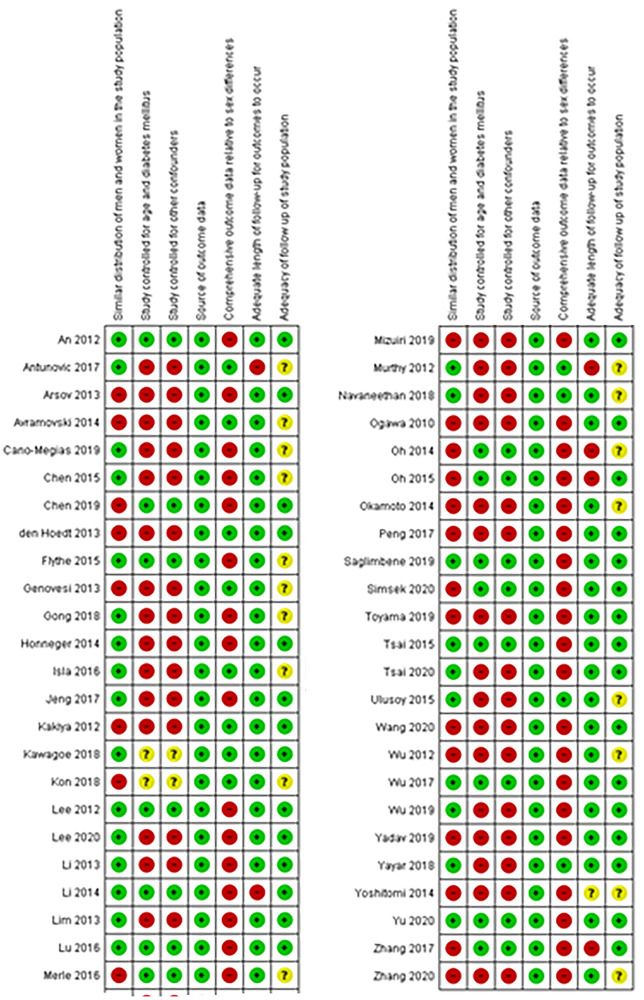
Presented in Fig 2 is a summary of the quality assessment of included studies, with review authors’ judgements about each risk of bias item for each included study in Fig 3. Additional information regarding the risk of bias for each study is provided in S2 Table.
Fig 2. Risk of bias graph.
Review authors’ judgements about each risk of bias item as presented as percentages across all included studies.
Fig 3. Risk of bias summary.
Review authors’ judgements about each risk of bias item for each included study.
In total, 28 studies had a cumulative quality score ≥ four [17, 19, 21, 22, 25, 28–36, 38–41, 43, 49, 51–54, 58–60], and were thus considered high quality. The most frequent limitations observed were a lack of comprehensive sex-stratified data, unequal distribution of men and women in the study population, lack of adjustment for age and diabetes, lack of adjustment for other confounders and covariates, e.g., history of CVD, hypertension and haemoglobin level, and a lack of information regarding patient follow-up (see Figs 2 and 3). The outcome data source was consistent across studies, mainly through patient medical records or data linkage to mortality registries.
Sex and overall cardiovascular mortality
The HR of men for cardiovascular mortality relative to women with 95% CI were reported in 38 out of the 48 studies, with 23 unadjusted and 15 adjusted HRs (Table 2). The remaining ten studies reported the number/proportion of cardiovascular deaths stratified by sex (Table 3), which were used to calculate the rate ratio (RR). 23 out of the 48 included studies reported a higher cardiovascular mortality rate in men than women, with risk estimates ranging from 1.16 to 3.51 (Tables 2 and 3) [18–20, 25, 30, 35, 37, 38, 42, 45, 55, 57, 58, 60–62]. However, 18 of the 48 studies showed that men had a lower risk of cardiovascular mortality compared to women, with risk estimates ranging from 0.29 to 0.93 (Tables 2 and 3) [23, 24, 34, 39, 44, 46, 47, 49, 53, 54, 59].
Table 2. Summary of reported risk estimates of male sex for cardiovascular mortality.
| Study | Unadjusted | Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI Lower Limit | 95% CI Upper Limit | P-value | HR | 95% CI Lower Limit | 95% CI Upper limit | P-value | Adjusted Variables | |
| Wang 2020 | 1.36 | 0.46 | 4.05 | 0.58 | - | - | - | - | n/a |
| Zhang 2020 | 0.93 | 0.49 | 1.75 | 0.81 | - | - | - | - | n/a |
| Yu 2020 | - | - | - | - | 1.49 | 0.75 | 2.95 | 0.25 | Age, dialysis vintage in months, diastolic BP, CHF, CHD, Arrhythmia, Arrhythmia with a pacemaker, Previous stroke, DM, Primary gout, Systemic vasculitis or lupus nephritis, Cancer, Vascular access types, others |
| Lee 2020 | 0.86 | 0.52 | 1.42 | 0.56 | - | - | - | - | n/a |
| Tsai 2020 | 1.21 | 0.50 | 2.29 | 0.67 | - | - | - | - | n/a |
| Simsek 2020 | - | - | - | - | 2.79 | 1.38 | 5.62 | 0.00 | Previous CHD, Anaemia, HTN, Urinary albumin to creatinine ratio >30 mg/g, Log N-terminal pro-brain natriuretic peptide, eGFR |
| Toyama 2019 | 1.04 | 0.52 | 12.51 | 0.36 | - | - | - | - | n/a |
| Mizuiri 2019 | 0.83 | 0.46 | 1.54 | 0.54 | - | - | - | - | n/a |
| Chen 2019 | 1.81 | 0.96 | 3.42 | 0.07 | 1.23 | 0.61 | 2.49 | 0.57 | Age, Chest Thoracic Ratio, Aortic Calcification score |
| Yadav 2019 | 0.88 | 0.50 | 1.59 | 0.68 | - | - | - | - | n/a |
| Cano-Megias 2019 | 1.19 | 0.57 | 2.50 | 0.65 | - | - | - | - | n/a |
| Wu [28] | 1.03 | 0.46 | 2.30 | 0.95 | - | - | - | - | n/a |
| Saglimbene [29] | - | - | - | - | 1.16 | 0.98 | 1.37 | 0.08 | Age, DM, MI, education, smoker, vascular access type, body mass index, albumin, Charlson comorbidity score, phosphorus level, calcium level, haemoglobin, KT/V (index to quantify haemodialysis treatment adequacy), fibre daily intake, energy intake |
| Gong [48] | 0.69 | 0.29 | 1.64 | 0.40 | - | - | - | - | n/a |
| Zhang [25] | - | - | - | - | 2.77 | 1.11 | 6.94 | 0.03 | Age, DM, coronary heart disease, dialysis vintage, vascular access, sST2, NT-proBNP, hs-cTnT, hs-CRP, haemoglobin, serum albumin, leukocyte count, serum urea, serum creatinine, uric acid, body mass index, systolic BP, diastolic BP |
| Wu [49] | - | - | - | - | 0.40 | 0.05 | 3.02 | 0.38 | Age, DM, duration of PD, CVD, MBP, BMI, albumin, phosphorous, HDL, aortic arch calcification |
| Peng [50] | 0.70 | 0.34 | 1.42 | 0.32 | - | - | - | - | n/a |
| Jeng [32] | 0.96 | 0.51 | 1.79 | 0.89 | - | - | - | - | n/a |
| Isla [17] | 0.83 | 0.39 | 1.78 | - | - | - | - | - | n/a |
| Lu [33] | - | - | - | - | 1.28 | 0.61 | 2.66 | - | Age, DM, HTN, endothelial progenitor cells, current smoker, dialysis efficiency, haemoglobin, Harrell’s concordance |
| Merle [22] | - | - | - | - | 1.05 | 0.67 | 1.64 | 0.84 | Age, DM, hypertension, smoking, prevalent cardiovascular events (cerebrovascular disease, ischemic heart disease, heart failure, and peripheral artery disease) |
| Chen [37] | 3.51 | 1 | 12.31 | 0.05 | - | - | - | - | n/a |
| Flythe [36] | - | - | - | - | 1.19 | 1.04 | 1.35 | - | Age, DM, race, CAD, HF, vascular access type, albumin, phosphorus level, haemoglobin, equilibrated Kt/V, dialytic vintage, prescribed treatment time (minutes), intradialytic weight gain, post-dialysis weight, pre-dialysis systolic BP, missed treatments |
| Tsai [35] | 1.13 | 0.75 | 1.70 | 0.54 | 1.87 | 1.11 | 3.16 | 0.02 | Age, DM, CVD, BP, albumin, triglyceride cholesterol, Kt/v, cardiomegaly, Ca-P product, peripheral arterial occlusion disease |
| Oh [51] | 0.68 | 0.32 | 1.45 | 0.32 | 0.69 | 0.32 | 1.49 | 0.34 | Age, white blood cell count |
| Yoshitomi [61] | 2.82 | 0.95 | 12.09 | 0.06 | - | - | - | - | n/a |
| Okamoto [56] | 1.19 | 0.42 | 3.34 | 0.33 | - | - | - | - | n/a |
| Li [38] | 2.25 | 0.99 | 5.10 | 0.05 | 2.06 | 0.89 | 4.75 | 0.09 | Age, DM, CVD, pulmonary hypertension, duration of HD, pre-HD BP, serum phosphorus, urea reduction ratio, systolic dysfunction |
| Honneger Bloch [39] | 0.66 | 0.35 | 1.24 | 0.20 | - | - | - | - | n/a |
| Oh [24] | 1.09 | 0.50 | 2.34 | 0.83 | 0.62 | 0.18 | 2.10 | 0.44 | Age, DM, HTN |
| Arsov [42] | 2.44 | 1.05 | 5.64 | 0.04 | - | - | - | - | n/a |
| Lim [40] | 0.95 | 0.58 | 1.54 | 0.83 | - | - | - | - | n/a |
| Li [52] | 1.31 | 0.68 | 2.54 | 0.42 | - | - | - | - | n/a |
| Murthy [18] | 1.71 | 1.11 | 2.63 | 0.01 | - | - | - | - | n/a |
| An [54] | - | - | - | - | 0.29 | 0.09 | 0.90 | 0.03 | Age, diabetic nephropathy, history of CVD, albumin level, neutrophil to lymphocyte ratio |
| Wu [15] | 0.71 | 0.27 | 1.83 | 0.47 | - | - | - | - | n/a |
| Lee [53] | - | - | - | - | 0.55 | 0.25 | 1.21 | >0.05 | Age, DM, CVD, smoking, lipid-lowering therapy calcium phosphorous product, albumin, log hs-CRP, baseline aortic arch calcification |
| Ogawa [23] | 0.59 | 0.25 | 1.37 | 0.22 | - | - | - | - | n/a |
Table 3. Summary of additional data from studies not reporting risk estimates.
| Study | Men | Women | RR of men for cardiovascular mortality | 95% (CI) lower limit | 95% CI upper limit | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| CVD deaths (n) | Sample size (n) | Rate of CVD death in/1000 person-years | CVD deaths (n) | Sample size (n) | Rate of CVD death/1000 person-years | |||||
| Yayar [30] | 13 | 36 | 361.11 | 8 | 46 | 173.91 | 2.08 | 0.96 | 4.46 | 0.06 |
| Kawagoe [31] | 29 | 770 | 37.66 | 25 | 540 | 46.29 | 0.81 | 0.48 | 1.37 | 0.44 |
| Kon [62] | 31 | 14,810 | 2.09 | 12 | 12,509 | 0.95 | 2.18 | 1.11 | 4.24 | 0.02 |
| Navaneethan [21] | 1851 | 15,112 | 122.49 | 1817 | 19,597 | 92.71 | 1.30 | 1.20 | 1.36 | <0.01 |
| Antunovic [20] | 6 | 30 | 200.00 | 4 | 32 | 125.00 | 1.60 | 0.50 | 5.11 | 0.43 |
| Ulusoy [34] | 20 | 140 | 142.86 | 20 | 98 | 204.08 | 0.66 | 0.37 | 1.15 | 0.15 |
| Avramovski [26] | 10 | 53 | 188.68 | 7 | 27 | 259.25 | 0.73 | 0.31 | 1.69 | 0.46 |
| Genovesi [27] | 13 | 79 | 164.56 | 7 | 43 | 162.79 | 1.01 | 0.43 | 2.34 | 0.98 |
| den Hoedt [41] | 50 | 445 | 112.36 | 24 | 269 | 89.21 | 1.26 | 0.79 | 2.00 | 0.33 |
| Kakiya [43] | 22 | 313 | 70.29 | 13 | 181 | 71.82 | 0.98 | 0.50 | 1.89 | 0.95 |
After combining the reported and calculated risk estimates (HR/RR) in a random-effects meta-analysis, the overall risk estimate indicated that men with CKD were marginally at higher risk of cardiovascular mortality compared to women with CKD (RE 1.13, 95%CI 1.03–1.25), with moderate heterogeneity (I2 = 35.89%; see Fig 4).
Fig 4. Forest plot of meta-analysis of risk estimates of all included studies.
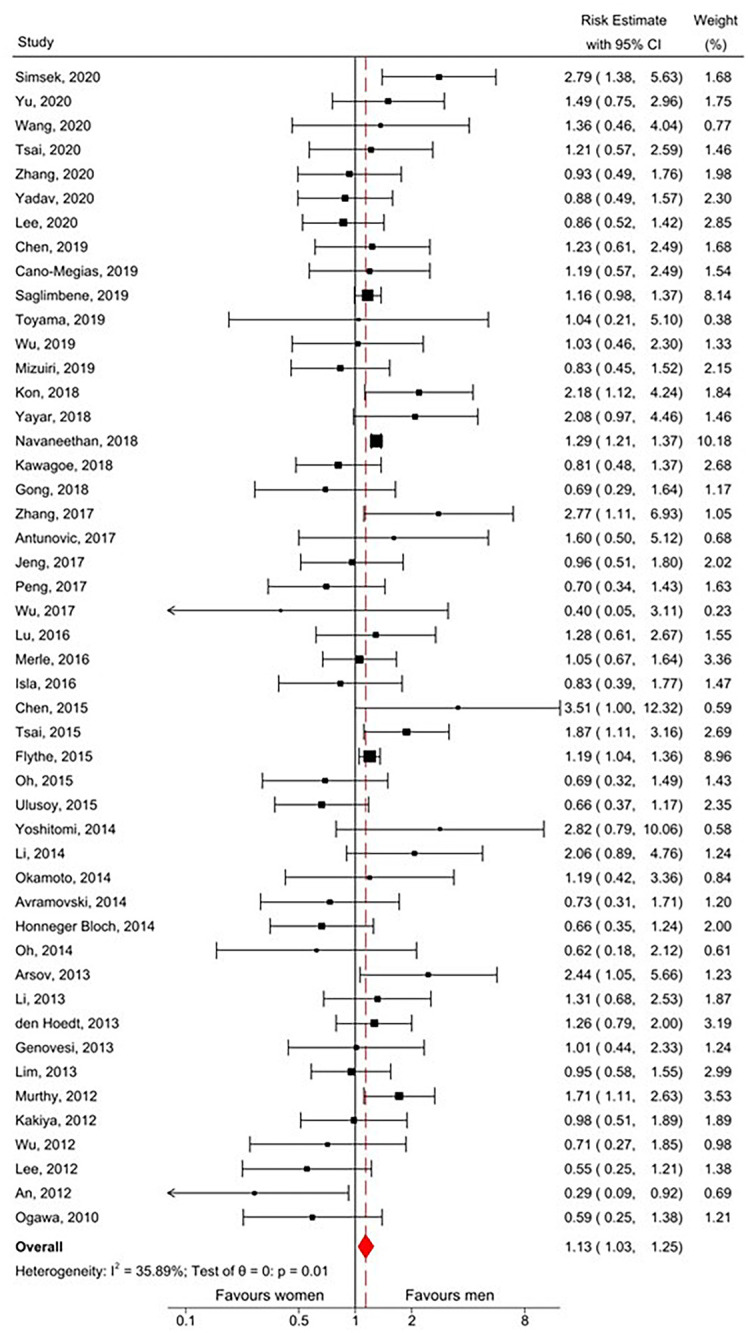
CI, confidence interval. I2, Measure of heterogeneity. θ, Risk estimate. p, p-value.
Subgroup analysis
Table 4 summarises the results of the subgroup analyses. There was no statistically significant difference in risk estimate between any of the subgroups. When restricted to the 28 high-quality studies alone, risk was elevated for men (RE 1.18 95%CI 1.08, 1.27) with very low heterogeneity between studies (I2 = 0%).
Table 4. Risk estimate (RE) of cardiovascular disease, stratified by study characteristics.
| Subgroup | Studies (n) | RE (95% CI) | I2 (%) |
|---|---|---|---|
| Overall | 48 | 1.13 (1.03, 1.25) | 35.89 |
| Sample size | |||
| >1000 | 6 | 1.23 (1.15, 1.33) | 17.31 |
| 100–999 | 37 | 1.09 (0.94, 1.26) | 33.40 |
| <100 | 5 | 1.18 (0.77, 1.81) | 24.32 |
| Length of follow-up (years) | |||
| >5 years | 8 | 1.20 (0.90, 1.59) | 20.19 |
| 2–5 | 35 | 1.10 (0.99, 1.22) | 35.13 |
| <2 | 5 | 1.41 (0.84, 2.37) | 54.80 |
| Men: women ratio | |||
| 1.2–2.8 | 30 | 1.09 (0.98, 1.21) | 8.17 |
| 0.9–1.1 | 12 | 1.16 (0.93, 1.44) | 25.65 |
| 0.4–0.8 | 6 | 1.41 (1.01, 1.96) | 25.60 |
| Geographical distribution | |||
| Europe | 12 | 1.20 (0.97, 1.48) | 37.30 |
| China | 11 | 1.18 (0.85, 1.63) | 41.17 |
| Taiwan | 9 | 1.10 (0.87, 1.38) | 9.59 |
| Japan | 8 | 1.07 (0.76, 1.50) | 34.22 |
| Korea | 3 | 0.62 (0.37, 1.02) | 0.00 |
| United States | 3 | 1.28 (1.21, 1.35) | 0.00 |
| Australia and New Zealand | 1 | 0.66 (0.35, 1.24) | - |
| Africa | 1 | 0.83 (0.39, 1.77) | - |
| Study quality | |||
| High | 28 | 1.18 (1.08, 1.27) | 0.00 |
| Low | 20 | 1.04 (0.86, 1.26) | 42.32 |
| Source of risk estimate | |||
| HR | 38 | 1.13 (1.03, 1.25) | 35.93 |
| RR | 10 | 1.16 (0.93, 1.44) | 42,70 |
| By dialysis modality | |||
| HD | 30 | 1.14(1.05, 1.24) | 0.00 |
| PD | 8 | 0.76 (0.55, 1.05) | 13.09 |
| Both HD, PD | 3 | 1.30 (0.80, 2.13) | 34.89 |
| No dialysis | 6 | 1.34 (1.00, 1.80) | 45.27 |
| Adjustment for other risk factors (only includes studies where HR was reported) | |||
| Adjusted | 15 | 1.21 (0.97, 1.50) | 60.50 |
| Unadjusted | 23 | 1.03 (0.88, 1.21) | 12.13 |
Note: I2 = I2 statistic for the measurement of heterogeneity among risk estimates.
Subgroup analysis by measure of association (HR versus RR) revealed that there was no meaningful difference in estimate of sex effect on the method of calculation [HR 1.13 (95%CI 1.03–1.25) vs. RR 1.16 (95%CI 0.93–1.44)]. Subgroup analysis by adjusted versus unadjusted HRs revealed that the association did not reach statistical significance for either group [adjusted HR 1.21 (95%CI 0.97–1.50) vs. unadjusted HR 1.03 (95%CI 0.88–1.21)]. The direction of association varied between countries with the highest increased risk for men reported in studies from the United States (RE 1.28 95%CI 1.21–1.35). In all Korean studies, the point estimate for risk indicated greater mortality in women, although this did not reach statistical significance (RE 0.62, 95%CI 0.37–1.02; Fig 5). Analysis stratified by male-to-female ratio showed that CVD mortality was only greater in studies which included more women (M: F ratio 0.4–0.8; RE 1.43 95%CI 1.15–1.78). Additional analysis by dialysis modality suggested that peritoneal dialysis was associated with lower cardiovascular mortality risk among men; however, this did not reach statistical significance (RE 0.76, 95% CI 0.55–1.05; Fig 6).
Fig 5. Forest plot of subgroup analysis by country of risk estimates of all included studies.
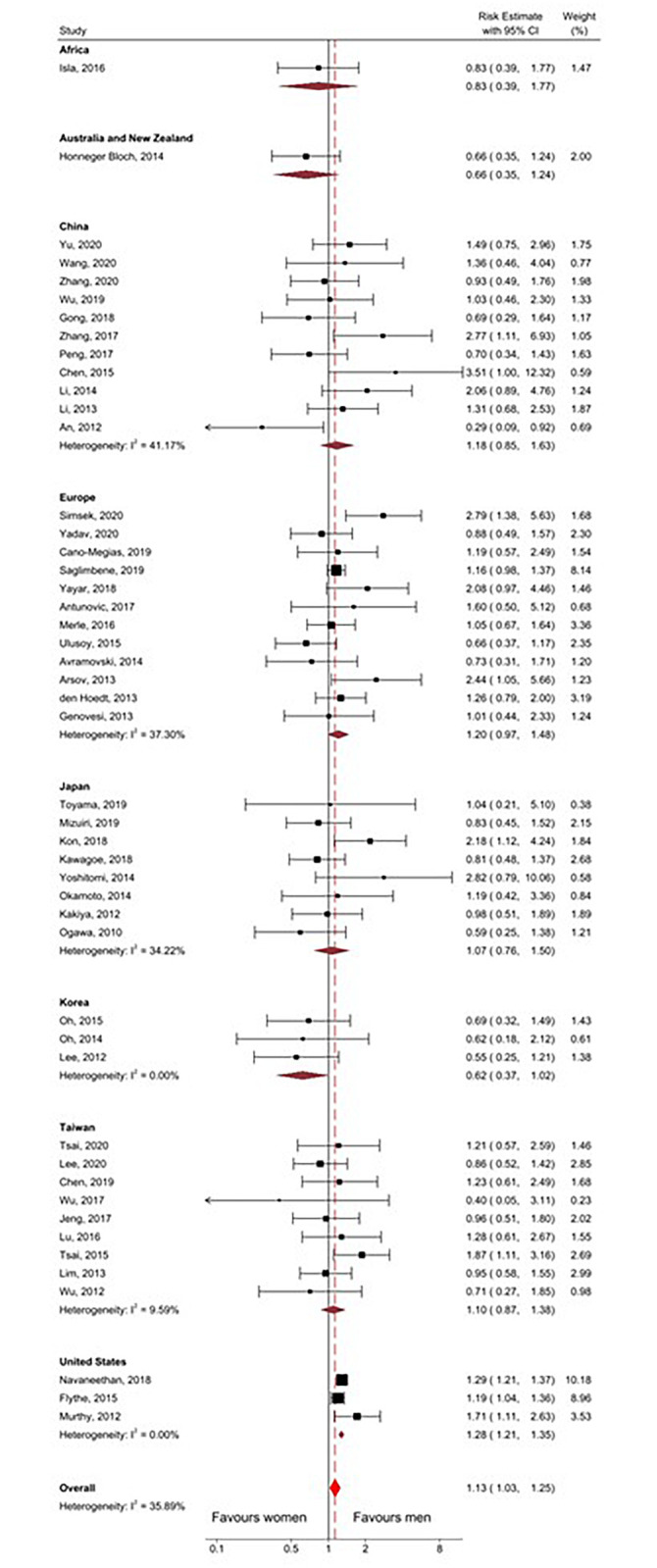
CI, confidence interval. I2, Measure of heterogeneity.
Fig 6. Forest plot of subgroup analysis by dialysis modality of risk estimates of all included studies.
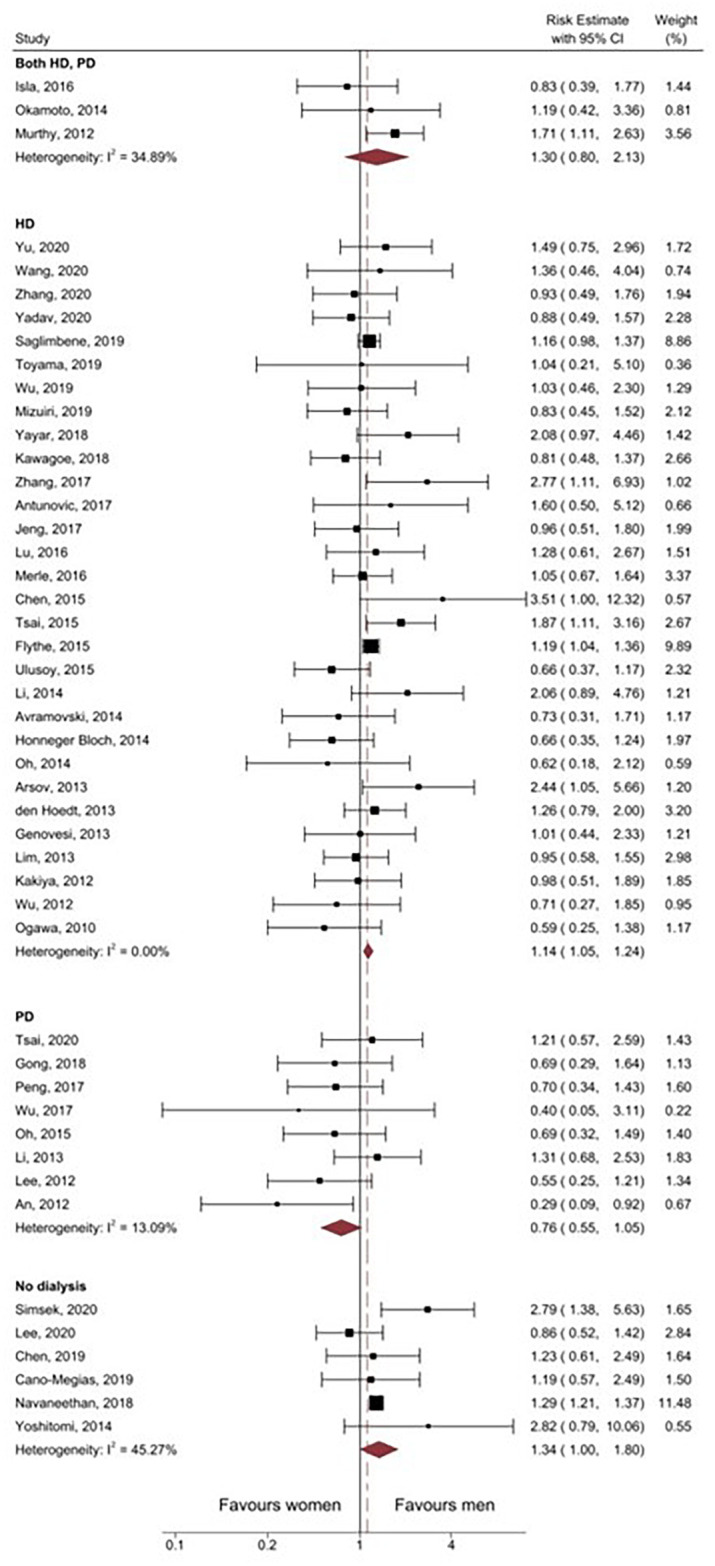
CI, confidence interval. I2, Measure of heterogeneity. HD, Haemodialysis. PD, Peritoneal dialysis.
Publication bias
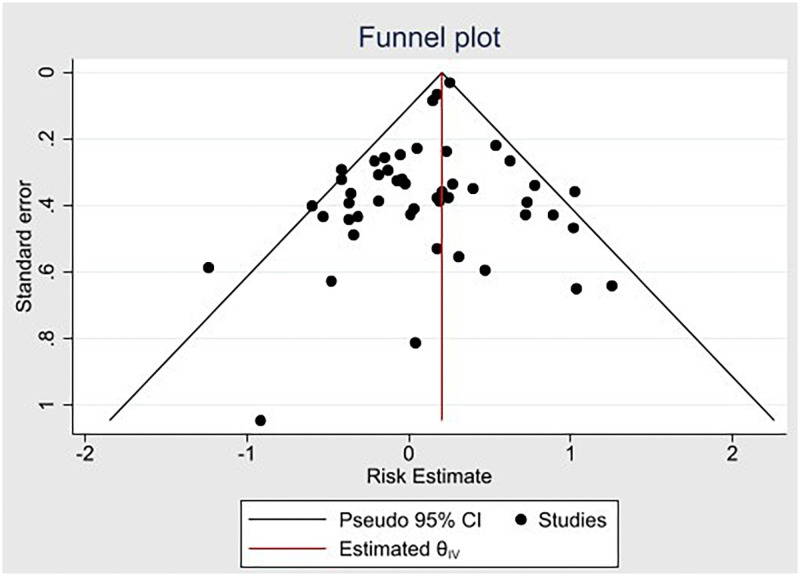
Fig 7 shows the funnel plot (with pseudo 95% CI) used to assess publication bias, with the y-axis representing standard error of effect estimate and x-axis representing individual study effect estimates. Scattering of the effect estimates demonstrates a wide range of standard errors, and the symmetrical shape shows that there was no significant publication bias.
Fig 7. Funnel plot showing publication bias across all studies.
CI, confidence interval. Estimated θIV, Estimated effect-size line.
Discussion
This systematic review and meta-analysis provide a comprehensive analysis of the association of sex and cardiovascular mortality among CKD patients with patient data collected from 2004 onwards. Data from 48 original publications encompassing a large total study population and lengthy follow-up time showed men were at marginally higher risk of cardiovascular mortality than women among the CKD population, with borderline significance. Though this significance was lost when considering only calculated risk ratios, combining the two groups (pre-calculated and calculated) showed borderline significance. Subgroup analysis of only adjusted rates (HR) also showed a marginally higher risk in men, though the association was not statistically significant.
Contrary to the combined result, subgroup analysis by male-to-female ratio showed a comparable risk of cardiovascular mortality among men and women when the distribution of men and women were primarily equal or favoured male participant, suggesting that a higher proportion of either men or women in the study population may bias the overall association. Country-based subgroup analysis indicated that men with CKD in Korea may have less risk of cardiovascular mortality compared to women with CKD; however, the association was not statistically significant. Several studies have shown that although myocardial infarction incidence is higher in Korean men in the overall Korean population, in-hospital mortality for MI is higher in women [63–65]. For our meta-analysis, there were only three studies conducted in Korea with all patient recruitment and follow-up completed by the end of 2012/early 2013. It would be important to consider if this relationship exists currently and when conducted on a large study population. Contrasting the observations from the Korean studies, our analysis clearly supports the assertion that male patients with CKD from the United States are at elevated risk of cardiovascular mortality. This subgroup analysis included two very large-scale studies (>10000 subjects each) [21, 36] and one moderately sized study (100–999) [18], which on balance had an even ratio of male to female participants and included both dialysis-dependent and non-dialysis-dependent CKD patients. The reasons underlying this clearly elevated risk are unknown; however, may relate to known co-morbidities such as hypertension, diabetes, or coronary artery disease, which were prevalent in these studies. Finally, subgroup analysis by dialysis modality revealed that men had higher cardiovascular mortality risk when considering only haemodialysis or non-dialysis-dependent patients whereas there was equivalent risk when only peritoneal dialysis patients are considered. Peritoneal dialysis, relative to haemodialysis, is associated with an increased risk of mortality [66, 67] and therefore further research is required to understand why our subgroup analysis demonstrated that there was no difference in cardiovascular mortality risk when patients received peritoneal dialysis i.e., was risk actually reduced in male patients receiving peritoneal dialysis relative to haemodialysis or increased in female patients.
Historically, reports of cardiovascular mortality risk within the general population have been much higher in men, with age-adjusted cardiovascular mortality reported to be as much as 80% higher in men [6, 68, 69]. However, this increased cardiovascular protection in women within the general population cannot be extrapolated to women with CKD. Our findings suggest that given that cardiovascular risk was only marginally higher in men, the protective effect of female sex is significantly reduced in women with CKD. In support of this, previous meta-analyses looking at the association of sex with cardiovascular mortality among both the general population and CKD cohorts showed that although men in both groups had higher cardiovascular mortality at all levels of eGFR, the slope of the risk relationship for cardiovascular mortality rose more rapidly in women than in men with decreasing eGFR [7]. Similarly, in a cohort study looking at incident adult dialysis patients, women receiving dialysis had a higher cardiovascular mortality risk than women in the general population (eGFR > 90) [8]. Future studies are required to comprehensively compare the relative risk of cardiovascular mortality for men and women with CKD relative to the general population.
There were several strengths to this systematic review: 1. the literature search included several large databases with the search criteria designed to identify as many relevant articles as possible; 2. a proportion of the study selection, data extraction and quality assessment were conducted in duplicate by separate reviewers to reduce reporting bias; 3. none of the included studies addressed sex differences specifically in their primary study objectives and, therefore, there was less potential for selection and publication bias with respect to our study question. Because the included studies’ primary study objectives were different from our research question, we adapted the Risk of Bias tool to evaluate our research question’s quality of data rather than the reported outcomes in the original study; and finally, 4. our study was widely representative of the ESRD disease population, allowing us to determine whether sex differences in cardiovascular mortality exist based on dialysis modality.
There are some limitations to our study that need to be taken into consideration. Notably, most studies did not report sex-stratified proportions of cardiovascular deaths or sex-stratified cardiovascular mortality rates. To overcome this pre-calculated HR from these studies were used in the meta-analysis. In the ten studies where source data was available, RR was calculated, and subgroup analysis based on the measure of association (HR versus RR) conducted to assess whether the method of measure of association contributed to differences in study outcome. This analysis showed similar results regardless of the method of assessment. Cause-specific cardiovascular mortality was only reported in three studies [21, 27, 43], and no subgroup analysis on the cause of cardiovascular death could be performed. In addition, there was some heterogeneity among risk/rate estimates from the included studies, possibly due to some bias at the study level. Possible sources of bias included unequal distribution of men and women and variation in follow-up time. However, subgroup analysis by variables was done to assess these sources of heterogeneity.
In conclusion, the findings from this systematic review and meta-analysis, covering study populations with substantial follow-up, show that male sex is marginally associated with higher cardiovascular mortality among CKD patients. Although men with CKD showed overall higher cardiovascular mortality risk than women with CKD, the increased risk was minimal, and appropriate risk awareness is necessary for both sexes to reduce exposure to cardiovascular risk factors, facilitate early diagnosis of complications, and ensure better cardiovascular health outcomes for both sexes.
Supporting information
(PDF)
(DOCX)
(PDF)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its S1 Checklist, S1 and S2 Tables and S1–S3 Data.
Funding Statement
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript”. The source of funding for this project was “The Hillcrest Foundation”.
References
- 1.Schoolwerth AC, Engelgau MM, Hostetter TH, Rufo KH, Chianchiano D, McClellan WM, et al. Chronic kidney disease: a public health problem that needs a public health action plan. Prev Chronic Dis. 2006;3(2):A57. Epub 2006/03/17. . [PMC free article] [PubMed] [Google Scholar]
- 2.Fraser SDS, Roderick PJ. Kidney disease in the Global Burden of Disease Study 2017. Nature Reviews Nephrology. 2019;15(4):193–4. doi: 10.1038/s41581-019-0120-0 [DOI] [PubMed] [Google Scholar]
- 3.Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet. 2018;392(10159):2052–90. Epub 2018/10/21. doi: 10.1016/S0140-6736(18)31694-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Lullo L, House A, Gorini A, Santoboni A, Russo D, Ronco C. Chronic kidney disease and cardiovascular complications. Heart Fail Rev. 2015;20(3):259–72. Epub 2014/10/26. doi: 10.1007/s10741-014-9460-9 . [DOI] [PubMed] [Google Scholar]
- 5.Liabeuf S, Desjardins L, Diouf M, Temmar M, Renard C, Choukroun G, et al. The Addition of Vascular Calcification Scores to Traditional Risk Factors Improves Cardiovascular Risk Assessment in Patients with Chronic Kidney Disease. PLoS One. 2015;10(7):e0131707. Epub 2015/07/17. doi: 10.1371/journal.pone.0131707 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mikkola TS, Gissler M, Merikukka M, Tuomikoski P, Ylikorkala O. Sex Differences in Age-Related Cardiovascular Mortality. PLOS ONE. 2013;8(5):e63347. doi: 10.1371/journal.pone.0063347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nitsch D, Grams M, Sang Y, Black C, Cirillo M, Djurdjev O, et al. Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis. Bmj. 2013;346:f324. Epub 2013/01/31. doi: 10.1136/bmj.f324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrero JJ, de Jager DJ, Verduijn M, Ravani P, De Meester J, Heaf JG, et al. Cardiovascular and noncardiovascular mortality among men and women starting dialysis. Clin J Am Soc Nephrol. 2011;6(7):1722–30. Epub 2011/07/08. doi: 10.2215/CJN.11331210 . [DOI] [PubMed] [Google Scholar]
- 9.Meisinger C, Doring A, Lowel H. Chronic kidney disease and risk of incident myocardial infarction and all-cause and cardiovascular disease mortality in middle-aged men and women from the general population. Eur Heart J. 2006;27(10):1245–50. Epub 2006/04/14. doi: 10.1093/eurheartj/ehi880 . [DOI] [PubMed] [Google Scholar]
- 10.Guajardo I, Ayer A, Johnson AD, Ganz P, Mills C, Donovan C, et al. Sex differences in vascular dysfunction and cardiovascular outcomes: The cardiac, endothelial function, and arterial stiffness in ESRD (CERES) study. Hemodial Int. 2018;22(1):93–102. Epub 2017/03/09. doi: 10.1111/hdi.12544 . [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Medicine. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Kidney Foundation. KDOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification and Stratification. Am J Kidney Dis. 2002;39:S1–S000. [PubMed] [Google Scholar]
- 13.GA Wells, B Shea, D O’Connell, J Peterson, V Welch, M Losos, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [1/5/2019]. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 14.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). 2019. [DOI] [PMC free article] [PubMed]
- 15.Wu IW, Hsu KH, Hsu HJ, Lee CC, Sun CY, Tsai CJ, et al. Serum free p-cresyl sulfate levels predict cardiovascular and all-cause mortality in elderly hemodialysis patients—a prospective cohort study. Nephrology Dialysis Transplantation. 2012;27(3):1169–75. 10.1093/ndt/gfr453. . [DOI] [PubMed] [Google Scholar]
- 16.Toyama T, Kasama S, Sato M, Sano H, Ueda T, Sasaki T, et al. Most Important Prognostic Values to Predict Major Adverse Cardiovascular, Cerebrovascular, and Renal Events in Patients with Chronic Kidney Disease Including Hemodialysis for 2 Years. Cardiology. 2019;142(1):14–23. Epub 2019/03/14. doi: 10.1159/000496330 . [DOI] [PubMed] [Google Scholar]
- 17.Isla RAT, Ameh OI, Mapiye D, Swanepoel CR, Bello AK, Ratsela AR, et al. Baseline predictors of mortality among predominantly rural-dwelling end-stage renal disease patients on chronic dialysis therapies in limpopo, South Africa. PLoS One. 2016;11(6). doi: 10.1371/journal.pone.0156642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Dorbala S, et al. Coronary vascular dysfunction and prognosis in patients with chronic kidney disease. JACC Cardiovasc Imaging. 2012;5(10):1025–34. doi: 10.1016/j.jcmg.2012.06.007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu Y, Xiong Y, Zhang C, Fu M, Li Y, Fu P. Vascular Access Type Was Not Associated with Mortality and the Predictors for Cardiovascular Death in Elderly Chinese Patients on Hemodialysis. Blood Purification. 2020;49(1–2):63–70. doi: 10.1159/000502941 [DOI] [PubMed] [Google Scholar]
- 20.Antunovic T, Stefanovic A, Gligorovic Barhanovic N, Miljkovic M, Radunovic D, Ivanisevic J, et al. Prooxidant-antioxidant balance, hsTnI and hsCRP: mortality prediction in haemodialysis patients, two-year follow-up. Renal Failure. 2017;39(1):491–9. 10.1080/0886022X.2017.1323645. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navaneethan SD, Schold JD, Walther CP, Arrigain S, Jolly SE, Virani SS, et al. High-density lipoprotein cholesterol and causes of death in chronic kidney disease. Journal of Clinical Lipidology. 2018;12(4):1061–71.e7. 10.1016/j.jacl.2018.03.085. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merle E, Roth H, London GM, Jean G, Hannedouche T, Bouchet JL, et al. Low parathyroid hormone status induced by high dialysate calcium is an independent risk factor for cardiovascular death in hemodialysis patients. Kidney Int. 2016;89(3):666–74. doi: 10.1016/j.kint.2015.12.001 . [DOI] [PubMed] [Google Scholar]
- 23.Ogawa T, Ishida H, Akamatsu M, Matsuda N, Fujiu A, Ito K, et al. Progression of aortic arch calcification and all-cause and cardiovascular mortality in chronic hemodialysis patients. International urology and nephrology. 2010;42(1):187–94. doi: 10.1007/s11255-009-9574-5 [DOI] [PubMed] [Google Scholar]
- 24.Oh HJ, Lee MJ, Lee HS, Park JT, Han SH, Yoo TH, et al. NT-proBNP: is it a more significant risk factor for mortality than troponin T in incident hemodialysis patients? Medicine. 2014;93(27):e241. 10.1097/MD.0000000000000241. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Z, Shen B, Cao X, Liu Z, Chen X, Nie Y, et al. Increased Soluble Suppression of Tumorigenicity 2 Level Predicts All-Cause and Cardiovascular Mortality in Maintenance Hemodialysis Patients: A Prospective Cohort Study. Blood Purification. 2017;43(1–3):37–45. doi: 10.1159/000452924 [DOI] [PubMed] [Google Scholar]
- 26.Avramovski P, Janakievska P, Sotiroski K, Zafirova-Ivanovska B, Sikole A. Aortic pulse wave velocity is a strong predictor of all—cause and cardiovascular mortality in chronic dialysis patients. Renal Failure. 2014;36(2):176–86. 10.3109/0886022X.2013.843359. . [DOI] [PubMed] [Google Scholar]
- 27.Genovesi S, Rossi E, Nava M, Riva H, De Franceschi S, Fabbrini P, et al. A case series of chronic haemodialysis patients: mortality, sudden death, and QT interval. Europace. 2013;15(7):1025–33. doi: 10.1093/europace/eus412 . [DOI] [PubMed] [Google Scholar]
- 28.Wu L, Cai K, Luo Q, Wang L, Hong Y. Baseline Serum Magnesium Level and Its Variability in Maintenance Hemodialysis Patients: Associations with Mortality. Kidney and Blood Pressure Research. 2019;44(2):222–32. doi: 10.1159/000498957 [DOI] [PubMed] [Google Scholar]
- 29.Saglimbene VM, Wong G, Ruospo M, Palmer SC, Campbell K, Larsen VG, et al. Dietary n-3 polyunsaturated fatty acid intake and all-cause and cardiovascular mortality in adults on hemodialysis: The DIET-HD multinational cohort study. Clinical Nutrition. 2019;38(1):429–37. doi: 10.1016/j.clnu.2017.11.020 [DOI] [PubMed] [Google Scholar]
- 30.Yayar O, Eser B, Kilic H. Relation between high serum hepcidin-25 level and subclinical atherosclerosis and cardiovascular mortality in hemodialysis patients. Anatolian Journal of Cardiology. 2018;19(2):117–22. 10.14744/AnatolJCardiol.2017.8019. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawagoe C, Sato Y, Toida T, Nakagawa H, Yamashita Y, Fukuda A, et al. N-terminal-pro-B-type-natriuretic peptide associated with 2-year mortality from both cardiovascular and non-cardiovascular origins in prevalent chronic hemodialysis patients. Renal Failure. 2018;40(1):127–34. 10.1080/0886022X.2018.1437047. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeng Y, Lim PS, Wu MY, Tseng TY, Chen CH, Chen HP, et al. Proportions of Proinflammatory Monocytes Are Important Predictors of Mortality Risk in Hemodialysis Patients. Mediators of Inflammation. 2017;2017. doi: 10.1155/2017/1070959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu CL, Leu JG, Liu WC, Zheng CM, Lin YF, Shyu JF, et al. Endothelial progenitor cells predict long-term mortality in hemodialysis patients. International Journal of Medical Sciences. 2016;13(3):240–7. doi: 10.7150/ijms.14209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ulusoy S, Ozkan G, Mentese A, Guvercin B, Caner Karahan S, Yavuz A, et al. A new predictor of mortality in hemodialysis patients; Tenascin-C. 2015;1:54–60. [DOI] [PubMed] [Google Scholar]
- 35.Tsai MH, Liou HH, Leu JG, Yen MF, Chen HH. Sites of peripheral artery occlusive disease as a predictor for all-cause and cardiovascular mortality in chronic hemodialysis. PLoS One. 2015;10(6). doi: 10.1371/journal.pone.0128968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flythe JE, Kshirsagar AV, Falk RJ, Brunelli SM. Associations of Posthemodialysis Weights above and below Target Weight with All-Cause and Cardiovascular Mortality. Clinical Journal of The American Society of Nephrology: CJASN. 2015;10(5):808–16. 10.2215/CJN.10201014. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen XN, Chen ZJ, Ma XB, Ding B, Ling HW, Shi ZW, et al. Aortic artery and cardiac valve calcification are associated with mortality in Chinese hemodialysis patients: A 3.5 years follow-up. Chinese Medical Journal. 2015;128(20):2764–71. doi: 10.4103/0366-6999.167315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Z, Liu S, Liang X, Wang W, Fei H, Hu P, et al. Pulmonary hypertension as an independent predictor of cardiovascular mortality and events in hemodialysis patients. International Urology and Nephrology. 2014;46:141–9. doi: 10.1007/s11255-013-0486-z . [DOI] [PubMed] [Google Scholar]
- 39.Honneger Bloch S, Semple D, Sidhu K, Stewart R, Pilmore H. Prognostic value and long-term variation of high sensitivity troponin T in clinically stable haemodialysis patients. New Zealand Medical Journal. 2014;127(1402):97–109. . [PubMed] [Google Scholar]
- 40.Lim PS, Jeng Y, Wu MY, Pai MA, Wu TK, Liu CS, et al. Serum Oxidized Albumin and Cardiovascular Mortality in Normoalbuminemic Hemodialysis Patients: A Cohort Study. PLoS One. 2013;8(7). doi: 10.1371/journal.pone.0070822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.den Hoedt CH, Bots ML, Grooteman MP, Mazairac AH, Penne EL, van der Weerd NC, et al. Should we still focus that much on cardiovascular mortality in end stage renal disease patients? The CONvective TRAnsport STudy. PLoS One. 2013;8(4):e61155. doi: 10.1371/journal.pone.0061155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arsov S, Trajceska L, van Oeveren W, Smit AJ, Dzekova P, Stegmayr B, et al. Increase in skin autofluorescence and release of heart-type fatty acid binding protein in plasma predicts mortality of hemodialysis patients. Artificial Organs. 2013;37(7):E114–E22. doi: 10.1111/aor.12078 [DOI] [PubMed] [Google Scholar]
- 43.Kakiya R, Shoji T, Hayashi T, Tatsumi-Shimomura N, Tsujimoto Y, Tabata T, et al. Decreased serum adrenal androgen dehydroepiandrosterone sulfate and mortality in hemodialysis patients. Nephrology Dialysis Transplantation. 2012;27(10):3915–22. 10.1093/ndt/gfs162. . [DOI] [PubMed] [Google Scholar]
- 44.Zhang P, Zou JZ, Chen J, Tan X, Xiang FF, Shen B, et al. Association of trimethylamine N-Oxide with cardiovascular and all-cause mortality in hemodialysis patients. Renal Failure. 2020;42(1):1004–14. doi: 10.1080/0886022X.2020.1822868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Z, Chen Z, Yu H, Ma X, Zhang C, Qu B, et al. Superior prognostic value of soluble suppression of tumorigenicity 2 for the short-term mortality of maintenance hemodialysis patients compared with NT-proBNP: a prospective cohort study. Renal Failure. 2020;42(1):523–30. doi: 10.1080/0886022X.2020.1767648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yadav R, Gerrickens MWM, Scheltinga MR. Abnormal Finger Pressures Prior to Primary Hemodialysis Access Construction Predict Overall Mortality and Cardiovascular Mortality in End-Stage Renal Disease Patients. European Journal of Vascular and Endovascular Surgery. 2019;58 (6 Supplement 3):e691. 10.1016/j.ejvs.2019.09.210. [DOI] [Google Scholar]
- 47.Mizuiri S, Nishizawa Y, Yamashita K, Naito T, Ono K, Tanji C, et al. Hypomagnesemia is not an independent risk factor for mortality in Japanese maintenance hemodialysis patients. Int Urol Nephrol. 2019;51(6):1043–52. Epub 2019/04/13. doi: 10.1007/s11255-019-02073-w . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gong L, Zheng D, Yuan J, Cao L, Ni Z, Fang W. Elevated levels of serum sclerostin are linked to adverse cardiovascular outcomes in peritoneal dialysis patients. International Urology & Nephrology. 2018;50(5):955–61. 10.1007/s11255-018-1795-z [DOI] [PubMed] [Google Scholar]
- 49.Wu CF, Lee YF, Lee WJ, Su CT, Lee LJ, Wu KD, et al. Severe aortic arch calcification predicts mortality in patients undergoing peritoneal dialysis. Journal of the Formosan Medical Association. 2017;116(5):366–72. 10.1016/j.jfma.2016.06.006. . [DOI] [PubMed] [Google Scholar]
- 50.Peng F, Chen W, Zhou W, Li P, Niu H, Chen Y, et al. Low prognostic nutritional index associated with cardiovascular disease mortality in incident peritoneal dialysis patients. International Urology & Nephrology. 2017;49(6):1095–101. 10.1007/s11255-017-1531-0. . [DOI] [PubMed] [Google Scholar]
- 51.Oh HJ, Lee MJ, Kwon YE, Park KS, Park JT, Han SH, et al. Which Biomarker is the Best for Predicting Mortality in Incident Peritoneal Dialysis Patients: NT-ProBNP, Cardiac TnT, or hsCRP? Medicine (united states). 2015;94(44):e1636. doi: 10.1097/MD.0000000000001636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y, Zhang L, Gu Y, Hao C, Zhu T. Insulin resistance as a predictor of cardiovascular disease in patients on peritoneal dialysis. Peritoneal Dialysis International. 2013;33(4):411–8. 10.3747/pdi.2012.00037. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee MJ, Shin DH, Kim SJ, Oh HJ, Yoo DE, Ko KI, et al. Progression of Aortic Arch Calcification Over 1 Year Is an Independent Predictor of Mortality in Incident Peritoneal Dialysis Patients. PLoS One. 2012;7(11). doi: 10.1371/journal.pone.0048793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.An X, Mao HP, Wei X, Chen JH, Yang X, Li ZB, et al. Elevated neutrophil to lymphocyte ratio predicts overall and cardiovascular mortality in maintenance peritoneal dialysis patients. International urology and nephrology. 2012;44(5):1521–8. doi: 10.1007/s11255-012-0130-3 [DOI] [PubMed] [Google Scholar]
- 55.Tsai CH, Huang JW, Lin C, Ma HP, Lo MT, Liu LYD, et al. Heart Rhythm Complexity Predicts Long-Term Cardiovascular Outcomes in Peritoneal Dialysis Patients: A Prospective Cohort Study. Journal of the American Heart Association. 2020;9(2). 10.1161/JAHA.119.013036. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okamoto T, Morimoto S, Ikenoue T, Furumatsu Y, Ichihara A. Visceral fat level is an independent risk factor for cardiovascular mortality in hemodialysis patients. American Journal of Nephrology. 2014;39(2):122–9. 10.1159/000358335. . [DOI] [PubMed] [Google Scholar]
- 57.Cano-Megías M, Guisado-Vasco P, Bouarich H, de Arriba-de la Fuente G, de Sequera-Ortiz P, Álvarez-Sanz C, et al. Coronary calcification as a predictor of cardiovascular mortality in advanced chronic kidney disease: a prospective long-term follow-up study. BMC Nephrol. 2019;20(1):188. Epub 2019/05/30. doi: 10.1186/s12882-019-1367-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen SC, Teh M, Huang JC, Wu PY, Chen CY, Tsai YC, et al. Increased Aortic Arch Calcification and Cardiomegaly is Associated with Rapid Renal Progression and Increased Cardiovascular Mortality in Chronic Kidney Disease. Sci Rep. 2019;9(1):5354. Epub 2019/03/31. doi: 10.1038/s41598-019-41841-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee WH, Hsu PC, Chu CY, Chen SC, Chen YC, Lee MK, et al. Upstroke time as a novel predictor of mortality in patients with chronic kidney disease. Diagnostics. 2020;10(6). 10.3390/diagnostics10060422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simsek MA, Degertekin M, Cabbar AT, Hunuk B, Akturk S, Erdogmus S, et al. NT-proBNP level in stage 3–4 chronic kidney disease and mortality in long-term follow-up: HAPPY study subgroup analysis. Turk Kardiyoloji Dernegi Arsivi. 2020;48(5):454–60. 10.5543/tkda.2020.57746 [DOI] [PubMed] [Google Scholar]
- 61.Yoshitomi R, Nakayama M, Ura Y, Kuma K, Nishimoto H, Fukui A, et al. Ankle-brachial blood pressure index predicts cardiovascular events and mortality in Japanese patients with chronic kidney disease not on dialysis. Hypertension Research—Clinical & Experimental. 2014;37(12):1050–5. 10.1038/hr.2014.120. . [DOI] [PubMed] [Google Scholar]
- 62.Kon S, Konta T, Ichikawa K, Asahi K, Yamagata K, Fujimoto S, et al. Association between renal function and cardiovascular and all-cause mortality in the community-based elderly population: results from the Specific Health Check and Guidance Program in Japan. Clinical & Experimental Nephrology. 2018;22(2):346–52. 10.1007/s10157-017-1455-0. . [DOI] [PubMed] [Google Scholar]
- 63.Hong JS, Kang HC, Lee SH, Kim J. Long-term trend in the incidence of acute myocardial infarction in Korea: 1997–2007. Korean Circ J. 2009;39(11):467–76. Epub 2009/12/10. doi: 10.4070/kcj.2009.39.11.467 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hong J-S, Kang H-C. Sex Differences in the Treatment and Outcome of Korean Patients With Acute Myocardial Infarction Using the Korean National Health Insurance Claims Database. Medicine. 2015;94(35):e1401. doi: 10.1097/MD.0000000000001401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park JS, Kim YJ, Shin DG, Jeong MH, Ahn YK, Chung WS, et al. Gender differences in clinical features and in-hospital outcomes in ST-segment elevation acute myocardial infarction: from the Korean Acute Myocardial Infarction Registry (KAMIR) study. Clin Cardiol. 2010;33(8):E1–6. Epub 2010/07/01. doi: 10.1002/clc.20557 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Banshodani M, Kawanishi H, Moriishi M, Shintaku S, Tsuchiya S. Association between Dialysis Modality and Cardiovascular Diseases: A Comparison between Peritoneal Dialysis and Hemodialysis. Blood Purif. 2020;49(3):302–9. Epub 2019/12/19. doi: 10.1159/000504040 . [DOI] [PubMed] [Google Scholar]
- 67.Han SS, Park JY, Kang S, Kim KH, Ryu DR, Kim H, et al. Dialysis Modality and Mortality in the Elderly: A Meta-Analysis. Clin J Am Soc Nephrol. 2015;10(6):983–93. Epub 2015/05/06. doi: 10.2215/CJN.05160514 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mosca L, Barrett-Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124(19):2145–54. Epub 2011/11/09. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mosca L, Hammond G, Mochari-Greenberger H, Towfighi A, Albert MA. Fifteen-year trends in awareness of heart disease in women: results of a 2012 American Heart Association national survey. Circulation. 2013;127(11):1254–63, e1–29. Epub 2013/02/23. doi: 10.1161/CIR.0b013e318287cf2f . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(DOCX)
(PDF)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its S1 Checklist, S1 and S2 Tables and S1–S3 Data.