Abstract
Background and Objectives:
Ureters are at risk of injury in settings of inflammation and distorted anatomy. The use of a fluorescent dye can improve intraoperative ureteral identification without the need for any additional invasive procedures. Our team has previously described the development of a preclinical ureter-specific dye, UL-766, tested in a rat model. Here, we present the use of the fluorescent dye during laparoscopy to assist in ureteral identification in a swine model with an inflamed abdomen; the results of this study serve as proof of feasibility for use in the setting of tissue edema and erythema.
Study Design/Materials and Methods:
With institutional approval, two 20–25 kg pigs underwent abdominal surgery with the use of a Food and Drug Administration-approved fluorescence laparoscopic system. Using standard laparoscopy, inflammation was induced with sharp and blunt dissection and irritation was induced with gauze. The animals were allowed to recover and returned to the operating room after 7 days. Images of the inflamed right retroperitoneum, with fluorescence imaging, turned on, were taken before and after intravenous injection of the novel fluorescent dye at 120 μg/kg. The time until fluorescence visualization of the ureters was measured, and the fluorescent signal was measured for up to 4 hours from the time of the initial dye injection. Partial and complete transection of ureteral injuries was made by scissors and monitored under both standard video and fluorescence laparoscopy.
Results:
Inflammation reduced the certainty of ureter identification by white light alone. Despite surrounding tissue erythema and edema, ureteral visualization under fluorescence laparoscopy was achieved within 5–10 minutes after dye injection. The fluorescent signal remained visible for at least 4 hours after injection, and the fluorescent dye showed a partial ureteral injury that would not have been observed under standard laparoscopy.
Conclusions:
UL-766 is a preclinical fluorescent dye useful for the intraoperative identification of the ureters and ureteral injuries in an inflamed abdomen. With the acquisition of additional preclinical data, this novel dye can be a valuable tool during laparoscopic abdominal and pelvic surgeries. Lasers Surg. Med. © 2019 Wiley Periodicals, Inc.
Keywords: ureter identification, near-infrared fluorescent dye, inflammatory pig model, fluorescence laparoscopy
INTRODUCTION
Iatrogenic ureteral injuries occur during abdominal and pelvic surgeries; however, visualization of the ureters is often obscured by retroperitoneal fat, making identification more difficult. Furthermore, it becomes more difficult in patients with active abdominal inflammation, a history of radiation, or anatomic distortion due to surgical history [1-3]. Although it is considered a relatively rare complication, the risk of iatrogenic ureteral injury has been reported as being as high as 7.6% [3-6]. Ureteral injuries are associated with significant postoperative morbidities including acute renal insufficiency, urinary fistula, sepsis, and mortality [2,3,7,8]. They are also associated with prolonged hospital stays, increased healthcare costs as well as the requirement for more procedures and operative interventions.
Given the significant complications associated with iatrogenic ureteral injuries, particularly those diagnosed postoperatively, it is of utmost importance to identify the ureters in order to avoid unintended injury. With the diminished tactile sensation in laparoscopic surgery, lighted ureteral stents have been used by some surgeons to aid in ureteral identification. Overall, ureteral stents are invasive and costly, and their use is not without complications, including hematuria, urinary tract infection, and ureteral perforation [2,3,6,8]. Ureteral stent placement also requires foresight, as it is an additional procedure that must be performed before the primary abdominal surgery, and sometimes, the need for ureteral stenting may not be appreciated until after the primary surgery has begun.
There is a clear need for a fast and noninvasive method to aid in ureteral identification, particularly one that can be used at any point during the operation. With the recent market availability of fluorescence laparoscopy, fluorescent dyes are becoming a valuable tool for anatomic identification during minimally invasive surgery. There has been increasing interest in the discovery of an ideal near-infrared (NIR) fluorescent dye to aid in intraoperative ureteral visualization, and a recent review by Slooter et al showed many novel NIR intravenous dyes in various stages of development [9]. Furthermore, preclinical experiences have been described using simple rat or swine models with IRdye800CW by Korb et al. [5], CW800-CA by Schols et al. [10], and UreterGlow by Mahalingam et al. [11] For clinical use, only two experimental dyes have been listed in early clinical trials for feasibility and dosing: ZW800-1 and IRDye CW800 [9]. Otherwise, current clinical experiences using intravenous dyes for ureteral identification have been limited to low-dose methylene blue [9,12], although its low brightness and NIR emission properties make it less optimal [9].
In their early application, these novel dyes have mostly been tested in the context of normal porcine ureters [4,5,10,11]. However, with little obscuring periureteral fat and a thin retroperitoneum, ureteral identification is relatively easy in swines. It remains unclear how much benefit a fluorescent ureter dye can provide in the setting of abdominal and pelvic inflammation, where tissue erythema and edema can significantly interfere with identification.
Our team has previously described the development of a renally-cleared, fluorescent dye, ureter-label (UL)-766 [13]. Previously tested in a rat model, this dye was shown to have a good contrast/background ratio (CBR), given its high specificity for the urinary tract [13]. The dye was previously tested with a custom-built fluorescent imaging system. In this study, we aim to test the application of the UL-766 dye in a laparoscopic setting utilizing a Food and Drug Administration (FDA) approved fluorescent laparoscopy system. Furthermore, we aim to assess the feasibility of this dye in visualizing ureters in the setting of inflammatory tissue.
MATERIALS AND METHODS
UL-766 Dye
The synthesis of the UL-766 dye was previously described by Cha et al. [13]. For this study, the dye was synthesized at the National Cancer Institute (Frederick, Maryland) and stored at −20°C until required for the animal experiments. The dye was then brought to room temperature and diluted in sterile water until ready for use at our research animal facility at the Children’s National Medical Center (Washington, DC).
Laparoscopic System
A recent, FDA-cleared laparoscopic fluorescence imaging system (Model-L; InTheSmart Incorporated, Palo Alto, CA) was used in the animal studies. This system is capable of concurrent white light and NIR imaging with a dual light source (ITSEL1711; InTheSmart Incorporated, Palo Alto, CA). This laparoscopic fluorescence imaging system can present a standard laparoscopy video as well as a fusion video, with the NIR signal overlaid with a blue-violet color if the infrared light source is turned on. A NIR-only image is presented in a box in the lower-left corner of the laparoscopy video. This laparoscopy system is compatible with standard laparoscopes commonly used in the United States; we used a 10-mm 30° laparoscope from Karl Storz (Storz 26003BA; Karl Storz Endoscopy-America Inc., El Segundo, CA).
Animal Study
This feasibility animal study was conducted, with approval from the Children’s National Health System IACUC (protocol #30591). Two female Yorkshire pigs (weight 20–30 kg) were used for the study. The animals were acclimated in our facility for at least 24 hours before surgery and were fasted for 12 hours before any surgery. In the initial procedure, inflammation was induced in the right retroperitoneum. Each pig was intubated and placed under isoflurane anesthesia before being positioned in the left lateral decubitus position. The abdomen was prepped with betadine and then draped in a standard sterile fashion. The heart rate, cardiac rhythm, blood oxygen saturation, and rectal temperature were continuously monitored. One 12-mm trocar was placed superior to the umbilicus as the camera port, with three 5-mm trocars placed in the right abdomen under laparoscopic visualization. In the initial surgery, only standard laparoscopy (RGB video) was used. At the right retroperitoneum, using blunt dissection and electrocautery, the right ureter was carefully dissected from its surrounding tissue. This started at the level of the right inferior renal margin down to the ureteral insertion into the bladder. Care was taken to not devitalize the ureter from perfusing vessels. Sterile surgical gauze was then rolled and brought into the abdomen through the 12 mm port site. The gauze roll was then used to further abrade the ureter and surrounding retroperitoneal tissue with strokes over the tissue until petechial hemorrhages were seen. After sufficient abrasion, the gauze and trocars were removed. The abdominal fascia and skin were then closed with 2-0 and 4-0 vicryl sutures, respectively. The pigs were allowed to recover from anesthesia and return to their cages. Diet was resumed ad lib 12 hours after surgery. Intramuscular buprenorphine was given at 0.1mg/kg for 72 hours and as needed for postoperative analgesia.
Inflammation was allowed to develop, and the pigs were returned to the operating room 7 days after the initial surgery. The pigs were intubated, anesthetized, prepped, and draped in the same fashion as before. The right retroperitoneum was illuminated with both NIR and white light during laparoscopy. Standard RGB and fluorescent fusion images were obtained for comparison. The UL-766 dye was diluted in 10ml of sterile water and given at 120 μg/kg by injection into an ear vein catheter with running normal saline infusion at a rate of 20ml/kg/h. Once visualization of the ureters was achieved by the UL-766 dye, the ureter was dissected from its surrounding tissue, starting from the level of the inferior renal pole to the insertion into the bladder. The fluorescent signal was monitored for up to 4 hours after the initial injection. At the end of the experiment, ureteral injuries, by partial and complete transection, were made by scissors with confirmation as an effluence of urine into the peritoneum. The animals were then euthanized as per the IACUC approved protocol.
The CBR is defined as the ratio of fluorescence intensity at the structure of interest to that of nearby tissue. The CBR of the UL-766 dye was calculated as
where the background intensity is the fluorescent signal measured at the site of the retroperitoneal tissue. Essentially, the CBR helps describe how well the signal is localized to a specific tissue. A dye with a high CBR creates a strong contrast of minimal to no fluorescent signal at the surrounding tissues against the highly fluorescent target.
RESULTS
No complications were seen, and both animals survived until euthanasia at the end of the second surgery. Both animals tolerated the intravenous injection of UL-766 dye without acute changes in heart rate, oxygenation, or temperature. In the first pig, visualization of the ureter was achieved 7 minutes after dye injection. Visualization of the ureter was fully achieved 5 minutes after dye injection in the second pig. As expected, the fluorescent signal from the ureters was only visible when urine was present within the ureteral lumen. The fluorescent bolus of urine was seen to travel from the renal pelvis along the length of the ureter and into the bladder. Similar to our previous animal studies, the fluorescent signal was seen in the kidneys before the presence of ureteral signals. As urine collects in the bladder, this signal increase was expected. The peak CBRs at these organs are listed in Table 1. Of note, are the high average CBR values at the ureter, 38.56 and 14.5, at 10 minutes and 4 hours, respectively, after dye injection.
TABLE 1.
Contrast-Background-Ratios of the Right Kidney, Ureter, and Bladder Measured 10 minutes and 4 hours after Initial UL-766 Dye Injection During Laparoscopic Right Ureteral Dissection With Inflamed Retroperitoneal Tissue
| Time after injection | ||
|---|---|---|
| 10 minutes | 4 hours | |
| Kidney | 17.96 | 11.20 |
| Ureter | 38.56 | 14.50 |
| Bladder | 1.49 | 2.98 |
The creation of retroperitoneal inflammation was successful, and significant erythema and edema were observed in the local tissue surrounding the ureters in both animals (Fig. 1). The abrasion was sufficient to create inflammatory tissue adhesion between critical structures, including the ureter, bowel, and retroperitoneal tissue. In pig 2, we discovered that the inflammation was enough to have caused an adhesive band development that led to a mild degree of obstruction at the ureter, just distal to the renal pelvis. The fluorescent signal was used to help identify the ureter location during dissection (Fig. 2). In both pigs, the inflamed ureters were successfully isolated from the surrounding tissue. Complete ureteral transection was performed in the first pig, and partial ureteral transection was performed in the second pig. Visualization of ureteral injury is shown by a fluorescent signal in the peritoneal space (Figs. 3 and 4).
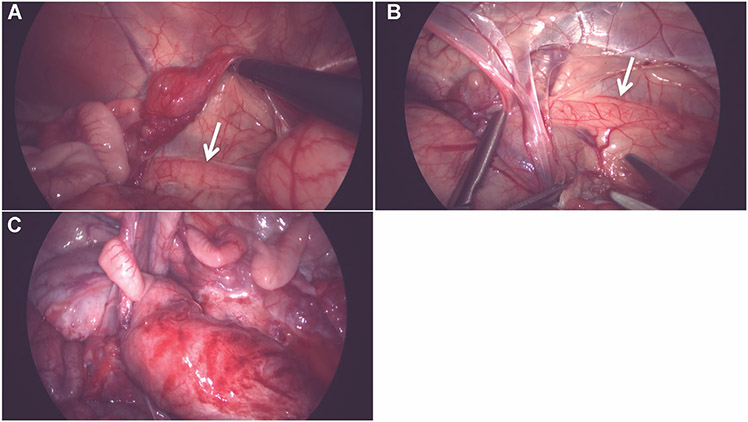
Fig. 1.
Standard laparoscopy images of the right retroperitoneum, showing little periureteral fat and overlying tissue, making ureteral identification relatively easy (A and B). However, after inflammation (C), erythema and tissue edema make ureter location more difficult to ascertain.
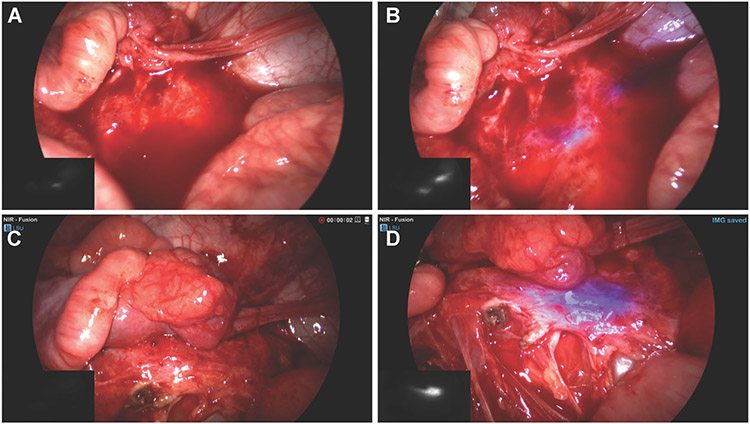
Fig. 2.
(A) Standard RGB image of right retroperitoneal tissue with obscuring bloody peritoneal fluid. (B) Fusion near-infrared (NIR) image showing a fluorescent signal identifying the ureter despite bloody peritoneal fluid. (C) Erythematous and edematous right retroperitoneal tissue without an identifiable ureteral structure. (D) Fusion NIR image identifying the location of the ureter within the erythematous tissue as urine peristalses through.
Fig. 3.
Creation of complete ureteral transection. The proximal ureter stump is shown with fluorescence overlay in violet color. The signal derives from fluorescent urine flowing into the peritoneum.
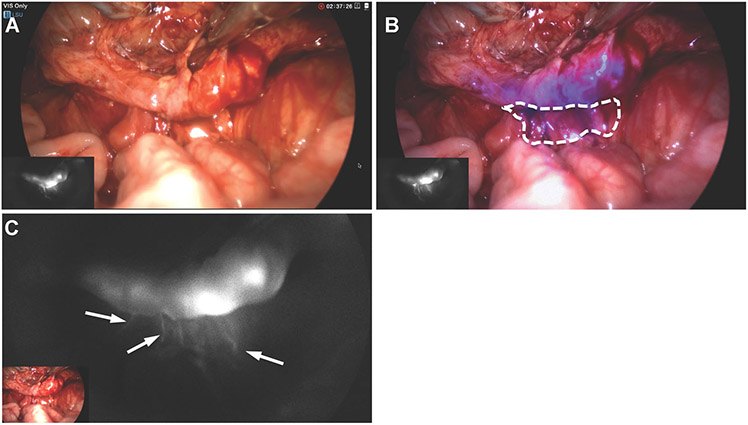
Fig. 4.
Partial transection of the ureter was created after dissecting free from surrounding tissue. Plain white light imaging does not clearly show the presence of transection (A). But the presence of extraureteral fluorescent signals (B and C) indicate that the presence of urine leakage.
DISCUSSION
Fluorescent dyes have a NIR emission spectrum, usually between 650 and 900 nm [14,15]. At this range, there is little tissue autofluorescence, and clinically available fluorescence imaging systems take advantage of these qualities for real-time intra-operative imaging. Despite the availability of these systems, only two NIR ureter-specific dyes are in early clinical trials, while the rest remain in preclinical testing. Whereas early testing has shown the efficacies of these novel dyes, with some groups testing various doses or imaging distances with the laparoscope [4,16], few groups have shown their applicability in clinically relevant situations, such as abdominal inflammation and distorted anatomy.
As described in our previous work, the chemical design of the UL-766 dye provides its water-solubility, allowing for predominant renal clearance with high selectivity and CBRs at the urinary tract [13]. This confers the strong fluorescent signal seen in the urine. Our past experience did not elucidate the dye’s applicability in the setting of inflammation and distorted anatomy as shown in this study. This is also our first experience using the dye with a clinically available fluorescent laparoscopy system.
In our experience, there is little retroperitoneal fat in the pig, making ureter identification relatively easy (Fig. 1A and B). Expectedly, all preclinical tests of previously published novel fluorescent dyes were presented in animals with normal anatomy [4,5,10,11,17]. It would be difficult to replicate a model similar to the human retroperitoneum, which often has a thick layer of fat overlying the ureters and obscuring their identification. Instead, we attempted to mimic an often-encountered scenario with acute abdominal inflammation to test the feasibility of ureteral identification with our novel UL-766 dye. Given that our previous experience is limited to a rat model, this experiment shows the use of this dye in a large animal while utilizing an FDA-approved laparoscopy system. Overall, we were successful in creating acute inflammation around the right ureter by dissection and careful abrasion of the surrounding tissues. At the second procedure, each pig had developed significant erythema and tissue adhesion to the retroperitoneum that made ureteral identification more difficult than during the initial procedure (Fig. 1C).
While the fluorescent signal is seen only when urine is present in the lumen of the ureters, observation of the urine bolus moving through the ureter provided guidance for the location of the ureter throughout the course of the surgery. With its high CBR, one could clearly delineate the ureter despite being concealed by bloody peritoneal fluid (Fig. 2A and B) and thickened erythematous tissue (Fig. 2C and D). Specifically, in the second pig, the fluorescent signal helped the identification of an adhesive band at the proximal ureter that partially obstructed the flow of urine during surgery. Under fluorescence guidance, the renal pelvis was exposed and the ureter was followed distally until the area of obstruction. This was visualized by the lack of movement of fluorescent urine distally. The adhesive band causing the obstruction was then ligated, subsequently allowing for better urinary flow, as visualized by larger boluses of urine down the ureter. This scenario showed the potential value of using a ureter-specific fluorescent dye for ureterolysis.
Ureteral obstruction can occur with retroperitoneal fibrosis or severe endometriosis due to compression by external inflammatory tissue, and intervention often requires ureterolysis to free the obstruction [18,19]. Furthermore, ureterolysis may need to be performed in benign gynecologic diseases such as adnexal masses and myomas [20]. On the other hand, ureteral injuries have been reported to occur most often with laparoscopic hysterectomies during surgeries for benign gynecologic diseases [21,22]. Thus, the use of a ureter-specific dye may be able to reduce ureteral injuries during many types of abdominal and pelvic surgeries.
Although ureteral injury prevention is critical, the identification of injury during the initial operation may be even more important. Intraoperative ureteral injuries are often missed during the initial surgery, with delayed diagnosis in up to 67–87% of injuries, and are associated with significant morbidity [2,4,7,8]. While ureteral stents, lighted or unlighted, have the potential to show an injury, they cannot be easily placed on-demand during an operation without the assistance of a urology colleague, bringing in new equipment into the operating room, and breaking the already placed sterile field. A better solution to improve intraoperative ureteral injury diagnosis may rest on the use of ureter-specific fluorescent dyes such as UL-766. In the current study, complete ureteral transection was purposefully made after the ureter was freed from the retroperitoneum. With the NIR overlay, the fluorescent signal from the free-flowing urine into the peritoneal cavity helped show the location of the proximal ureteral stump (Fig. 3). Furthermore, the identification of a partial ureteral transection makes this technique even more useful. In our experiment, despite the purposeful attempt at creating a partial transection with scissors, it was unclear if an injury was made under plain white light. Expectedly, this would be similar to that of an injury in a real operating room, and only with fluorescence imaging did it become obvious that there was, in fact, a partial ureteral injury as confirmed by the visualization of extraluminal fluorescent signals (Fig. 4B and C).
In this study, we present our preclinical experience of using a novel, ureter-specific, fluorescent dye to improve the identification and visualization of ureters during laparoscopic abdominal surgery in a porcine model with an inflamed retroperitoneum. Not only is the UL-766 dye compatible with a clinically available fluorescence laparoscopy system, but this dye also aided in the identification of ureters despite overlying erythematous and inflamed tissue. The results show the potential value of ureter specific fluorescent dyes in both routine and emergency abdominal surgeries, where inflamed tissues or distorted anatomy may obscure the visualization of ureters.
There are certain drawbacks to the use of this dye. Given that the fluorescent signal is only in the urine and not the urothelium, surgeons will have to wait for peristalsis of urine through the ureter in order to visualize the complete structure. This issue has also been noted by others testing ureter dyes [4]. An obvious solution is to optimize intravenous fluid infusion for maximal urine excretion. In cases of adhesive bands causing obstruction, such as that observed in our second pig experiment, we started at the renal pelvis and followed the collection of fluorescent urine in the ureter until there was a reduction in signal. Gentle tissue traction allowed for some urine to pass through and show the trajectory of the ureter.
This is an initial feasibility study to test the utility of the UL-766 dye for use with an FDA approved laparoscopy system. Although we showed the benefits of this dye in ureteral identification despite a background of inflammation, we did not create a model that closer mimicked the greater periureteral fat seen in humans. Thus, as the penetration of NIR emissions is limited to about 1 cm, the true application of this dye in clinical practice still remains to be elucidated [15]. In addition, we did not test different doses to observe how the concentration of dye would affect operative visualization. To the best of our knowledge, this is the first experiment to test the applicability of fluorescence ureterography in an acutely inflamed porcine abdomen. Despite the lack of periureteral fat in pigs, this model shows a scenario where ureter visualization by white light alone is insufficient due to the edematous and erythematous surrounding tissue. This study highlights the potential of the UL-766 dye for use in most abdominal and pelvic surgeries where anatomy distortion, inflammation, and ureteral injury are a concern.
CONCLUSION
UL-766 is a novel fluorescent dye with high specificity for the urinary tract system. With a clinically available fluorescence laparoscopic system, the use of this dye can be a valuable aid in ureter visualization as well as reduce the risk of iatrogenic ureteral injuries, particularly in patients with active abdominal inflammation or anatomical distortion due to surgical history. With additional preclinical studies, the usefulness of this dye in clinical practice can be fully delineated.
ACKNOWLEDGEMENT
Funding Sources: Sheikh Zayed Institute for Pediatric Surgical Innovation (SPF30306-44215) and InTheSmartUSA (Grant 30004721). ML and MS acknowledge support from the Intramural Research Program of the National Institutes of Health (NIH), NCI-CCR, NIH.
REFERENCES
- 1.Yeung TM, Volpi D, Tullis IDC, et al. Identifying ureters in situ under fluorescence during laparoscopic and open colorectal surgery. Ann Surg 2016;263:e1–e2. [DOI] [PubMed] [Google Scholar]
- 2.Palaniappa NC. Incidence of iatrogenic ureteral injury after laparoscopic colectomy. Arch Surg 2012;147:267–271. [DOI] [PubMed] [Google Scholar]
- 3.Coakley KM, Kasten KR, Sims SM, Prasad T, Heniford BT, Davis BR. Prophylactic ureteral catheters for colectomy: A national surgical quality improvement program-based analysis. Dis Colon Rectum 2018;61:84–88. [DOI] [PubMed] [Google Scholar]
- 4.Al-Taher M, van den Bos J, Schols RM, Kubat B, Bouvy ND, Stassen LPS. Evaluation of a novel dye for near-infrared fluorescence delineation of the ureters during laparoscopy. BJS Open 2018;2:254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korb ML, Huh WK, Boone JD, et al. Laparoscopic fluorescent visualization of the ureter with intravenous IRDye800CW. J Minim Invasive Gynecol 2015;22:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyan WP Jr., Lavy D, Dinallo A, et al. Lighted ureteral stents in laparoscopic colorectal surgery; a five-year experience. Ann Transl Med 2017;5:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackwell RH, Kirshenbaum EJ, Shah AS, Kuo PC, Gupta GN, Turk TMT. Complications of recognized and unrecognized Iatrogenic ureteral injury at time of hysterectomy: A population based analysis. J Urol 2018;199:1540–1545. [DOI] [PubMed] [Google Scholar]
- 8.Burks FN, Santucci RA. Management of iatrogenic ureteral injury. Ther Adv Urol 2014;6:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slooter MD, Janssen A, Bemelman WA, Tanis PJ, Hompes R. Currently available and experimental dyes for intraoperative near-infrared fluorescence imaging of the ureters: A systematic review. Tech Coloproctol 2019;23:305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schols RM, Lodewick TM, Bouvy ND, van Dam GM, Dejong CH, Stassen LP. Application of a new dye for near-infrared fluorescence laparoscopy of the ureters: Demonstration in a pig model. Dis Colon Rectum 2014;57:407–411. [DOI] [PubMed] [Google Scholar]
- 11.Mahalingam SM, Dip F, Castillo M, et al. Intraoperative ureter visualization using a novel near-infrared fluorescent dye. Mol Pharm 2018;15:3442–3447. [DOI] [PubMed] [Google Scholar]
- 12.Verbeek FP, van der Vorst JR, Schaafsma BE, et al. Intraoperative near infrared fluorescence guided identification of the ureters using low dose methylene blue: A first in human experience. J Urol 2013;190:574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cha J, Nani RR, Luciano MP, et al. A chemically stable fluorescent marker of the ureter. Bioorg Med Chem Lett 2018;28:2741–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kusano M, Kokudo N, Toi M, Kaibori M. ICG fluorescence imaging and navigation surgery. Japan: Springer; 2016. [Google Scholar]
- 15.Vahrmeijer AL, Hutteman M, van der Vorst JR, van de Velde CJ, Frangioni JV. Image-guided cancer surgery using near-infrared fluorescence. Nat Rev Clin Oncol 2013;10:507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Bos J, Al-Taher M, Bouvy ND, Stassen LPS. Near-infrared fluorescence laparoscopy of the ureter with three preclinical dyes in a pig model. Surg Endosc 2019;33:986–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyun H, Henary M, Gao T, et al. 700-nm Zwitterionic near-infrared fluorophores for dual-channel image-guided surgery. Mol Imaging Biol 2016;18:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miranda-Mendoza I, Kovoor E, Nassif J, Ferreira H, Wattiez A. Laparoscopic surgery for severe ureteric endometriosis. Eur J Obstet Gynecol Reprod Biol 2012;165:275–279. [DOI] [PubMed] [Google Scholar]
- 19.Srinivasan AK, Richstone L, Permpongkosol S, Kavoussi LR. Comparison of laparoscopic with open approach for ureterolysis in patients with retroperitoneal fibrosis. J Urol 2008;179:1875–1878. [DOI] [PubMed] [Google Scholar]
- 20.Jan H, Ghai V. Ureterolysis in total laparoscopic hysterectomy. J Minim Invasive Gynecol 2018;25:S133. [DOI] [PubMed] [Google Scholar]
- 21.Wong JMK, Bortoletto P, Tolentino J, Jung MJ, Milad MP. Urinary tract injury in gynecologic laparoscopy for benign indication: A systematic review. Obstet Gynecol 2018;131: 100–108. [DOI] [PubMed] [Google Scholar]
- 22.Adelman MR, Bardsley TR, Sharp HT. Urinary tract injuries in laparoscopic hysterectomy: A systematic review. J Minim Invasive Gynecol 2014;21:558–566. [DOI] [PubMed] [Google Scholar]