Abstract
Diffuse malignant mesothelioma of the pleura (MPM) is a highly aggressive tumour that typically is associated with short survival. CD70 and CD27 belong to the tumour necrosis factor (TNF) and the TNF receptor (TNFR) superfamily, respectively. Under physiological conditions, the tightly regulated interaction between CD70 and CD27 plays a co-stimulatory role in promoting T-cell expansion and differentiation through the NFκB pathway. Aberrantly high CD70 expression has been documented in haematological and solid malignancies in association with immune evasion in malignant cells. In this study, 172 well-characterised primary diffuse MPM tumours including epithelioid (n =145), biphasic (n =15), and sarcomatoid (n =12) histotypes were evaluated immunohistochemically for CD70, CD27, CD3, CD4, CD8, CD56, PDCD1 (PD-1), and FOXP3 expression. Twenty per cent (34/172) of the mesothelioma cells expressed CD70 on the cell membrane. Overall survival was significantly decreased in the cohort of patients with CD70-expressing tumour cells (p <0.01). Patients with MPM containing a higher number of CD3+ (p <0.01), CD4+ (p <0.01), CD8+ (p <0.01), or FOXP3+ (p <0.01) tumour-infiltrating lymphoid cells (TILs) showed significantly worse clinical outcomes. As potential independent risk factors for MPM patients, multivariate Cox proportional hazards regression analysis revealed CD70 expression on mesothelioma cells [hazard ratio (HR) 2.25; p =0.010], higher FOXP3+ TILs (HR 2.81; p =0.004), and higher CD3+ TIL accumulation (HR 6.12; p <0.001). In contrast, as a potential independent favourable factor, higher CD27+ TIL accumulation (HR 0.48; p =0.037) was identified. In vitro experiments and an immunodeficient mouse model revealed that CD70 enhances the invasiveness of MPM cells through MET–ERK axis activation. Further analyses in syngeneic mouse models demonstrated possible roles for CD70 in immune evasion. Collectively, these findings suggest that the CD70–CD27 pathway enhances the malignant phenotypes of MPM and diminishes anti-tumor immune response in patients with these neoplasms. These markers might be useful in MPM for prognostic evaluations as well as targeted therapeutics.
Keywords: malignant pleural mesothelioma, immunohistochemistry, CD70, CD27, PDCD1 (PD-1), FOXP3, MET, invasion, immune evasion
Introduction
Malignant mesothelioma is an aggressive neoplasm arising from the mesothelial cells of the pleura, peritoneum, and pericardium [1]. Up to 80% of all cases are defined as malignant pleural mesothelioma (MPM) derived from pleural mesothelial cells, and MPM patients usually have a short survival [2]. Additional therapeutics such as immune checkpoint modulators are of utmost importance to improve the prognosis of MPM patients.
CD70 is a type II transmembrane surface antigen belonging to the tumour necrosis factor (TNF) superfamily (TNFSF). This transmembrane protein comprises 193 amino acids with a C-terminal TNF homology domain that identifies CD70 as a member of the TNFSF [3,4]. Under physiological conditions, the expression of CD70 is tightly regulated, and CD70 is only transiently expressed on activated T cells, B cells, and mature dendritic cells [5,6].
CD27 is a member of the tumour necrosis factor receptor superfamily (TNFRSF) that contains two complete and one incomplete cysteine-rich domains that are characteristic of the TNFRSF [3,4]. CD27 is a co-stimulatory immune checkpoint receptor that is constitutively expressed by a broad range of T cells (naïve, αβ, γδ, and memory T cells), NK cells, and B cells. The CD70–CD27 signalling pathway plays a co-stimulatory role in promoting T-cell expansion and differentiation through activation of the NFκB pathway under physiological conditions [6-8].
In contrast to the limited CD70 expression in normal tissues, aberrant CD70 expression has been documented in haematological tumours [8] and solid malignancies such as renal cell carcinoma [9] and glioblastoma [10]. In haematological malignancies, CD70 overexpression has been implicated in accelerated tumour cell proliferation and survival through its interaction with CD27, which is co-expressed on tumour cells [11,12]. Furthermore, CD70 expression was associated with a poor clinical outcome in B-cell lymphoma [13]. In solid malignancies such as glioblastomas and melanomas, CD70 has been implicated to regulate tumour cell migration and invasion with ERK phosphorylation through unknown signalling [14,15]. However, the expression of CD70 and CD27, as well as their correlations with patient survival, has never been studied in MPM patients.
FOXP3+ regulatory T-cells (Tregs) suppress aberrant immune responses against self-antigens under physiological conditions. Under neoplastic conditions, they also suppress the anti-tumour immune response, and the presence of Tregs within the tumour microenvironment accelerates tumour immune evasion [16]. CD70 expression on the tumour cells has been suggested to enhance immune evasion and accelerate tumour growth through several distinct mechanisms, including Treg expansion, weaker tumour-specific T-cell responses, and increased angiogenesis [8,10,17-22].
CD274 (PD-L1) has been identified as a cell-surface glycoprotein from the B7 family [23]. PDCD1 (CD279, PD-1), a physiological receptor for CD274, belongs to the immunoglobulin superfamily and is mainly expressed on activated T cells as well as non-T lymphocytes such as B cells and natural killer (NK) cells, though only upon induction [24,25]. In physiological conditions, the CD274/PDCD1 axis is crucial for the modulation to reduce collateral tissue damage from the inflammatory response as well as for the maintenance of self-tolerance to avoid autoimmune diseases in the immune system [26,27]. When T cells are exposed to chronic antigen stimulation such as chronic viral infection or cancer, high-level expression of PDCD1 is induced and leads to T-cell exhaustion or anergy [28]. Recently, our group reported that the expressions of ALCAM (CD166) and CD274 independently predict a shorter survival in MPM [29].
This study examines the expression statuses of CD70, CD27, CD3, CD4, CD8, CD56, PDCD1 (PD-1), and FOXP3 in MPM cells and tumour-infiltrating lymphocytes (TILs). Additionally, the associations of these molecules with clinicopathological parameters and clinical outcome were analysed to assess the potential of these molecules for clinical use. Further in vitro and in vivo experiments were performed to uncover the tumour-related biological importance of CD70 expression in MPM cells.
Materials and methods
Tumour samples
Thirty MPMs were included on IRB-approved protocols at the National Institutes of Health (NIH) and informed consent was obtained from these patients. One hundred and forty-two additional anonymised MPM samples were collected. This project was completed under the Office of Human Subject Research Exemption for anonymised specimens. All of the tumours were extensively characterised clinically and histopathologically. A previously performed immunohistochemical analysis revealed that all tumours were positive for the cytokeratin cocktail AE1/AE3, calretinin, and/or WT1 without TTF-1 or CEA expression [29]. In the present study, all tumours were diffuse (multiple) mesothelioma, and no localised mesothelioma was included.
Immunohistochemistry
Tumour samples derived from surgical specimens were assembled into multi-tumour blocks containing up to 40 rectangular tissue samples as previously described [30]. The size of the tumour samples was estimated to exceed the size of a single 0.6mm2 core by a factor of 10–15.
Immunohistochemistry was performed using a Leica Bond-Max (Leica Biosystems, Bannockburn, IL, USA), Ventana BenchMark XT or ULTRA automated immunostainer (Roche Diagnostics, Basel, Switzerland). A Leica Refine (Leica Biosystems) or OptiView (Roche Diagnostics) detection kit was used to detect signals in human tissue samples. For the sequential double staining, the additional antibody was visualised using Fast Red chromogen. An iVIEW detection kit (Roche Diagnostics) with a biotinylated anti-rabbit secondary antibody (Sigma-Aldrich Japan, Tokyo, Japan) at a 1:800 dilution was used for mouse tissue samples. The origins and dilutions of the primary antibodies are summarised in supplementary material, Table S1. In mesothelioma cells, CD70 immunoreactivity (cell membrane) was evaluated at a detection cut-off of 5% (supplementary material, Figure S1). The number of CD27-, CD3-, CD4-, CD8-, CD56-, PDCD1-, or FOXP3-positive TILs was counted in high-power fields (HPFs, 400×).
Statistical analysis
All statistical analyses were performed using EZR version 1.32 software [31]. The chi-square, Fisher’s exact, or Mann–Whitney U-test was performed to investigate statistical correlations between categorical data. Univariate Kaplan–Meier survival estimates with the log-rank test were used to analyse the prognostic value of the categorical data for MPM patient overall survival. To analyse the associations between survival and other factors, Cox proportional hazards regression analysis was performed. The initial model included age (< 65 versus ≥ 65 years old), sex (male versus female), tumour histology (epithelial versus biphasic versus sarcomatoid), and data from tumour immunohistochemical staining including CD70 expression (positive versus negative). The cut-off values for TILs were as follows: CD27 (15, 75th percentile), CD3 (3, 25th percentile), CD4 (28, 50th percentile), CD8 (7, 25th percentile), CD56 (1, 50th percentile), PDCD1 (3, 75th percentile), and FOXP3 (19, 75th percentile). Backward elimination with a threshold of p = 0.05 was used to select variables for the final model. Patients with missing information were eliminated from the statistical analysis of that parameter.
Cells and reagents
The human immortalised mesothelial cell line MeT-5A and six human malignant mesothelioma cell lines were kindly provided by Dr Yoshitaka Sekido (Aichi Cancer Center Research Institute). The mouse mesothelioma cell line AB1 was obtained from CellBank Australia (Westmead, Australia). Cells derived from humans were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% foetal bovine serum (FBS). AB1 cells were maintained in RPMI 1640 medium supplemented with 5% FBS and 25 mM HEPES. Stable CD70-transfected and control LacZ-transfected cell lines were established using the vector pcDNA3.1 (Invitrogen/Thermo Fisher Scientific, Waltham, MA, USA) containing the CD70 or LacZ cDNA sequence followed by IRES2 and the puromycin resistance gene (supplementary material, Figure S2A). Formalin-fixed, p araffin-embedded (F FPE) sections of H2052CD70 and H2052LacZ were used as positive and negative controls, respectively, for CD70 immunohistochemistry (supplementary material, Figure S2B). SGX-523, a selective MET inhibitor, was from Selleck Biotech (Tokyo, Japan).
FACS analysis and immunoblotting assays
In FACS analyses, an FITC-conjugated anti-CD70 antibody (BioLegend, Inc, San Diego, CA, USA) was applied to harvested cells at a dilution of 1:20 for 1 h on ice. After washing and staining with 7-AAD (Beckman Coulter, Inc, Brea, CA, USA), the cells were analysed using a FACSCanto II flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Collected data were analysed by FlowJo 7.6.5 software (Tomy Digital Biology Co, Ltd, Taito-ku, Japan).
Whole-cell lysates were prepared and subjected to immunoblotting analyses using a previously reported procedure [32-34]. Information about the antibodies used in the immunoblot assays is summarised in supplementary material, Table S2. Signal intensity was measured by ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Migration and invasion assays
Migration and invasion assays were performed using the Falcon® Permeable Support for 24-Well Plate with 8.0 μm Transparent PET Membrane and Corning® BioCoat™ Matrigel® Invasion Chambers with 8.0 μm PET Membrane (Corning, Corning, NY, USA) according to the manufacturer’s instructions. For migration and invasion assays, 1 × 104 or 2 × 104 cells per chamber, respectively, were used. Ten per cent FBS was used as a chemoattractant. After incubating, the cells were fixed using 100% methanol at room temperature and stained with Giemsa’s stain. The number of migrated or invaded cells was counted under a microscope. The anti-CD70 antibody (Clone #301731; R&D Systems/Thermo Fisher Scientific, Waltham, MA, USA) was added to the culture medium at 20 μg/ml for the prevention of CD70–CD27 interactions (supplementary material, Figure S3).
Animal models
All animal procedures were approved by the Animal Ethics Committee of Aichi Medical University. An in vivo tumour formation assay with immunodeficient mice was carried out as follows: 2 × 106 H2052 cells constitutively expressing CD70 or LacZ were injected into the peritoneal cavity of 6-week-old NOD/SCID mice (Charles River Laboratories Japan Inc, Yokohama, Japan). Four months later, the mice were euthanised. Tumour foci over 2mm in diameter were counted under a stereoscopic microscope.
For the first syngeneic mouse experiment, 1 × 106 CD70-expressing AB1 cells or control cells were injected into the right thorax of 6-week-old BALB/c mice (n=6; CLEA Japan, Inc, Meguro, Japan). The mice were monitored for up to 25 days. AB1LacZ-tranplanted mice were euthanised on days 15 and 25.
The second syngeneic mouse experiment was performed to compare the characteristics of TILs within tumour microenvironments; 5 × 105 CD70-expressing AB1 cells and 5 × 106 control cells were injected into the right thorax of 6-week-old BALB/c mice (n=3). All of the mice were euthanised on day 12. In all of the mouse models, the lesions and/or surrounding organs were harvested and subjected to histopathological examination.
Results
Expression of CD70, CD27, CD3, CD4, CD8, CD56, PDCD1, and FOXP3 in MPM
Representative images for haematoxylin and eosin (H&E) staining and immunohistochemistry including calretinin are shown in Figure 1. Thirty-four of 172 mesothelioma samples (20%) showed variable CD70 expression on the cell membrane (with 5–100% of all tumour cells staining positive; median 20%; supplementary material, Figure S1) (representative images in Figure 1C,F). The clinical, pathological, and immunohistochemical features of the analysed tumours are summarised in Table 1 according to the tumour cell CD70 expression on the cytomembrane. CD70 positivity tended to occur in older patients (mean age 62.9 ± 11.0 years). CD70-positive tumours contained significantly higher numbers of FOXP3+ TILs than CD70-negative tumours (p = 0.0024). CD70-positive tumours tended to contain higher numbers of CD27+ (p = 0.049), CD3+ (p = 0.050) or PDCD1+ (p = 0.079) TILs. Notably, no mesothelioma cells expressed CD27, PDCD1 or FOXP3.
Figure 1.
Representative images of H&E and immunostaining for malignant pleural mesothelioma samples. (A–C) H&E (A), calretinin (B), and CD70 (C) in an epithelioid MPM case. Diffuse membranous and cytoplasmic CD70 expression was observed. (D–F) H&E (D), calretinin (E), and CD70 (F) in a biphasic MPM case. Cytoplasmic CD70 expression was observed in spindle-shaped MPM cells. (G–I) Mesothelioma-infiltrating lymphoid cells showed expression of CD27 (G), PDCD1 (H), and FOXP3 (I). Inset: double staining for FOXP3 (brown) and CD4 (Red). Note that Tregs co-expressing FOXP3 and CD4 were detected (arrowheads). Bar=50 μm.
Table 1.
Characteristics of malignant pleural mesotheliomas with or without CD70 expression
| CD70 expression |
||||
|---|---|---|---|---|
| Total No. 172 (100%) [100%] |
Positive 34 (20%) [100%] |
Negative 138 (80%) [100%] |
P value | |
| Sex | 0.15* | |||
| Male | 98 (100%) [69%] | 23 (23%) [82%] | 75 (77%) [66%] | |
| Female | 44 (100%) [31%] | 5 (11%) [18%] | 39 (89%) [34%] | |
| Age, years (mean±SD) | 59.2±11.9 | 62.9±11.0 | 58.3±11.9 | 0.069† |
| Histology | 0.89‡ | |||
| Epithelial | 145 (100%) [84%] | 28 (19%) [82%] | 117 (81%) [85%] | |
| Biphasic | 15 (100%) [9%] | 3 (20%) [9%] | 12 (80%) [9%] | |
| Sarcomatoid | 12 (100%) [7%] | 3 (25%) [9%] | 9 (75%) [7%] | |
| CD27+ TILs (/HPF) | 4 (2–11.75) | 6 (3–15) | 4 (1–10) | 0.049§ |
| CD3+ TILs (/HPF) | 8.5 (2–24) | 15.5 (5.25–28.5) | 6.5 (2–23.25) | 0.50§ |
| CD4+ TILs (/HPF) | 25 (13–48.5) | 33 (14–48) | 23 (12–48) | 0.33§ |
| CD8+ TILs (/HPF) | 15 (7–32.75) | 22 (10.5–45.75) | 12.5 (7–28.75) | 0.069§ |
| CD56+ TILs (/HPF) | 1 (0–3) | 0 (0–1) | 1 (0–5) | 0.059§ |
| PDCD1+ TILs (/HPF) | 1 (0–2) | 1 (0–4) | 1 (0–2) | 0.079§ |
| FOXP3+ TILs (/HPF) | 4 (1–12.5) | 9.5 (4–20.75) | 3 (1–10.5) | 0.0024§ |
TILs, tumour-infiltrating lymphocytes; HPF, high-power field (×400).
P values were calculated by the chi-square test for CD70 expression.
t-test was used to compare the means of age.
P values were calculated by Fisher’s exact test for CD70 expression.
Mann–Whitney U-test was used for analyses. Data are shown as median (25–75 percentiles). The Bonferroni-corrected P value for significance was p =0.005 (0.05/10).
Survival analyses of mesothelioma patients
The characteristics of the 63 mesothelioma patients analysed for survival are summarised in Table 2. The patients were followed up for up to 120 months. Survival was significantly shorter in the patients with mesothelioma CD70 expression on the cytomembrane (6.5 months median versus 18.0 months without CD70 expression or with cytoplasmic labelling only; p<0.01; Figure 2A and supplementary material, Figure S4A). Consistent with these findings, the analysis of The Cancer Genome Atlas (TCGA) data revealed a significant inverse association between CD70 expression and overall survival in MPM patients (supplementary material, Figure S4B). Significant associations were found between patient survival and the higher number of CD3+ (p<0.01), CD4+ (p<0.01), CD8+ (p<0.01), and FOXP3+ (p<0.01) TILs (Figure 2C-E,H). The multivariate Cox proportional hazards regression analysis revealed CD70 expression on mesothelioma cells [hazard ratio (HR) 2.25; 95% confidence interval (CI) 1.21–4.17; p = 0.010], higher FOXP3+ (HR 2.81; 95% CI 1.39–5.66; p = 0.0040), and CD3+ TIL accumulation (HR 6.12; 95% CI 2.64–14.2; p < 0.001) as potential independent risk factors. In contrast, higher CD27+ TIL accumulation (HR 0.48; 95% CI 0.25–0.96; p = 0.037) was identified as a potential independent favourable factor in mesothelioma patients (Table 3).
Table 2.
Characteristics of the 63 patients analysed for survival
| Age, years | |
| Mean | 61.0±10.6 |
| Median (range) | 61 (27–87) |
| Sex, No. (%) | |
| Male | 47 (75) |
| Female | 16 (25) |
| Histology, No. (%) | |
| Epithelial | 53 (84) |
| Biphasic | 7 (11) |
| Sarcomatoid | 3 (5) |
| Tumour positive for membranous CD70, No. (%) | 20 (32) |
| CD27+ TILs (/HPF) | 6 (3–15) |
| CD3+ TILs (/HPF) | 11 (3–29.75) |
| CD4+ TILs (/HPF) | 28 (9–61.75) |
| CD8+ TILs (/HPF) | 16 (7–34) |
| CD56+ TILs (/HPF) | 1 (0–6) |
| PDCD1+ TILs (/HPF) | 1 (0–3) |
| FOXP3+ TILs (/HPF) | 6 (2–19) |
Data for TILs are shown as median (25–75 percentiles). TILs, tumour-infiltrating lymphocytes; HPF, high-power field (×400).
Figure 2.
Overall survival of MPM patients classified by their CD70, CD27, CD3, CD4, CD8, CD56, PDCD1, and FOXP3 expression patterns. (A) Kaplan–Meier curves for patients with mesothelioma with or without CD70 expression in the cell membrane. (B–H) Kaplan–Meier curves for patients grouped according to their CD27 (B), CD3 (C), CD4 (D), CD8 (E), CD56 (F), PDCD1 (G), and FOXP3 (H) expression pattern in mesothelioma-infiltrating lymphoid cells.
Table 3.
Cox hazard ratio of mesothelioma patients
| Hazard ratio |
95% CI |
|||
|---|---|---|---|---|
| Min | Max | P value | ||
| CD27+ TILs (15≥/HPF) | 0.48 | 0.25 | 0.96 | 0.037 |
| Tumour CD70 expression | 2.25 | 1.21 | 4.17 | 0.010 |
| FOXP3+ TILs (19≥/HPF) | 2.81 | 1.39 | 5.66 | 0.004 |
| CD3+ TILs (3≥/HPF) | 6.12 | 2.64 | 14.2 | <0.001 |
Cox proportional hazards regression analysis was performed to analyse the association of survival and other factors. The initial model included age, sex, tumour histology, and data from immunohistochemical staining for CD70, CD27, CD3, CD4, CD8, CD56, PDCD1, and FOXP3 expression in tumours or TILs. A backward elimination with a threshold of p =0.05 was used to select variables in the final model. TILs, tumour-infiltrating lymphocytes; HPF, high-power field (×400).
CD70 regulates the migration and invasion of mesothelial and mesothelioma cells
Additional experiments were performed to examine the effects of CD70 expression in MPM cells. Among immortalised or malignant human mesothelial cell lines, CD70 was uniquely expressed on the cell surface of the ACC-MESO-1 cells (Figure 3). CD27, a unique physiological receptor for CD70, was expressed below detectable levels in all of the cells analysed in the present study.
Figure 3.
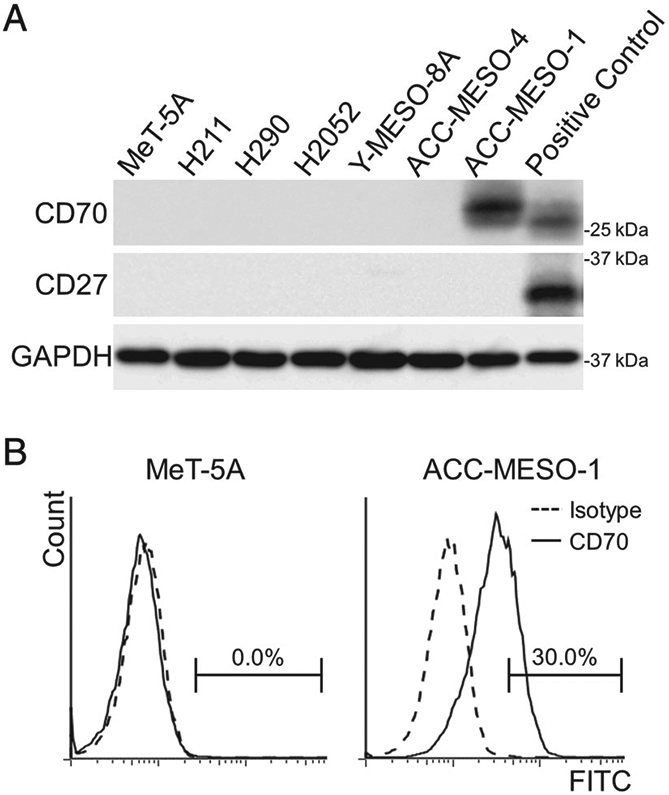
CD70 and CD27 expression in mesothelial cells. (A) Immunoblotting analyses for CD70 and CD27. For positive controls, CD70- and CD27-transfected Met-5A cells were used. (B) FACS analyses identifying CD70 expression on the cell surface of ACC-MES-1 cells.
Transient transfection of two different siRNAs targeting CD70 significantly reduced the migration and invasion of ACC-MESO-1 cells without modulating cellular proliferation and apoptosis (Figure 4 and supplementary material, Figure S5). To further examine this issue, CD70 was ectopically expressed in MeT-5a (SV40T immortalised mesothelial cells) and H2052 MPM cells. Compared with LacZ transfected controls, CD70 protein levels were markedly increased in MeT-5A and H2052 CD70 transfectants. CD70 overexpression coincided with increased phospho-ERK levels in both cell lines; the MEK1/2 inhibitor UO126 abrogated CD70-associated increases in phospho-ERK levels in these cells (Figure 5A). Additional experiments demonstrated that ectopic expression of CD70 increased the migration and invasion of MeT-5A and H2052 cells, which could be significantly ameliorated by UO126 (Figure 5B,C). In contrast, the addition of CD70 blocking antibody did not alter the migration, invasion, or expression of phospho-ERK in CD70-expressing MPM cells (supplementary material, Figure S6). Interestingly, the expression levels of MET and phospho-MET correlated with the CD70 expression levels (Figure 5D and supplementary material, Figure S5A). Furthermore, SGX-523, a selective MET inhibitor, suppressed phospho-ERK expression to almost the baseline level without modulating MET expression (Figure 5E). Based on these observations, we concluded that the CD70-driven ERK activation was due to the activated MET–ERK signalling by stabilised MET protein.
Figure 4.
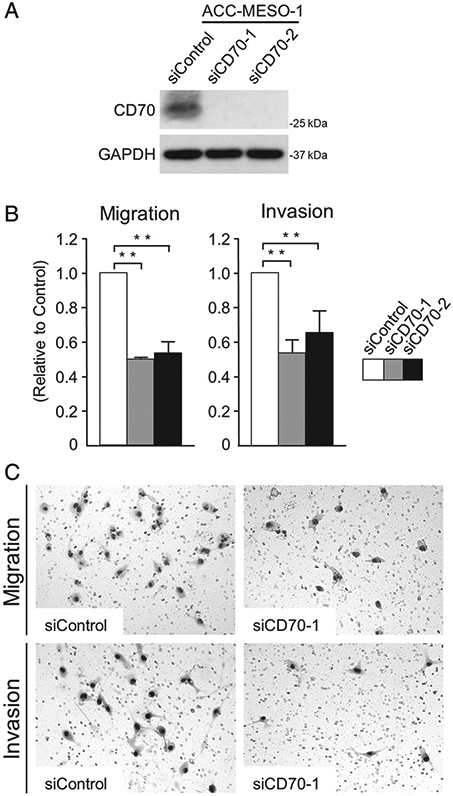
Knockdown of CD70 expression suppressed the migration and invasion of ACC-MESO-1 cells. (A) Immunoblotting analyses for CD70 with or without CD70 expression knockdown. (B, C) Results (B) and representative images (C) for migration and invasion assays. Assays were performed in triplicate. Data are shown as the mean±SD. **p <0.01.
Figure 5.
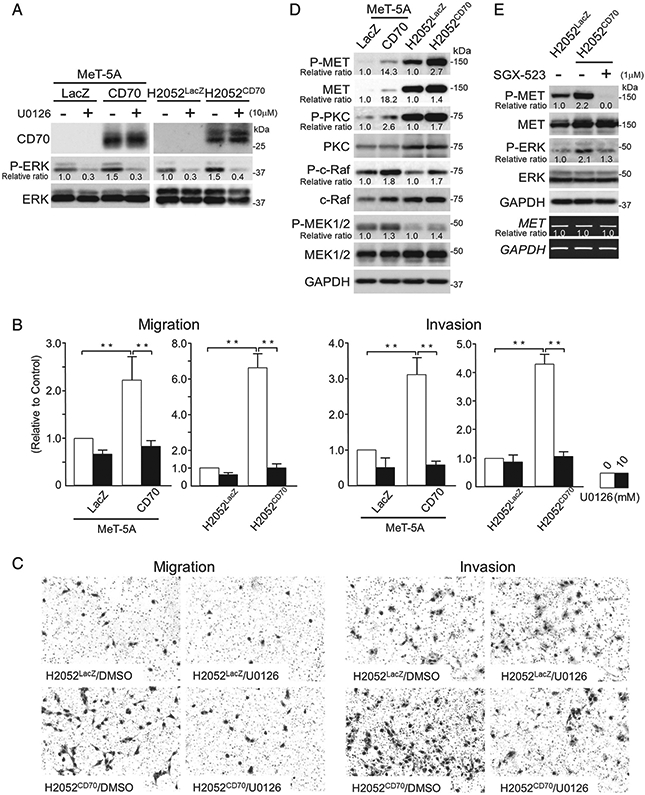
Forced CD70 expression enhanced the migration and invasion of Met-5A and H2052 cells in an ERK-dependent manner. (A) Immunoblotting analyses for Met-5A and H2052 cells with or without CD70 expression and U0126 treatment. (B, C) Results (B) and representative images (C) for migration (left) and invasion (right) assays. Assays were performed in triplicate. Data are shown as the mean±SD **p <0.01. (D) Immunoblotting analyses for Met-5A and H2052 cells with or without CD70 expression. Accumulations of MET, phospho-MET, phospho-PKC, phospho-c-Raf, and phospho-MEK1/2 are evident. (E) SGX-523, a MET inhibitor, down-regulated the CD70-induced phospho-ERK.
CD70 worsens the prognosis of mesothelioma-transplanted mice via enhanced invasiveness and immune evasion
H2052CD70 cells formed invasive and proliferative tumour masses in the peritoneal cavity of immunodeficient mice. In contrast, control H2052LacZ cells formed fewer, smaller, and less invasive lesions with central necrosis (Figure 6A-C).
Figure 6.
CD70 enhanced the tumourigenicity of MPM cells in mouse models. (A, B) H2052CD70 cells formed a significantly higher number of proliferative tumours than H2052LacZ cells in immunodeficient mice. Bar=1 cm. *p <0.05. (C) H2052CD70 cells showed invasion into the pancreas. In contrast, H2052LacZ cells formed small and less invasive tumour foci with central necrosis. Inset: CD70 immunohistochemistry. Bar=1 mm. (D, E) Representative photographs (D) and Kaplan–Meier curves (E) of AB1CD70- and AB1LacZ-transplanted BALB/c mice are shown. AB1CD70 cells formed highly aggressive tumour masses with a significantly worse prognosis. Bar=5 mm. (F) Tumours formed by AB1CD70 (seven foci) contained significantly higher numbers of FOXP3+, PDCD1+, and HVCR2+ TILs than those from AB1LacZ (three foci). *p <0.05.
In the first syngeneic mouse model, five out of six AB1CD70-transplanted mice died of tumour on days 10–11 after intra-thoracic tumour transplantation. The last AB1CD70-transplanted mouse was sacrificed on day 12. The transplanted AB1CD70 cells formed massive tumours invading the mediastinum, diaphragm, and opposite side of the thorax. In contrast, no lesions were found during the histopathological examination of control AB1LacZ cells-transplanted mice on day 25 (Figure 6D,E and supplementary material, Figure S7A).
In the second syngeneic mouse transplantation experiment, all of the mice were alive at day 12. No significant difference in the tumour diameter was found between AB1CD70 and AB1LacZ (supplementary material, Figure S7B). The tumours originating from AB1CD70 contained a significantly higher number of FOXP3+, PDCD1+, and HAVCR2 (TIM-3)+ TILs within their microenvironment (Figure 6F).
Discussion
In the present study, 172 diffuse malignant pleural mesotheliomas (MPMs) were immunohistochemically evaluated for the expression of CD70, CD27, PDCD1, FOXP3, and other lymphoid markers to assess their impacts on clinicopathological parameters and survival. Furthermore, experiments in vitro and in vivo were performed to uncover the tumour-related biological importance of CD70 expression in MPM cells.
CD70 was variably expressed on MPM cells in 20% (34/172) of the cases, associating with a significantly shorter survival (6.5 months median, p < 0.01). Many types of TILs were accumulated in MPM tissues at variable levels. Patients with MPM containing a higher number of CD3+ (11.0 months median, p < 0.01), CD4+ (10.0 months median, p < 0.01), CD8+ (11.5 months median, p < 0.01) or FOXP3+ (4.0 months median, p < 0.01) TILs showed a significantly s horter survival. From the multivariate Cox proportional hazards regression analysis, tumour CD70 expression (HR 2.25, p=0.010) and a higher number of FOXP3+ (HR 2.81, p=0.004) and CD3+ TILs (HR 6.12, p<0.001) were identified as potential independent risk factors, whereas a higher number of CD27+ TILs (HR 0.48, p=0.037) was identified as a potential independent favourable factor. In the present study, the Cox proportional hazards regression model did not include data regarding asbestos exposure, smoking, tumour staging, or treatment modality, due to the limited clinical information and samples, many of which were small biopsy specimens non-informative for staging purposes. This is a major limitation of the analysis. However, our review of TCGA did not reveal a significant association between CD70 expression and the stage of MPM (data not shown). Further study of a larger number of MPM patients and their respective clinical treatments and outcomes might establish a better prognostic system.
In haematological malignancies, it has been reported that the co-expression of CD70 and CD27 on tumour cells plays a significant role in tumour cell expansion, tumour cell survival, and leukaemia stem/progenitor cell maintenance [11,35-38]. Tumours expressing CD70 alone have been reported to enhance malignant phenotypes of tumour cells with ERK phosphorylation, though the mechanism is unknown [11,12,14,15]. In the present study, CD70 enhanced the ERK-dependent migration and invasion of MPM cells expressing CD27 at undetectable levels. To best of our knowledge, the present study is the first to uncover that CD70 stabilises MET protein for the activation of MET–ERK signalling in mesothelial and MPM cells. MET–ERK signalling, indispensable for the malignant phenotypes of MPM cells, was intriguingly activated by CD70 expression [39]. It is, however, unclear whether CD70 directly interacts with MET for its stabilisation, which should be elucidated in the near future.
CD70 expression on tumour cells has also been suggested to contribute to the immune evasion of tumour cells through (1) T-cell apoptosis [10,19-21]; (2) Treg expansion [8,17,18]; and (3) T-cell exhaustion [22]. It was reported that CD70-expressing tumour cells, such as those in renal cell carcinoma and glioblastoma, can induce apoptosis in T cells under experimental conditions [10,19,20]. In this process, binding of the pro-apoptotic protein Siva to the cytoplasmic tail of CD27 is believed to mediate apoptosis through caspase activation [20,21]. On the one hand, patients with CD70-positive MPM tended to have a higher number of CD27+ TILs than CD70-negative patients (p = 0.07). On the other hand, the higher number of CD27+ TILs (HR 0.48, p < 0.001) was identified as a potential independent favourable factor. These results might suggest that CD27-positive TILs have a certain role in the MPM microenvironment. Harnessing such a mechanism could help in tumour immunotherapies including the use of CD27-agonistic monoclonal antibodies (mAbs).
Tregs can be induced (iTreg) from CD4+CD25− naïve cells upon T-cell receptor stimulation or stimulation by interleukin-2 (Il-2) and TGF-β, while naturally occurring CD4+CD25+ Tregs (nTreg) arise in the thymus. FOXP3 expression has been shown to be crucial to the development and immunosuppressive function of Tregs [40]. Recently, increased amounts of iTregs have been identified within CD70-positive tumour microenvironments. Moreover, the CD70–CD27 pathway-dependent induction of Tregs and the promotion of tumour growth based on weakened tumour-specific T -cell responses have been identified [ 8,17,18]. Here, CD70-positive MPM contained a significantly higher number of FOXP3+ TILs than CD70-negative MPM (p = 0.0024), and patients with positive tumours showed significantly worse clinical outcomes than patients with negative tumours (p <0.01). Additionally, in our syngeneic mouse model, a significantly higher number of FOXP3+ TILs (p <0.05) was found in AB1CD70 tumours than in AB1LacZ tumours. Based on these observations, inhibition of Tregs induced by tumour-expressed CD70 might be a good therapeutic approach.
T-cell exhaustion has been reported in different types of CD70-positive tumours. Reduced naïve and central memory T-cell numbers but elevated effector memory T-cell numbers have been identified in CD70-positive renal cell carcinoma [41]. In follicular lymphoma, it was reported that CD70+ T cells appear to express high levels of PDCD1 and HAVCR2, indicating an exhausted phenotype similar to the phenotypes driven by TGF-β that are associated with worse clinical outcomes [22]. In the present study, variable PDCD1+ TIL numbers were observed within the MPM microenvironment. In our human studies, PDCD1+ TILs showed tendencies to associate with CD70 expression in MPM (p=0.079) and worse clinical outcomes (p=0.069). Furthermore, significantly higher numbers of PDCD1+ (p<0.05) and HAVCR2+ TILs (p<0.05) were found in AB1CD70 tumours than in AB1LacZ tumours. Thus, CD70-positive MPM might induce dysfunction in immune cells within the tumour microenvironment by exhausting TILs.
mAbs blocking immune checkpoint pathways such as the CTLA-4 and PDCD1 axes have been introduced for cancer therapy and show significant anti-cancer effects [42-44]. However, monotherapy with these immune checkpoint inhibitors fails to completely eradicate tumour cells. Based on the limited expression in normal tissues and aberrant expression of CD70 by tumour cells, CD70–CD27 axis-targeting mAbs such as SGN-75 (vorsetuzumab mafodotin, a humanised mAb conjugated with the cytotoxic agent non-cleavable monomethyl auristatin F), ARGX-110 (a blocking antibody causing antibody-dependent cellular cytotoxicity), and CDX1127 (varlilumab, an agonistic antibody specific for CD27) have been developed and tested in clinical trials [6,45-47]. Recently, it has been reported that an agonistic anti-CD27 antibody can synergise with a blocking antibody against CD274 to restore the function of CD8+ effector T cells [48]. Based on the CD274 [29] and CD70 expression patterns in MPM, combination therapies targeting the CD274–PDCD1 and CD70–CD27 axes might be considered for patients with MPM.
In conclusion, the present study demonstrated that the expression of CD70 on pleural mesothelioma cells was associated with a shorter survival. The molecular and animal studies demonstrated that CD70 worsened the prognosis of MPM patients via enhanced invasiveness and immune evasion. CD70 and the other lymphoid markers demonstrated here could be useful in prognosis and treatment planning, including the CD70–CD27 pathway-targeted therapy, in MPM patients. Combination therapies targeting the CD274–PDCD1 and CD70–CD27 axes might be a therapy candidate for MPM patients.
Supplementary Material
Figure S1. CD70 expression in 172 malignant pleural mesothelioma patients
Figure S2. CD70 expression in CD70 or LacZ transfectants
Figure S3. Anti-CD70 antibody blocked CD70–CD27 interaction
Figure S4. Overall survival of malignant pleural mesothelioma cases according to CD70 expression
Figure S5. Knockdown of CD70 suppressed MET expression but did not modulate apoptosis or cellular proliferation of ACC-MESO-1
Figure S6. Anti-CD70 antibody did not modulate migration, invasion, or expression of MET and phospho-ERK in CD70-expressing mesothelioma cells
Figure S7. Supplementary data for animal experiments
Table S1. Antibodies and conditions for immunohistochemistry
Table S2. Antibodies and dilutions for immunoblotting analyses
Acknowledgements
We thank Dr Yoshitaka Sekido (Aichi Cancer Center Research Institute) for providing human mesothelioma cell lines. Human malignant lymphoma cell lines were kindly provided by Dr Akinobu Ota and Dr Yoshitaka Hosokawa (Aichi Medical University). We also thank Ms Kazuko Tanimizu, Ms Shino Kojima, and Mr Naoki Igari for their assistance with tissue preparation and immunohistochemical staining. We had support for manuscript editing from Ms Yukiko Kuru (Aichi Medical University). This work was supported as part of a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (to SI; 17K08706) and by the National Cancer Institute’s intramural research programme.
Footnotes
No conflicts of interest were declared.
References
- 1.Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med 2005; 353: 1591–1603. [DOI] [PubMed] [Google Scholar]
- 2.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003; 21: 2636–2644. [DOI] [PubMed] [Google Scholar]
- 3.Bodmer JL, Schneider P, Tschopp J. The molecular architecture of the TNF superfamily. Trends Biochem Sci 2002; 27: 19–26. [DOI] [PubMed] [Google Scholar]
- 4.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 2001; 104: 487–501. [DOI] [PubMed] [Google Scholar]
- 5.Nolte MA, van Olffen RW, van Gisbergen KP, et al. Timing and tuning of CD27–CD70 interactions: the impact of signal strength in setting the balance between adaptive responses and immunopathology. Immunol Rev 2009; 229: 216–231. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs J, Deschoolmeester V, Zwaenepoel K, et al. CD70: an emerging target in cancer immunotherapy. Pharmacol Ther 2015; 155: 1–10. [DOI] [PubMed] [Google Scholar]
- 7.Duggleby RC, Shaw TN, Jarvis LB, et al. CD27 expression discriminates between regulatory and non-regulatory cells after expansion of human peripheral blood CD4+ CD25+ cells. Immunology 2007; 121: 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang ZZ, Novak AJ, Ziesmer SC, et al. CD70+ non-Hodgkin lymphoma B cells induce Foxp3 expression and regulatory function in intratumoral CD4+CD25− T cells. Blood 2007; 110: 2537–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Law CL, Gordon KA, Toki BE, et al. Lymphocyte activation antigen CD70 expressed by renal cell carcinoma is a potential therapeutic target for anti-CD70 antibody–drug conjugates. Cancer Res 2006; 66: 2328–2337. [DOI] [PubMed] [Google Scholar]
- 10.Wischhusen J, Jung G, Radovanovic I, et al. Identification of CD70-mediated apoptosis of immune effector cells as a novel immune escape pathway of human glioblastoma. Cancer Res 2002; 62: 2592–2599. [PubMed] [Google Scholar]
- 11.Nilsson A, de Milito A, Mowafi F, et al. Expression of CD27–CD70 on early B cell progenitors in the bone marrow: implication for diagnosis and therapy of childhood ALL. Exp Hematol 2005; 33: 1500–1507. [DOI] [PubMed] [Google Scholar]
- 12.Ranheim EA, Cantwell MJ, Kipps TJ. Expression of CD27 and its ligand, CD70, on chronic lymphocytic leukemia B cells. Blood 1995; 85: 3556–3565. [PubMed] [Google Scholar]
- 13.Bertrand P, Maingonnat C, Penther D, et al. The costimulatory molecule CD70 is regulated by distinct molecular mechanisms and is associated with overall survival in diffuse large B-cell lymphoma. Genes Chromosomes Cancer 2013; 52: 764–774. [DOI] [PubMed] [Google Scholar]
- 14.Ge H, Mu L, Jin L, et al. Tumor associated CD70 expression is involved in promoting tumor migration and macrophage infiltration in GBM. Int J Cancer 2017; 141: 1434–1444. [DOI] [PubMed] [Google Scholar]
- 15.Pich C, Sarrabayrouse G, Teiti I, et al. Melanoma-expressed CD70 is involved in invasion and metastasis. Br J Cancer 2016; 114: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res 2017; 27: 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Claus C, Riether C, Schurch C, et al. CD27 signaling increases the frequency of regulatory T cells and promotes tumor growth. Cancer Res 2012; 72: 3664–3676. [DOI] [PubMed] [Google Scholar]
- 18.Jak M, Mous R, Remmerswaal EB, et al. Enhanced formation and survival of CD4+ CD25hi Foxp3+ T-cells in chronic lymphocytic leukemia. Leuk Lymphoma 2009; 50: 788–801. [DOI] [PubMed] [Google Scholar]
- 19.Chahlavi A, Rayman P, Richmond AL, et al. Glioblastomas induce T-lymphocyte death by two distinct pathways involving gangliosides and CD70. Cancer Res 2005; 65: 5428–5438. [DOI] [PubMed] [Google Scholar]
- 20.Diegmann J, Junker K, Loncarevic IF, et al. Immune escape for renal cell carcinoma: CD70 mediates apoptosis in lymphocytes. Neoplasia 2006; 8: 933–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prasad KV, Ao Z, Yoon Y, et al. CD27, a member of the tumor necrosis factor receptor family, induces apoptosis and binds to Siva, a proapoptotic protein. Proc Natl Acad Sci U S A 1997; 94: 6346–6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang ZZ, Grote DM, Xiu B, et al. TGF-beta upregulates CD70 expression and induces exhaustion of effector memory T cells in B-cell non-Hodgkin’s lymphoma. Leukemia 2014; 28: 1872–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong H, Zhu G, Tamada K, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999; 5: 1365–1369. [DOI] [PubMed] [Google Scholar]
- 24.Fanoni D, Tavecchio S, Recalcati S, et al. New monoclonal antibodies against B-cell antigens: possible new strategies for diagnosis of primary cutaneous B-cell lymphomas. Immunol Lett 2011; 134: 157–160. [DOI] [PubMed] [Google Scholar]
- 25.Terme M, Ullrich E, Aymeric L, et al. IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer Res 2011; 71: 5393–5399. [DOI] [PubMed] [Google Scholar]
- 26.Nishimura H, Honjo T. PD-1: an inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol 2001; 22: 265–268. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura H, Nose M, Hiai H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999; 11: 141–151. [DOI] [PubMed] [Google Scholar]
- 28.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev 2008; 224: 166–182. [DOI] [PubMed] [Google Scholar]
- 29.Inaguma S, Lasota J, Wang Z, et al. Expression of ALCAM (CD166) and PD-L1 (CD274) independently predicts shorter survival in malignant pleural mesothelioma. Hum Pathol 2018; 71: 1–7.28811252 [Google Scholar]
- 30.Miettinen M A simple method for generating multitissue blocks without special equipment. Appl Immunohistochem Mol Morphol 2012; 20: 410–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanda Y Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013; 48: 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inaguma S, Kasai K, Ikeda H. GLI1 facilitates the migration and invasion of pancreatic cancer cells through MUC5AC-mediated attenuation of E-cadherin. Oncogene 2011; 30: 714–723. [DOI] [PubMed] [Google Scholar]
- 33.Inaguma S, Riku M, Hashimoto M, et al. GLI1 interferes with the DNA mismatch repair system in pancreatic cancer through BHLHE41-mediated suppression of MLH1. Cancer Res 2013; 73: 7313–7323. [DOI] [PubMed] [Google Scholar]
- 34.Inaguma S, Ito H, Riku M, et al. Addiction of pancreatic cancer cells to zinc-finger transcription factor ZIC2. Oncotarget 2015; 6: 28257–28268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goto N, Tsurumi H, Takemura M, et al. Serum soluble CD27 level is associated with outcome in patients with diffuse large B-cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone. Leuk Lymphoma 2012; 53: 1494–1500. [DOI] [PubMed] [Google Scholar]
- 36.Lens SM, Drillenburg P, den Drijver BF, et al. Aberrant expression and reverse signalling of CD70 on malignant B cells. Br J Haematol 1999; 106: 491–503. [DOI] [PubMed] [Google Scholar]
- 37.Riether C, Schurch CM, Buhrer ED, et al. CD70/CD27 signaling promotes blast stemness and is a viable therapeutic target in acute myeloid leukemia. J Exp Med 2017; 214: 359–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schurch C, Riether C, Matter MS, et al. CD27 signaling on chronic myelogenous leukemia stem cells activates Wnt target genes and promotes disease progression. J Clin Invest 2012; 122: 624–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawaguchi K, Murakami H, Taniguchi T, et al. Combined inhibition of MET and EGFR suppresses proliferation of malignant mesothelioma cells. Carcinogenesis 2009; 30: 1097–1105. [DOI] [PubMed] [Google Scholar]
- 40.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003; 299: 1057–1061. [PubMed] [Google Scholar]
- 41.Wang QJ, Hanada K, Robbins PF, et al. Distinctive features of the differentiated phenotype and infiltration of tumor-reactive lymphocytes in clear cell renal cell carcinoma. Cancer Res 2012; 72: 6119–6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 2015; 372: 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tannir NM, Forero-Torres A, Ramchandren R, et al. Phase I dose-escalation study of SGN-75 in patients with CD70-positive relapsed/refractory non-Hodgkin lymphoma or metastatic renal cell carcinoma. Invest New Drugs 2014; 32: 1246–1257. [DOI] [PubMed] [Google Scholar]
- 46.Silence K, Dreier T, Moshir M, et al. ARGX-110, a highly potent antibody targeting CD70, eliminates tumors via both enhanced ADCC and immune checkpoint blockade. MAbs 2014; 6: 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wajant H Therapeutic targeting of CD70 and CD27. Expert Opin Ther Targets 2016; 20: 959–973. [DOI] [PubMed] [Google Scholar]
- 48.Buchan SL, Manzo T, Flutter B, et al. OX40- and CD27-mediated costimulation synergizes with anti-PD-L1 blockade by forcing exhausted CD8+ T cells to exit quiescence. J Immunol 2015; 194: 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. CD70 expression in 172 malignant pleural mesothelioma patients
Figure S2. CD70 expression in CD70 or LacZ transfectants
Figure S3. Anti-CD70 antibody blocked CD70–CD27 interaction
Figure S4. Overall survival of malignant pleural mesothelioma cases according to CD70 expression
Figure S5. Knockdown of CD70 suppressed MET expression but did not modulate apoptosis or cellular proliferation of ACC-MESO-1
Figure S6. Anti-CD70 antibody did not modulate migration, invasion, or expression of MET and phospho-ERK in CD70-expressing mesothelioma cells
Figure S7. Supplementary data for animal experiments
Table S1. Antibodies and conditions for immunohistochemistry
Table S2. Antibodies and dilutions for immunoblotting analyses





