Abstract
Obstructive sleep apnea (OSA), independently of obesity (OBS), predisposes to insulin resistance (IR) for largely unknown reasons. Because OSA-related intermittent hypoxia triggers lipolysis, overnight increases in circulating free fatty acids (FFAs) including palmitic acid (PA) may lead to ectopic intramuscular lipid accumulation potentially contributing to IR. Using 3-T-1H-magnetic resonance spectroscopy, we therefore compared intramyocellular and extramyocellular lipid (IMCL and EMCL) in the vastus lateralis muscle at approximately 7 am between 26 male patients with moderate-to-severe OSA (17 obese, 9 nonobese) and 23 healthy male controls (12 obese, 11 nonobese). Fiber type composition was evaluated by muscle biopsies. Moreover, we measured fasted FFAs including PA, glycated hemoglobin A1c, thigh subcutaneous fat volume (ScFAT, 1.5-T magnetic resonance tomography), and maximal oxygen uptake (VO2max). Fourteen patients were reassessed after continuous positive airway pressure (CPAP) therapy. Total FFAs and PA were significantly (by 178% and 166%) higher in OSA patients vs controls and correlated with the apnea-hypopnea index (AHI) (r ≥ 0.45, P < .01). Moreover, IMCL and EMCL were 55% (P < .05) and 40% (P < .05) higher in OSA patients, that is, 114% and 103% in nonobese, 24.4% and 8.4% in obese participants (with higher control levels). Overall, PA, FFAs (minus PA), and ScFAT significantly contributed to IMCL (multiple r = 0.568, P = .002). CPAP significantly decreased EMCL (–26%) and, by trend only, IMCL, total FFAs, and PA. Muscle fiber composition was unaffected by OSA or CPAP. Increases in IMCL and EMCL are detectable at approximately 7 am in OSA patients and are partly attributable to overnight FFA excesses and high ScFAT or body mass index. CPAP decreases FFAs and IMCL by trend but significantly reduces EMCL.
Keywords: ectopic fat, intermittent hypoxia, diabetes, obesity, aerobic capacity
Obstructive sleep apnea syndrome (OSA) and obesity (OBS) are closely associated conditions that both impose severe metabolic as well as cardiovascular risks [1-6]. The prevalence of moderate to severe OSA (as defined by an apnea-hypopnea index [AHI] > 15 h–1) in male adults aged 50 to 70 years reaches at least 17% and is largely dependent on OBS, that is, it increases to an alarming 29.9% or even 56% with a body mass index (BMI) of 30 to 40 or greater than 40 kg m−2, respectively [7-9]. According to a more recent large, community-based study, these numbers may still represent an underestimation [10]. OBS is considered to promote OSA directly via parapharyngeal or thoracic fat or indirectly through increases in circulating cytokines, insulin, or leptin as implicated in impaired sleep quality/quantity and ventilatory control [2, 11, 12]; by contrast, OSA-related sleep fragmentation and chronic intermittent hypoxia (CIH) may contribute to weight gain and metabolic disorders possibly involving novel factors like peripheral chemoreceptor dysfunction. Despite such complex interaction with OBS, OSA independently predisposes to hypertension [13], increased carotid intima media thickness (cIMT) [14], coronary heart disease [15, 16], myocardial infarction [17], stroke [18-20], or death [4, 5]. More recently, an OBS-independent association between OSA and insulin resistance (IR) and type 2 diabetes [2, 6, 21] was shown to be causal by a randomized controlled trial demonstrating that IR was significantly improved through (supervised) 8 hours of continuous positive airway pressure (CPAP) per night [22]. However, the common adherence of 4 hours CPAP per night under real-world conditions may not have a significant benefit for IR [1, 19, 23, 24].
Until now, the specific link between OSA and IR has remained unclear and obscured by a large overlap of OSA- and OBS-associated factors of IR like the proinflammatory/oxidative milieu [25-27], dyslipidemia, and hepatic steatosis [28]. OSA may, however, involve OBS-independent, unique pathophysiological triggers through CIH, sympathetic activation, periodic sways in intrathoracic and O2/CO2 partial pressures, and sleep fragmentation. These factors result in massive increases in circulating catecholamines, atrial natriuretic peptide, and angiotensin II [1, 2], low nitric oxide availability, for example, via low endothelial NO synthetase expression, increased nitrotyrosine formation [27, 29], in addition to increases in vascular endothelial growth factor expression [30, 31]. Therefore, the mechanisms with OSA and OBS leading to IR, dyslipidemia, and inflammation may be different, as illustrated by the separate or combined effects of CPAP and weight loss intervention [23]. Indeed, IR could be experimentally induced in lean healthy volunteers through 5 hours of CIH corresponding to an AHI of 24.3 h–1 [32-34]. In lean mice, IR could be triggered by sleep fragmentation [35], and IR caused by CIH was successfully prevented by alpha-adrenergic (but not beta-adrenergic) blockade [36, 37]. The increase of circulating catecholamines through exaggerated and repetitive sympathetic activation (especially with OSA-related long-term facilitation of the peripheral (carotid) O2 chemoreflex) may well result in excessive lipolysis [38] as demonstrated by numerous peaks in plasma free fatty acids (FFAs) during the first 4 hours of sleep in OSA patients on interruption of CPAP therapy [39]. Earlier studies already demonstrated that OSA-related FFA increases can be prevented by oxygen supply in patients with heart failure [40] and that FFA infusion in lean individuals caused skeletal lipid accumulation and IR [41].
It was therefore hypothesized by Gu et al [38] that OSA could trigger a “perfect storm” of FFAs, leading to ectopic lipid accumulation in skeletal muscle fibers (in addition to endothelium and hepatocytes), where it is an established factor of peripheral IR [42, 43]. Increased intramyocellular lipid (IMCL) may indeed lead to decreases in insulin signaling to protein kinase B, mitochondrial density, and function among other metabolic perturbations that are considered to be in line with a “lipotoxic” concept, or the more recent, “gridlock” theory of IR, an insufficient switching between competing, overloaded substrates [44-46]. Moreover, increases in extramyocellular lipid (EMCL) may also become critical for IR, as EMCL may recruit inflammatory cells, especially macrophages, expressing paracrine factors of IR like tumor necrosis factor (TNF) [47, 48]. TNF along with other inflammatory cytokines may impair microvascular regulation and therefore the delivery of insulin and glucose to myofibers (the “microvascular” concept of IR) [49].
To date, studies on IMCL and EMCL in OSA are rare and limited to 1.5-Tesla (T) 1H-magnetic resonance spectroscopy (1H-MRS), which provides rather limited separation of IMCL and EMCL signals. A recent cross-sectional study showed significantly lower IMCL and EMCL of the vastus lateralis muscle in patients with severe OSA and impaired glycemic control as compared to healthy controls [50]. A previous 1.5 T 1H-MRS study detected no difference in either IMCL or insulin sensitivity in obese OSA patients under regular CPAP treatment compared to irregular CPAP treatment use over 12 weeks [51].
In our study we hypothesized, in line with others [38, 42, 43], that using 3-T 1H-MRS and taking measures at around 7 am, that is, immediately after overnight lipolytic episodes, will allow us to detect significant increases of IMCL and EMCL (primary outcome) in OSA patients compared to healthy controls that might be related to higher circulating FFA levels. In addition to this cross-sectional comparison we also evaluated whether 3 to 5 months of home-based CPAP would affect (normalize) IMCL, EMCL, and FFAs. Muscle biopsies were obtained to control for fiber type composition in both the cross-sectional and the longitudinal comparison, as the lipid content has been shown to depend on fiber type [52].
Materials and Methods
Study Population and Design
Twenty-six male patients with hitherto untreated moderate to severe OSA (AHI > 15 h–1) were recruited from the outpatient clinic of the Department of the Sleep Medicine of the University Clinics of Marburg after home cardiorespiratory polygraphy for eligibility. The patients were assigned to an obese stratum (BMI ≥ 30 kg m−2, n = 17; BMI range, 30.0-36.6 kg m−2) or to a nonobese stratum with or without overweight (BMI < 30 kg m−2, n = 9; BMI range: 21.0-28.4 kg m−2) (Table 1). Twenty-three healthy men of similar age without a history of snoring or sleep apnea were recruited by public announcement to an obese control stratum (n = 12; BMI range, 30.0-38.0 kg m−2) or to a nonobese control stratum with or without overweight (n = 11; BMI range: 21.1-29.4 kg m−2) (see Table 1). Prior to inclusion all participants underwent 2 overnight polysomnographies in the hospital to assess the severity of OSA. After an overnight fast (second stationary night), all participants underwent 3-T 1H-MRS for IMCL and EMCL at around 7 am and provided a venous blood sampling between 7 and 9 am. At least 2 hours after breakfast, the participants’ right thigh subcutaneous fat volume (ScFAT) was determined by 1.5-T magnetic resonance tomography and their maximal oxygen uptake (VO2max) was measured. Muscle biopsies were obtained several hours thereafter (to allow for blood flow normalization). Any study-related delay in the initiation of CPAP therapy was excluded by individual adjustments in the time schedule for outpatients. Fourteen OSA patients agreed to a longitudinal evaluation after 3 to 5 months of home-based CPAP therapy on the variables IMCL, EMCL, blood parameters, VO2max, and muscle biopsy histomorphometry. Informed oral and written consent was obtained from all participants. This study was approved by the local ethical committee of the medical faculty (FB20) of the University of Marburg (63/11, September 12, 2011) and complied with good laboratory and medical practice and the amended Declaration of Helsinki (1996).
Table 1.
Anthropometry, polysomnography, vascular risk factors, free fatty acids, intramyocellular lipid content and extramyocellular lipid content as well as maximal oxygen uptake and physical activity of untreated obstructive sleep apnea patients and controls and their respective nonobese and obese strata
| All | Nonobese | Obese | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | OSA | Control | OSA | Control | OSA | ANOVA | ||
| No. | 23 | 26 | 11 | 9 | 12 | 17 | 49 | |
| Age | y | 45.4 ± 1.2 | 48.8 ± 1.4 | 45.0 ± 1.7 | 52.2 ± 1.7b | 45.8 ± 1.7 | 47.0 ± 1.7d | a |
| Body ht | cm | 180.2 ± 1.2 | 176.2 ± 3.0 | 182.1 ± 1.8 | 180.0 ± 2.5 | 178.5 ± 1.6 | 174.2 ± 4.4 | |
| Body wt | kg | 94.4 ± 2.5 | 102.1 ± 4.5 | 84.3 ± 1.8 | 83.1 ± 2.8 | 103.7 ± 2.3f | 112.2 ± 5.3f | f |
| BMI | kg m−2 | 29.3 ± 0.9 | 30.9 ± 0.9 | 25.8 ± 0.7 | 25.6 ± 0.8 | 32.5 ± 0.6f | 33.7 ± 0.5f | f |
| Subcutaneous fat, thigh | cm3 | 3766 ± 279 | 4064 ± 226 | 2693 ± 181 | 3050 ± 242 | 4481 ± 307 | 4605 ± 218 | f |
| AHI | h–1 | 5.3 ± 0.8 | 48.8 ± 4.6c | 5.8 ± 1.3 | 41.8 ± 6.8c | 4.8 ± 1.0 | 52.8 ± 6.1c | c |
| Time < 90% SaO2 | min | 1.86 ± 0.79 | 64.5 ± 15.0c | 0.81 ± 0.43 | 47.32 ± 17.46b | 2.82 ± 1.43 | 73.6 ± 21.0c | c |
| Mean SaO2 | % | 94.3 ± 0.2 | 92.5 ± 0.4b | 94.2 ± 0.2 | 92.9 ± 0.5a | 94.3 ± 0.3 | 92.2 ± 0.5b | c |
| Heart rate at rest | min–1 | 64.8 ± 2.0 | 72.0 ± 2.2a | 68.5 ± 3.3 | 70.4 ± 4.7 | 61.4 ± 2.0 | 72.9 ± 2.4a | a |
| BP systolic | mm Hg | 137.5 ± 2.0 | 146.6 ± 3.4a | 136.0 ± 1.4 | 136.8 ± 5.5 | 138.8 ± 2.7 | 151.6 ± 3.1a,d | a,d |
| BP diastolic | mm Hg | 88.0 ± 1.1 | 94.4 ± 2.0b | 86.9 ± 1.5 | 89.6 ± 3.2 | 89.0 ± 1.7 | 97.1 ± 2.3b | a,d |
| cIMT | mm | 0.62 ± 0.02 | 0.70 ± 0.03a | 0.61 ± 0.03 | 0.71 ± 0.06 | 0.63 ± 0.03 | 0.70 ± 0.03 | a |
| HbA1c | % | 5.50 ± 0.05 | 5.70 ± 0.08a | 5.48 ± 0.06 | 5.71 ± 0.06a | 5.50 ± 0.09 | 5.70 ± 0.12 | a |
| Glucose, fasted | mg 100 mL–1 | 92.3 ± 1.5 | 98.5 ± 2.5a | 93.3 ± 1.9 | 98.0 ± 4.3 | 91.3 ± 2.3 | 98.8 ± 3.1 | |
| Insulin, fasted | µU mL–1 | 7.69 ± 1.25 | 12.35 ± 1.51a | 5.45 ± 0.98 | 8.64 ± 2.12 | 9.93 ± 2.15 | 14.21 ± 1.88 | d |
| HOMA-IR | 1.77 ± 0.30 | 3.04 ± 0.39a | 1.24 ± 0.22 | 2.15 ± 0.59 | 2.30 ± 0.53 | 3.48 ± 0.47 | a,d | |
| TNF, circulating | pg mL–1 | 9.01 ± 1.16 | 6.93 ± 0.28 | 8.93 ± 2.27 | 7.56 ± 0.66 | 9.08 ± 0.74 | 6.58 ± 0.19b | |
| HDL | mg 100 mL–1 | 48.3 ± 2.0 | 42.8 ± 2.7 | 51.5 ± 3.2 | 42.9 ± 2.5 | 45.3 ± 2.3 | 42.8 ± 4.0 | |
| LDL | mg 100 mL–1 | 138.4 ± 6.6 | 138.2 ± 9.6 | 153.7 ± 6.4 | 147.0 ± 17.2 | 124.4 ± 9.7d | 133.3 ± 11.6 | |
| Total cholesterol | mg 100 mL–1 | 203.3 ± 8.0 | 214.1 ± 7.8 | 219.6 ± 8.9 | 229.0 ± 15.2 | 188.4 ± 11.1d | 205.8 ± 8.3 | d |
| Triglycerides | mg 100 mL–1 | 110.1 ± 12.7 | 165.8 ± 21.5 | 95.6 ± 12.6 | 214.7 ± 40.9a | 123.3 ± 21.2 | 138.4 ± 22.7 | a,g |
| Total FFAs, C12-C24 | mg mL–1 | 5.29 ± 0.31 | 14.72 ± 3.33b | 5.05 ± 0.33 | 16.95 ± 6.75a | 5.51 ± 0.53 | 13.54 ± 3.76 | a |
| Palmitic acid, 16:0 | mg mL–1 | 1.11 ± 0.07 | 2.96 ± 0.67a | 1.02 ± 0.07 | 3.47 ± 1.37a | 1.21 ± 0.12 | 2.69 ± 0.74 | a |
| IMCL | a.u. | 13.55 ± 2.03 | 21.06 ± 2.68a | 9.06 ± 1.15 | 19.36 ± 3.91a | 17.66 ± 3.39d | 21.97 ± 3.61 | a |
| EMCL | a.u. | 15.89 ± 2.39 | 22.22 ± 2.04a | 11.81 ± 1.80 | 24.00 ± 3.83b | 19.64 ± 4.07 | 21.28 ± 2.44 | a |
| VO2max | l min–1 | 3.24 ± 0.13 | 2.79 ± 0.16a | 3.51 ± 0.16 | 2.43 ± 0.27b | 2.94 ± 0.18d | 2.95 ± 0.20 | a,g |
| VO2max/kg | mL min–1 kg–1 | 35.4 ± 2.1 | 28.5 ± 1.5b | 41.9 ± 2.5 | 29.5 ± 3.2b | 28.3 ± 1.6f | 28.0 ± 1.6 | b,e,h |
| Physical activity | h wk–1 | 2.59 ± 0.63 | 1.00 0.27a | 3.86 ± 0.24 | 1.11 ± 1.76 | 1.42 ± 0.50 | 0.94 ± 0.30 | a,d |
Data represent mean ± SEM.
Abbreviations: AHI, apnea-hypopnea index; ANOVA, analysis of variance; a.u., arbitrary units; BMI, body mass index; BP, brachial arterial blood pressure; cIMT, carotid intima media thickness; EMCL, extramyocellular lipid content; FFAs, free fatty acids; HbA1c, glycated hemoglobin A1c; HDL, high-density lipoprotein; HOMA-IR, homeostasis assessment model index of insulin resistance (see “Materials and Methods”); IMCL, intramyocellular lipid content; LDL, low-density lipoprotein; OSA, obstructive sleep apnea; SaO2, oxygen saturation; TNF, tumor necrosis factor; VO2max, maximal oxygen uptake with or without normalization for body weight in kg.
a P less than .05; bP less than .01; cP less than .001 OSA vs control.
d P less than .05; eP less than .01; fP less than .001 obese vs nonobese.
g P less than .05; hP less than .01 for interaction of factor OSA and obesity by ANOVA (for details see “Materials and Methods”).
All participants underwent an initial medical assessment that included a detailed medical history including physical activity, physical examination, postabsorptive venous routine blood parameters, bilateral brachial blood pressure measurement by the Riva-Rocci method after 20 minutes of rest, ultrasonic measurement of cIMT by the Logiq E9 system and 9D-L-scanner (GE), pulmonary function, and a 12-lead electrocardiogram at rest.
VO2max was assessed by an incremental cycle ergometer test using the Ergoline 100 system with workload increments of 25 W every 2 minutes starting from 50 W until exhaustion. VO2max was calculated as the maximum of mean values covering intervals of 10 seconds using the ZAN 680 breath-by-breath spirometry system (ZAN Ferraris Cardiorespiratory) for measurement of tidal volume, breathing frequency, as well inspiratory and end-tidal gas fractions. Continuous measurements of this system furthermore included a 12-lead electrocardiogram and left index fingertip oximetry data (Nonin 7500, nSpire Health).
Exclusion criteria: Age younger than 35 or older than 65 years; smoking or any history of smoking; any medication; any vitamin or antioxidant supplementation; central or mixed sleep apnea determined by polysomnography; prior CPAP treatment; type 2 diabetes mellitus or glycated hemoglobin A1c (HbA1c) greater than 7.0; preexisting arterial hypertension or a resting (20 minutes) systolic brachial arterial blood pressure of more than 180 or diastolic of greater than 115 mm Hg; any known cardiac, respiratory, or other internal, neurological or psychiatric, immunological, or inflammatory diseases; any alcohol or drug abuse; missing written consent; or any reported coagulopathy or susceptibility to keloid development with regard to skin incision for muscle biopsy.
Inpatient Polysomnography
The polysomnography on 2 consecutive nights was performed at the nationally licensed Department of Sleep Medicine, University Clinics of Marburg, using the SIDAS-GS-System (Fa. Stimotron Medizinische Geräte GmbH). The first night was to familiarize the participants with the equipment and the environment. Data obtained during the second night were used to determine AHI (during total sleep time) of obstructive, central nervous, or mixed origin, arousal index (n/h total sleep time) mean, and minimal O2 saturation (SaO2) in rapid eye movement (REM) or non-REM sleep phases, cumulative time (minutes) spent at SaO2 less than 90% (Tu90%), less than 80% or less than 70% as well as time (minutes) and fraction (%) of sleep stages in REM and non-REM (1, 2 or 3, 4) among other parameters. On night 3 or 4 at the sleep center (ie, after baseline measurements within this study), the OSA patients were introduced to optimized CPAP treatment. After 3 to 5 months of home-based CPAP treatment, 14 OSA patients were reevaluated in the hospital regarding their treatment effectiveness and compliance before reassessment of study parameters.
Venous Blood Parameters
Antecubital venous blood samples were obtained after an overnight fast and routinely processed by the Central Laboratory of the University Clinics of Marburg for plasma levels of HbA1c (HPLC, Tosoh G8, Tosoh Bioscience), glucose, as well as insulin (Cobas 8000, Roche) for calculation of the homeostasis model assessment index of IR (HOMA-IR, “insulin (µU/mL) × glucose (mg/100 mL)/405”), furthermore, triglycerides, total cholesterol, low-density lipoprotein, high-density lipoprotein, as well as other routine laboratory screening parameters for hepatic, renal, and muscular diseases (AU5800 chemistry analyzers, Beckman Coulter), myoglobin, total plasma homocysteine, thyrotropin, 3,5,3′-triiodothyronine, and thyroxine (DxI 800, Beckman Coulter), hemoglobin, hematocrit, and differential hemogram (XN system, Sysmex) as well as TNF (Immulite 1000, Siemens). The intra-assay coefficient of variation was less than 5% for all parameters.
For measurement of plasma levels of total FFAs (C12-C24) including palmitic acid (PA, C16:0), sample preparation and detection of fatty acid methyl esters was performed according to the method of Bicalho et al [53] using gas chromatography/mass spectrometry (Agilent Technologies, 7890 B) with a coefficient of variation less than 5% as well.
3-Tesla 1H-Magnetic Resonance Spectroscopy for Measurements of Intramyocellular Lipid and Extramyocellular Lipid
The 1H-MRS scans of IMCL and EMCL were obtained by the 3-T MR scanner Siemens Magnetom Trio at around maximal convexity of the right vastus lateralis muscle (on maximal knee extension) at 7 am in overnight-fasted supine participants in a head-out position. Post-CPAP remeasurement was located 5 cm proximal or distal (at random order) of the pre-CPAP measurement to avoid interference with the first muscle biopsy (see the following sections). The spectra were acquired using a single-voxel spectroscopy (SVS, repetition time: 2000 ms; echo time: 20 ms; averages: 176; volume of interest: 20 × 20 × 20 mm3; TM: 10 ms; flip angle: 90°; water saturation; shimming < 40 Hz full width half maximum. All spectra were analyzed and postprocessed by JMRUI (v5), Amares (http://www.jmrui.eu). The following substances were identified: IMCL (1.3 ppm), EMCL (1.5 ppm), and creatine (3.03 ppm). The IMCL and EMCL signals were normalized for the creatine signal.
1.5-T Magnetic Resonance Tomography for Volugraphy of Thigh Subcutaneous Fat Volume
The total ScFAT of the right thigh was quantified between the trochanter minor and the upper patella margin by a 1.5-T whole-body MR scanner (Siemens Espree). The images were acquired using a T2 turbo-spin-echo sequence (TSE, repetition time = 5490 ms, echo time = 91 ms, slice thickness = 4.0 mm, interslice gap = 0%) with composing algorithm: spine (syngo MR B17). The software OSIRIX (OSIRIX v.6.0.2 32-bit, Pixmeo) with segmentation plugin (Segmentation 1.0.xr plugin, Chimaera GmbH) was used for the volugraphy of ScFAT in all volunteers.
Skeletal Muscle Fiber Histomorphology and Composition
In 25 OSA patients and 20 controls muscle biopsies were obtained from the vastus lateralis muscle at the exact site of 1H-MRS by conchotome technique through an 8-mm skin and fascia incision after local anesthesia and disinfection, immediately shock-frozen in liquid N2-cooled isopentane, and stored at –80 °C. Transverse 7 µm-cryosections (cryostat microtome Hyrax C60, Carl Zeiss AG) were used for histochemical identification of fiber type 1, 2a, or 2x using the acid-sensitive myofibrillar adenosine triphosphatase (ATPase) staining (adenosine triphosphatase, Sigma-Aldrich Co LLC) after preincubation at pH 4.6 (5 minutes, room temperature) as previously described [54]. Fiber-type–specific cross-sectional area and composition (percentage of type 1, 2a, and 2x fiber count and overall area) were analyzed in rectangular tissue areas with more than 100 fibers by applying ImageJ (Scion Image, National Institutes of Health) to digitized images with 200-fold magnification obtained by the Zeiss Axio Imager.M2 microscope (Carl Zeiss AG combined with Axio-Cam HRc/AxioVision,).
Statistics
Continuous variables were presented as means ± SEM for the total group of control individuals and OSA patients (before and after CPAP treatment) as well as for their nonobese and obese strata. Cross-sectional analyses of the separate impact of untreated OSA, OBS, or their interaction were performed by 2-factorial analysis of variance (ANOVA). The unpaired t test or, whenever adequate according to a Kolmogorov-Smirnov test within groups and strata, the Mann-Whitney U test was used (post hoc) to detect significant differences between groups or strata. Significant OSA- and OBS-related differences were indicated by the symbols “*” and “#,” respectively, in the figures (for tables see legends). The t test for paired observation was used to assess significant individual changes pre- and post-CPAP treatment (indicated by §), in the figures (for tables see legends). Bivariate correlations between individual parameters (eg, between FFAs and AHI) were described by scatter plots with identification of groups and strata, regression line, Pearson correlation coefficient r, and corresponding P values or presented by r and P values in the text. Multivariable regression was used to evaluate the contribution of likely relevant factors to IMCL (and EMCL), for example, by combining the factors total FFAs (minus PA) and PA plasma levels together with ScFAT (or BMI). To illustrate overall prediction of IMCL by contributors identified to be independent and significant, IMCL (y-axis) was plotted against the predictive term (x-axis) derived from the multivariable regression equation together with the regression line and indication of multiple r and P values. A P level less than .05 was considered statistically significant. Owing to the exploratory rather than confirmatory use of statistical analysis, no Bonferroni correction was used. SPSS software version 22.0 (IBM) was used for all statistical procedures.
Results
Cross-Sectional Comparison at Baseline
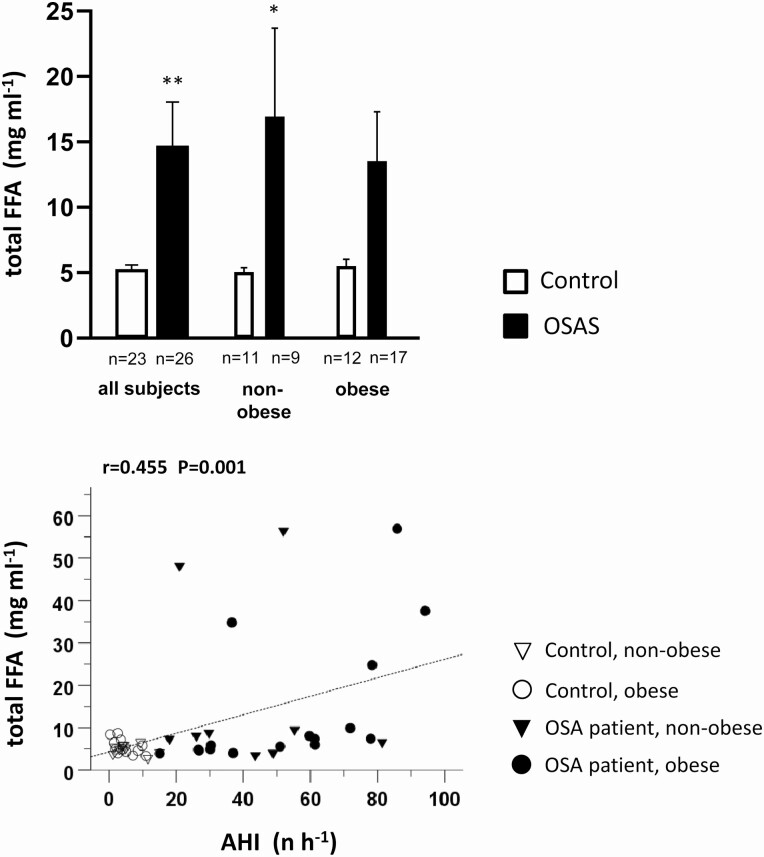
In general, the anthropometric parameters (see Table 1, P values of 2-factorial ANOVA on the right side) were similar between the total group of OSA patients and control participants and between their respective nonobese or obese strata. However, owing to their rare eligibility, the 9 nonobese OSA patients had a significantly (by 7.2 and 5.2 years) higher age than nonobese controls and obese OSA patients, respectively. The total thigh ScFAT showed no significant difference between OSA and controls; it was, however, significantly affected by OBS. OSA patients showed an AHI between 15.1 and 94.2 h–1. They were exposed to a significantly longer Tu90% (64.5 ± 15.0 minutes; controls: 1.86 ± 0.79 minutes) and a significantly (by 1.2%) lower mean SaO2. OSA was associated with a moderately but significantly higher systolic and diastolic blood pressure, with the latter also being affected by OBS, whereas higher cIMT was attributable solely to OSA. Moreover, OSA was associated with a moderate but significant increase in HbA1c, whereas fasted glucose, insulin, and HOMA-IR-index were increased both with OSA and OBS. Circulating plasma TNF levels showed no OSA- or OBS-related differences. Within the lipid profile, increased plasma triglyceride levels were associated with OSA, especially in the nonobese strata, whereas total cholesterol was lower with OBS as detectable within controls. There was a significant increase in plasma levels of FFAs (by 178%) and PA (by 166%) with OSA but not with OBS (see Table 1, Figure 1 upper panel) with these increases being more accentuated in the nonobese (by 236% and 246%) than in the obese (by 146% and 122%) strata.
Figure 1.
Upper panel: Total free fatty acid (FFA) plasma levels between 7 am and 9 am in obstructive sleep apnea (OSA) patients and healthy control individuals and their nonobese and obese strata. Mean values ± SEM, *P less than .05; **P less than .01 OSA vs control. Lower panel: Correlation of total FFA plasma levels to apnea-hypopnea index (AHI) within the total study population with indication of the individual assignment to the 2 groups, OSA and controls, and their nonobese or obese strata as well as of Pearson correlation coefficient r and P.
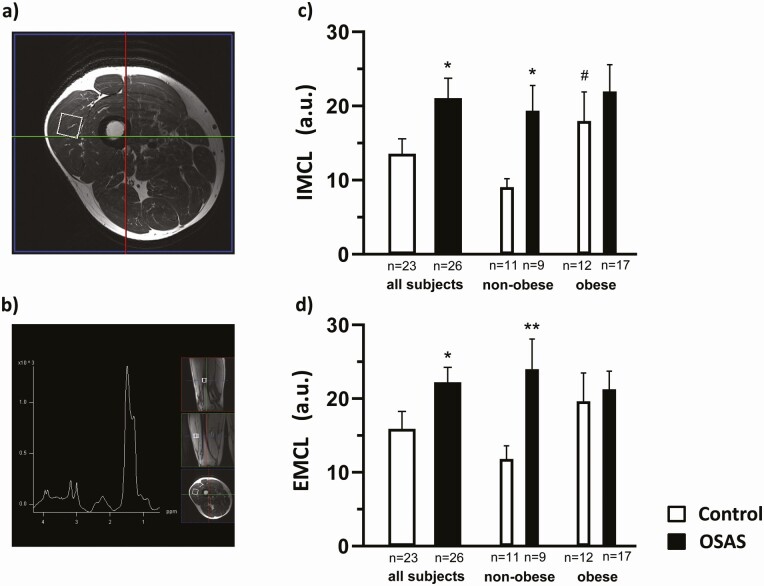
As a main finding, IMCL and EMCL were found to be significantly, by approximately 55% and 40%, respectively, higher in the total group of OSA as compared to the control group (Figure 2, see Table 1). Thereby, notably, the nonobese OSA patients showed massive increases of IMCL (114%) and EMCL (103%) compared to their nonobese controls. In contrast, in obese OSA patients increases of 24.4% and 8.4%, respectively, were observed compared to their obese controls, notably, with prevailing high IMCL and EMCL. The overall impact of OBS on IMCL and EMCL did not reach significance but obviously increased IMCL by 95% (P < .05) and EMCL by 66% (P > .05) in obese compared to nonobese OSA-free control participants. Thereby, interestingly, no significant additional (or interactive) muscular lipid accumulation arose from OBS within the group of OSA patients according to ANOVA (see Figure 2, Table 1).
Figure 2.
1H-magnetic resonance spectroscopy (1H-MRS, 3 Tesla) of intramyocellular and extramyocellular lipid (IMCL and EMCL) accumulation in the right vastus lateralis muscle at 7 am of overnight fasted obstructive sleep apnea (OSA) patients compared to healthy control individuals. A, Localization of the 1H-MRS voxel within an MR tomographic image obtained at maximal convexity of the vastus lateralis muscle was marked for muscle biopsy. B, 1H-MR spectrum with peaks relating to IMCL (1.3 ppm), EMCL (1.5 ppm), and creatine (3.03 ppm). C and D, Mean ± SEM IMCL and EMCL values, respectively, presented for the 2 groups of OSA patients and control individuals (left) and their respective nonobese (middle) and obese strata (right). Mean values ± SEM, *P less than .05; **P less than .01 OSA vs control; # P less than .05, obese vs nonobese strata. For 2-factorial analysis of variance, see Table 1 and “Materials and Methods.”
While VO2max in absolute terms was significantly reduced solely with OSA, VO2max normalized to body weight was also lower with OBS in addition to OSA and there was an interaction of OSA and OBS with both these parameters. Lower physical activity per week was found to be associated with OSA and OBS (see Table 1).
Skeletal muscle histomorphometry in biopsies largely excluded any significant impact of the factors OSA or OBS on type-specific fiber fraction (see Table 2). However, owing to enlarged fiber size, a significantly larger fractional area of type 2x fiber was observed with OBS, which correlated with BMI (r = 0.38, P = .01), likely reflecting some muscular adaptation to implicit body weight–related training.
Table 2.
Vastus lateralis muscle fiber composition of untreated obstructive sleep apnea patients and controls as well as their respective nonobese and obese strata
| All | Nonobese | Obese | ||||||
|---|---|---|---|---|---|---|---|---|
| Controls | OSA | Controls | OSA | Controls | OSA | ANOVA | ||
| No. | 20 | 25 | 10 | 8 | 10 | 17 | 45 | |
| Fiber count fraction—type 1 | % | 39.5 ± 3.1 | 40.6 ± 2.4 | 41.7 ± 4.5 | 36.3 ± 4.0 | 37.3 ± 4.3 | 42.6 ± 2.9 | |
| Fiber count fraction—type 2a | % | 31.5 ± 3.6 | 29.0 ± 1.9 | 35.5 ± 6.1 | 32.6 ± 3.5 | 27.6 ± 3.6 | 27.3 ± 2.2 | |
| Fiber count fraction—type 2x | % | 29.0 ± 3.0 | 30.8 ± 2.2 | 22.7 ± 3.9 | 31.1 ± 3.9 | 35.3 ± 3.6 | 30.6 ± 2.8 | |
| Fractional area—type1 | % | 39.3 ± 3.2 | 39.6 ± 2.3 | 41.8 ± 4.7 | 36.3 ± 4.4 | 36.8 ± 4.4 | 41.1 ± 2.6 | |
| Fractional area—type 2a | % | 34.6 ± 3.7 | 30.3 ± 2.1 | 38.8 ± 6.4 | 35.9 ± 3.9 | 30.4 ± 3.7 | 27.7 ± 2.3 | |
| Fractional area—type 2x | % | 26.1 ± 2.9 | 30.1 ± 2.1 | 19.3 ± 3.3 | 27.8 ± 3.0 | 32.8 ± 3.8 | 31.2 ± 2.8 | a |
Data represent mean ± SEM. No significant differences between groups or strata were detected by the unpaired t test or, whenever adequate, the Mann-Whitney U test. Overall, ANOVA detected a significant impact of obesity (aP < .05) on fractional area of type 2x fibers.
Abbreviations: ANOVA, analysis of variance; OSA, obstructive sleep apnea.
Correlations at Baseline
There were significant correlations of total FFAs and of PA to AHI (r = 0.455, P < .01 and r = 0.445, P < .01, respectively; see Figure 1 lower panel) as well as to Tu90% (r = 0.415, P < .05 and r = 0.407, P < .05, not shown), but not, however, to BMI (r = 0.076, P > .604 and r = 0.066, P = .661) within the total study population. IMCL and EMCL were overall unrelated to AHI (r = 0.18, P = .210 and r = 0.20, P = .168, respectively). There was, however, a correlation between IMCL and Tu90%, which indicates cumulative hypoxic stress (r = 0.331, P = .042), but not between EMCL and Tu90% (r = 0.319, P = .051). In addition, IMCL but not EMCL was significantly related to BMI (r = 0.333, P = .019) and to ScFAT (r = 0.447, P = .002). Within the nonobese strata, the IMCL and EMCL both were considerably closer correlated to AHI (r = 0.590, P = .006 and r = 0.446, P = .049, respectively) and to Tu90% than the obese strata. At the same time, the obese strata showed a closer correlation between IMCL and ScFAT (r = 0.534, P = .004) than the nonobese strata. A positive correlation between IMCL and plasma levels of total FFAs or of PA existed within the nonobese strata (r = 0.606, P = .005 or r = 0.642, P = .003, respectively), but not within the obese strata or the study population in total. In the nonobese strata, EMCL also showed significant correlations to plasma levels of total FFAs and PA (r = 0.675, P = .002 or r = 0.703, P = .001); the correlations were also significant within the total study population. Moreover, the nonobese, but not the obese strata, showed a significant positive correlation between IMCL and plasma levels of triglycerides (r = 0.448, P = .047). Regarding muscle histology, IMCL or EMCL were unrelated to type 1 fiber fraction (% count or % area), except for a significant negative correlation between EMCL and % type 1 fiber count. Furthermore, there was a significant negative correlation between both IMCL and EMCL to VO2max per kg body weight within the total study population and the nonobese strata.
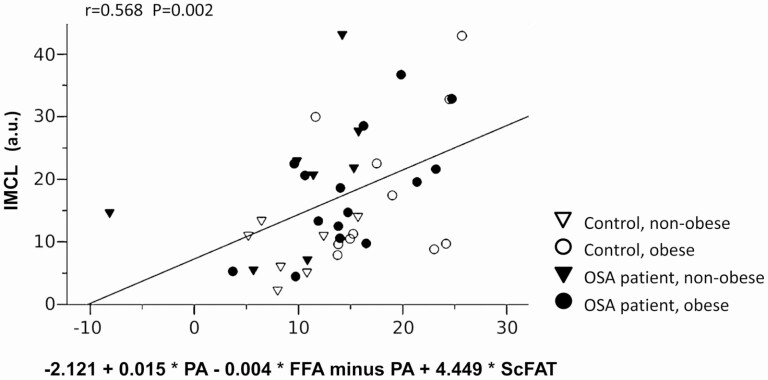
To explore factors predominantly contributing to high IMCL, we conducted a multivariable regression analysis considering the aforementioned factors given. The inclusion of 1) PA and 2) total FFAs (minus PA) plasma concentration (affected by OSA, ie, related to AHI) on the one hand and 3) ScFAT (affected by OBS, ie, related to BMI: r = 0.765, P < .001) on the other hand into the regression equation yielded a multiple r = 0.568 (P = .002) for IMCL prediction within the total study population with a significant independent contribution of each of the factors 1) (P = .013), 2) (P = .016) and 3) (P = .001) (Figure 3; for multivariable predictive term see the x-axis: –2.121 + 0.015 * PA-0.004 * FFAs minus PA + 4.449 * ScFAT). Thereby, importantly, an inclusion of the 3 potential (bias) factors VO2max, age, and type 1 muscle fiber fraction (alone or combined) into the regression equation yielded no significant independent contribution to IMCL, that is, no improved prediction. When applying this regression approach to the nonobese strata (with high OSA-related FFA and PA increases) solely, a stronger prediction of IMCL was found (multiple r = 0.853, P = .002), with a significant contribution for each of these factors 1) (P = .007), 2) (P = .013), and 3) (P = .032), respectively (multivariate predictive term: –8.881 + 0.017 * PA-0.004 * FFAs minus PA + 6.712 * ScFAT, not shown). Again, VO2max per kg body weight, age, and/or muscle histology had no significant additional impact (bias) on inclusion into the regression equation. Notably, replacing ScFAT by BMI in the regression equation yielded a slightly weaker prediction within the total study population (multiple r = 0.425, P = .034; factor 1): P = .060, 2): P = .062, and 3): P = .016) and within the nonobese strata (multiple r = 0.775, P = .003; factor 1): P = .009, 2), P = .019, and 3): P = .770).
Figure 3.
Scatter plot representing explorative multivariable prediction of intramyocellular lipid (IMCL) (y-axis) by the combination of total fatty acid (FFA) and palmitic acid (PA) as well as thigh subcutaneous fat volume (ScFAT) according to regression analysis (x-axis) within the total study population (for details see “Materials and Methods” and “Results”).
In case of EMCL multivariate regression approach including these 3 variables, that is PA, FFAs (minus PA), and ScFAT, resulted in a prediction with a multiple of r = 0.529 (P = .006) in the total study population with the independent contribution of each the factors 1) (P = .038), 2) (P = .060), and 3) (P = .022), respectively (not shown).
Overall, HbA1c showed no significant bivariate correlation to IMCL (r = –0.178, P = .226) or to VO2max (r = 0.260, P = .085). Interestingly, however, HbA1c was significantly positively related to IMCL (r = 0.593, P = .006) - beside being negatively related to VO2max (r = −0.522, P = .022) - within the nonobese participants, whereas within the obese strata, HbA1c showed a significant negative correlation to IMCL (r = –0.400, P = .035). According to an explorative multivariable regression including all participants, the combination of IMCL and VO2max resulted in a multiple r = 0.428 (P = .014) with significant contributions of both factors (P = .019 and P = .009, respectively). The HbA1c prediction was improved by inclusion of AHI (multiple r = 0.589, P = .001), while BMI or age made no significant contribution to the prediction.
Notably, VO2max per body weight was found to be related to OSA severity in terms of AHI (r = –0.335, P = .026) as well as to OBS in terms of BMI (r = –0.542, P = .000) or ScFAT (r = –0.579, P = .000) (see also ANOVA, Table 1). Regarding vascular risks through OSA, we noted a correlation between cIMT and IMCL (r = 0.37, P = .011), EMCL (r = 0.35, P = .017), AHI (r = 0.34, P = .023), and Tu90% (r = 0.42, P = .003). There was a highly significant, close correlation between IMCL and EMCL within the total study population (r = 0.73, P = .000) as well as within the groups or strata.
Effect of Continuous Positive Airway Pressure Therapy
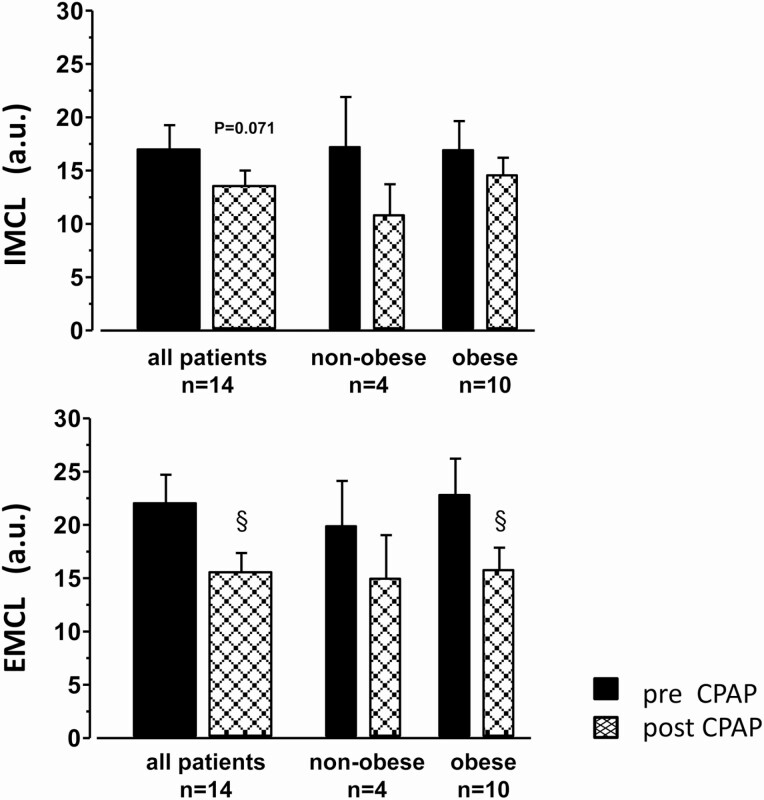
Several months of CPAP (4.3 ± 0.5 hours per night, in 4 nonobese and 10 obese patients) improved AHI and Tu90%, and mean SaO2 (Table 3), not only compared to the pretreatment status but also compared to the healthy control group (see Table 1). Although CPAP treatment had no significant effect on BMI or ScFAT, it significantly lowered heart rate—a bona fide marker of resting sympathetic activity—by more than 6 beats/min (P = .034, Table 3) Moreover, patients on CPAP showed a trend toward lower systolic and diastolic blood pressure (–6 mm Hg) as well as reduced cIMT. Notably, CPAP failed to significantly improve HbA1c, postabsorptive glucose, insulin, HOMA-IR, TNF, as well as lipid plasma levels. The total FFAs and PA plasma levels were found to decrease nonsignificantly by 27% and 24%, respectively, on CPAP therapy. There was a concomitant trend toward decreased IMCL (–22% overall, –37% in nonobese, –16% in obese) and a significant reduction in EMCL (–26% overall, –25% in nonobese, –27% in obese) with CPAP treatment (Fig. 4). For comparison, a complete reversal of OSA-induced IMCL and EMCL through CPAP to respective control levels (in terms of percentage of levels in OSA patients, Table 1) would require IMCL to decrease by –36% overall, by –54% in nonobese, and by –20% in obese patients. EMCL would need to decrease by –28% overall, by –51% in nonobese, and by –8% in obese patients. Thus, CPAP only partly reversed IMCL but completely reversed EMCL at least in obese patients.
Table 3.
Effect of continuous positive airway pressure on sleep parameter, resting heart rate, and aerobic capacity
| Pre-CPAP | Post-CPAP | ||
|---|---|---|---|
| No. | 14 | 14 | |
| AHI | h–1 | 49.4 ± 7.2 | 3.7 ± 1.4c |
| Time < 90% SaO2 | min | 75.4 ± 25.4 | 8.7 ± 7.1a |
| Mean SaO2 | % | 92.1 ± 0.6 | 94.6 ± 0.4b |
| Heart rate at rest | min–1 | 72.5 ± 2.5 | 65.9 ± 2.1a |
| VO2max/kg body wt | mL min–1 kg–1 | 29.8 ± 2.2 | 25.2 ± 1.3 |
Data represent mean ± SEM
Abbreviations: AHI, apnea-hypopnea index; CPAP, continuous positive airway pressure; SaO2, oxygen saturation; VO2max, maximal oxygen uptake.
a P less than .05; bP less than .01; cP less than .001 by paired t test comparing post- vs pre-CPAP values.
Figure 4.
Effect of 3 to 5 months of home-based continuous positive airway pressure (CPAP) on intramyocellular lipid (IMCL) (upper panel) and extramyocellular lipid (EMCL) (lower panel) in vastus lateralis muscle of 14 obstructive sleep apnea patients. Mean ± SEM of pre- and post-CPAP values are presented for the total group (left) and its nonobese (middle) as well as its obese stratum (right). § for P less than .05 by t test for paired observations post- vs pre-CPAP.
Notably, CPAP significantly changed neither VO2max per body weight (P = .058) nor the amount physical activity in OSA patients, which might have been due to the small number of volunteers enrolled. Furthermore, none of the histomorphological parameters of the vastus lateralis muscle biopsies were affected by CPAP.
Discussion
The present 3-T 1H-MRS study, which, notably, was conducted in the early morning (7 am), provides first evidence that moderate to severe OSA is independently of OBS associated with significant increases both of IMCL and EMCL in the vastus lateralis muscle as compared to healthy controls with exclusion of these OSA grades. Importantly, substantial OSA-related increases of IMCL and EMCL may occur especially in nonobese OSA, since healthy obese individuals tend to have already increased IMCL and EMCL [43]. Moreover, in line with a hypothesis, that OSA- that is, CIH-related lipolysis may increase circulating FFAs and thus contribute to high IMCL [38-40], we presently demonstrate that total FFAs and especially PA (as a factor of IR in healthy humans [41, 42]) i) are significantly increased with OSA (see Table 1, Figure 1) and ii) significantly correlate with AHI as a measure of OSA severity (see Fig. 1). Furthermore, total FFAs (minus PA) and PA plasma levels both contribute significantly and independently of each other to the IMCL (multiple r = 0.568, P = .002) when controlled for thigh ScFAT as a measure of OBS. Replacing ScFAT by BMI, which reflects both fat and muscle mass, still resulted in significant correlation (multiple r = 0.425, P = .034). In either approach, there was no significant independent influence (ie, bias) of age, VO2max, or muscle histology to explain IMCL variability.
When we considered the nonobese strata alone, the 3 factors, that is, FFAs (minus PA) and PA plasma levels as well as ScFAT gave an improved multivariate IMCL prediction (multiple r = 0.853, P = .002) whereby each parameter showed a significant independent contribution. Including the more readily available BMI instead of ScFAT in the regression equation resulted in significant multivariate prediction as well (multiple r = 0.775, P = .003).
The present evidence from CPAP treatment for a causal role of OSA in increased IMCL and EMCL should be considered as preliminary, because only EMCL, not however, IMCL, FFA, and PA were significantly reduced. A distinct weakness of the study was the small size of the treatment group, the short duration of treatment applied with the usual poor adherence, and the lack of a sham intervention group. However, the trends observed may warrant further studies.
Notably, OSA-related lipolytic increases in plasma FFAs and in IMCL (and EMCL) may be massive but transient [39], that is, detectable only shortly after overnight CIH stress. In the course of the day they may subside depending on metabolic demand and activity [52]. This point may, at least in part, explain previous negative studies on IMCL in OSA patients [50, 51]. The rapid decrease in FFAs between 7 am and 10 am, as reported in untreated OSA patients [39], may have contributed to the rather large variability in FFAs (and IMCL) presently observed within this time interval.
Interestingly, there was a massive increase in plasma triglycerides with OSA that occurred especially in nonobese participants (214.7 ± 40.9 vs 95.6 ± 12.6 mg 100 mL–1), suggesting that overnight increases in circulating FFAs may exceed muscle or liver uptake capacity and may therefore be associated with re-esterification of FFAs (FFA-triacylglycerol recycling). Diurnal variations in FFAs and IMCL in OSA patients should therefore be considered in future studies.
Another important confounding factor of IMCL (and EMCL) obviously is OBS itself [43] as presently indicated by a trend toward higher IMCL in obese than in nonobese healthy controls. Interestingly, the combination of OBS with OSA yielded no essential further increase in IMCL or EMCL, which implies that the effect of OSA on IMCL or EMCL or its reversal by CPAP may be confounded by prevailing OBS. This interference of OBS was controlled for by our multivariable regression analysis in our study. Our data indicated that ScFAT or BMI, along with FFAs, made significant contributions to the prediction of IMCL and EMCL within the total study population. As a study limitation, our analysis was limited to BMI and ScFAT and could not assess the potentially more critical role of truncal fat distribution for IMCL and EMCL in OSA. In view of the high OSA prevalence in obese participants, it remains to be seen to what extent well-documented increases in IMCL with OBS may be in fact attributable to OSA not previously taken into consideration [43].
The “lipotoxic” role of increased IMCL in IR has been extensively discussed and reviewed and appears not to be uniformly applicable because of factors like OBS, physical activity (“athlete paradox”), as well as dynamic metabolism of triacylglycerides and diacylglycerides including ceramide formation [55]. Since our study did not contain a gold-standard parameter for peripheral IR, the potential muscular metabolic contributions to HbA1c in the total study population were examined in a hypothesis-generating manner: We were able to show by multivariable regression that IMCL and VO2max with (multiple r = 0.589, P = .001) or without (r = 0.428, P = .014) AHI were significant factors for the prediction of HbA1c, whereas BMI or age made no significant contributions. Thereby, notably, the bivariate correlation of HbA1c to IMCL was positive in nonobese (r = 0.593, P = .006), but negative in obese participants (r = –0.400, P = .035), which may not generally support a monocausal lipotoxic concept of IMCL as a missing link between OSA and IR [38, 41, 42] and warrants further studies regarding other relevant metabolic factors [55]. The significant role of VO2max (reduced both with OSA and OBS) may point at inactivity-related factors such as endothelial dysfunction, capillary rarefication (in case of OBS [56]), or other reported factors of IR [49, 57] not presently determined. It is important, however, that with our results we could largely rule out a role for changes in skeletal muscle fiber composition, in particular changes in the type 1 fiber fraction, which would affect IR [57], for example, via mitochondrial capacity. Notably, however, there was an OBS-related significant increase in type 2x fiber fractional area that may require consideration in future studies on IR in obese and nonobese OSA patients (see Table 2).
Moreover, the present data suggest that accumulation of EMCL (ie, intermyocellular or perimuscular/perivascular fat) may similarly be attributable to increased total FFAs, PA, and ScFAT (multiple r = 0.529, P = .006). Interestingly, EMCL has been suggested to contribute to IR as well: Dietary lipid overload or OBS leads to skeletal muscle infiltration of inflammatory macrophages and T cells (stimulated by factors like monocyte-chemotactic-protein-1 (MCP1 = CCL2), CD11a or palmitate) that is clearly related to IR in humans as well as in animal models [47, 48, 58-62]. Such inflammation (along with endothelial lipid accumulation) may contribute to a microvascular, that is, a nonmyocellular cause of IR, such as impaired insulin-related vasodilation or transendothelial insulin transport, both of which delay glucose uptake of skeletal muscle [49, 63]. Skeletal muscle IR may even be predicted by perivascular fat at the site of large conduit arteries [64].
In summary, the present 3-T 1H-MRS study observed significant increases in the early morning of circulating FFAs and of IMCL and EMCL that appear to be interrelated and all attributable to OSA rather than to OBS (controlled for by ScFAT or BMI). The correlation of FFAs and especially PA to the severity of OSA (AHI) in combination with a CPAP treatment effect, at least on EMCL, supports the part of the concept of Gu et al [38] that dynamic increases in circulating FFAs lead to ectopic lipid accumulation with OSA [39, 40]. As possible triggers for ectopic lipid accumulation, the role of sympathetic activation through repeated hypoxic (and hypercapnic) stimulation of peripheral chemoreceptors (which tend to be hypersensitive with OSA [2]) and the role other lipolytic factors such as elevated cortisol levels warrant further studies. Whether there is a role of high IMCL and inactivity-related factors determining VO2max for IR, as presently suggested by the association with HbA1c, requires studies with gold-standard IR measurements.
Acknowledgments
We gratefully acknowledge the expert laboratory assistance of Claudia Keppler, Michael Dreher, and Irmgard Dammshäuser, and furthermore the support of v. Behring-Röntgen-Stiftung (Project Mo. 580071). We also would like to thank the Core Facility “BrainImaging” of the Medical Faculty of the Philipps-University of Marburg for the support of this project. Moreover, we thank Dr Regina Conradt, Cambridge, UK, for linguistic adaptation of this manuscript.
Financial Support: This work was supported by von Behring-Röntgen-Stiftung (Project No. 580071).
Glossary
Abbreviations:
- 1H-MRS
1H-magnetic resonance spectroscopy;
- AHI
apnea-hypopnea index;
- BMI
body mass index;
- CIH
chronic intermittent hypoxia;
- cIMT
carotid intima media thickness;
- CPAP
continuous positive airway pressure;
- EMCL
extramyocellular lipid;
- FFA
free fatty acid;
- HbA1c
, glycated hemoglobin A1c;
- HOMA-IR
homeostasis model assessment of insulin resistance;
- IMCL
intramyocellular lipid;
- IR
insulin resistance;
- OBS
obesity;
- OSA
obstructive sleep apnea;
- PA
palmitic acid;
- REM
rapid eye movement;
- ScFAT
thigh subcutaneous fat volume;
- TNF
tumor necrosis factor;
- Tu90%
cumulative time (minutes) spent at SaO2 less than 90%;
- VO2max
maximal oxygen uptake
Additional Information
Disclosures: These authors have nothing to disclose.
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69(7):841-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046-1053. [DOI] [PubMed] [Google Scholar]
- 4. Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J Clin Sleep Med. 2014;10(4):355-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kendzerska T, Gershon AS, Hawker G, Leung RS, Tomlinson G. Obstructive sleep apnea and risk of cardiovascular events and all-cause mortality: a decade-long historical cohort study. PloS Med. 2014;11(2):e1001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kendzerska T, Gershon AS, Hawker G, Tomlinson G, Leung RS. Obstructive sleep apnea and incident diabetes. A historical cohort study. Am J Respir Crit Care Med. 2014;190(2):218-225. [DOI] [PubMed] [Google Scholar]
- 7. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217-1239. [DOI] [PubMed] [Google Scholar]
- 9. Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol (1985). 2005;99(4):1592-1599. [DOI] [PubMed] [Google Scholar]
- 10. Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pugliese G, Barrea L, Laudisio D, et al. Sleep apnea, obesity, and disturbed glucose homeostasis: epidemiologic evidence, biologic insights, and therapeutic strategies. Curr Obes Rep. 2020;9(1):30-38. [DOI] [PubMed] [Google Scholar]
- 12. Sacramento JF, Andrzejewski K, Melo BF, Ribeiro MJ, Obeso A, Conde SV. Exploring the mediators that promote carotid body dysfunction in type 2 diabetes and obesity related syndromes. Int J Mol Sci. 2020;21(15):5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378-1384. [DOI] [PubMed] [Google Scholar]
- 14. Gunnarsson SI, Peppard PE, Korcarz CE, et al. Obstructive sleep apnea is associated with future subclinical carotid artery disease: thirteen-year follow-up from the Wisconsin Sleep Cohort. Arterioscler Thromb Vasc Biol. 2014;34(10):2338-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the Sleep Heart Health Study. Circulation. 2010;122(4):352-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hla KM, Young T, Hagen EW, et al. Coronary heart disease incidence in sleep disordered breathing: the Wisconsin Sleep Cohort Study. Sleep. 2015;38(5):677-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hung J, Whitford EG, Parsons RW, Hillman DR. Association of sleep apnoea with myocardial infarction in men. Lancet. 1990;336(8710):261-264. [DOI] [PubMed] [Google Scholar]
- 18. Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2010;182(2):269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim Y, Koo YS, Lee HY, Lee SY. Can continuous positive airway pressure reduce the risk of stroke in obstructive sleep apnea patients? A systematic review and meta-analysis. PloS One. 2016;11(1):e0146317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yaggi H, Mohsenin V. Obstructive sleep apnoea and stroke. Lancet Neurol. 2004;3(6):333-342. [DOI] [PubMed] [Google Scholar]
- 21. Pamidi S, Tasali E. Obstructive sleep apnea and type 2 diabetes: is there a link? Front Neurol. 2012;3:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pamidi S, Wroblewski K, Stepien M, et al. Eight hours of nightly continuous positive airway pressure treatment of obstructive sleep apnea improves glucose metabolism in patients with prediabetes. a randomized controlled trial. Am J Respir Crit Care Med. 2015;192(1):96-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chirinos JA, Gurubhagavatula I, Teff K, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med. 2014;370(24):2265-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McEvoy RD, Antic NA, Heeley E, et al. ; SAVE Investigators and Coordinators . CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919-931. [DOI] [PubMed] [Google Scholar]
- 25. Arnaud C, Beguin PC, Lantuejoul S, et al. The inflammatory preatherosclerotic remodeling induced by intermittent hypoxia is attenuated by RANTES/CCL5 inhibition. Am J Respir Crit Care Med. 2011;184(6):724-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bauters F, Rietzschel ER, Hertegonne KB, Chirinos JA. The link between obstructive sleep apnea and cardiovascular disease. Curr Atheroscler Rep. 2016;18(1):1. [DOI] [PubMed] [Google Scholar]
- 27. Schulz R, Schmidt D, Blum A, et al. Decreased plasma levels of nitric oxide derivatives in obstructive sleep apnoea: response to CPAP therapy. Thorax. 2000;55(12):1046-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mesarwi OA, Loomba R, Malhotra A. Obstructive sleep apnea, hypoxia, and nonalcoholic fatty liver disease. Am J Respir Crit Care Med. 2019;199(7):830-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jelic S, Lederer DJ, Adams T, et al. Vascular inflammation in obesity and sleep apnea. Circulation. 2010;121(8):1014-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schulz R, Hummel C, Heinemann S, Seeger W, Grimminger F. Serum levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea and severe nighttime hypoxia. Am J Respir Crit Care Med. 2002;165(1):67-70. [DOI] [PubMed] [Google Scholar]
- 31. Takahashi S, Nakamura Y, Nishijima T, Sakurai S, Inoue H. Essential roles of angiotensin II in vascular endothelial growth factor expression in sleep apnea syndrome. Respir Med. 2005;99(9):1125-1131. [DOI] [PubMed] [Google Scholar]
- 32. Louis M, Punjabi NM. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. J Appl Physiol (1985). 2009;106(5):1538-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105(3):1044-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137(1):95-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baud MO, Magistretti PJ, Petit JM. Sustained sleep fragmentation affects brain temperature, food intake and glucose tolerance in mice. J Sleep Res. 2013;22(1):3-12. [DOI] [PubMed] [Google Scholar]
- 36. Iiyori N, Alonso LC, Li J, et al. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med. 2007;175(8):851-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jun JC, Shin MK, Devera R, et al. Intermittent hypoxia-induced glucose intolerance is abolished by α-adrenergic blockade or adrenal medullectomy. Am J Physiol Endocrinol Metab. 2014;307(11):E1073-E1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gu C, Younas H, Jun JC. Sleep apnea: an overlooked cause of lipotoxicity? Med Hypotheses. 2017;108:161-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chopra S, Rathore A, Younas H, et al. Obstructive sleep apnea dynamically increases nocturnal plasma free fatty acids, glucose, and cortisol during sleep. J Clin Endocrinol Metab. 2017;102(9):3172-3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jun JC, Drager LF, Najjar SS, et al. Effects of sleep apnea on nocturnal free fatty acids in subjects with heart failure. Sleep. 2011;34(9):1207-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boden G, Lebed B, Schatz M, Homko C, Lemieux S. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes. 2001;50(7):1612-1617. [DOI] [PubMed] [Google Scholar]
- 42. Tumova J, Andel M, Trnka J. Excess of free fatty acids as a cause of metabolic dysfunction in skeletal muscle. Physiol Res. 2016;65(2):193-207. [DOI] [PubMed] [Google Scholar]
- 43. Yki-Järvinen H. Ectopic fat accumulation: an important cause of insulin resistance in humans. J R Soc Med. 2002;95(Suppl 42):39-45. [PMC free article] [PubMed] [Google Scholar]
- 44. Lettieri-Barbato D, Cannata SM, Casagrande V, Ciriolo MR, Aquilano K. Time-controlled fasting prevents aging-like mitochondrial changes induced by persistent dietary fat overload in skeletal muscle. PloS One. 2018;13(5):e0195912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Muoio DM. Metabolic inflexibility: when mitochondrial indecision leads to metabolic gridlock. Cell. 2014;159(6):1253-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Morino K, Petersen KF, Dufour S, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115(12):3587-3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fink LN, Costford SR, Lee YS, et al. Pro-inflammatory macrophages increase in skeletal muscle of high fat-fed mice and correlate with metabolic risk markers in humans. Obesity (Silver Spring). 2014;22(3):747-757. [DOI] [PubMed] [Google Scholar]
- 48. Khan IM, Perrard XY, Brunner G, et al. Intermuscular and perimuscular fat expansion in obesity correlates with skeletal muscle T cell and macrophage infiltration and insulin resistance. Int J Obes (Lond). 2015;39(11):1607-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Barrett EJ, Eggleston EM, Inyard AC, et al. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia. 2009;52(5):752-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chien MY, Lee PL, Yu CW, Wei SY, Shih TT. Intramyocellular lipids, insulin resistance, and functional performance in patients with severe obstructive sleep apnea. Nat Sci Sleep. 2020;12:69-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Trenell MI, Ward JA, Yee BJ, et al. Influence of constant positive airway pressure therapy on lipid storage, muscle metabolism and insulin action in obese patients with severe obstructive sleep apnoea syndrome. Diabetes Obes Metab. 2007;9(5):679-687. [DOI] [PubMed] [Google Scholar]
- 52. De Bock K, Dresselaers T, Kiens B, Richter EA, Van Hecke P, Hespel P. Evaluation of intramyocellular lipid breakdown during exercise by biochemical assay, NMR spectroscopy, and Oil Red O staining. Am J Physiol Endocrinol Metab. 2007;293(1):E428-E434. [DOI] [PubMed] [Google Scholar]
- 53. Bicalho B, David F, Rumplel K, Kindt E, Sandra P. Creating a fatty acid methyl ester database for lipid profiling in a single drop of human blood using high resolution capillary gas chromatography and mass spectrometry. J Chromatogr A. 2008;1211(1-2):120-128. [DOI] [PubMed] [Google Scholar]
- 54. Weber MA, Kinscherf R, Krakowski-Roosen H, et al. Myoglobin plasma level related to muscle mass and fiber composition: a clinical marker of muscle wasting? J Mol Med (Berl). 2007;85(8):887-896. [DOI] [PubMed] [Google Scholar]
- 55. Gemmink A, Goodpaster BH, Schrauwen P, Hesselink MKC. Intramyocellular lipid droplets and insulin sensitivity, the human perspective. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862(10 Pt B):1242-1249. [DOI] [PubMed] [Google Scholar]
- 56. Frisbee JC, Goodwill AG, Frisbee SJ, et al. Distinct temporal phases of microvascular rarefaction in skeletal muscle of obese Zucker rats. Am J Physiol Heart Circ Physiol. 2014;307(12):H1714-H1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lillioja S, Young AA, Culter CL, et al. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest. 1987;80(2):415-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Patsouris D, Cao JJ, Vial G, et al. Insulin resistance is associated with MCP1-mediated macrophage accumulation in skeletal muscle in mice and humans. PloS One. 2014;9(10):e110653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Talbot NA, Wheeler-Jones CP, Cleasby ME. Palmitoleic acid prevents palmitic acid-induced macrophage activation and consequent p38 MAPK-mediated skeletal muscle insulin resistance. Mol Cell Endocrinol. 2014;393(1-2):129-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41(1):36-48. [DOI] [PubMed] [Google Scholar]
- 61. Li P, Da Young O, Bandyopadhyay G, et al. LTB4 promotes insulin resistance in obese mice by acting on macrophages, hepatocytes and myocytes. Nat Med. 2015;21(3):239-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hong EG, Ko HJ, Cho YR, et al. Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes. 2009;58(11):2525-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kusters YHAM, Barrett EJ. Muscle microvasculature’s structure and functional specialization facilitate muscle metabolism. Am J Physiol Endocrin Metab. 2016;310(6):E379-E387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rittig K, Staib K, Machann J, et al. Perivascular fatty tissue at the brachial artery is linked to insulin resistance but not to local endothelial dysfunction. Diabetologia. 2008;51(11):2093-2099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.