Abstract
Background
Around 25% of the world population was affected by the metabolic-related fatty liver disorder. Hepatic steatosis is frequently observed in conjunction with hypertension, obesity comorbidities, and diabetes. We evaluate the hepatic steatosis frequency found in chest CT exams of COVID-19-positive cases compared to non-infected controls and evaluate the related increased prevalence and severity of COVID.
Results
Our research includes 355 subjects, 158 with positive PCR for COVID-19 (case group) and 197 with negative PCR and negative CT chest (control group). The mean age in the positive group was 50.6 ± 16 years, and in the control, it was 41.3 ± 16 years (p < 0.001). Our study consists of 321 men (90.5%) and 34 women (9.5%). The number of males in both cases and control groups was greater. In the case group, 93% men vs. 6.9% women, while in controls, 88.3% men vs.11.6% women, p < 0.001. CT revealed normal results in 55.5% of individuals (i.e., CORADs 1) and abnormal findings in 45.5% of participants (i.e., CORADs 2–5). In abnormal scan, CO-RADs 2 was 13.92%, while CO-RADs 3–4 were 20.89% of cases. CO-RADs 5 comprised 65.19% of all cases. Approximately 42.6% of cases had severe disease (CT score ≥ 20), all of them were CO-RADs 5. The PCR-positive class had a greater prevalence of hepatic steatosis than controls (28.5% vs.12.2%, p < 0.001). CO-RADs 2 represented 11.1%, CO-RADs 3–4 represented 15.6%, and CO-RADs 5 represented 73.3% in the hepatic steatosis cases. The mean hepatic attenuation value in the case group was 46.79 ± 12.68 and in the control group 53.34 ± 10.28 (p < 0.001). When comparing patients with a higher severity score (CT score ≥ 20) to those with non-severe pneumonia, it was discovered that hepatic steatosis is more prevalent (73.2% vs. 26.8%).
Conclusions
Steatosis was shown to be substantially more prevalent in COVID-19-positive individuals. There is a relation among metabolic syndrome, steatosis of the liver, and obesity, as well as the COVID-19 severity.
Keywords: Fatty liver, Computed tomography, COVID-19
Key points
High-resolution computed tomography aids clinicians in evaluating lung affection in COVID-positive cases.
Fatty liver and obesity are rising globally.
Fatty liver and metabolic syndrome are significant predisposing parameters for COVID-19 infection and increase disease’s severity.
Background
The World Health Organization declared coronavirus disease 2019 (COVID-19) as a pandemic on March 11, 2020 [1]. By May 2021, around 165,772,430 reported cases and 3,437,545 mortalities had occurred (https://www.who.org). COVID-19 is symptomatized by fever and dry cough, and the infection is diagnosed by a real-time reverse transcription-polymerase chain reaction (RT-PCR) test [2, 3]. Due to the rise in global cases, other symptoms like constipation, diarrhea, abdominal pain, and vomiting have risen; these are associated with abnormal renal and liver functions, and D-Dimer levels [4, 5]
COVID-19 can impact other organs besides the respiratory system, like the cardiovascular system, kidneys, liver, and coagulation system [6–10].
Diabetes, age, metabolic syndrome, hypertension, and obesity are all risk parameters for severe/critical illness and death [11–13].
People with obesity and type 2 diabetes are at greater risk for non-alcoholic fatty liver disease NAFLD, that worsen these disorders. It has been linked to an inflammatory response (increased neutrophil-to-lymphocyte ratio [NLR]) and subsequent poor outcomes in COVID-19-infected cases [14].
NAFLD has risen over the last two decades, affecting around 24% of the individual [15, 16]. NAFLD is a complex process with hepatic and extrahepatic pathophysiology and clinical symptoms. It leads to ectopic fatty substrate deposits in the liver, ranging from simple steatosis without inflammation to steatohepatitis, which causes cirrhosis and fibrosis [15].
CT affects the care of COVID-19 individuals because it aids in the early discovery and diagnosis, particularly in cases when the RT-PCR result is false-negative [16].
COVID-19 chest CT results are typically multifocal bilateral, mostly peripheral subpleural round, ground-glass opacities with or without patchy consolidations affecting mostly the posterior lower lobes [17]. Additionally, airway changes, reversed halo sign, and crazy paving patterns can be detected [18]. The Radiological Society of North America (RSNA) defined four categories on reporting chest CT findings in COVID-19 pneumonia: (1) typical features that are usually reported in COVID-19, (2) indeterminate features that are not characteristic of COVID-19 pneumonia, (3) atypical features that are uncommon in COVID-19 pneumonia but can occur with other infections, and (4) negative for lung inflammation with no lung results denoting infection. Chest CT may be negative in the early stages of COVID-19 infection [19].
We frequently include the upper abdomen in the regular CT scan of the chest conducted to assess cases with COVID-19 pneumonia, so that most of the liver and spleen can be viewed and examined [20].
The regular liver appears slightly more attenuated on non-contrast CT than the blood and spleen, and the intrahepatic arteries present as hypo-attenuated structures. Although histopathological analysis and liver biopsy are the gold measure for determining hepatic steatosis, they are invasive procedures. As a result, numerous studies have examined non-invasive alternatives to liver biopsy utilizing CT imaging [21].
Unenhanced CT liver attenuation alone is highly specific for moderate to severe hepatic steatosis, obviating the requirement for verification by biopsy [22].
Numerous approaches have been used for evaluating hepatic steatosis by computed tomography; the most important of which is determining the liver’s attenuation value. In the non-enhanced phase, the region of interest is set in the right hepatic lobe; if it is less than 40 HU, this indicates moderate hepatic steatosis with a fat liver percentage greater than 30% [23, 24]. Another way for assessing hepatic steatosis is to compare the area of interest in the splenic parenchyma to the liver, when we find the attenuation of liver is at least 10 HU less than that of the spleen. Several studies have demonstrated that non-enhanced CT has a great sensitivity (from 43 to 95%) and a great specificity (from 90 to 100%) for detecting hepatic steatosis [25–27].
Methods
This retrospective study was conducted at our institution’s Radiology Department from May 1, 2020, to June 1, 2020. Approval was acquired from the Institution’s Ethics and Research Committee. Informed consent was taken.
Inclusion criteria
Our research included 355 subjects who presented with flu-like symptoms and were suspected of being infected with COVID-19. They underwent PCR checking and chest CT for COVID-19. For all, we utilize the same 64-slice CT scanner (Siemens Healthcare, Germany).
The case group included 158 subjects (PCR positive for COVID-19), while the control group consisted of 197 subjects with a negative PCR test. It is widely established that false-negative RT-PCR can happen in infected individuals, but CT chest may reveal disease signs (positive CT). Therefore, to ensure the control group’s negativity, we checked their CT chest and retained only those who had a negative CT chest (PCR-negative and chest CT-negative pattern).
Two radiologists with over 10 years of expertise interpreted the CT chest.
CT evaluation involved identifying the areas of ground-glass opacities, crazy-paving patterns (ground-glass opacities with interlobular septal thickening), atelectatic bands, and consolidations. CT results were divided into five classes using the RSNA Expert Consensus Criteria [28], as well as the COVID-19 Reporting and Data System (CO-RADs) from the COVID-19 Working Group of the Dutch Radiological Society [29]. CT results are graded according to these grading methods as normal, inconsistent, or typical of COVID-19 pneumonia. The severity of lung affection (CT severity index) was measured as per Yang et al. A CT score of more than 20/40 indicates serious illness and is typically related to a poor prognosis [30].
We assess hepatic steatosis in our research by determining the attenuation of liver value. The area of interest (with an average area of 10 cm2) was located in the right hepatic lobe (between segments VI and VII), chosen area away from the biliary tree, vessels, or focal lesions. We examine one slice and define the liver as fatty if the HU reading is less than 40.
Statistical analysis
SPSS (Statistical Package for the Social Sciences; SPSS Inc., Chicago, IL, USA) release 25 was utilized for all statistical calculations. Standard deviations and means are used to describe quantitative data, while percentages are used to indicate qualitative data. Per the variable distribution, we employed the t-Student and chi-square checks. A p-value below 0.05 was considered significant.
Results
Characteristics of study group (Table 1)
Table 1.
Demographic comparison parameters and statistics between the two groups
| Parameter | Case group Positive PCR N = 158 |
Control group Negative PCR/negative chest CT N = 197 |
|---|---|---|
| Age | 50.6 ± 16 years | 41.3 ± 16 years |
| Sex | ||
| Male | N = 147 (93%) | N = 174 (88.3%) |
| Female | N = 11 (6.9%) | N = 23 (11.6%) |
| Steatosis | 28.5% | 12.2% |
| HU | 46.79 ± 12.68 | 53.34 ± 10.28 |
| CO-RADs | CO-RADs 2, N = 22 (13.92%) | CO-RADs 1, N = 197 (55.5%) |
| CO-RADs 3–4, N = 33 (20.89%) | ||
| CO-RADs 5, N = 103 (65.19%) | ||
Our research includes 355 subjects, 158 with positive PCR for COVID-19 (case group) and 197 with negative PCR and negative CT chest (control group). The mean age in the positive group was 50.6 ± 16 years, and in the control, it was 41.3 ± 16 years (p < 0.001).
Our study comprised 321 men (90.5%) and 34 women (9.5%). The number of males in both cases and control groups was greater. In the case group, 93% men vs. 6.9% women, while in controls, 88.3% men vs.11.6% women, p < 0.001.
CT demonstrated normal results in 55.5% of individuals (i.e., CORADs 1) and abnormal findings in 45.5% of participants (i.e., CORADs 2–5). In abnormal scan, CO-RADs 2 was 13.92%, while CO-RADs 3–4 were 20.89% of cases. CO-RADs 5 comprised 65.19% of all cases. Approximately 42.6% of cases had severe disease (CT score ≥ 20); all of them were CO-RADs 5.
Association with steatosis (Figs. 1, 2, 3, 4, and 5)
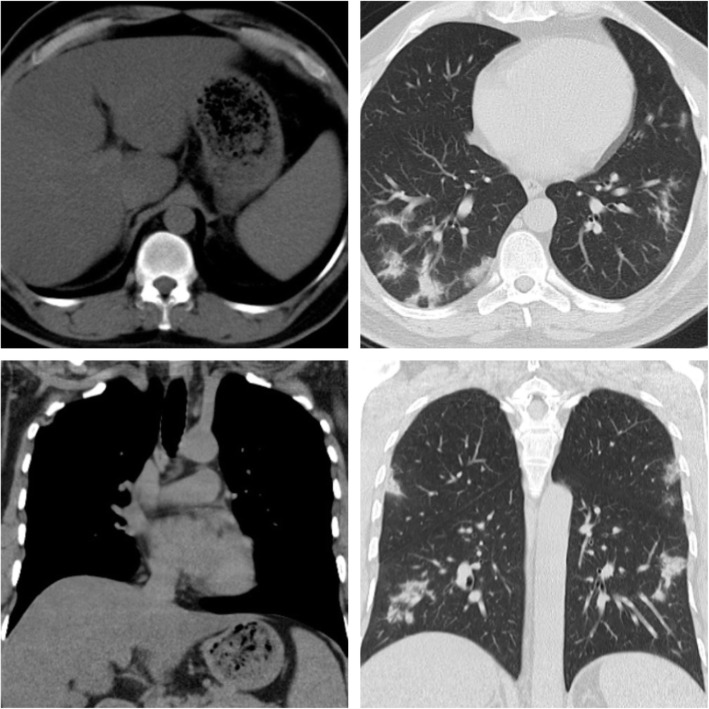
Fig. 1.
Male patient, 45 years old. CT chest shows multiple bilateral pulmonary patchy ground-glass opacities, reported as CORADs 5. Upper abdominal cuts show hepatic steatosis
Fig. 2.
Female patient, 28 years old. CT chest shows multiple pulmonary ground-glass opacities mounting to consolidative patches, reported as CORADs 5. Upper abdominal cuts show diffuse low parenchymal attenuation of the liver denoting fatty infiltration
Fig. 3.
Male patient, 36 years old. The first scan after few days of symptoms shows just a small patchy ground-glass opacity in the middle lobe. The second scan after 3 weeks for follow-up shows a progressive course with multiple pulmonary ground-glass opacities. CT cuts of the upper abdomen show a fatty liver
Fig. 4.
Male patient, 64 years. CT chest shows bilateral lower lobar subpleural patchy ground-glass opacities with underlying interlobular septal thickening and atelectatic bands, reported as CORADs 5. Upper abdominal cuts show fatty hepatomegaly
Fig. 5.
Female patient, 58 years old. The first scan shows multiple subpleural ground-glass opacities on follow-up after 3 months. CT shows multiple subpleural parenchymal bands. Upper abdominal cuts show fatty hepatomegaly
The PCR-positive group had a greater prevalence of hepatic steatosis than controls (28.5% vs.12.2%, p < 0.001). CO-RADs 2 represented 11.1%, CO-RADs 3–4 represented 15.6%, and CO-RADs 5 represented 73.3% in the hepatic steatosis cases.
The mean hepatic attenuation value in the case group was 46.79 ± 12.68, and in the control group, 53.34 ± 10.28 (p < 0.001).
When comparing patients with a higher severity score (CT score ≥ 20) to those with non-severe pneumonia, it was discovered that hepatic steatosis is more prevalent (73.2% vs. 26.8%).
Discussion
In 2016, the World Health Organization reported that 1.9 billion adults were overweight, with over 650 million being obese [31]. Obesity is the most major and significant risk factor in developing hepatic steatosis in adults and children [32].
Obesity is thought to be a condition of low-grade systemic inflammation that has been linked to a variety of metabolic diseases like type 2 diabetes mellitus and dyslipidemia. It can alter immunological responses, causing the immune system more sensitive to infection development [33].
Due to its endocrine roles and the release of various adipokines and proinflammatory cytokines like leptin, interleukin 6, C-reactive protein, visceral adipose tissue, and TNF are more metabolically active than subcutaneous adipose tissue [34, 35]. It is well documented that raised IL-6 levels are related to chronic inflammatory airway disorder. Numerous studies have discovered greater IL-6 concentrations in post-mortem specimens from COVID-19 cases [36, 37]. Leptin has been linked with airway reactivity, and current research indicates that leptin concentrations are increased in COVID-19 cases with significant pulmonary inflammation [38, 39].
COVID-19 invades human cells through binding with angiotensin-converting enzyme 2, and some research shows that the renin-angiotensin-aldosterone system’s imbalanced activity in obese individuals contributes to this pathogenesis. Because ACE2 expression is greater in adipose tissue than in lung tissue, and because ACE2 in lung tissue is known to be the primary entry point for SARS-CoV-2, this increases the sensitivity of obese patients to infection [40].
Additionally, obese individuals have impaired B and T cell responses due to changes in the quantity and function of lymphocytes, resulting in an increased vulnerability to viral infection. In virally infected obese individuals, the inflammatory response is dysregulated, resulting in a reduction and delay in macrophage activation [41]. Obesity can promote antiviral resistance as well [42].
NLR, a measure of systemic inflammation, was considerably elevated and related to poorer results in cases infected with COVID-19 [43]. There is a substantial correlation between this ratio and the severity of liver fibrosis in people with NAFLD. Current research has established that this association affects the COVID-19-induced inflammatory storm, which is associated with raised death and morbidity. In cases infected with COVID-19, liver injury occurs following lung injury [44]. This destruction could be caused by the overactivation of Kupffer cells, the production of a cytotoxic T cell response produced by the virus, or the production of a dysregulated innate immune response [45]. In these cases, post-mortem liver biopsies revealed microvascular steatosis [44].
According to Zheng et al., individuals with metabolic-associated fatty liver disease and obesity had a sixfold raised chance of developing severe COVID-19 infection [46]. Another research indicated that populations with metabolically related fatty liver disease (MAFLD) have a fourfold greater risk of developing severe forms of COVID-19 [47].
Per Palomar-Lever et al.’s findings, the combination of obesity and hepatic steatosis led to a significant relationship with serious illness, implying a synergic connection between both [20].
Medeiros et al. concluded that the steatosis prevalence on CT was greater in confirmed COVID pneumonia cases than in the control. This is important for radiologists because liver steatosis can be easily assessed and verified by any radiologist reading a chest CT. Moreover, this data can be added to the clinical data available to clinicians [48].
In research conducted in New York, cases with a body mass index (BMI) of ≥ 30 had a higher chance of acute care hospitalization, and those with a BMI of ≥ 35 had a higher risk of intensive care unit admission [49].
Additionally, it was observed that cases with NAFLD had a greater rate of progression to severe illness and poorer findings in COVID19 [44, 50, 51].
Ji et al. studied NAFLD in 202 cases with COVID-19 using the hepatic steatosis index based on ALT, AST, body mass index, gender, presence of diabetes, and/or an ultrasound examination. They discovered that preexisting comorbidities and NAFLD were linked with COVID-19 progression [44].
According to Zhou et al., the risk of severe COVID-19 increases fourfold when metabolic-related fatty liver disease is present [52].
As with the previous research, univariate and multivariate analyses suggested that individuals with NAFLD had an increased risk of disease progression. Comorbidities like diabetes mellitus, hypertension, coronary artery disease, and COPD are identified as additional risk parameters for COVID-19 progression [44, 53].
Petersen et al. used low-dose computed tomography and post-processing software to measure body fat distribution particularly visceral adipose tissue and upper abdominal circumference in COVID patients and found that these two parameters significantly increase the likelihood of COVID-19 severe courses [54].
Parlak et al. found that chest CT, which is critical for diagnosing COVID-19, can provide data about the disease’s prognosis and that fatty liver is a significant indicator of a bad prognosis and may be easily spotted on chest CT used for COVID-19 diagnosis [55].
Limitations
Other significant variables like hypertension, lipid profile, diabetes, weight, obesity, body mass index, and liver function were not evaluated. Hence, a correlation between these variables and hepatic steatosis could not be established.
Conclusion
In confirmed COVID-19 cases, our research demonstrates a considerably greater frequency of hepatic steatosis by CT as compared to controls. There is a correlation among metabolic syndrome, steatosis of the liver, and obesity, as well as the severity of COVID-19.
Acknowledgements
Not applicable.
Abbreviations
- RT-PCR
Real-time reverse transcription polymerase chain reaction
- COVID-19
Coronavirus disease of 2019
- MAFLD
Metabolic fatty liver disease
- NLR
Neutrophil-to-lymphocyte ratio
- NAFLD
Non-alcoholic fatty liver disease
- IL-6
Interleukin 6
- HU
Hounsfield unit
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- ACE2
Angiotensin-converting enzyme 2
- HS
Hepatic steatosis
Authors’ contributions
AA wrote the manuscript and was responsible for the correspondence to the journal. MH collected the patient data and participated in its design. SH contributed to the image processing and collection of the patient’s images. AE participated in the design of the study, performed the statistical analysis and participated in its design and coordination, and helped to draft the manuscript.All authors have read and approved the final manuscript.
Funding
This work has not received any funding sources.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the ethical committee of “Theodor Bilharz Institute” institutional review board with ethical committee approval number FWA 10609. An informed written consent was taken from all patients.
Consent for publication
An informed written consent was taken from all subjects.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Watanabe M, Risi R, Tuccinardi D, Baquero CJ, Manfrini S, Gnessi L (2020) Obesity and SARS-CoV-2: a population to safeguard. Diabetes Metab Res Rev. 10.1002/dmrr.3325 [DOI] [PubMed]
- 2.Kanne JP (2020) Chest CT findings in 2019 novel coronavirus (2019-nCoV) infections from Wuhan, China: key points for the radiologist. Radiology 295(1):16–17. 10.1148/radiol.2020200241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoon SH, Lee KH, Kim JY, Lee YK, Ko H, Kim KH, Park CM, Kim YH (2020) Chest radiographic and CT findings of the 2019 novel coronavirus disease (COVID-19): analysis of nine patients treated in Korea. Korean J Radiol 21:494–500. 10.3348/kjr.2020.0132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cholankeril G, Podboy A, Aivaliotis VI, Tarlow B, Pham EA, Spencer SP, Kim D, Hsing A, Ahmed A (2020) High prevalence of concurrent gastrointestinal manifestations in patients with SARS-CoV-2: early experience from California. Gastroenterology. 159(2):775–777. 10.1053/j.gastro.2020.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo S, Zhang X, Xu H (2020) Don’t overlook digestive symptoms in patients with 2019 novel coronavirus disease (COVID-19). Clin Gastroenterol Hepatol 18(7):1636–1637. 10.1016/j.cgh.2020.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han H, Xie L, Liu R et al (2020) Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China [published online ahead of print, 2020 march 31]. J Med Virol. 10.1002/jmv.25809 [DOI] [PMC free article] [PubMed]
- 7.Ammirati E, Wang DW (2020) SARS-CoV-2 inflames the heart. The importance of awareness of myocardial injury in COVID-19 patients [published online ahead of print, 2020 April 6]. Int J Cardiol S0167-5273(20)31669-7. 10.1016/j.ijcard.2020.03.086 [DOI] [PMC free article] [PubMed]
- 8.Zhang C, Shi L, Wang FS (2020) Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol 5(5):428–430. 10.1016/S2468-1253(20)30057-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durvasula R, Wellington T, McNamara E, Watnick S (2020) COVID-19 and kidney failure in the acute care setting: our experience from Seattle [published online ahead of print, 2020 April 7]. Am J Kidney Dis S0272-6386(20)30618-1. 10.1053/j.ajkd.2020.04.001 [DOI] [PMC free article] [PubMed]
- 10.Dolhnikoff M, Duarte-Neto AN, de Almeida Monteiro RA et al (2020) Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19 [published online ahead of print, 2020 April 15]. J Thromb Haemost. 10.1111/jth.14844 [DOI] [PMC free article] [PubMed]
- 11.Wu Z, McGoogan JM (2020) Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 323(13):1239–1242. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 12.Mantovani A, Byrne CD, Zheng MH, Targher G (2020) Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: a meta-analysis of observational studies. Nutr Metab Cardiovasc Dis 30(8):1236–1248. 10.1016/j.numecd.2020.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Targher G, Mantovani A, Wang XB, Yan HD, Sun QF, Pan KH, Byrne CD, Zheng KI, Chen YP, Eslam M, George J, Zheng MH (2020) Patients with diabetes are at higher risk for severe illness from COVID-19. Diabetes Metab 46(4):335–337. 10.1016/j.diabet.2020.05.001 In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Targher G, Mantovani A, Byrne CD, Wang XB, Yan HD, Sun QF, Pan KH, Zheng KI, Chen YP, Eslam M, George J, Zheng MH (2020) Detrimental effects of metabolic dysfunction-associated fatty liver disease and increased neutrophil-to-lymphocyte ratio on severity of COVID-19. Diabetes Metab 46(6):505–507. 10.1016/j.diabet.2020.06.001 In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angulo P (2002) Nonalcoholic fatty liver disease. N Engl J Med 346(16):1221–1231. 10.1056/NEJMra011775 [DOI] [PubMed] [Google Scholar]
- 16.Yang W, Sirajuddin A, Zhang X, Liu G, Teng Z, Zhao S et al (2020) The role of imaging in 2019 novel coronavirus (COVID-19). Eur Radiol. 10.1007/s00330-020-06827-4 [DOI] [PMC free article] [PubMed]
- 17.Zu ZY, Jiang MD, Xu PP, Chen W, Ni QQ, Lu GM, Zhang LJ (2020) Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology 296(2):E15–E25. 10.1148/radiol.2020200490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye Z, Zhang Y, Wang Y, Huang Z, Song B (2020) Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol 30(8):4381–4389. 10.1007/s00330-020-06801-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simpson S, Kay FU, Abbara S, Bhalla S, Chung JH, Chung M, Henry TS, Kanne JP, Kligerman S, Ko JP, Litt H (2020) Radiological Society of North America expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. Radiol Cardiothoracic Imaging 2(2):e200152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palomar-Lever A, Barraza G, Galicia-Alba J, Echeverri-Bolaños M, Escarria-Panesso R, Padua-Barrios J, Halabe-Cherem J, Hernandez-Molina G, Chargoy-Loustaunau TN, Kimura-Hayama E (2020) Hepatic steatosis as an independent risk factor for severe disease in patients with COVID-19: a computed tomography study. JGH Open 4(6):1102–1107. 10.1002/jgh3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamer OW, Aguirre DA, Casola G, Sirlin CB (2005) Imaging features of perivascular fatty infiltration of the liver: initial observations. Radiology 237:159–169. 10.1148/radiol.2371041580 [DOI] [PubMed] [Google Scholar]
- 22.Pickhardt PJ, Park SH, Hahn L, Lee SG, Bae KT, Yu ES (2012) Specificity of unenhanced CT for non-invasive diagnosis of hepatic steatosis: implications for the investigation of the natural history of incidental steatosis. Eur Radiol 22(5):1075–1082. 10.1007/s00330-011-2349-2 [DOI] [PubMed] [Google Scholar]
- 23.Lawrence DA, Oliva IB, Israel GM (2012) Detection of hepatic steatosis on contrast-enhanced CT images: diagnostic accuracy of identification of areas of presumed focal fatty sparing. Am J Roentgenol 199:44–47. 10.2214/AJR.11.7838 [DOI] [PubMed] [Google Scholar]
- 24.Wells MM, Li Z, Addeman B, Mckenzie CA, Mujoomdar A, Beaton M et al (2016) Computed tomography measurement of hepatic steatosis: prevalence of hepatic steatosis in a Canadian population. Can J Gastroenterol Hepatol 2016:4930987. 10.1155/2016/4930987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monjardim RDF, Costa DMC, Romano RFT, Salvadori PS, dos Santos J, de VC VAAC et al (2013) Diagnosis of hepatic steatosis by contrast-enhanced abdominal computed tomography. Radiol Bras 46:134–138. 10.1590/S0100-39842013000300005 [Google Scholar]
- 26.Ma X, Holalkere NS, Avinash KR, Mino-Kenudson M, Hahn PF, Sahani DV (2009) Imaging-based quantification of hepatic fat: methods and clinical applications. Radiographics 29:1253–1277. 10.1148/rg.295085186 [DOI] [PubMed] [Google Scholar]
- 27.Boyce CJ, Pickhardt PJ, Kim DH, Taylor AJ, Winter TC, Bruce RJ et al (2010) Hepatic steatosis (fatty liver disease) in asymptomatic adults identified by unenhanced low-dose CT. Am J Roentgenol 194:623–628. 10.2214/AJR.09.2590 [DOI] [PubMed] [Google Scholar]
- 28.Simpson S, Kay FU, Abbara S, Bhalla S, Chung JH, Chung M (2020) Radiological Society of North America expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. Radiology 2:2. 10.1148/ryct.2020200152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prokop M, van Everdingen W, van Rees VT, Jet Quarles van Ufford JT, Stöger L et al (2020) CO-RADS - a categorical CT assessment scheme for patients with suspected COVID-19: definition and evaluation. Radiology. 10.1148/radiol.2020201473 [DOI] [PMC free article] [PubMed]
- 30.Yang R, Li X, Liu H et al (2020) Chest CT severity score: an imaging tool for assessing severe COVID-19. Radiology 2:2. 10.1148/ryct.2020200047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill JJ (2018) Obesity: an emerging threat. J Natl Black Nurses Assoc 29(2):36–39 [PubMed] [Google Scholar]
- 32.Festi D, Colecchia A, Sacco T, Bondi M, Roda E, Marchesini G (2004) Hepatic steatosis in obese patients: clinical aspects and prognostic significance. Obes Rev 5:27–42. 10.1111/j.1467-789x.2004.00126.x [DOI] [PubMed] [Google Scholar]
- 33.Dhurandhar NV, Bailey D, Thomas D (2015) Interaction of obesity and infections. Obes Rev 16(12):1017–1029. 10.1111/obr.12320 [DOI] [PubMed] [Google Scholar]
- 34.Muscogiuri G, Pugliese G, Barrea L, Savastano S, Colao A (2020) Comentary: obesity: the “Achilles heel” for COVID-19? Metabolism. 108:154251. 10.1016/j.metabol.2020.154251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ritter A, Friemel A, Fornoff F, Adjan M, Solbach C, Yuan J, Louwen F (2015) Characterization of adipose-derived stem cells from subcutaneous and visceral adipose tissues and their function in breast cancer cells. Oncotarget. 6(33):34475–34493. 10.18632/oncotarget.5922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z et al (2020) Clinical course and risk parameters for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 395(10229):1054–1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park YS, Kwon HT, Hwang SS, Choi SH, Cho YM, Lee J, Yim JJ (2011) Impact of visceral adiposity measured by abdominal computed tomography on pulmonary function. J Korean Med Sci 26(6):771–777. 10.3346/jkms.2011.26.6.771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sideleva O, Suratt BT, Black KE, Tharp WG, Pratley RE, Forgione P, Dienz O, Irvin CG, Dixon AE (2012) Obesity and asthma: an inflammatory disease of adipose tissue not the airway. Am J Respir Crit Care Med 186(7):598–605. 10.1164/rccm.201203-0573OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ et al (2020) Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol Ahead of print [DOI] [PMC free article] [PubMed]
- 40.Lavie CJ, Sanchis-Gomar F, Henry BM, Lippi G (2020) COVID-19 and obesity: links and risks. Expert Rev Endocrinol Metab. 10.1080/17446651.2020.1767589 [DOI] [PubMed]
- 41.Kim J, Nam JH (2020) Insight into the relationship between obesity induced low-level chronic inflammation and COVID-19 infection. Int J Obes 44(7):1541–1542. 10.1038/s41366-020-0602-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petrakis D, Margină D, Tsarouhas K, Tekos F, Stan M, Nikitovic D et al (2020) Obesity - a risk factor for increased COVID-19 prevalence, severity and lethality (review). Mol Med Rep 22:9–19. 10.3892/mmr.2020.11127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lagunas-Rangel FA (2020) Neutrophil-to-lymphocyte ratio and lymphocyteto-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. J Med Virol 92(10):1733–1734. 10.1002/jmv.25819 In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ji D, Qin E, Xu J et al (2020) Non-alcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J Hepatol 8:451–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE (2008) Increased hepatic and circulating interleukin-6 levels in human non-alcoholic steatohepatitis. Am J Gastroenterol 103(6):1372–1379. 10.1111/j.1572-0241.2007.01774.x [DOI] [PubMed] [Google Scholar]
- 46.Zheng KI, Gao F, Wang XB, Sun QF, Pan KH, Wang TY et al (2020) Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154244. [DOI] [PMC free article] [PubMed]
- 47.Gao F, Zheng KI, Wang X-B, Yan H-D, Sun Q-F, Pan K-H et al (2020) Metabolic associated fatty liver disease increases COVID-19 disease severity in non-diabetic patients. J Gastroenterol Hepatol. 10.1111/jgh.15112
- 48.Medeiros AK, Barbisan CC, Cruz IR, Araújo EM, Libânio BB, Albuquerque KS, Torres US (2020) Higher frequency of hepatic steatosis at CT among COVID-19-positive patients. Abdom Radiol (NY) 45(9):2748–2754. 10.1007/s00261-020-02648-7 Epub 2020 July 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lighter J, Phillips M, Hochman S, Sterling S, Johnson D, Francois F et al (2020) Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis 15:2019–2020. 10.1093/cid/ciaa4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, Su Y, Ma Z, Zhang Y, Li Z, He Q, Liu L, Fu Y, Chen J (2020) COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy 75(7):1742–1752. 10.1111/all.14309 [DOI] [PubMed] [Google Scholar]
- 51.Garrido I, Liberal R, Macedo G (2020) Review article: COVID-19 and liver disease-what we know on may 1 2020. Aliment Pharmacol Ther 52(2):267–275. 10.1111/apt.15813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou YJ, Zheng KI, Wang XB, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, George J, Zheng MH (2020) Metabolic-associated fatty liver disease is associated with severity of COVID-19. Liver Int 40(9):2160–2163. 10.1111/liv.14575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Portincasa P, Krawczyk M, Smyk W, Lammert F, Di Ciaula A (2020) COVID-19 and non-alcoholic fatty liver disease: two intersecting pandemics. Eur J Clin Investig 50:e13338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petersen A, Bressem K, Albrecht J, Thieß H, Vahldiek J, Hamm B, Makowski M, Niehues A, Niehues S, Adams L (2020) The role of visceral adiposity in the severity of COVID-19: highlights from a unicenter cross-sectional pilot study in Germany. Metabolism. 110:154317. 10.1016/j.metabol.2020.154317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parlak S, Çıvgın E, Beşler MS, Kayıpmaz AE (2021) The effect of hepatic steatosis on COVID-19 severity: chest computed tomography findings. Saudi J Gastroenterol 27(2):105–110. 10.4103/sjg.sjg_540_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.