Abstract
Background
Acute kidney injury (AKI) is a disease that negatively affects patient prognosis and requires early diagnosis and treatment. Biomarkers that predict AKI are needed for early diagnosis of this disease.
Methods
We compared the AKI group and the non‐AKI group in patients who were admitted to our critical care intensive care unit (ICU) and conducted a comparative study focusing on urinary neutrophil gelatinase‐associated lipocalin (U‐NGAL) and serum procalcitonin (PCT).
Results
Seventy‐one out of 106 ICU inpatients were diagnosed with AKI in accordance with the Kidney Disease: Improving Global Outcomes (KDIGO) criteria. Among the patients who were diagnosed with AKI stages 1 to 3, 94.4% of all patients reached the maximum stage by day 5 after admission. Comparing the non‐AKI group and AKI stage 1 to 3 on days 1 to 3 after admission, U‐NGAL and PCT levels in the stage 3 group were significantly higher than those in the non‐AKI group. Additionally, in receiver operating characteristic curve (ROC) analysis on days 1–3 after admission, U‐NGAL and PCT levels can be used as biomarkers for the diagnosis of AKI, and in particular, AKI stage 3 can be predicted and diagnosed with high accuracy. U‐NGAL and PCT levels were also significantly higher in AKI due to sepsis and acute pancreatitis and due to sepsis, respectively.
Conclusions
Measuring U‐NGAL and PCT levels as biomarkers for AKI may further improve the accuracy of AKI diagnosis in critical care ICU.
Keywords: acute kidney Injury, NGAL, pancreatitis, procalcitonin, sepsis
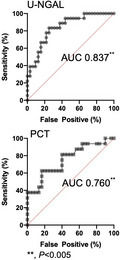
In receiver operating characteristic curve (ROC) analysis, urinary neutrophil gelatinase‐associated lipocalin (U‐NGAL) and serum procalcitonin (PCT) levels can be used as biomarkers for the diagnosis of acute kidney injury (AKI) after critical care ICU admission. The figures show the ROC analysis and area under the curve (AUC) of U‐NGAL and PCT in AKI stage 3 patients on day 2 after admission.
1. INTRODUCTION
Acute kidney injury (AKI) is a serious disease that is directly related to the patient's prognosis, and it has been reported that the mortality rate increases due to AKI complications. 1 Therefore, early diagnosis and early treatment of AKI are necessary, and consensus definitions and classifications have become important. The Risk, Injury, Failure, Loss of kidney function and End stage of kidney disease (RIFLE) classification in 2004, 2 the Acute Kidney Injury Network (AKIN) classification in 2007, 3 and the Kidney Disease: Improving Global Outcomes (KDIGO) classification in 2012 4 have been reported. For AKI diagnostic criteria and staging, KDIGO clinical practice guidelines using changes in serum creatinine (Cr) and urine volume criteria have been proposed. Because it was reported to take 24–48 h from kidney damage to an increase in serum Cr, it is difficult to use serum Cr alone as a marker for early diagnosis. 5 , 6 , 7 In addition to renal damage, AKI that is not accompanied by factors such as dehydration or oliguria is clinically seen as a decrease in urine volume. Thus, a diagnosis of renal disorder accompanied by a rapid decrease in renal function such as AKI requires a diagnosis using a marker that reflects the renal disorder at an early stage. We divided intensive care unit (ICU) inpatients into an AKI group and a non‐AKI group, and compared urinary neutrophil gelatinase‐associated lipocalin (U‐NGAL) levels, serum procalcitonin (PCT) levels, and other clinical test results. In this study, we confirmed that U‐NGAL and PCT levels were excellent AKI biomarkers. We also reported on the three diseases that cause AKI (acute pancreatitis, sepsis, and pneumonia).
2. MATERIALS AND METHODS
2.1. Study population and classification of acute kidney injury
An observational study was conducted in adult patients who were treated at the critical care ICU at Fukuoka University Hospital from April to October 2018. The subjects were 126 patients whose U‐NGAL and PCT levels were measured three times or more within 7 days after entering the ICU. Seventeen cases of cardiopulmonary arrest and three cases of renal replacement therapy due to renal failure were excluded. AKI was defined as having occurred by 7 days after admission, and patients were classified into a non‐AKI group (35 patients) and AKI group (71 patients) on the basis of the KDIGO diagnostic criteria. For a diagnosis of AKI, Cr and urine volume in accordance with the KDIGO clinical practice guidelines were used, and the basal Cr value was calculated using the Modification of Diet in Renal Disease (MDRD) formula. 8 , 9 The AKI group was further classified into AKI stage 1 to 3 groups on the basis of the day of ICU admission. AKI stage was defined in accordance with KDIGO, as follows: Stage 1 was 1.5–1.9‐times the reference serum Cr or an increase of ≥0.3 mg/dl within 48 h, and urine volume <0.5 ml/kg/h for 6–12 h; stage 2 was 2.0–2.9‐times the reference serum Cr or urine volume <0.5 ml/kg/h over 12 h; and stage 3 was 3.0‐times the reference serum Cr or an increase of ≥4.0 mg/dl, or urine volume <0.3 ml/kg/h over 24 h. In the AKI group, the number of days that were required to reach the maximum stage of AKI was classified into three categories (1 to 2 days, 3 to 5 days, and 6 to 7 days), and the number of patients in each stage was compared.
For days 1–3 after ICU admission, U‐NGAL, PCT, and serum Cr, C‐reactive protein (CRP), blood urea nitrogen (UN), white blood cells (WBC), and lactic acid (LA) were compared between the non‐AKI, AKI, and AKI stage 1–3 groups. Furthermore, for acute pancreatitis, sepsis, and pneumonia, U‐NGAL and PCT were compared between the AKI and the non‐AKI groups. Pneumonia was defined as not included in sepsis in clinical diagnosis.
This study was approved by the Ethics Committee at Medical Sciences, Fukuoka University (2018M016).
2.2. Laboratory measurements
U‐NGAL was measured using U‐NGAL kit (Abbott Japan Co., Ltd.), UN was measured using a Pureauto S UN‐L® kit (Sekisui Medical Co., Ltd.), Cr was measured using a Pureauto S CRE‐N® kit (Sekisui Medical Co., Ltd.), CRP was measured using a CRP latex X2 kit (Denka Seiken Co., Ltd.), lactic acid (LA) was measured using Determiner LA kit (Hitachi Chemical Diagnostics Systems Co., Ltd.), and PCT was measured using a Elecsys® BRAHMS Procalcitonin kit (Roche Diagnostics K.K.). For the equipment that was used, U‐NGAL was measured using the ARCHITECT i1000SR (Abbott Japan Co.); BUN, Cr, CRP, and LA were measured using the automatic analyzer TBA‐c16000 (Canon Medical Systems Co., Ltd.); PCT was measured using a Cobas 6000 (Roche Diagnostics K.K.); and WBCs were measured using a XN‐1000 (Sysmex Co., Ltd.).
2.3. Statistical analysis
To compare the non‐AKI and AKI groups, the Shapiro‐Wilk test was used to confirm whether the data were normality distributed. The data did not follow a normal distribution, so the Mann‐Whitney test was performed. The numerical values are presented as the median and the interquartile range. Additionally, when there were fewer than 20 cases and the U comparison target was a ratio rather than a continuous variable, Fisher's exact test was performed. To compare AKI stages 1 to 3 and the non‐AKI group, the Kruskal‐Wallis (Steel multiple comparison) test was performed because the data did not follow a normal distribution. The significance level was p < 0.05. The AKI diagnostic ability of U‐NGAL, PCT, and Cr was evaluated using a receiver operating characteristic (ROC) analysis. The ROC curve was created by changing the cutoff value and plotting the true‐positive rate on the vertical axis and the false‐positive rate on the horizontal axis. AKI diagnostic ability was evaluated using the area under the curve (AUC) of the ROC (AUC‐ROC). EZR (ver 1.41, Saitama Medical Center, Jichi Medical University), which is a user interface of R (the R Foundation for Statistical Computing), was used for statistical analysis. The ROC curve was created using Prism 8 (GraphPad Software Inc.).
3. RESULTS
3.1. Comparison between non‐AKI and AKI groups
One hundred six critical care ICU inpatients were divided into the following two groups: the non‐AKI group (35 patients) and the AKI group (71 patients). Comparisons were made between the groups. As shown in Table 1, 56.3% were men and 43.7% were women in the AKI group, there was no difference in age, gender, body mass index, and basal Cr between the two groups. When compared by disease, the AKI group had four cases of acute pancreatitis, 18 cases of sepsis, and ten cases of pneumonia, and these three diseases accounted for 45.1% of the AKI group. There were nine deaths during hospitalization (9/71; 12.7%) in the AKI group and two in the non‐AKI group (2/35; 5.7%), which was not significant difference between the two groups (Table 1).
TABLE 1.
Clinical characteristics and prognosis of all patients, non‐AKI patients, and AKI patients
| Characteristics | All patients | non‐AKI patients | AKI patients | p‐value |
|---|---|---|---|---|
| n = 106 | n = 35 | n = 71 | ||
| Male sex, n (%) | 58 (54.7) | 18 (51.4) | 40 (56.3) | 0.787 |
| Age (years) | 72 (63–80) | 71 (65–79) | 73 (63–80) | 0.704 |
| Body mass index (kg/m2) | 21.7 (19.2–24.8) | 20.7 (18.5–22.7) | 22.2 (19.5–25.6) | 0.073 |
| Baseline serum creatinine (mg/dl) | 0.80 (0.60–0.83) | 0.79 (0.61–0.83) | 0.80 (0.60–0.83) | 0.901 |
| Diseases, n (%) | ||||
| Cerebrovascular disease | 42 (39.6) | 25 (71.4) | 17 (23.9) | |
| Pneumonia | 12 (11.3) | 2 (5.7) | 10 (14.1) | |
| Heart disease | 10 (9.4) | 4 (11.4) | 6 (8.5) | |
| Sepsis | 20 (18.9) | 2 (5.7) | 18 (25.4) | |
| Acute pancreatitis | 4 (3.8) | 0 (0) | 4 (5.6) | |
| Others | 18 (17.0) | 2 (5.7) | 16 (22.5) | |
| Outcome, n (%) | ||||
| Discharge | 95 (89.6) | 33 (94.3) | 62 (87.3) | 0.332 |
| Death | 11 (10.4) | 2 (5.7) | 9 (12.7) | |
Values are expressed as the median and interquartile range or number. We calculated the baseline serum creatinine using the Modification of Diet in Renal Disease (MDRD) equation.
Abbreviation: AKI; acute kidney injury.
3.2. Time required to reach KDIGO maximum stage
Figure 1 shows the number of patients with the AKI maximum stage in accordance with the KDIGO diagnostic criteria for each length of admission (1 to 2, 3 to 5, or 6 to 7 days). There were 43 (60.0%) patients who reached the AKI maximum stage on days 1 and 2, 24 (33.8%) patients on days 3 and 5, and four (5.6%) patients on days 6 to 7.
FIGURE 1.
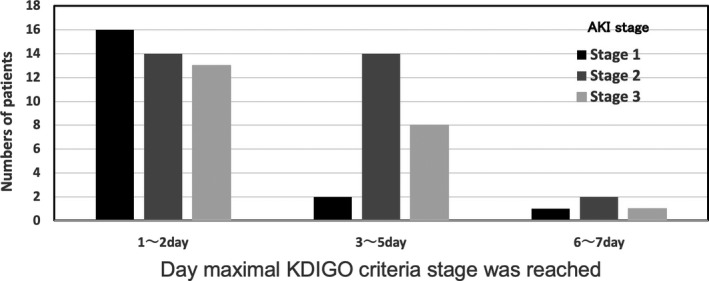
AKI stage and the day that the maximal KDIGO criteria stage was reached. AKI stage means the maximal stage within the first 7 d after the patient was transferred to the ICU. AKI, acute kidney injury; ICU, intensive care unit; KIDGO, Kidney Disease: Improving Global Outcomes
3.3. Comparison of the non‐AKI group with the AKI group and AKI stage 1–3 groups
The non‐AKI group and the AKI group were compared on days 1 to 3 of hospitalization, and there was a significant difference between the U‐NGAL, PCT, Cr, and UN for all 3 days (Table 2). Among the 71 patients in the AKI group, there were 17, 32, and 22 in stages 1, 2, and 3, respectively. When the non‐AKI group was compared with the AKI stage 1 to 3 groups, U‐NGAL was significantly different in the AKI stage 3 group for all 3 days, it increased from 161.3 to 375.4 ng/ml in the AKI stage 3 group, and it increased from 25.3 to 39.9 ng/ml in the non‐AKI group (Table 3 a–c). PCT showed a significant difference in AKI stage 2 only on day 1 (p < 0.05) and was significantly higher in the AKI stage 3 group than in the non‐AKI group for 3 days (Table 3 a–c). Serum Cr was significantly different in all AKI stage 1–3 groups (p < 0.05) for all 3 days, increased from 0.91 to 2.48 mg/dl in AKI 1–3 groups, and increased from 0.66 to 0.72 mg/dl in non‐AKI groups (p < 0.005). U‐NGAL and PCT showed high values at AKI stage 3, and U‐NGAL showed a significantly higher value in the stage 3 group compared with the other two groups (p < 0.005), which was significantly different from that of the non‐AKI group on days 2 and 3 (Table 3 a–c).
TABLE 2.
Comparison of clinical markers for non‐AKI and AKI patients from days 1–3 after admission.
| Day 1 | Day 2 | Day 3 | ||||
|---|---|---|---|---|---|---|
| non‐AKI | AKI | non‐AKI | AKI | non‐AKI | AKI | |
| U‐NGAL (ng/ml) | 30.9 (18.8–72.7) | 81.7* (29.2–278.8) | 25.3 (14.8–83.2) | 103.5** (31.6–276.3) | 39.9 (20.7–77.8) | 111.1** (43.2–374.3) |
| PCT (ng/ml) | 0.12 (0.04–0.29) | 0.54** (0.12–5.00) | 0.34 (0.16–0.84) | 1.18* (0.22–7.48) | 0.23 (0.11–0.85) | 1.56** (0.26–4.94) |
| Cr (mg/dl) | 0.72 (0.57–0.82) | 1.13** (0.79–1.75) | 0.68 (0.55–0.80) | 1.08** (0.80–1.74) | 0.66 (0.50–0.73) | 1.10** (0.72–1.64) |
| UN (mg/dl) | 17 (13–20) | 25** (17–42) | 16 (13–21) | 28** (18–44) | 16 (12–23) | 33** (22–49) |
| CRP (mg/dl) | 0.38 (0.12–2.37) | 3.74* (0.35–16.56) | 3.19 (1.24–14.60) | 7.74* (2.97–20.83) | 10.68 (6.41–18.98) | 14.80 (8.33–20.68) |
| WBC (103/ml) | 11.4 (8.2–12.7) | 13.0 (9.0–16.4) | 9.1 (8.1–12.8) | 12.5 (8.0–17.0) | 9.2 (7.5–11.3) | 11.0* (8.7–15.7) |
| LA (mg/dl) | 17.1 (11.2–28.5) | 19.8 (12.7–30.1) | 9.7 (8.0–13.7) | 12.7 (8.3–17.4) | 8.7 (7.2–9.7) | 10.0* (7.9–13.2) |
| urine volume (ml/kg/h) | ND | ND | 1.02 (0.83–1.95) | 0.60** (0.40–1.11) | 0.94 (0.75–1.42) | 0.69** (0.45–0.99) |
The numbers are presented as the median value and the 25th–75th percentile.
Abbreviations: AKI, acute kidney injury; Cr, creatinine; CRP, C‐reactive protein; LA, lactic acid; ND, not determined; PCT, procalcitonin; UN, blood urea nitrogen; U‐NGAL, urinary neutrophil gelatinase‐associated lipocalin; WBC, white blood cell.
p < 0.05 vs. non‐AKI
p < 0.005 vs. non‐AKI
TABLE 3.
Comparison of clinical markers between non‐AKI patients and AKI stage 1–3 patients on days 1–3 after admission
| (a) Day 1 | ||||
|---|---|---|---|---|
| non‐AKI n=35 | AKI stage 1 n=17 | AK I stage 2 n=32 | AKI stage 3 n = 22 | |
| U‐NGAL (ng/ml) | 30.9 (18.8–72.7) | 55.2 (33.2–206.7) | 63.7 (15.8–211.1) | 161.3* (68.0–652.2) |
| PCT (ng/ml) | 0.12 (0.04–0.29) | 0.55 (0.08–15.60) | 0.74* (0.11–4.02) | 0.34* (0.30–5.54) |
| Cr (mg/dl) | 0.72 (0.57–0.82) | 1.12** (0.97–1.30) | 1.07** (0.77–1.23) | 2.20** (0.74–3.07) |
| UN (mg/dl) | 17 (13–20) | 25** (21–34) | 20 (15–30) | 40** (21–64) |
| CRP (mg/dl) | 0.38 (0.12–2.37) | 2.11 (0.26–15.50) | 5.57 (0.12–18.58) | 2.68* (1.01–12.02) |
| WBC (103/ml) | 11.4 (8.2–12.7) | 12.7 (8.9–16.4) | 14.0* (9.5–18.4) | 11.9 (7.3–15.6) |
| LA (mg/dl) | 17.1 (11.2–28.5) | 19.8 (12.7–32.8) | 22.0 (12.6–28.7) | 17.7 (13.7–27.5) |
| (b) Day 2 | ||||
|---|---|---|---|---|
| non‐AKI n = 35 | AKI stage 1 n = 17 | AKI stage 2 n = 32 | AKI stage 3 n = 22 | |
| U‐NGAL (ng/ml) | 25.3 (14.8–83.2) | 61.0 (39.8–171.6) | 81.6 (25.2–159.2) | 296.2** (111.9–2683.3) |
| PCT (ng/ml) | 0.34 (0.16–0.84) | 0.33 (0.16–6.30) | 1.72 (0.16–4.34) | 1.46* (0.54–15.19) |
| Cr (mg/dl) | 0.68 (0.55–0.80) | 0.97** (0.80–1.14) | 1.02** (0.77–1.51) | 2.48** (1.07–2.73) |
| UN (mg/dl) | 16 (13–21) | 27** (18–36) | 23* (17–41) | 39** (28–62) |
| CRP (mg/dl) | 3.19 (1.24–14.60) | 7.72 (1.65–12.10) | 7.64 (2.97–20.80) | 7.80* (5.12–21.86) |
| WBC (103/ml) | 9.1 (8.1–12.8) | 11.6 (8.6–14.1) | 13.1 (8.6–18.3) | 11.8 (6.9–16.6) |
| LA (mg/dl) | 9.7 (8.0–13.7) | 9.0 (7.8–18.3) | 13.4 (9.2–15.0) | 13.5 (9.4–23.0) |
| (c) Day 3 | ||||
|---|---|---|---|---|
| non‐AKI n = 35 | AKI stage 1 n = 17 | AKI stage 2 n = 32 | AKI stage 3 n = 22 | |
| U‐NGAL (ng/ml) | 39.9 (20.7–77.8) | 68.7 (37.1–141.9) | 101.7 (26.4–131.3) | 375.4** (138.9–1141.9) |
| PCT (ng/ml) | 0.23 (0.11–0.85) | 0.64 (0.23–5.63) | 0.68 (0.20–2.17) | 2.07** (1.18–21.63) |
| Cr (mg/dl) | 0.66 (0.50–0.73) | 0.91** (0.70–1.23) | 1.02** (0.70–1.48) | 2.05** (1.27–2.96) |
| UN (mg/dl) | 16 (12–23) | 29* (18–34) | 30** (22–37) | 47** (38–68) |
| CRP (mg/dl) | 10.68 (6.41–18.98) | 8.74 (7.62–19.27) | 14.20 (9.85–19.09) | 19.72* (10.04–27.67) |
| WBC (103/ml) | 9.2 (7.5–11.3) | 10.6 (9.1–12.2) | 10.7 (8.1–14.6) | 14.7* (9.7–17.3) |
| LA (mg/dl) | 8.7 (7.2–9.7) | 8.0 (6.7–10.8) | 9.9 (8.4–12.0) | 11.8 (9.4–16.0) |
The numbers are presented as the median value and 25th–75th percentile. Groups were compared by Kruskal‐Wallis and Steel‐adjusted Mann‐Whitney U tests.
Abbreviations: AKI, acute kidney injury; Cr, creatinine; CRP, C‐reactive protein; LA, lactic acid; PCT, procalcitonin; UN, blood urea nitrogen; U‐NGAL, urinary neutrophil gelatinase‐associated lipocalin; WBC, white blood cell.
p<0.05 vs. non‐AKI
p<0.005 vs. non‐AKI
3.4. ROC analysis for the non‐AKI group and AKI stage 1–3 groups
The AKI predictive ability of U‐NGAL, PCT, and Cr was analyzed using ROC curve analysis. As shown in Figure 2, U‐NGAL and PCT had a significant predictive ability for AKI stage 3 on day 2 of hospitalization. As shown in Table 4, the AUC‐ROC of all AKI groups was 0.671 to 0.723 for U‐NGAL, 0.662 to 0.751 for PCT, and 0.798 to 0.828 for Cr during days 1 to 3 after admission. The AUC‐ROC of the AKI stage 3 group was significantly higher, with 0.752 to 0.917 for U‐NGAL and 0.760 to 0.848 for PCT. In particular, the U‐NGAL AUC‐ROC (0.837 to 0.917) on days 2 and 3 was as high as the Cr AUC‐ROC (0.837 to 0.931) (p < 0.005; Table 4).
FIGURE 2.
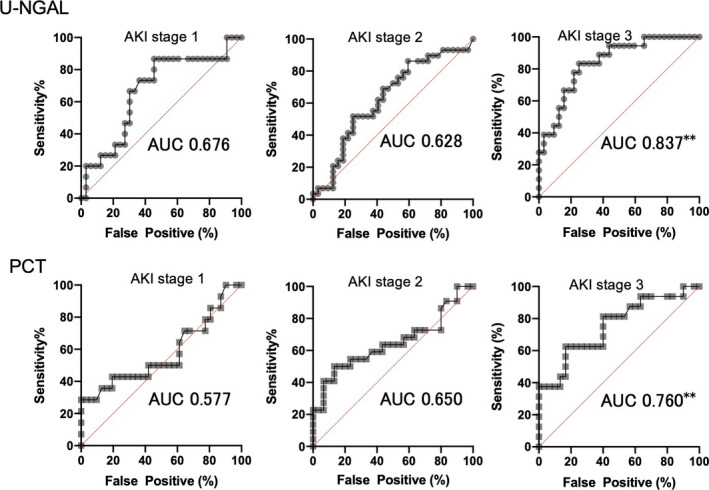
ROC curves of U‐NGAL and PCT in AKI stages 1 to 3 on the 2nd day of ICU admission. **p < 0.005; AUC, area under the curve. AKI, acute kidney injury; ROC, receiver operating characteristics curve; U‐NGAL, urinary neutrophil gelatinase‐associated lipocalin; PCT, procalcitonin; ICU, intensive care unit; AUC, area under the curve
TABLE 4.
The diagnostic properties of U‐NAGL, procalcitonin, and creatinine stratified by the length of hospital stay in all AKI stages, AKI stage 3, and three diseases
| Day | All AKI stages (n = 71) | Stage 3 of AKI (n = 22) | Three diseases (n = 32) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC (95%CI) | Cutoff | Sensitivity (%) | Specificity (%) | AUC (95%CI) | Cutoff | Sensitivity (%) | Specificity (%) | AUC (95%CI) | Cutoff | Sensitivity (%) | Specificity (%) | ||
| U‐NGAL | 1 | 0.671* (0.555–0.787) | 38.1 ng/ml | 70.7 | 67.9 | 0.752* (0.594–0.910) | 128.3 ng/ml | 68.4 | 82.1 | 0.865** (0.770–0.960) | 52.7ng/ml | 96.3 | 67.9 |
| 2 | 0.700** (0.585–0.816) | 54.4 ng/ml | 64.5 | 71.0 | 0.837** (0.724–0.949) | 69.8 ng/ml | 74.2 | 83.3 | 0.841** (0.740–0.941) | 56.6 ng/ml | 84.6 | 71.0 | |
| 3 | 0.723** (0.621–0.825) | 98.7 ng/ml | 60.9 | 78.8 | 0.917** (0.838–0.995) | 129.1ng/ml | 90.0 | 87.9 | 0.878** (0.789–0.967) | 104.7ng/ml | 89.3 | 78.8 | |
| PCT | 1 | 0.751** (0.622–0.880) | 0.21 ng/ml | 69.8 | 73.7 | 0.787* (0.630–0.945) | 0.30 ng/ml | 76.9 | 73.7 | 0.848** (0.731–0.965) | 0.30 ng/ml | 86.4 | 73.7 |
| 2 | 0.662* (0.544–0.779) | 1.39 ng/ml | 50.0 | 82.8 | 0.760* (0.606–0.913) | 1.39 ng/ml | 62.5 | 82.8 | 0.808** (0.683–0.933) | 1.51 ng/ml | 66.7 | 82.8 | |
| 3 | 0.729** (0.622–0.835) | 0.46 ng/ml | 67.2 | 71.0 | 0.848** (0.738–0.957) | 1.09 ng/ml | 77.8 | 80.6 | 0.832** (0.729–0.936) | 0.41 ng/ml | 84.0 | 67.7 | |
| Cr | 1 | 0.798** (0.713–0.882) | 0.95mg/dl | 68.6 | 91.2 | 0.783** (0.626–0.939) | 1.11 mg/dl | 71.4 | 97.1 | 0.802** (0.686–0.917) | 0.96 mg/dl | 68.8 | 91.2 |
| 2 | 0.806** (0.724–0.887) | 0.97 mg/dl | 62.0 | 97.1 | 0.837** (0.702–0.972) | 1.05 mg/dl | 77.3 | 97.1 | 0.871** (0.771–0.972) | 0.91 mg/dL | 81.2 | 91.4 | |
| 3 | 0.828** (0.752–0.905) | 0.91 mg/dl | 67.1 | 100.0 | 0.931** (0.836–1.000) | 0.96 mg/dl | 90.5 | 100.0 | 0.879** (0.783–0.976) | 0.89 mg/dl | 78.1 | 97.1 | |
The three diseases refer to pneumonia, sepsis, and acute pancreatitis. The AUC values are presented as the median value and the 25th–75th percentile.
Abbreviations: AKI, acute kidney injury; Cr, creatinine; PCT, procalcitonin; U‐NGAL, urinary neutrophil gelatinase‐associated lipocalin.
p<0.05 vs. non‐AKI
p<0.005 vs. non‐AKI
3.5. Comparison of U‐NGAL and PCT in AKI group due to three diseases
The median AUC‐ROC for AKI prediction in the three diseases (pneumonia, sepsis, and acute pancreatitis) was 0.841 to 0.878 for U‐NGAL and 0.808 to 0.848 for PCT, which were significantly higher for all three days (p < 0.005). In AKI due to the three diseases, U‐NGAL and PCT AUC‐ROC were larger than those of all AKI (Table 4). Figure 3 a and b shows a comparison of U‐NGAL and PCT in the AKI group and non‐AKI group due to three diseases on days 1 and 3 after hospitalization. U‐NGAL was significantly higher in sepsis and acute pancreatitis, and PCT was higher in sepsis.
FIGURE 3.
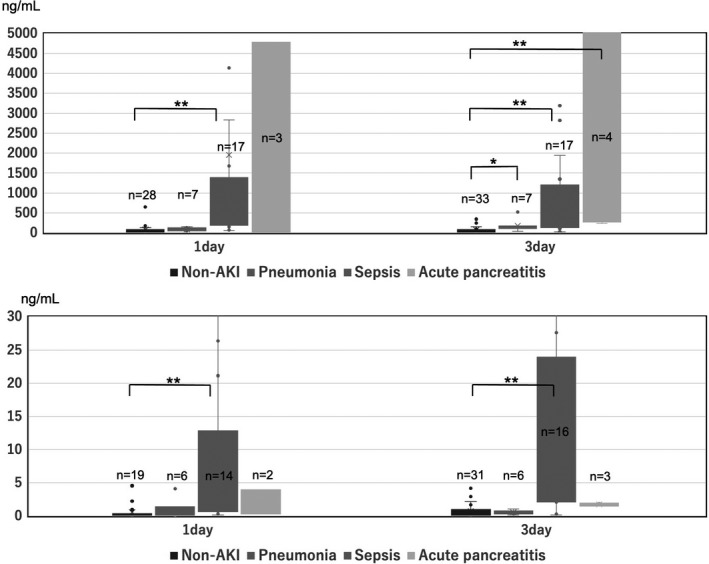
(A) Comparison of U‐NGAL between patients in the AKI subgroup and the non‐AKI group. AKI subgroup means pneumonia, sepsis, and acute pancreatitis.*p < 0.05 vs. non‐AKI; **, p < 0.005 vs. non‐AKI. AKI, acute kidney injury; U‐NGAL, urinary neutrophil gelatinase‐associated lipocalin. (B) Comparison of PCT between patients with AKI subgroup and the non‐AKI group. AKI subgroup means pneumonia and sepsis and acute pancreatitis. *p < 0.05 vs. non‐AKI; **p < 0.005 vs. non‐AKI. AKI, acute kidney injury; PCT, procalcitonin
4. DISCUSSION
To diagnose a renal disorder accompanied by a rapid renal function decline, an AKI biomarker that reflects the renal disorder at an early stage is required. AKI biomarkers have been discovered and used in clinical practice, and studies that predict an early diagnosis and prognosis have been reported. 10 , 11 , 12 , 13 In this study, we verified the usefulness of U‐NGAL, PCT, and routine laboratory test results as AKI biomarkers in hospitalized adult ICU patients in the emergency department.
The medical history of patients in the non‐AKI group and the AKI group showed that 56.3% were men and 43.7% were women in the AKI group, and there was no difference in age, gender, mortality rate, and basal Cr between the two groups. Three diseases (acute pancreatitis, sepsis, and pneumonia) that are thought to be accompanied by a systemic inflammatory response accounted for 45.1% of the AKI group. In particular, 18 of 20 patients with sepsis had AKI. The kidney is one of the organs that is most commonly affected by sepsis, and AKI is associated with sepsis in 47% of patients. 14 AKI is a potentially fatal complication of sepsis, and it contributes to increased morbidity and mortality. AKI is also associated with the development of chronic kidney disease. 15
An early diagnosis of AKI was considered to be important because 94.4% of the patients developed AKI by 5 days after admission to the ICU and reached the maximum AKI KDIGO stage in this study. In the comparison between the non‐AKI group and the AKI group on days 1 to 3 after hospitalization, there was a significant difference in U‐NGAL, PCT, and Cr for all 3 days (p < 0.05). In a comparison of these three markers between the non‐AKI group and the AKI stage 1 to 3 groups, all three markers in the AKI stage 3 group were significantly higher than those of the non‐AKI group on days 1 to 3. Additionally, PCT levels in the stage 2 group on day 1 and serum Cr in all stages on all 3 days were significantly higher than those in the non‐AKI group. Because AKI was diagnosed on the basis of serum Cr levels using the KDIGO diagnostic criteria, it was expected that the serum Cr level was significantly higher in the AKI group in this study.
We analyzed the AUC‐ROC values to confirm the usefulness of the AKI diagnostic ability of U‐NGAL and PCT levels in the early stages of hospitalization. Thus, the U‐NGAL and PCT values for days 1 to 3 after hospitalization had a median AUC‐ROC of 0.66 or higher, which was useful for making a diagnosis of AKI. In particular, the U‐NGAL AUC‐ROC on day 2 in the AKI stage 3 group was as high as 0.837, which shows a high AKI diagnostic ability that is equivalent to that of serum Cr levels.
NGAL has been identified as a predictive biomarker for AKI. For example, studies in adults who developed AKI after cardiac surgery reported a significant increase in urine and/or plasma NGAL 1–3 h after surgery. 7 , 16 , 17 , 18 , 19 Patients with acute ischemic stroke with AKI were also shown to have high urine and plasma NGAL levels. 20 NGAL appears to be most sensitive and specific in homogeneous patient populations with temporally predictable forms of AKI. Published studies have also identified age as an effective modifier of NGAL’s performance as an AKI biomarker, with better predictive ability in children (overall AUC‐ROC, 0.93) than in adults (AUC‐ROC, 0.78). 10 In studies of adult intensive care patients, plasma NGAL concentrations on admission were a very good to outstanding biomarker for the development of AKI within the next 2 days, with AUC‐ROC ranging from 0.78 to 0.92. 21 , 22 However, in a more mixed population of all the critical care patient admissions, the U‐NGAL on admission was only moderately predictive of AKI with an AUC‐ROC of 0.71. 23
NGAL measurements may be influenced by several coexisting variables such as chronic kidney disease, hypertension, systemic infections, inflammatory conditions, anemia, hypoxia, and malignancies. 24 , 25 , 26 , 27 , 28 In the critical care ICU, patients with septic AKI have the highest levels of both plasma and urine NGAL compared with those of patients with non‐septic AKI, which can increase the heterogeneity of results. 21 , 29 , 30 The variable performance of biomarkers such as NGAL in the critical care setting may also be because this patient population is extremely heterogeneous, and the etiology and timing of AKI is often unclear. A high proportion of patients may have already sustained AKI on admission to the ICU. Although sepsis accounts for a large part of AKI in the critical care ICU, other etiologies include exposure to nephrotoxins, hypotension, kidney ischemia, mechanical ventilation, and multi‐organ disease. Each of these etiologies has a different intensity at different times and is associated with different injury mechanisms that may act synergistically. Despite the many variables, a meta‐analysis revealed an overall AUC‐ROC of 0.73 to predict AKI when NGAL was measured within 6 h of clinical contact with critically ill patients, and AKI was defined as a >50% increase in serum Cr. 31 The predictive performance of NGAL also depends on the definition of AKI that is used and on the severity of AKI. For example, the predictive value of plasma NGAL postcardiac surgery was higher for more severe AKI (increase in serum Cr >50%; mean AUC‐ROC, 0.79) compared with less severe AKI (increase in serum Cr >25%; mean AUC‐ROC, 0.65). Similarly, the discriminatory ability of NGAL for AKI increased with the increasing severity, as classified by the RIFLE criteria. Thus, the AUC‐ROC improved progressively for discrimination of the R (0.72), I (0.79), and F (0.80) categories of AKI. 32
During severe bacterial infections and sepsis, serum concentrations of PCT, which are produced mainly in human peripheral blood mononuclear cells and modulated by bacterial lipopolysaccharides and sepsis‐related cytokines, 33 increase up to 1,000‐fold. 34 PCT has recently been suggested as a diagnostic marker of systemic bacterial infection and sepsis, 35 which are the leading causes of AKI in critically ill patients. 36 , 37 , 38 , 39 PCT may be an early biomarker of AKI because PCT increases 3–4 h after a systemic inflammatory response, 40 but Cr increases 24–48 h after renal injury.
There are few comparative studies of the diagnostic ability of NGAL and PCT for AKI in adult critical care ICU patients. The U‐NGAL concentration was reported to potentially predict AKI in patients with sepsis in the emergency department. 30 Siew et al. showed that U‐NGAL diagnosed AKI with an AUC‐ROC value of 0.64 to 0.71. 23 Chun et al. showed that serum PCT levels were significantly higher in patients with AKI than in those without AKI (p < 0.001). 39 Additionally, Nakamura et al. reported that the AUC‐ROC value of PCT for an AKI diagnosis based on the RIFLE standard was 0.857 or higher. 41 Although the diagnostic criteria for AKI have changed, the above‐mentioned reports show that U‐NGAL and PCT are useful for AKI diagnosis with almost the same accuracy. In this study, U‐NGAL levels were higher in the acute pancreatitis and sepsis group and PCT levels were higher in the sepsis group when U‐NGAL and PCT were compared for the three diseases (pneumonia, sepsis, and acute pancreatitis).
This study has some limitations. First, this is an observational study with a small number of patients at a single center. Second, patients whose U‐NGAL and PCT levels were measured participated in this study. Third, the study began with ICU admission and the date of onset of the current disease was unknown. Fourth, this limitation is the use of the KDIGO classification based on elevated serum Cr levels as the definition of AKI. The definition of AKI in published studies has been based largely on serum Cr increases, which raises the issue of using a flawed outcome variable to analyze the performance of a novel assay. Using AKI as defined by a change in serum Cr could cause inaccuracy in the biomarker assay due to either false negatives (true tubular injury but no significant change in serum Cr) or false positives (absence of true tubular injury, but an increase in serum Cr due to pre‐renal causes or several other variables). Studies of biomarkers such as NGAL or PCT for the diagnosis of AKI may have yielded different results had there been a true gold standard for AKI.
However, this study is considered to provide important data to show the AKI diagnostic potential of U‐NGAL and PCT levels in clinical practice.
In conclusion, U‐NGAL and serum PCT levels have been shown to be useful in diagnosing AKI in critical care ICU patients. Because an AKI diagnosis that was defined by the KDIGO classification on the basis of serum Cr level fluctuations was made, it did not exceed the serum Cr levels, but U‐NGAL and serum PCT showed sufficient AKI diagnostic ability.
In particular, the diagnostic ability of severe AKI at the early stage of hospitalization was equivalent to that of serum Cr for both U‐NGAL and serum PCT. In addition, it was suggested that U‐NGAL and serum PCT are useful for diagnosing AKI due to sepsis and pancreatitis.
DATA AVAILABILITY STATEMENT
Due to the nature of this research, original supporting data are not available for other studies. The authors confirm that the data supporting the findings of this study are available within the article.
REFERENCES
- 1. Mehta RL, Pascual MT, Gruta CG, Zhuang S, Chertow GM. Refining Predictive Models in Critically Ill Patients with Acute Renal Failure. J Amer Soc Nephrol. 2002;13(5):1350‐1357. 10.1097/01.ASN.0000014692.19351.52 [DOI] [PubMed] [Google Scholar]
- 2. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative w. Acute renal failure ‐ definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204‐12. 10.1186/cc2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. 10.1186/cc5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kellum JA, Lameire N, Aspelin P, et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1‐138. Available from: https://kdigo.org/wp‐content/uploads/2016/10/KDIGO‐2012‐AKI‐Guideline‐English.pdf [Google Scholar]
- 5. Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15(6):1597‐1605. 10.1097/01.ASN.0000130340.93930.DD [DOI] [PubMed] [Google Scholar]
- 6. Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase‐associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231‐1238. 10.1016/S0140-6736(05)74811-X [DOI] [PubMed] [Google Scholar]
- 7. Wagener G, Jan M, Kim M, et al. Association between increases in urinary neutrophil gelatinase–associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology. 2006;105(3):485‐491. 10.1097/00000542-200609000-00011 [DOI] [PubMed] [Google Scholar]
- 8. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461‐470. 10.7326/0003-4819-130-6-199903160-00002 [DOI] [PubMed] [Google Scholar]
- 9. Pickering JW, Endre ZH. Back‐calculating baseline creatinine with MDRD misclassifies acute kidney injury in the intensive care unit. Clin J Am Soc Nephrol. 2010;5(7):1165‐1173. 10.2215/CJN.08531109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Devarajan P. Review: Neutrophil gelatinase‐associated lipocalin: A troponin‐like biomarker for human acute kidney injury. Nephrology. 2010;15(4):419‐428. 10.1111/j.1440-1797.2010.01317.x [DOI] [PubMed] [Google Scholar]
- 11. Klein SJ, Brandtner AK, Lehner GF, et al. Biomarkers for prediction of renal replacement therapy in acute kidney injury: a systematic review and meta‐analysis. Intensive Care Med. 2018;44(3):323‐336. 10.1007/s00134-018-5126-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Amaral Pedroso L, Nobre V, Carneiro D, et al. Acute kidney injury biomarkers in the critically ill. Clin Chim Acta. 2020;508:170‐178. 10.1016/j.cca.2020.05.024 [DOI] [PubMed] [Google Scholar]
- 13. Wang RR, He M, Ou XF, Xie XQ, Kang Y. The predictive value of RDW in AKI and mortality in patients with traumatic brain injury. J Clin Lab Anal. 2020;34(9):e23373. 10.1002/jcla.23373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singbartl K, Kellum JA. AKI in the ICU: definition, epidemiology, risk stratification, and outcomes. Kidney Int. 2012;81(9):819‐825. 10.1038/ki.2011.339 [DOI] [PubMed] [Google Scholar]
- 15. Doyle JF, Forni LG. Acute kidney injury: short‐term and long‐term effects. Crit Care. 2016;20(1):188. 10.1186/s13054-016-1353-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wagener G, Gubitosa G, Wang S, Borregaard N, Kim M, Lee HT. Urinary neutrophil gelatinase‐associated lipocalin and acute kidney injury after cardiac surgery. Am J Kidney Dis. 2008;52(3):425‐433. 10.1053/j.ajkd.2008.05.018 [DOI] [PubMed] [Google Scholar]
- 17. Xin C, Yulong X, Yu C, Changchun C, Feng Z, Xinwei M. Urine neutrophil gelatinase‐associated lipocalin and interleukin‐18 predict acute kidney injury after cardiac surgery. Ren Fail. 2008;30(9):904‐913. 10.1080/08860220802359089 [DOI] [PubMed] [Google Scholar]
- 18. Tuladhar SM, Puntmann VO, Soni M, Punjabi PP, Bogle RG. Rapid detection of acute kidney injury by plasma and urinary neutrophil gelatinase‐associated lipocalin after cardiopulmonary bypass. J Cardiovasc Pharmacol. 2009;53(3):261‐266. 10.1097/FJC.0b013e31819d6139 [DOI] [PubMed] [Google Scholar]
- 19. Haase M, Bellomo R, Devarajan P, et al. Novel Biomarkers Early Predict the Severity of Acute Kidney Injury After Cardiac Surgery in Adults. Ann Thorac Surg. 2009;88(1):124‐130. 10.1016/j.athoracsur.2009.04.023 [DOI] [PubMed] [Google Scholar]
- 20. Xiao W, Chen W, Hu H, Huang X, Luo Y. The clinical significance of neutrophil gelatinase‐associated lipocalin in ischemic stroke patients with acute kidney injury. J Clin Lab Anal. 2019;33(6):e22907. 10.1002/jcla.22907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cruz DN, de Cal M, Garzotto F, et al. Plasma neutrophil gelatinase‐associated lipocalin is an early biomarker for acute kidney injury in an adult ICU population. Intensive Care Med. 2010;36(3):444‐451. Available from http://link.springer.com/10.1007/s00134‐009‐1711‐1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Constantin JM, Futier E, Perbet S, et al. Plasma neutrophil gelatinase‐associated lipocalin is an early marker of acute kidney injury in adult critically ill patients: a prospective study. J Crit Care. 2010;25(1):176.e1‐176.e6. 10.1016/j.jcrc.2009.05.010 [DOI] [PubMed] [Google Scholar]
- 23. Siew ED, Ware LB, Gebretsadik T, et al. Urine neutrophil gelatinase‐associated lipocalin moderately predicts acute kidney injury in critically ill adults. J Am Soc Nephrol. 2009;20(8):1823‐1832. 10.1681/ASN.2008070673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Devarajan P. The promise of biomarkers for personalized renal cancer care. Kidney Int. 2010;77(9):755‐757. 10.1038/ki.2010.26 [DOI] [PubMed] [Google Scholar]
- 25. Mitsnefes MM, Kathman TS, Mishra J, et al. Serum neutrophil gelatinase‐associated lipocalin as a marker of renal function in children with chronic kidney disease. Pediatr Nephrol. 2007;22(1):101‐108. http://link.springer.com/10.1007/s00467‐006‐0244‐x [DOI] [PubMed] [Google Scholar]
- 26. Malyszko J, Bachorzewska‐Gajewska H, Malyszko JS, Pawlak K, Dobrzycki S. Serum neutrophil gelatinase‐associated lipocalin as a marker of renal function in hypertensive and normotensive patients with coronary artery disease. Nephrology. 2008;13(2):153‐156. 10.1111/j.1440-1797.2007.00899.x [DOI] [PubMed] [Google Scholar]
- 27. Malyszko J, Bachorzewska‐Gajewska H, Sitniewska E, Malyszko JS, Poniatowski B, Dobrzycki S. Serum neutrophil gelatinase‐associated lipocalin as a marker of renal function in non‐diabetic patients with stage 2‐4 chronic kidney disease. Ren Fail. 2008;30(6):625‐628. 10.1080/08860220802134607 [DOI] [PubMed] [Google Scholar]
- 28. Cho CH, Yoon J, Kim DS, Kim SJ, Sung HJ, Lee SR. Association of peripheral blood neutrophil gelatinase‐associated lipocalin levels with bone marrow neutrophil gelatinase‐associated lipocalin levels and neutrophil count in hematologic malignancy. J Clin Lab Anal. 2019;33(6):e22920. 10.1002/jcla.22920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aydoǧdu M, Gursel G, Sancak B, et al. The use of plasma and urine neutrophil gelatinase associated lipocalin (NGAL) and Cystatin C in early diagnosis of septic acute kidney injury in critically ill patients. Dis Markers. 2013;34(4):237‐246. 10.3233/DMA-130966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park HS, Kim JW, Lee KR, et al. Urinary neutrophil gelatinase‐associated lipocalin as a biomarker of acute kidney injury in sepsis patients in the emergency department. Clin Chim Acta. 2019;495:552‐555. 10.1016/j.cca.2019.06.005 [DOI] [PubMed] [Google Scholar]
- 31. Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase‐Fielitz A, Group NM‐aI . Accuracy of neutrophil gelatinase‐associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta‐analysis. Am J Kidney Dis. 2009;54(6):1012‐1024. 10.1053/j.ajkd.2009.07.020 [DOI] [PubMed] [Google Scholar]
- 32. Haase‐Fielitz A, Bellomo R, Devarajan P, et al. The predictive performance of plasma neutrophil gelatinase‐associated lipocalin (NGAL) increases with grade of acute kidney injury. Nephrol Dial Transplant. 2009;24(11):3349‐3354. 10.1093/ndt/gfp234 [DOI] [PubMed] [Google Scholar]
- 33. Oberhoffer M, Stonans I, Russwurm S, et al. Procalcitonin expression in human peripheral blood mononuclear cells and its modulation by lipopolysaccharides and sepsis‐related cytokines in vitro. J Lab Clin Med. 1999;134(1):49‐55. 10.1016/S0022-2143(99)90053-7 [DOI] [PubMed] [Google Scholar]
- 34. Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341(8844):515‐518. 10.1016/0140-6736(93)90277-N [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Theodorou VP, Papaioannou VE, Tripsianis GA, et al. Procalcitonin and procalcitonin kinetics for diagnosis and prognosis of intravascular catheter‐related bloodstream infections in selected critically ill patients: a prospective observational study. BMC Infect Dis. 2012;12(1): 247. 10.1186/1471-2334-12-247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nie X, Wu B, He Y, et al. Serum procalcitonin predicts development of acute kidney injury in patients with suspected infection. Clin Chem Lab Med. 2013;51(8):1655‐1661. 10.1515/cclm-2012-0822 [DOI] [PubMed] [Google Scholar]
- 37. Huang HL, Nie X, Cai B, et al. Procalcitonin levels predict acute kidney injury and prognosis in acute pancreatitis: a prospective study. PLoS ONE. 2013;8(12):e82250. 10.1371/journal.pone.0082250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jeeha R, Skinner DL, De Vasconcellos K, Magula NP. Serum procalcitonin levels predict acute kidney injury in critically ill patients. Nephrology (Carlton). 2018;23(12):1090‐1095. 10.1111/nep.13174 [DOI] [PubMed] [Google Scholar]
- 39. Chun K, Chung W, Kim AJ, et al. Association between acute kidney injury and serum procalcitonin levels and their diagnostic usefulness in critically ill patients. Sci Rep. 2019;9(1):4777. 10.1038/s41598-019-41291-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang YT, Lai MY, Kan WC, Shiao CC. Independent predictive ability of procalcitonin of acute kidney injury among critically ill Patients. J Clin Med. 2020;9(6):1939. 10.3390/jcm9061939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nakamura Y, Murai A, Mizunuma M, et al. Potential use of procalcitonin as biomarker for bacterial sepsis in patients with or without acute kidney injury. J Infect Chemother. 2015;21(4):257‐263. 10.1016/j.jiac.2014.12.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to the nature of this research, original supporting data are not available for other studies. The authors confirm that the data supporting the findings of this study are available within the article.


