Abstract
Background
Negative regulatory T cells (Tregs) not only deplete effector T cells but also inhibit the clearance of HIV during infection, which may allow Tregs to be used as informative diagnostic markers. To facilitate both diagnosis and treatment, a thorough understanding of these regulators by characterizing them on temporal and spatial scales is strongly required.
Methods
Hundred HIV‐infected/AIDS patients, including 87 males, with an average age of 35.8 years, as well as 20 healthy controls, were enrolled. Flow cytometry was used to analyze CD3+T cells, CD4+T cells, and CD8+T cells to evaluate the immune status of the participants. Then, a group of representative negative regulatory T cells, including CD4+PD‐1+T cells, CD4+PD‐1highT cells, CD8+PD‐1+T cells, and CD4+CD25high Tregs was also analyzed to explore their effects on disease progression and intercorrelation.
Results
The percentages of CD4+PD‐1+T cells and CD4+CD25highTregs increased in patients with the same ultrahigh significance. Temporally, the patients with both intermediate‐stage and late‐stage disease had higher percentages of CD4+PD‐1+T cells; however, the percentage of CD4+CD25highTregs only increased in the patients with late‐stage disease. In addition, CD4+PD‐1+T cells but not CD4+CD25highTregs were negatively correlated with the absolute CD4+T cell count. Spatially, no correlations between CD4+PD‐1+T cells and CD4+CD25highTregs were observed, which suggests these Tregs function differently during immunosuppression.
Conclusions
This study characterized negative regulatory T cells in HIV‐infected/AIDS patients at both temporal and spatial scales and found that CD4+CD25+Tregs and CD4+PD‐1+T cells could be used as potential diagnostic markers for identifying different disease stages and monitoring disease progression.
Keywords: AIDS, CD4+CD25+Treg, CD4+PD‐1+T cell, diagnostic marker, negative regulatory T cells
At late‐stage (CD4+T cells <200/μl) and intermediate‐stage (CD4+T cells = 200–500/μl), CD4+PD‐1+T were higher than that at early stage (CD4+T cells >500/μl). The percentage of CD4+CD25highTregs in the group of CD4+T cells <200/μl was higher than those of the groups of CD4+T cells = 200–500/μl and CD4+T cells >500/μl. It reveals that the CD4+PD‐1+T cells increased in patients when their absolute CD4+ is less than 500/μl, while CD4+CD25highTregs only increased when absolute CD4+ count is lower than 500/μL. This new discovery indicated that CD4+CD25highTregs are generated at a later stage of HIV infection, which could be used as a clinical indicator for poor disease status. We also found the percentage of CD4+PD‐1+T had a negative correlation with absolute CD4+ count in the HIV infected/AIDS patients and the percentage of CD4+CD25highTregs was not correlated with absolute CD4+ count. This provides a new quantitative maker for evaluating the progress of immunosuppression.

1. INTRODUCTION
Acquired immune deficiency syndrome (AIDS), is an infectious disease caused by human immunodeficiency virus (HIV) and is associated with high fatality and propagation rates. HIV infection does not directly cause disease; however, the human immune system collapses when viruses damage CD4+T cells, which help coordinate immune responses by stimulating effector T cells. As a result, CD4+T cell numbers dramatically decrease, thereby depleting cellular immunity and increasing susceptibility to opportunistic infections and tumors. 1 , 2 , 3 In contrast to these CD4+T cells that stimulate the immune system, another subset of T cells, namely, negative regulatory T cells, which are represented by CD4+CD25+Tregs, act to suppress both the proliferation and function of effector T‐cell subsets, thus preventing autoimmune disease and controlling immunopathological damage. 4 , 5 , 6 , 7 Treg numbers are increased during HIV infection, other chronic virus infections, such as hepatitis C virus (HCV), hepatitis B virus (HBV), and Epstein‐Barr virus (EBV), and cancer, 8 , 9 , 10 , 11 and these Tregs limit patient responses to treatments. Recently, CD4+ T cells that express PD‐1, an immune checkpoint (IC) molecule, have been recognized as another category of negative regulatory T cells that can block TCR‐induced T‐cell proliferation and cytokine production 12 , 13 ; this phenomenon has been observed in tumor immunity, autoimmunity, transplantation, and infectious diseases. In HIV infection, high expression of programmed death receptor 1 (PD‐1) on CD4+T cells may represent a marker of T‐cell dysfunction 14 , 15 , 16 and HIV persistence. 17 , 18 , 19 These findings indicate that Tregs might be used as informative diagnostic markers. Moreover, the specific therapeutic removal or inhibition of Tregs has been shown to enhance immunity to a certain extent, but this approach still has limited clinical benefit. 20 , 21 , 22 , 23 , 24 Therefore, a thorough understanding of these regulators by characterizing them on temporal and spatial scales is strongly required.
To address this question, we measured the CD3+T cells, CD4+T cells, CD8+T cells, absolute CD4+T cell count, CD4+PD‐1+T cells, CD4+PD‐1highT cells, CD8+PD‐1+T cells, and CD4+CD25highTregs of 100 HIV‐infected/AIDS patients and in 20 controls. Temporally, the patients at both the intermediate stage and late stage had lower percentages of CD4+T cells but higher percentages of CD8+T cells and CD4+PD‐1+T cells among their lymphocytes, which allowed these cells to be used as markers for identifying disease beyond the early stage with intermediate or high levels of immunosuppression. Moreover, the percentage of CD4+CD25highTregs only increased in patients with late‐stage disease, providing a marker for uniquely identifying late‐stage disease with a high level of immunosuppression. In addition, CD4+PD‐1+T cells were negatively correlated with absolute CD4+T cell count, but no correlations between CD4+CD25highTregs and absolute CD4+T cell count were observed; thus, CD4+PD‐1+T cells were suitable for use as quantitative markers to monitor the progression of immunosuppression. Spatially, CD4+PD‐1+T cells were not correlated with CD4+CD25highTregs, which suggested that these Tregs function differently during immunosuppression; therefore, we should design a smart therapeutic strategy that either inhibits these cells individually or in combination with different degrees based on the stage of disease. In the future, we will further study Tregs at the single‐cell level to determine the mechanism underlying the immunosuppression in HIV.
2. MATERIALS AND METHODS
2.1. HIV‐infected/AIDS patients and healthy controls
One hundred HIV‐infected/AIDS patients were enrolled; these patients included 87 males and 13 females, with an average age of 35.8 years, ranging from 18 to 67 years. These patients were diagnosed according to the “Clinical diagnosis and treatment of AIDS” and “Treatment manual of free national antiretroviral drugs for AIDS.” Twenty healthy controls were enrolled in this study, including 16 males and 4 females, with an average age of 36.8 years, ranging from 20 to 60 years; the healthy controls were negative for markers of HBV, HCV, HDV, and tuberculosis infection. There was no significant difference in age or sex between the patients and healthy controls. Ethical approval was given by the Suzhou City Hospital Institutional Review Board. Based on the ethical approval, the patients were enrolled from January 1, 2012, to December 31, 2015, at the Fifth People's Hospital of Suzhou City after providing written consent.
2.2. Reagents and equipment
Simultest IMK‐lymphocyte kit, CD3‐FITC/CD8‐PE/CD 45‐PreCP/CD4‐APC, CD3‐APC, CD4‐PerCP, CD25‐FITC, Mouse IgG2a‐FITC, Mouse IgG1‐PE, and TRU‐COUNT tubes were purchased from BD Company (San Jose). PD‐1‐PE monoclonal antibody was purchased from eBioscience (San Diego). FACS Calibur was purchased from BD Company (San Jose).
2.3. Preparation of blood samples
Two milliliters of venous blood was collected from each HIV‐infected/AIDS patient and healthy control, transferred to a tube coated with the anticoagulant EDTA, and processed within 2 h.
2.4. Analysis of lymphocyte subsets
Immunofluorescence staining of lymphocytes was performed with the Simultest IMK‐Lymphocyte kit according to the manufacturer's protocol. All the incubations were carried out in the dark at room temperature. Briefly, we added 100 μl of whole blood into each of six test tubes and then combined the blood with 10 μl of six different antibody solutions, namely, CD45‐FITC/CD14‐PE, IgG1‐FITC/IgG2a‐PE, CD3‐FITC/CD19‐PE, CD3‐FITC/CD4‐PE, CD3‐FITC/CD8‐PE, CD3‐FITC/CD16+56‐PE. After incubation in the dark at room temperature for 15 min, we added 2 ml of 10% FACS lysis solution into each tube, mixed the solution, and incubated the samples for 15–20 min. The white blood cells were pelleted by centrifugation at 370 g for 5 min and washed with 2 ml of PBS solution. To obtain the number of T lymphocytes (CD3+T cells) and the percentages of T helper cells (CD3+CD4+T cells) and suppressor/cytotoxic T cells (CD3+CD8+T cells) among the white blood cells, total lymphocytes, and T lymphocytes, the pellets were suspended in 500 μl of PBS solution and analyzed using flow cytometry.
2.5. Analysis of the absolute counts of CD4+ and CD8+ T cells
We combined 10 μl of CD3‐FITC/CD8‐PE/CD45 ‐PreCP/CD4‐APC solution and 50 μl of whole blood in a TRU‐COUNT tube, mixed the samples, and incubated the samples for 15 min. Then, 500 μl of FACS lysis solution was added and incubation for 12 min. The absolute counts of CD4+T cells and CD8+T cells were analyzed using flow cytometry.
2.6. Analysis of CD4+CD25+ Tregs and CD4+PD‐1+ T cells
We added 100 μl of whole blood into a test tube and a control tube. Then, 5 μl of CD3‐APC, 10 μl of Mouse IgG1‐PE, 10 μl of CD4‐PerCP, and 10 μl of Mouse IgG2a‐FITC was sequentially added into the control tube, and 10 μl of CD25‐FITC, 10 μl of PD‐1‐PE, 10 μl of CD4‐PerCP, and 5 μl of CD3‐APC were sequentially added into the test tube. The two tubes were incubated for 15 min. We added 2 ml of 10% FACS lysis solution to the tubes, mixed the samples, and incubated the samples for 15–20 min. After centrifugation at 370 g for 5 min, the supernatants were discarded. The pellets were washed with 2 ml of PBS and finally resuspended in 500 μl of PBS solution. The CD4+CD25+ Tregs and CD4+PD‐1+ T cells were finally analyzed using flow cytometry.
2.7. Statistical analysis
The SPSS 13.0 software package was used to conduct all the statistical analyses. Statistical significance was accepted if a p‐value < 0.05 was obtained. GraphPad Prism 5 was used to generate the figures.
3. RESULTS AND DISCUSSION
To assess the immune status of the HIV‐infected/AIDS patients, we first analyzed their lymphocyte subsets using flow cytometry. As shown in Figure 1, the patients exhibited a lower percentage of CD4+T cells (Figure 1B) among their WBCs (4.294 ± 0.4789 vs. 6.595 ± 0.6714, p < 0.0001) but higher percentages of CD3+T cells(Figure 1A) and CD8+ T cells (Figure 1C) (25.79 ± 2.074 vs. 13.41 ± 1.474, p < 0.0001, 22.28 ± 2.226 vs. 6.481 ± 0.9498, p < 0.0001, respectively) than the controls. Regarding lymphocytes, the patients also had a lower percentage of CD4+ T cells (12.86 ± 1.44 vs. 34.11 ± 2.52, p < 0.0001) and a higher percentage of CD8+ T cells (58.31 ± 2.98 vs. 32.65 ± 2.94, p < 0.0001) than the controls, but no difference was observed in the percentage of CD3+T cells between the patients and controls. The percentage of CD4+T cells among CD3+T cells was lower in the patients than in the controls (18.83 ± 2.14 vs. 50.17 ± 3.44, p < 0.0001), whereas the percentage of CD8+T cells among CD3+T cells was higher (81.10 ± 2.14 vs. 49.80 ± 3.42, p < 0.0001). In summary, the HIV‐infected/AIDS patients recruited for this study indeed had dramatically decreased percentages of CD4+T cells and inverted ratios of CD4+T cells to CD8+T cells in the WBCs, lymphocytes, and CD3+T cells.
FIGURE 1.
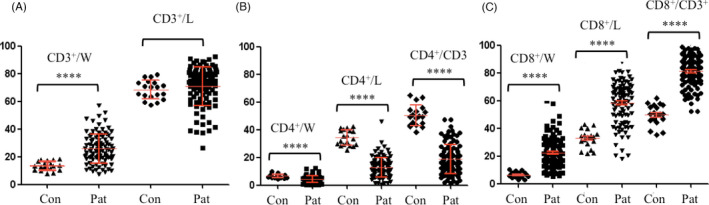
Analysis of T‐cell subsets in the peripheral blood of HIV‐infected/AIDS patients (n = 100) and healthy controls (n = 20). (A) CD3+T cells; (B) CD4+T cells (C) CD8+T cells. Con, healthy controls; Pat, HIV‐infected/AIDS patients. Con, controls; L, lymphocytes; Pat, patients; W, white blood cells. ****p < 0.0001
We then measured Tregs, including CD4+PD‐1+T cells, CD4+PD‐1highT cells, CD8+PD‐1+T cells, and CD4+CD25highTregs, among the lymphocytes of HIV‐infected/AIDS patients and controls using flow cytometry. The patients had much higher percentages of CD4+PD‐1+ T cells (Figure 2A) and CD4+CD25highTregs (Figure 2B) than the controls (57.17 ± 3.42 vs. 34.94 ± 4.07, p < 0.0001, 51.06 ± 2.89 vs. 26.65 ± 4.65, p < 0.0001, 7.48 ± 0.64 vs. 3.95 ± 1.11, p < 0.0001, respectively). The difference in CD4+PD‐1highT cells between the patients and controls was also significant (11.43 ± 1.37 vs. 6.71 ± 1.36, p < 0.01), but the significance was lower than that of the difference in CD4+PD‐1+T cells and CD4+CD25highTregs. In addition, PD‐1 expression on the surface of CD8+T cells was also higher in the patients (Figure 2A), this is similar to previous reports that Ag‐specific CD8+T cells are critical for controlling HIV infection but eventually undergo functional exhaustion partially because of the expression and signaling through the inhibitory PD‐1 receptor [28–30]. Since CD8+PD‐1+T cells were not the main focus here, we will investigate these cells in detail in the future.
FIGURE 2.
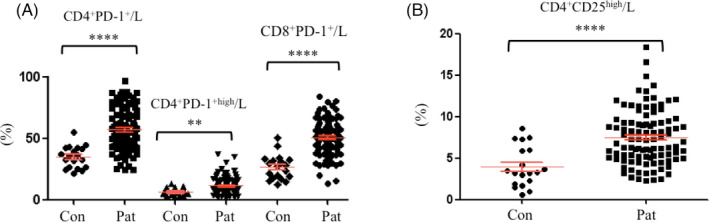
Measurement of (A) CD4+PD‐1+T, CD4+PD‐1highT and CD8+PD‐1+T, and (B) CD4+CD25highTregs in HIV‐infected/AIDS patients (n = 100) and healthy controls (n = 20). Con, healthy controls; Pat, HIV‐infected/AIDS patients. L, lymphocytes. **p < 0.01, ****p < 0.0001
To examine the trends of lymphocyte subsets on a temporal scale, we analyzed the CD3+T cells, CD4+T cells, and CD8+T cells of the HIV‐infected/AIDS patients on the basis of the stage of disease, including early‐stage disease (CD4+T cells >500/μl), intermediate‐stage disease (CD4+T cells = 200–500/μl), and late‐stage disease (CD4+T cells <200/μl). These three groups showed no difference in terms of the percentage of CD3+ T cells (Figure 3A); however, the 200–500/μl and 200–500/μl groups had significantly lower percentages of CD4+T cells among their lymphocytes than the >500/μl group (Figure 3B), and the first two groups had higher percentages of CD8+T cells among their lymphocytes than the last group (Figure 3C). These data suggested that HIV‐infected/AIDS patients at both the intermediate‐stage and late‐stage of disease had significantly decreased percentages of CD4+T cells and increased percentages of CD8+T cells among their lymphocytes.
FIGURE 3.
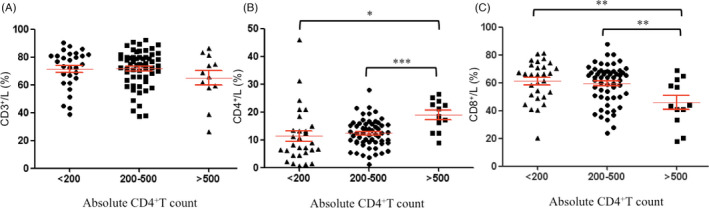
Analysis of T‐cell subsets of HIV‐infected/AIDS patients (n = 100) on absolute CD4+ count. L, lymphocytes. (A) The percentage of CD3+T cells in lymphocytes; (B) The percentage of CD4+T cells in lymphocytes; (C) The percentage of CD8+T cells in lymphocytes. *p < 0.05, **p < 0.01, ***p < 0.001
We then expanded the study at the temporal scale to Tregs by measuring the percentages of CD4+PD‐1+T cells and CD4+CD25high Tregs at different stages of disease due to their dramatic difference between the patients and controls. At the late stage (CD4+T cells <200/μl) and intermediate stage (CD4+T cells = 200‐500/μl) of disease, there were more CD4+PD‐1+T cells than at early stage (CD4+T cells >500/μl) (63.05 ± 6.20 vs. 42.28 + 9.76, p < 0.001, 57.55 ± 4.12 vs. 42.28 + 9.76, p < 0.05), but there was no difference between the CD4+T cells <200/μl and CD4+T cells = 200–500/μl groups and the CD4+T cells <200/μl group (Figure 4A). The percentage of CD4+CD25highTregs in the CD4+T cells <200/μl group was higher than that in the CD4+ T cells = 200–500/μl and CD4+T cells >500/μl groups (9.43 ± 1.54 vs. 6.77 ± 0.77, p < 0.001, 9.433 ± 1.54 vs. 6.62 ± 1.54, p < 0.05), but no difference was observed between the CD4+T cells = 200–500/μL and CD4+T cells >500/μl groups (Figure 4B). The percentage of CD4+PD‐1+T cells increased in the patients when their absolute CD4+T cell count was <500/μl, while the percentage of CD4+CD25highTregs only increased when the absolute CD4+T cell count was lower than 500/μl. This new discovery indicated that CD4+CD25highTregs are generated at a later stage of HIV infection, and these cells could be used as clinical markers of poor disease status.
FIGURE 4.
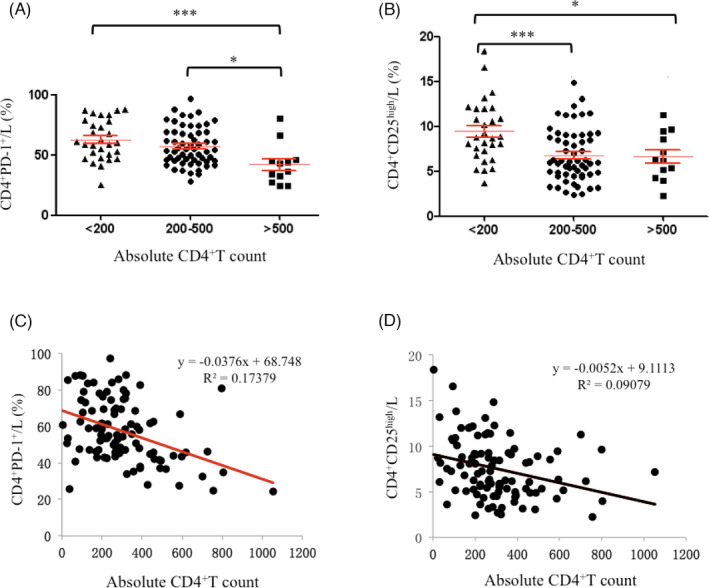
Analysis of CD4+PD‐1+T and CD4+CD25highTregs in HIV‐infected/AIDS patients (n = 100) on absolute CD4+ count (A) and the trend of CD4+PD‐1+T/L and CD4+CD25highTregs/L with the increase of absolute CD4+ count. L, lymphocytes. *p < 0.05, ***p < 0.001
Further studying the correlation between negative regulatory T cells and absolute CD4+T cell counts, we found that the percentage of CD4+PD‐1+T cells had a negative correlation with absolute CD4+T cell count in the HIV‐infected/AIDS patients (Figure 4C). In contrast, the percentage of CD4+CD25highTregs was not correlated with the absolute CD4+T cell count (Figure 4D). This finding provides a new quantitative marker for evaluating the progression of immunosuppression.
Finally, we characterized the spatial correlation between CD4+PD‐1+T cells and CD4+CD25highTregs. No obvious correlation was observed (Figure 5), and this finding will be very beneficial for developing suitable therapeutic strategies. It is known that antiretroviral therapy (ART) failure occurs mainly due to the persistence of HIV in a pool of infected CD4+T cells that express inhibitory molecules, such as PD‐1 and CD25. 4 , 5 , 6 , 7 , 17 , 18 , 19 Therefore, targeting this reservoir of latent HIV was essential for establishing a treatment for HIV 20 , 21 , 22 , 23 , 24 and one of the approaches was to increase the ability of these CD4+T cells to be eliminated by blocking either PD‐1 or CD25 to enhance HIV transcription. 25 The findings of our current study suggested that CD4+PD‐1+ T cells and CD4+CD25high Tregs possess different biological targets.
FIGURE 5.
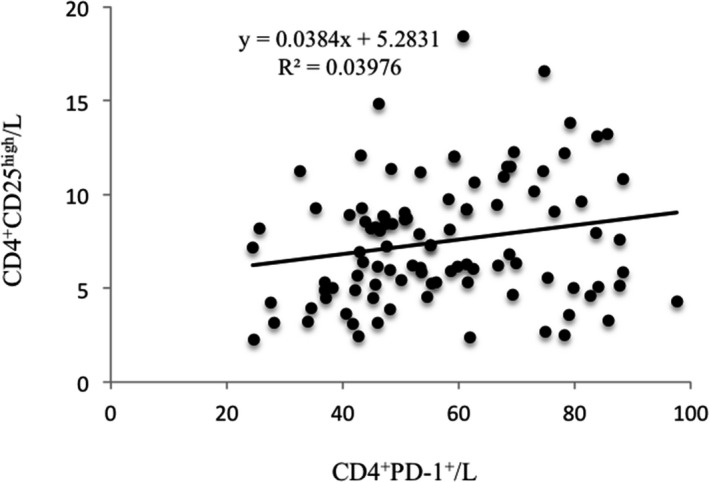
The correlation between the percentages of CD4+PD‐1+T and CD4+CD25highTregs in lymphocytes in HIV‐infected/AIDS patients (n = 100)
4. CONCLUSIONS
To conclude, we characterized Treg cells in HIV‐infected/AIDS patients at both temporal and spatial scales. We found that CD4+CD25+Tregs and CD4+PD‐1+T cells could be used as potential diagnostic markers for identifying different disease stages and for monitoring disease progression. Our findings are also very informative and illuminating for inhibition‐based treatments. Considering their different spatial and temporal functions, CD4+CD25+Tregs and CD4+PD‐1+T cells should be blocked either individually or in combination to different degrees based on the absolute CD4+T cell count. This approach will be beneficial for both maximizing drug efficacy and minimizing side effects, such as the immune‐related adverse events reported previously. 25 In the future, we will further study these negative regulatory T cells at the single‐cell level to determine the mechanism underlying the immunosuppression in HIV, such as with single‐cell RNA‐seq 26 and single‐cell TCR/BCR sequencing 27 experiments.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHORS’ CONTRIBUTIONS
PX, CLR, HC, HDZ, JCX, SFC, LLM, and MJW conceived the study. PX, CLR, HC, JCX, SFC, LLM, and MJW acquired and analyzed data. PX, CLR, and HC wrote the first draft of the study. HDZ and JCX contributed to the writing of the study. HDZ, JCX, and PX read and approved the final study.
ACKNOWLEDGEMENTS
This study was supported by grants from the Science and Technology Plan of Suzhou, China (SYS2020192, SYS2020190, SYS2018079, and SS2019074); Suzhou Gusu Health Talents Program of Suzhou (GSWS2019067); Young Medical Talents Program of Jiangsu (QNRC2016225); Natural Fund of Jiangsu Province, China (BZ2019017); National Natural Science Foundation, China (81900577).
Hui Chen, ChuanLu Ren, Hua Feng Song and LiLing Ma equally contributed to this work.
Contributor Information
HuiDan Zhang, Email: hdzhang@seas.harvard.edu.
Jun‐Chi Xu, Email: xuping19670822@126.com, Email: xujunchi19850504@126.com, Email: hdzhang@seas.harvard.edu.
Ping Xu, Email: xuping19670822@126.com.
DATA AVAILABILITY STATEMENT
The original data of this research are available from the corresponding author on request.
REFERENCES
- 1. Douek DC, Roederer M, Koup RA. Emerging concepts in the immunopathogenesis of AIDS. Annu Rev Med. 2009;60(1):471‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Doitsh G, Galloway N, Geng X, et al. Cell death by pyroptosis drives CD4 T‐cell depletion in HIV‐1 infection. Nature. 2014;505(7484):509‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Doitsh G, Greene WC. Dissecting how CD4 T cells are lost during HIV infection. Cell Host Microbe. 2016;19(3):280‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self‐tolerance maintained by activated T cells expressing IL‐2 receptor alpha‐chains(CD25). Breakdown of a single mechanism of self‐tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151‐1164. [PubMed] [Google Scholar]
- 5. Iwashiro M, Messer RJ, Peterson KE, Stromnes IM, Sugie T, Hasenkrug KJ. Immunosuppression by CD4+ regulatory T cells induced by chronic retroviral infection. Proc Natl Acad Sci USA. 2001;98(16):9226‐9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chinen T, Kannan AK, Levine AG, et al. An essential role for the IL‐2 receptor in Treg cell function. Nat Immunol. 2016;1322‐1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hasenkrug KJ, Chougnet CA, Dittmer U. Regulatory T cells in retroviral infections. PLoS Pathog. 2018;14(2):e1006776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Veiga‐Parga T, Sehrawat S, Rouse BT. Role of regulatory T cells during virus infection. Immunol Rev. 2013;255(1):182‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li S, Gowans EJ, Chougnet C, Plebanski M, Dittmer U. Natural regulatory T cells and persistent viral infection. J Virol. 2008;82(1):21‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Rinsho Ketsueki. 2014;55(10):2183‐2189. [PubMed] [Google Scholar]
- 11. Chang L, Workman CJ, Vignali D. Targeting regulatory T cells in tumors. FEBS J. 2016;283(14):2731‐2748. [DOI] [PubMed] [Google Scholar]
- 12. Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD‐1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carter L, Fouser L, Jussif J, et al. PD‐1: PD‐L inhibitory pathway affects both CD4+ and CD8+ T cells and is overcome by IL‐2. Eur J Immunol. 2002;32(3):634‐643. [DOI] [PubMed] [Google Scholar]
- 14. Day CL, Kaufmann DE, Kiepiela P, et al. PD‐1 expression on HIV‐specific T cells is associated with T‐cell exhaustion and disease progression. Nature. 2006;443(7109):350‐354. [DOI] [PubMed] [Google Scholar]
- 15. D'Souza M, Fontenot AP, Mack DG, et al. Programmed death 1 expression on HIV‐specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J Immunol. 2007;179(3):1979‐1987. [DOI] [PubMed] [Google Scholar]
- 16. Fromentin R, Bakeman W, Lawani MB, et al. CD4+ T cells expressing PD‐1, TIGIT and LAG‐3 contribute to HIV persistence during ART. PLoS Pathog. 2016;12(7):e1005761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khoury G, Fromentin R, Solomon A, et al. Human immunodeficiency virus persistence and T‐cell activation in blood, rectal, and lymph node tissue in human immunodeficiency virus‐infected individuals receiving suppressive antiretroviral therapy. J Infect Dis. 2017;215(6):911‐919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hiroyu H, Vivek J, Hunt PW, et al. Cell‐based measures of viral persistence are associated with immune activation and programmed cell death protein 1 (PD‐1)–expressing CD4+ T cells. J Infect Dis. 2013;208(1):50‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salisch NC, Kaufmann DE, Awad AS, et al. Inhibitory TCR coreceptor PD‐1 is a sensitive indicator of low‐level replication of SIV and HIV‐1. J Immunol. 2010;184(1):476‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brezar V, Ruffin N, Richert L, et al. Decreased HIV‐specific T‐regulatory responses are associated with effective DC‐vaccine induced immunity. PLoS Pathog. 2015;11(3):e1004752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kleinman AJ, Ranjit S, Ivona P, et al. Regulatory T cells as potential targets for HIV cure research. Front Immunol. 2018;9:734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Amancha PK, Hong JJ, Rogers K, et al. In vivo blockade of the PD‐1 pathway using soluble rPD‐1‐Fc enhances CD4+ and CD8+ T cell responses but has limited clinical benefit. J Immunol. 2013;191(12):6060‐6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Appay V, Lissina A, Sauce D. Enhancing SIV‐specific immunity in vivo by PD‐1 blockade. Nature. 2009;458(7235):206‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Porichis F, Kwon DS, Zupkosky J, et al. Responsiveness of HIV‐specific CD4 T cells to PD‐1 blockade. Blood. 2011;118(4):965‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boyer Z, Palmer S. Targeting immune checkpoint molecules to eliminate latent HIV. Front Immunol. 2018;9:2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vincent C, Treiber CD, Scott W. Cellular diversity in the Drosophila midbrain revealed by single‐cell transcriptomics. Elife. 2018;7:e34550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simone MD, Rossetti G, Pagani M. Single Cell TCR sequencing: techniques and future challenges. Front Immunol. 2018;9:1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original data of this research are available from the corresponding author on request.


