Abstract
Background
Circular RNA_0015278 (circ_0015278) inhibits the progression of several cancers and is greatly reduced in papillary thyroid carcinoma (PTC) tissues compared with benign thyroid lesions by microarray profiling. This study aimed to further investigate the correlation of circ_0015278 with tumor characteristics and prognosis in PTC patients.
Methods
Totally, 206 PTC patients who underwent tumor resection were retrospectively enrolled; subsequently, circ_0015278 expression in their tumor and adjacent tissues was detected by reverse transcriptional‐quantitative polymerase chain reaction. Besides, disease‐free survival (DFS) and overall survival (OS) were calculated.
Results
Circ_0015278 was reduced in tumor tissues compared with adjacent tissues (p < 0.001), and receiver operating characteristic analysis showed that it well discriminated tumor tissues from adjacent tissues (area under curve: 0.903, 95% confidence interval: 0.874–0.932). Besides, higher tumor circ_0015278 expression was correlated with absence of extrathyroidal invasion (p = 0.036), lower pathological tumor (pT) stage (p = 0.05), pathological node (pN) stage (p = 0.002), and pathological tumor‐node‐metastasis (pTNM) stage (p = 0.001). Moreover, higher tumor circ_0015278 expression was associated with a reduced relapse rate (p = 0.011), but not mortality rate (p = 0.110); meanwhile, it was also correlated with prolonged DFS (p = 0.017), but not OS (p = 0.136). Additionally, multivariate Cox's regression analyses showed that higher tumor circ_0015278 expression independently associated with favorable DFS (p = 0.026, hazard ratio = 0.529).
Conclusion
Circ_0015278 is reduced in tumor tissues, while its’ higher expression in tumor correlates with absence of extrathyroidal invasion, lower pT, pN, and pTNM stage, as well as prolonged DFS in PTC patients.
Keywords: circular RNA_0015278, disease‐free survival, overall survival, papillary thyroid carcinoma, tumor characteristics
Circular RNA_0015278 (circ_0015278) inhibits the progression of several cancers and is greatly reduced in papillary thyroid carcinoma (PTC) tissues compared with benign thyroid lesions by microarray profiling. This study aimed to further investigate the correlation of circ_0015278 with tumor characteristics and prognosis in PTC patients. Totally, 206 PTC patients who underwent tumor resection were retrospectively enrolled; subsequently, circ_0015278 expression in their tumor and adjacent tissues was detected by reverse transcriptional‐quantitative polymerase chain reaction. Besides, disease‐free survival (DFS) and overall survival (OS) were calculated. Circ_0015278 was reduced in tumor tissues compared with adjacent tissues (p < 0.001), and receiver operating characteristic analysis showed that it well discriminated tumor tissues from adjacent tissues (area under curve: 0.903, 95% confidence interval: 0.874–0.932). Besides, higher tumor circ_0015278 expression was correlated with absence of extrathyroidal invasion (p = 0.036), lower pathological tumor (pT) stage (p = 0.05), pathological node (pN) stage (p = 0.002), and pathological tumor‐node‐metastasis (pTNM) stage (p = 0.001). Moreover, higher tumor circ_0015278 expression was associated with a reduced relapse rate (p = 0.011), but not mortality rate (p = 0.110); meanwhile, it was also correlated with prolonged DFS (p = 0.017), but not OS (p = 0.136). Additionally, multivariate Cox's regression analyses showed that higher tumor circ_0015278 expression independently associated with favorable DFS (p = 0.026, hazard ratio = 0.529). Circ_0015278 is reduced in tumor tissues, while its higher expression in tumor correlates with absence of extrathyroidal invasion, lower pT, pN, and pTNM stage, as well as prolonged DFS in PTC patients.

1. INTRODUCTION
Papillary thyroid carcinoma (PTC) is the most prevalent endocrinal malignancy, which causes over 500 thousand newly diagnosed cases worldwide each year, and it is the fifth most common cancer in females. 1 , 2 Notably, the incidence of PTC has dramatically increased in the past few decades in several countries including China, which makes it a heavy burden on public health. 2 , 3 Generally, PTC is a type of cancer with a good prognosis: the 5‐year survival rate of localized PTC is over 95%; however, PTC patients with distant metastasis have a 5‐year survival rate of around 50%. 4 , 5 Besides, over a quarter of PTC patients suffer from PTC recurrence during long‐time follow‐up. 4 , 5 Therefore, it is urgent to search for novel biomarkers for the surveillance of disease progression and prognosis in PTC patients to potentially improve the management toward them.
Circular RNA (circRNA) is a type of non‐coding RNA that regulates various biological processes through sponging microRNAs and regulating RNA binding proteins. 6 , 7 Meanwhile, recent studies reveal that circRNAs regulate various signaling to modulate the pathogenesis and progression of cancers. 8 , 9 , 10 Besides, circRNA is regarded as a potential, clinical biomarker for it is stable and easy to extract. 11 , 12 In PTC, previous studies suggest that several circRNAs, including circ_0002111, circ_0006156, and circ_0137287, serve as clinical biomarkers and possess the potential to improve the management of PTC patients. 13 , 14 , 15 Regarding circ_0015278, it is a newly found circRNA that inhibits the progression of several cancers including non‐small cell lung cancer (NSCLC) and ovarian cancer. 16 , 17 Moreover, one previous study using microarray profiling finds that circ_0015278 is greatly reduced in PTC tumor tissues compared with benign thyroid lesions. 18 Taken these information together, we hypothesized that circ_0015278 could be a potential, clinical biomarker for PTC. However, no previous study had been conducted to explore that to the best of our knowledge.
In this study, we retrospectively enrolled 206 PTC patients who underwent resection. The aim of this study was to investigate the correlation of circ_0015278 with tumor characteristics and prognosis in PTC patients.
2. METHODS
2.1. Subjects
This study retrospectively analyzed 206 PTC patients treated by thyroidectomy in our hospital between March 2015 and February 2020. All patients with age above 18 years had a pathological diagnosis of PTC and met the following screening criteria: (i) surgically resected tumor tissue and adjacent tissue were well‐preserved and available for RNA isolation; (ii) the main clinicopathological data were complete; (iii) postoperative follow‐up data were available for survival analysis. Patients who received treatment for PTC before surgery or had a history of other malignant diseases were excluded from the study. Permission for the study was acquired from the Ethics Committee. The written informed consent was collected from the patients or guardians.
2.2. Data collection and specimen acquisition
The medical charts of PTC patients were reviewed for data collection. The data of age, gender, tumor size, extrathyroidal invasion status, pathological T (pT) stage, pathological N (pN) stage, pathological TNM (pTNM) stage, and adjuvant radioiodine therapy were extracted from the medical charts for study analysis. The clinical visit documents of patients were also reviewed, and the survival data were abstracted for calculation of disease‐free survival (DFS) and overall survival (OS). In addition, the tumor tissues and adjacent tissues corresponding to each patient were obtained from the sample library, which were all refrigerated and available for RNA determination.
2.3. Circ_0015278 determination
The tumor tissues and adjacent tissues were processed for the determination of circ_0015278 by reverse transcription‐quantitative polymerase chain reaction (RT‐qPCR). The procedures of RNA isolation, reverse transcription, as well as qPCR were conducted as described in a previous study. 18 qPCR was triplicated for each sample. The cycling conditions were presented as follows: 95°C, 2 min (1 cycle); 95°C, 5 s, 60°C, 10 s (40 cycles). The following kits were used in the RT‐qPCR: TRIzol reagent (Thermo Fisher Scientific); RNase R (Epicentre); iScript™ cDNA Synthesis Kit (with random primer) (Bio‐Rad); QuantiNova SYBR Green PCR Kit (QiaGen). GAPDH was severed as the internal control gene. The relative expression of circ_0015278 was calculated using the 2−ΔΔCt method. The primers were as follows: circ_0015278, forward primer (5′→3′): TGGCTGTGGCTGTGTTAGG; reverse primers (5′→3′): TTCATTGGTCTGTGGATCATATCG; GAPDH, forward primer (5′→3′): GACCACAGTCCATGCCATCAC; reverse primers (5′→3′): ACGCCTGCTTCACCACCTT.
2.4. Statistical analysis
Descriptive analysis for variable was carried out using number with proportion, mean value with standard deviation (SD), or median value with interquartile range (IQR). The circ_0015278 expression in the tumor tissues was classified into four grades according to the quantiles of relative expression: quantile 1 (Q1): 1%–25%; quantile 2 (Q2): 26%–50%; quantile 3 (Q3): 51%–75%; quantile 4 (Q4): 76%–100%. Difference analysis of circ_0015278 between tumor and adjacent tissues was performed by Wilcoxon signed‐rank test; the receiver operating characteristic (ROC) analysis was performed to evaluate the effect of circ_0015278 in distinguishing tumor tissues from adjacent tissues; the association between tumor circ_0015278 expression and clinical features was estimated by Linear‐by‐Linear Association or Spearman's rank correlation test; DFS and OS were compared among patients with different circ_0015278 expression grades using the log‐rank test. The prognostic value of circ_0015278 for PTC patients was assessed by univariate and multivariate Cox's proportional hazard regression analysis. SPSS 24.0 (IBM) and GraphPad Prism 7.02 (GraphPad Software Inc.) were used for data processing and chart making. p value < 0.05 was set as the threshold for statistical significance.
3. RESULTS
3.1. Description of patients’ characteristics
The enrolled PTC patients consisted of 50 (24.3%) males and 156 (75.7%) females with a mean age of 43.9 ± 11.3 years. Meanwhile, the mean tumor size was 3.5 ± 1.6 cm, and 84 (40.8%) patients had extrathyroidal invasion while 122 (59.2%) patients did not. Besides, 29 (14.1%) patients were of pT1 stage, 62 (30.1%) patients were of pT2 stage, 55 (26.7%) patients were of pT3 stage and 60 (29.1%) patients were of pT4 stage; 69 (33.5%) patients were of pN0 stage and 137 (66.5%) patients were of pN1 stage; 111 (53.9%) patients were of pTNM I stage, 16 (7.8%) patients were of pTNM II stage, 47 (22.8%) patients were of pTNM III stage and 32 (15.5%) patients were of pTNM IV stage. Moreover, 57 (27.7%) patients received adjuvant radioiodine therapy but 149 (72.3%) did not (Table 1).
TABLE 1.
Characteristics of patients with PTC
| Items | PTC patients (N = 206) |
|---|---|
| Age (years), mean ± SD | 43.9 ± 11.3 |
| Gender, no. (%) | |
| Male | 50 (24.3) |
| Female | 156 (75.7) |
| Tumor size (cm), mean ± SD | 3.5 ± 1.6 |
| Extrathyroidal invasion, no. (%) | |
| Present | 84 (40.8) |
| Absent | 122 (59.2) |
| pT stage, no. (%) | |
| pT1 | 29 (14.1) |
| pT2 | 62 (30.1) |
| pT3 | 55 (26.7) |
| pT4 | 60 (29.1) |
| pN stage, no. (%) | |
| pN0 | 69 (33.5) |
| pN1 | 137 (66.5) |
| pTNM stage, no. (%) | |
| I | 111 (53.9) |
| II | 16 (7.8) |
| III | 47 (22.8) |
| IV | 32 (15.5) |
| Adjuvant radioiodine therapy, no. (%) | |
| No | 149 (72.3) |
| Yes | 57 (27.7) |
Abbreviations: pN, pathological node; pT, pathological tumor; PTC, papillary thyroid carcinoma; pTNM, pathological tumor‐node‐metastasis; SD, standard deviation.
3.2. Comparison of circ_0015278 expression between tumor and adjacent tissues
Circ_0015278 expression was reduced in tumor tissues, median value: 0.342 (0.222–0.573) compared with adjacent tissues, median value: 1.001 (0.750–1.358) (p < 0.001) (Figure 1A). Besides, ROC analysis revealed that circ_0015278 presented good value in differentiating tumor tissues from adjacent tissues (AUC: 0.903, 95% CI: 0.847–0.932) (Figure 1B) in PTC patients.
FIGURE 1.
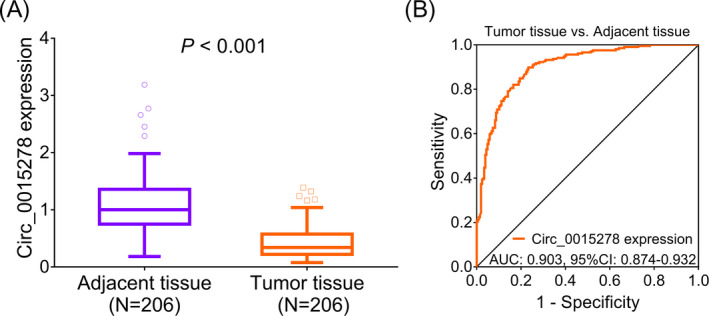
Circ_0015278 expression in PTC patients. A, Comparison of circ_0015278 between tumor tissues and adjacent tissues. B, Value of circ_0015278 in discriminating tumor tissues from adjacent tissues. Circ_0015278: circular RNA_0015278; AUC, area under curve; CI, confidence interval; PTC, papillary thyroid carcinoma
3.3. Correlation of tumor circ_0015278 expression with patients’ characteristics
Higher tumor circ_0015278 expression was correlated with age < 45 years (p = 0.021), absence of extrathyroidal invasion (p = 0.036), lower pT stage (p = 0.005), pN stage (p = 0.002), and pTNM stage (p = 0.001). However, no correlation was found in tumor circ_0015278 expression with gender (p = 0.404) or tumor size (p = 0.117) (Table 2) in PTC patients. Meanwhile, the correlation of tumor circ_0015278 with clinical characteristics in PTC patients was also present in Figure S1A–G.
TABLE 2.
Correlation of circ_0015278 expression with PTC patients’ characteristics
| Items | Circ_0015278 expression | ||||
|---|---|---|---|---|---|
| Q1 (1%−25%) (n = 52) | Q2 (26%−50%) (n = 51) | Q3 (51%−75%) (n = 52) | Q4 (76%−100%) (n = 51) | p Value | |
| Age, no. (%) | |||||
| <45 years | 23 (44.2) | 23 (45.1) | 26 (50.0) | 34 (66.7) | 0.021 |
| ≥45 years | 29 (55.8) | 28 (54.9) | 26 (50.0) | 17 (33.3) | |
| Gender, no. (%) | |||||
| Male | 12 (23.1) | 17 (33.3) | 11 (21.2) | 10 (19.6) | 0.404 |
| Female | 40 (76.9) | 34 (66.7) | 41 (78.8) | 41 (80.4) | |
| Tumor size, no. (%) | |||||
| ≤4 cm | 31 (59.6) | 30 (58.8) | 32 (61.5) | 38 (74.5) | 0.117 |
| >4 cm | 21 (40.4) | 21 (41.2) | 20 (38.5) | 13 (25.5) | |
| Extrathyroidal invasion, no. (%) | |||||
| Absent | 25 (48.1) | 29 (56.9) | 34 (65.4) | 34 (66.7) | 0.036 |
| Present | 27 (51.9) | 22 (43.1) | 18 (34.6) | 17 (33.3) | |
| pT stage, no. (%) | |||||
| pT1 | 9 (17.4) | 2 (3.9) | 7 (13.5) | 11 (21.6) | 0.005 |
| pT2 | 5 (9.6) | 20 (39.2) | 18 (34.6) | 19 (37.2) | |
| pT3 | 19 (36.5) | 13 (25.5) | 12 (23.1) | 11 (21.6) | |
| pT4 | 19 (36.5) | 16 (31.4) | 15 (28.8) | 10 (19.6) | |
| pN stage, no. (%) | |||||
| pN0 | 12 (23.1) | 13 (25.5) | 19 (36.5) | 25 (49.0) | 0.002 |
| pN1 | 40 (76.9) | 38 (74.5) | 33 (63.5) | 26 (51.0) | |
| pTNM stage, no. (%) | |||||
| I | 25 (48.1) | 23 (45.2) | 27 (51.9) | 36 (70.6) | 0.001 |
| II | 1 (1.9) | 4 (7.8) | 5 (9.6) | 6 (11.8) | |
| III | 15 (28.8) | 15 (29.4) | 8 (15.4) | 9 (17.6) | |
| IV | 11 (21.2) | 9 (17.6) | 12 (23.1) | 0 (0.0) | |
Abbreviations: pN, pathological node; pT, pathological tumor; PTC, papillary thyroid carcinoma; pTNM, pathological tumor‐node‐metastasis; Q1, quantile 1; Q2, quantile 2; Q3, quantile 3; Q4, quantile 4.
3.4. Correlation of tumor circ_0015278 expression with relapse and mortality rate
Higher tumor circ_0015278 expression was correlated with lower relapse rate (p = 0.011; Figure 2A). However, no correlation was found in tumor circ_0015278 expression with mortality rate (p = 0.110; Figure 2B) in PTC patients.
FIGURE 2.
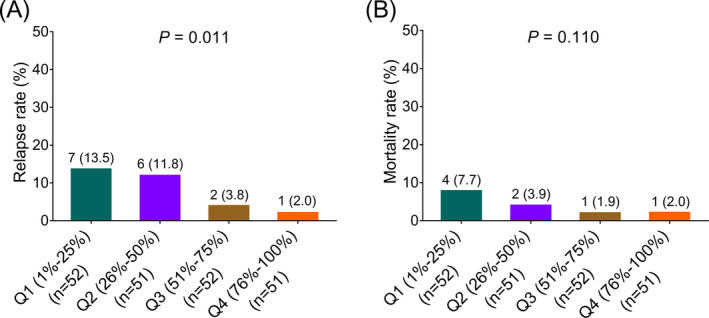
Relapse rate and mortality rate in PTC patients. A, Comparison of relapse rate among PTC patients with different quantiles of tumor circ_0015278 expressions. B, Comparison of mortality rate in PTC patients with different quantiles of tumor circ_0015278 expressions. PTC, papillary thyroid carcinoma; Q, quantile
3.5. Correlation of tumor circ_0015278 expression with DFS and OS
Higher tumor circ_0015278 expression was correlated with prolonged DFS (p = 0.017) (Figure 3A), while no correlation was found in it with OS (p = 0.136) (Figure 3B) in PTC patients. After adjustment by forward stepwise multivariate Cox's regression analyses, higher tumor circ_0015278 (p = 0.026, HR = 0.529) was independently correlated ameliorated DFS (Table 3), but not OS (Table 4). Besides, it was also found that higher pTNM stage (p = 0.017, HR = 1.766) was independently correlated with shorter DFS (Table 4), and tumor size (>4 cm vs. ≤4 cm) (p = 0.012, HR = 14.835) was independently correlated with unfavorable OS (Table 4) in PTC patients.
FIGURE 3.
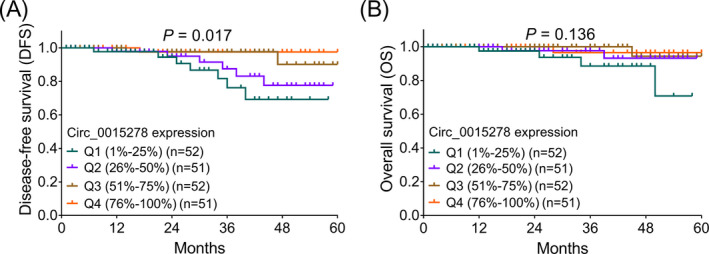
DFS and OS in PTC patients. A, Comparison of DFS among PTC patients with different quantiles of tumor circ_0015278 expressions. B, Comparison of OS in PTC patients with different quantiles of tumor circ_0015278 expressions. DFS, disease‐free survival; OS, overall survival; PTC, papillary thyroid carcinoma; Q, quantile
TABLE 3.
Cox's regression analysis of variables associated with DFS
| Parameters | Cox's proportional hazard regression model | |||
|---|---|---|---|---|
| p Value | HR | 95% CI | ||
| Lower | Higher | |||
| Univariate Cox's regression | ||||
| Higher circ_0015278 expression | 0.004 | 0.460 | 0.271 | 0.780 |
| Age (≥45 years vs. <45 years) | 0.012 | 6.737 | 1.531 | 29.655 |
| Gender (male vs. female) | 0.107 | 2.254 | 0.839 | 6.058 |
| Tumor size (>4 cm vs. ≤4 cm) | 0.052 | 2.671 | 0.990 | 7.206 |
| Extrathyroidal invasion (present vs. absent) | 0.080 | 2.472 | 0.898 | 6.809 |
| Higher pT stage | 0.036 | 1.775 | 1.037 | 3.040 |
| Higher pN stage | 0.067 | 3.999 | 0.908 | 17.606 |
| Higher pTNM stage | 0.002 | 2.014 | 1.284 | 3.159 |
| Adjuvant radioiodine therapy (yes vs. no) | 0.994 | 1.004 | 0.348 | 2.894 |
| Forward stepwise multivariate Cox's regression | ||||
| Higher circ_0015278 expression | 0.026 | 0.529 | 0.302 | 0.925 |
| Higher pTNM stage | 0.017 | 1.766 | 1.107 | 2.817 |
Abbreviations: CI, confidence interval; DFS, disease‐free survival; HR, hazards ratio; pN, pathological node; pT, pathological tumor; pTNM, pathological tumor‐node‐metastasis.
TABLE 4.
Cox's regression analysis of variables associated with OS.
| Parameters | Cox's proportional hazard regression model | |||
|---|---|---|---|---|
| p Value | HR | 95% CI | ||
| Lower | Higher | |||
| Univariate Cox's regression | ||||
| Higher circ_0015278 expression | 0.059 | 0.493 | 0.237 | 1.027 |
| Age (≥45 years vs. <45 years) | 0.092 | 6.074 | 0.747 | 49.398 |
| Gender (male vs. female) | 0.545 | 1.557 | 0.371 | 6.543 |
| Tumor size (>4 cm vs. ≤4 cm) | 0.012 | 14.835 | 1.815 | 121.243 |
| Extrathyroidal invasion (present vs. absent) | 0.057 | 4.751 | 0.953 | 23.682 |
| Higher pT stage | 0.040 | 2.546 | 1.043 | 6.216 |
| Higher pN stage | 0.188 | 4.091 | 0.502 | 33.325 |
| Higher pTNM stage | 0.029 | 2.081 | 1.080 | 4.009 |
| Adjuvant radioiodine therapy (yes vs. no) | 0.691 | 1.338 | 0.319 | 5.607 |
| Forward stepwise multivariate Cox's regression | ||||
| Tumor size (>4 cm vs. ≤4 cm) | 0.012 | 14.835 | 1.815 | 121.243 |
Abbreviations: CI, confidence interval; HR, hazards ratio; OS, overall survival; pN, pathological node; pT, pathological tumor; pTNM, pathological tumor‐node‐metastasis.
4. DISCUSSION
Circ_0015278 is a newly found circRNA that inhibits the progression of several cancers. 16 , 17 For instance, in NSCLC, circ_0015278 overexpression reduces cell proliferation through sponging microRNA‐1228 (miR‐1228). 16 In ovarian cancer, circ_0015278 represses cell proliferation and metastasis through miR‐1228/p53/epithelial‐mesenchymal transition (EMT) signaling. 17 Clinically, circ_0015278 is identified to be decreased in PTC tissues compared with benign thyroid lesions 18 ; while this finding should be verified with larger sample size. Therefore, we retrospectively enrolled 206 PTC patients and confirmed that circ_0015278 was reduced in PTC tissues compared with adjacent tissues. A possible explanation might be that: circ_0015278 high expression might serve as the sponge of miR‐1228 to enhance the activity of wild type p53 (as in ovarian cancer 17 ), and the latter one is the key suppressor of thyroid tumorigenesis. 19 Meanwhile, other previous studies also suggest that several circRNAs are dysregulated in PTC. 13 , 14 , 15
As to the correlation of circ_0015278 with the clinical characteristics of cancer patients, one previous study suggests that circ_0015278 is negatively correlated with lymph node metastasis in NSCLC patients. 16 In the present study, we found that higher tumor circ_0015278 was associated with absence of extrathyroidal invasion, lower pT stage, pN0 stage, and reduced pTNM stage in PTC patients. These findings could be explained by that: (1) circ_0015278 might suppress PTC growth (as in NSCLC 16 ), which resulted in lower pT stage; (2) circ_0015278 could sponge miR‐1228 to decrease the EMT of PTC cells (as in ovarian cancer cells 17 ); thus, it was correlated with absence of extrathyroidal invasion and pN0 stage; (3) circ_0015278 might regulate the miR‐1228/p53/EMT signaling to suppress PTC cell proliferation, migration, and invasion (as in ovarian cancer cells and NSCLC cells 16 , 17 ), and thus comprehensively repressing the progression of PTC. Therefore, higher circ_0015278 was correlated with lower pTNM stage in PTC patients.
The prognostic value of circ_0015278 in patients with NSCLC or ovarian cancer has been revealed by previous studies that circ_0015278 shows positive correlations with overall survival in patients with NSCLC or ovarian cancer. 16 , 17 While in PTC, no previous study had been conducted to explore that. Therefore, we conducted this study and found that higher tumor circ_0015278 was associated with lower relapse rate and higher DFS, and it was an independent predictive factor for higher DFS. However, no correlation was found in tumor circ_0015278 with mortality rate or OS, and it could not independently predict OS. These data might be explained by that: (1) circ_0015278 was correlated with absence of extrathyroidal invasion and pN0 stage, and the latter ones are potential risk factors for PTC recurrence 20 ; therefore, higher circ_0015278 was correlated with lower relapse rate, longer DFS, and it independently predicted favorable DFS; (2) higher circ_0015278 might increase drug sensitivity, thus enhancing the prognosis of PTC patients; (3) PTC is characterized by low mortality, 1 besides, considering that the follow‐up period of this study was not quite long, the difference of OS among PTC patients with different quantile of tumor circ_0015278 was not obvious enough to observe the statistical significance. Thus, no correlation was found in tumor circ_0015278 with OS in PTC patients in this study.
There were several limitations in this study. First, the long‐term follow‐up duration of this study was relatively short, and the long‐term prognostic value of circ_0015278 in PTC patients might be investigated further. Second, this study only investigated the clinical role of circ_0015278 from PTC tissues, while the clinical value of circ_0015278 from other sources such as peripheral blood, thyroid cyst fluid, or urine of PTC patients could be explored in further studies. Third, the molecular mechanisms of circ_0015278 in regulating the progression of PTC were not included in this study, which could be explored further.
Conclusively, circ_0015278 is insufficiently expressed in PTC tissues, and its higher expression correlates with absence of extrathyroidal invasion, decreased tumor stages, lower relapse rate, and prolonged DFS in PTC patients. The findings of this study imply circ_0015278 may serve as a potential biomarker to improve the management of PCT patients.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
Supporting information
Fig S1
ACKNOWLEDGEMENTS
Project plan of medical scientific research in Hebei Province in 2021 Approval number: 20210495.
Contributor Information
Huiling Liu, Email: jihuigan2454@163.com.
Lei Na, Email: ga28582884@163.com.
DATA AVAILABILITY STATEMENT
Data sharing was not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Seib CD, Sosa JA. Evolving understanding of the epidemiology of thyroid cancer. Endocrinol Metab Clin North Am. 2019;48(1):23‐35. [DOI] [PubMed] [Google Scholar]
- 2. Abdullah MI, Junit SM, Ng KL, Jayapalan JJ, Karikalan B, Hashim OH. Papillary thyroid cancer: genetic alterations and molecular biomarker investigations. Int J Med Sci. 2019;16(3):450‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roman BR, Morris LG, Davies L. The thyroid cancer epidemic, 2017 perspective. Curr Opin Endocrinol Diabetes Obes. 2017;24(5):332‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Filetti S, Durante C, Hartl D, et al. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐updagger. Ann Oncol. 2019;30(12):1856‐1883. [DOI] [PubMed] [Google Scholar]
- 5. Mansour J, Sagiv D, Alon E, Talmi Y. Prognostic value of lymph node ratio in metastatic papillary thyroid carcinoma. J Laryngol Otol. 2018;132(1):8‐13. [DOI] [PubMed] [Google Scholar]
- 6. Wang Y, Mo Y, Gong Z, et al. Circular RNAs in human cancer. Mol Cancer. 2017;16(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hansen TB, Jensen TI, Clausen BH, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495(7441):384‐388. [DOI] [PubMed] [Google Scholar]
- 8. Chao F, Song Z, Wang S, et al. Novel circular RNA circSOBP governs amoeboid migration through the regulation of the miR‐141‐3p/MYPT1/p‐MLC2 axis in prostate cancer. Clin Transl Med. 2021;11(3):e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen T, Wang X, Li C, et al. CircHIF1A regulated by FUS accelerates triple‐negative breast cancer progression by modulating NFIB expression and translocation. Oncogene. 2021;40(15):2756‐2771. [DOI] [PubMed] [Google Scholar]
- 10. Shen P, Yang T, Chen Q, et al. CircNEIL3 regulatory loop promotes pancreatic ductal adenocarcinoma progression via miRNA sponging and A‐to‐I RNA‐editing. Mol Cancer. 2021;20(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suzuki H, Zuo Y, Wang J, Zhang MQ, Malhotra A, Mayeda A. Characterization of RNase R‐digested cellular RNA source that consists of lariat and circular RNAs from pre‐mRNA splicing. Nucleic Acids Res. 2006;34(8):e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Y, Zheng Q, Bao C, et al. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25(8):981‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lan X, Cao J, Xu J, et al. Decreased expression of hsa_circ_0137287 predicts aggressive clinicopathologic characteristics in papillary thyroid carcinoma. J Clin Lab Anal. 2018;32(8):e22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu G, Zhou W, Pan X, et al. Circular RNA profiling reveals exosomal circ_0006156 as a novel biomarker in papillary thyroid cancer. Mol Ther Nucleic Acids. 2020;19:1134‐1144. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15. Du G, Ma R, Li H, et al. Increased expression of hsa_circ_0002111 and its clinical significance in papillary thyroid cancer. Front Oncol. 2021;11:644011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen D, Ma W, Ke Z, Xie F. CircRNA hsa_circ_100395 regulates miR‐1228/TCF21 pathway to inhibit lung cancer progression. Cell Cycle. 2018;17(16):2080‐2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li X, Lin S, Mo Z, et al. CircRNA_100395 inhibits cell proliferation and metastasis in ovarian cancer via regulating miR‐1228/p53/epithelial‐mesenchymal transition (EMT) axis. J Cancer. 2020;11(3):599‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peng N, Shi L, Zhang Q, Hu Y, Wang N, Ye H. Microarray profiling of circular RNAs in human papillary thyroid carcinoma. PLoS One. 2017;12(3):e0170287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Read ML, Seed RI, Fong JCW, et al. The PTTG1‐binding factor (PBF/PTTG1IP) regulates p53 activity in thyroid cells. Endocrinology. 2014;155(4):1222‐1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Llamas‐Olier AE, Cuellar DI, Buitrago G. Intermediate‐risk papillary thyroid cancer: risk factors for early recurrence in patients with excellent response to initial therapy. Thyroid. 2018;28(10):1311‐1317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Data Availability Statement
Data sharing was not applicable to this article as no datasets were generated or analyzed during the current study.


