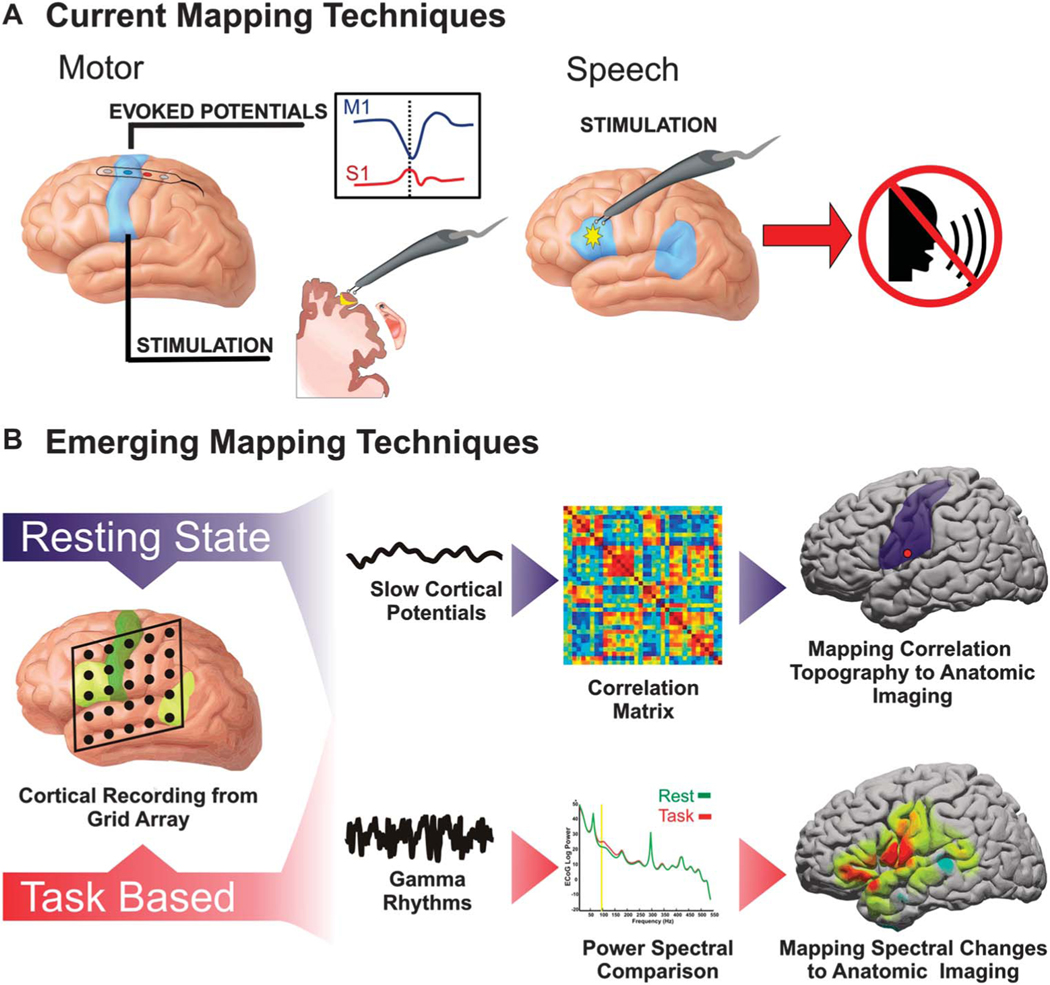
For surgically resectable lesions in proximity to motor and speech cortex, mapping eloquent cortex in and around the tumor is essential for optimizing clinical outcomes. This is especially true in the treatment of medically intractable epilepsy, tumors, or arteriovenous malformations.1–4 Because of the high degree of interpatient variability in the anatomic location of motor and language areas, correct localization of these sites is critical.5 The current gold standard for clinical mapping is electrocortical stimulation (ECS). There are, however, technical limitations to this technique that lessen its ease and utility to both the surgeon and the patient. ECS mapping can be complicated by the induction of afterdischarges and seizures, which can introduce a source of error in mapping and even possibly make mapping untenable.6 Although effective in identifying essential sites of functional cortex, stimulation is also relatively inefficient. Cortical locations must be interrogated in series, which can result in a prolonged mapping procedure. Another tool that is limited to identification of motor cortex is the use of somatosensory evoked potentials.7 This technique can identify the boundary between motor and sensory cortex in patients under general anesthesia. The procedure is safe, fast, and easy to apply in the operating room. In the presence of certain anesthetic agents, however, its sensitivity and specificity can sometimes be limited.8-10 Although considered a useful tool for identification of sensorimotor regions, the utility of somatosensory evoked potentials is limited to the sensorimotor cortex because of its unique anatomic and functional configuration. Efforts to extend the mapping potential of such cortical electrophysiological recordings to other eloquent areas and higher associative regions were explored by Sidney Goldring in the early 1990s.11 These more advanced approaches, however, have had limited success. Thus, taken together, ECS and the use of somatosensory evoked potentials have been the sole intraoperative techniques used for decades (summarized in Figure 1A).
FIGURE 1.
Summary of mapping techniques. A, the current methodologies to identify regions deemed eloquent, namely motor and speech cortex. Motor regions are currently identified with either passive localization using somatosensory evoked potentials associated with median nerve stimulation or with direct cortical stimulation. For somatosensory evoked potentials, there is typically a phase inversion between the primary motor cortex (M1) and primary sensory cortex (S1) indicative of central sulcus. Electrocortical stimulation is also used for motor localization. With the use of bipolar stimulators, the probe is moved from cortical site to cortical site until a motor response is elicited. B, functional localization accomplished by recording electrocorticographic brain signals from the surface of the cortex. Localization can be either purely passive, in which resting-state brain activity is used, or task based, in which a cortical change is associated with the performance of a cognitive action. Resting-state mapping relies on the correlation of infraslow rhythms known as slow cortical potentials. Task-based mapping typically relies on modulation of amplitude of high-frequency rhythms known as gamma rhythms.
With the advent of fast computing, real-time analysis of brain signals for brain computer interface applications, and advances in the understanding of cortical physiology, new techniques are emerging that will play a role in neurosurgical brain mapping. Fundamentally, these approaches enable the surgeon to listen passively to brain activity (whether during a task or at rest) to localize eloquent cortical regions. The advantages of passive recording approaches include the ability to interrogate multiple cortical regions simultaneously without the risk of afterdischarges seen with stimulation. Additionally, with some of the “task-independent” approaches, functional localization can be done without the need for patient participation or even consciousness. This article reviews some of the emerging science and applied technology research that is underway and how they will potentially affect the practice of a neurosurgeon.
CORTICAL PHYSIOLOGY RELEVANT TO SURGICAL BRAIN MAPPING
Task-Related Cortical Physiology
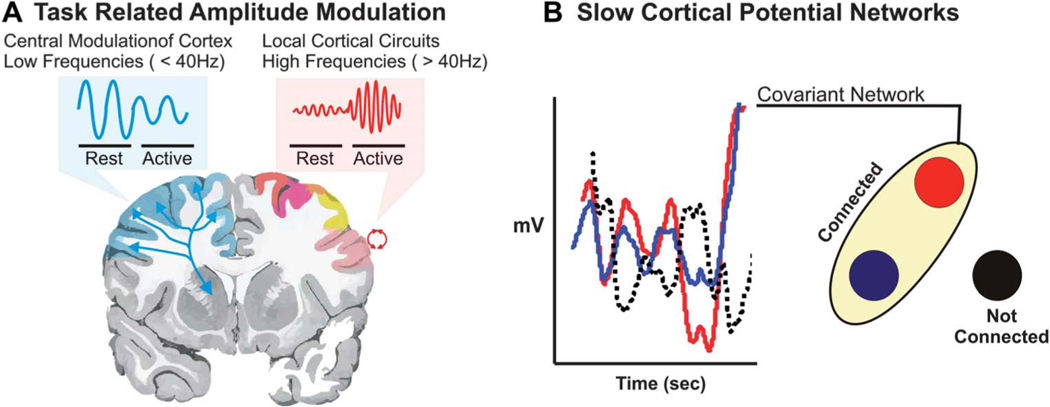
Recently, additional methods have been explored that rely on the use of passively recorded electrocorticographic changes in activity associated with a given stimulus or cognitive task. Amplitude modulation in different frequency bands has been used to identify focal areas associated with cortical activation. The brain generates oscillating electrical potentials over a broad range of frequencies that show characteristic task-related changes (Figure 2A). Commonly, these have been described in the context of sensorimotor cortical activation. Relatively low frequencies in the mu (8–12 Hz) and beta (18–26 Hz) ranges are thought to be produced by thalamocortical circuits that decrease in amplitude in association with actual or imagined movements.12–15 In general, these changes in lower-frequency bands tend to have a large spatial distribution and hence are only modestly specific to the type of function. Activity in the gamma frequency range (> 40 Hz) is thought to be produced by local cortical circuits.16 The gamma activity focally increases with cortical activation. Because of the anatomically constrained nature of high-frequency amplitude modulation, these gamma rhythms have been focused on as the optimal signal for use in clinical brain mapping.
FIGURE 2.
Summary of pertinent mapping physiologies. A, task-based mapping relies largely on modulation of brain oscillations > 40 Hz. These are known as gamma rhythms. These higher frequencies are typically associated with smaller cortical ensembles and show an increase in amplitude when active. Lower frequencies are thought to represent thalamocortical interactions. These rhythms typically have a broader cortical topography and decrease in amplitude when the cortex becomes active. B, resting-state mapping relies on the interaction of different cortical sites to define networks. These networks are identified by having a similar time series in infraslow rhythms, known as slow cortical potentials. If they have similar time series as indicated by the red and blue lines, they are connected; if they are not correlated or are anti-correlated (dotted line), then these sites are not functionally connected.
Resting-State Cortical Physiology
Resting-state networks were first identified with functional magnetic resonance imaging (fMRI). Spontaneous blood oxygen level-dependent fluctuations are low-frequency (< 0.1 Hz) oscillations in neuronal activity that are anatomically correlated within distinct functional networks.17,18 First reported by Biswal et al,18 there is strong coherence, which is reproducibly present between the left and right somatomotor cortices,19 between language areas,20,21 and between numerous other functional regions in the absence of task performance. Also of note, resting-state network topographies have been shown to be quite similar to the topographies associated with task-evoked fMRI responses.22 These resting-state networks have been used in preliminary investigations to preoperatively map motor regions adjacent to brain tumors.23 The cortical physiology that most correlates with these state-invariant functional structures is a cortical network defined by very-low-frequency oscillations (< 0.5 Hz), known as slow cortical potentials (SCPs).24 It appears likely that the negative shift of these SCPs reflects slow rhythmic depolarization of apical dendrites in superficial cortical layers25,26 and is thought to likely represent endogenous fluctuations of cortical excitability within functional systems. Correlated depolarization of different cortical sites is thought to represent connections between different regions of the brain. These connected sites (ie, networks) are represented by correlated time series of the SCPs. More generally, infraslow rhythms have been shown to play an important role in defining the fundamental architecture and organization of the cortex as they relate to both functional networks and pathological networks.27–30 In humans, fMRI blood oxygen level–dependent and SCP sensorimotor networks are stable throughout sleep/wake cycles and have been shown to be stable under anesthesia in both primates and humans.31–33 Investigations by He et al24 looking at electrocorticographic frequencies ranging from < 0.5 to 200 Hz demonstrated that the rhythms that were most closely correlated with the fMRI resting-state sensorimotor network were the power envelope of gamma rhythms (during wakefulness and rapid-eye-movement sleep) and the SCPs (during wakefulness, rapid-eye-movement sleep, and slow-wave sleep).
MAPPING APPLICATIONS OF PASSIVELY ACQUIRED CORTICAL SIGNALS
Task-Based Mapping
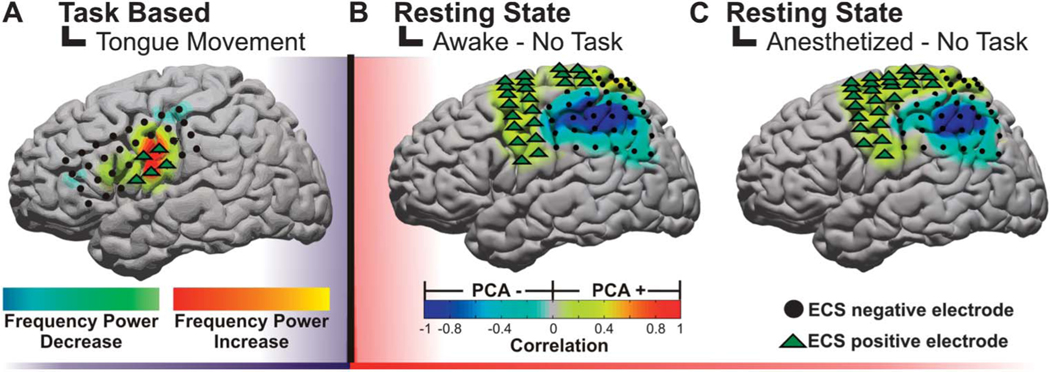
A substantial amount of work has been performed investigating the use of task-related electrocorticographic signals for surgical brain mapping. Although the earliest efforts demonstrated high- and low-frequency amplitude modulations associated with a task,34–36 the use of this approach as a clinical tool, which was expressly compared with stimulation, arrived several years later. The clinical mapping technique is known as electrocortical frequency alteration mapping (EFAM).37,38 The approach uses a block design in which the task is alternated with rest, and consistent changes in both low-frequency (8–32 Hz) and high-frequency (75–100 Hz) power are statistically identified. In this case, the crucial benefit of EFAM is its broader spectrum of tasks that can be reliably and rigorously mapped compared with ECS. When applied to motor localization, the method has performed well with a high sensitivity (88.9%−100%) and a lower specificity (79.0%−82.6%) for identifying electrodes with either hand or tongue ECS motor responses. The high-frequency band had a lower sensitivity (72.7%−88.9%) and a higher specificity (92.4%−94.9%) in correlating with the same respective ECS-positive electrodes (example in Figure 3A).38 When used for speech, the results were more variable. EFAM had an 83.9% sensitivity and a 40.4% specificity in identifying any language site when considering both high- and low-frequency bands. EFAM using the high-frequency bands alone was more sensitive in identifying the Wernicke area (100%) than the Broca area (72.2%). The higher-frequency rhythms were uniquely suited to identifying the Wernicke area, whereas a combination of the high and low frequencies was important for Broca localization. The variability in localizing essential speech sites with electrocorticographic recordings has been seen across multiple studies.39–41
FIGURE 3.
Examples of electrocorticographic brain mapping. A, example of cortical topography of a gamma power alteration associated with a subject protruding his or her tongue. The positive motor stimulation sites are represented by green triangles. B, example of resting-state networks derived from a principal component analysis (PCA) while the patient is awake. C, example of resting-state networks derived from a PCA while the patient is under anesthesia. Notably, these networks are preserved regardless of level of consciousness and can be derived in the absence of task. ECS, electrocortical stimulation.
Although an advancement in the practical use of cortical physiology, the block design of the cognitive paradigms still required offline signal analysis, and the application of multiple cognitive paradigms could limit the level of patient participation. Furthermore, this could be time-inefficient in the intraoperative, awake craniotomy setting. To address this shortcoming, a procedure known as SIGFRIED (signal modeling for real-time identification and event detection) was developed for motor and speech mapping. It was originally developed during extraoperative brain mapping42 to reduce this need for expert oversight and to speed the time needed for functional localization. In the extraoperative setting, compared with ECS maps, a next-neighbor evaluation showed no false negatives and only 0.46% and 1.10% false positives for hand and tongue maps, respectively.43 SIGFRIED accomplishes increased efficiency of localization by implementing a detection-based approach based on a gaussian mixture model of recorded brain signals. Rather than using a discrimination-based approach, in which an active condition is alternated with a rest condition and then subsequently analyzed, SIGFRIED creates a statistical model of baseline brain activity and then subsequently detects significant deviations from that baseline. This is done in an automated process that does not require the definition of any signal processing parameters by the clinician. The process requires 3 steps: (1) recording a baseline signal in the absence of overt motor or speech activity, (2) building a statistical model based on the baseline signal, and (3) detecting significant differences from the baseline signal model during cue-directed activity. This enables a quasi–real-time assessment of cortical changes that is shown on a topographical display and as a result obviates the need for post hoc analysis by an expert. This method has reliably predicted cortical stimulation sites and has been used effectively in awake craniotomies.43,44
Taken together, these findings demonstrate that using task-based cortical signals, most notably higher-frequency gamma rhythms, can be a useful supplement to current mapping techniques. Although in no way a replacement for stimulation, these methods can provide a risk-free rapid approach to identify high-probability functional sites associated with speech and motor function.
Resting-State Mapping
The requirement for awake participation excludes a large number of patients who could benefit from both the stimulation-based and electrocorticography-based mapping procedures. In the extraoperative setting (when a subdural electrode array has been implanted), children and patients with mental status impairments from any cause may be unable to participate in the language or motor protocol. In the intraoperative setting (ie, awake craniotomies), language mapping may be impossible if the patient is medically unstable or has a compromised airway. Resting-state networks, on the other hand, present an emerging means to passively identify functional cortex in such patients.23 Recent studies using resting-state cortical physiology may provide an ideal means of identifying eloquent regions without the need for patient cooperation or even that they be awake. With fMRI, these resting-state networks have been used in preliminary investigations to preoperatively map motor regions adjacent to brain tumors.23 As mentioned, the cortical physiology that most correlates with these state-invariant functional structures is a cortical network defined by very-low-frequency oscillations (< 0.5 Hz), known as SCPs.24 Given that these networks are durable through sleep/wake cycles and anesthesia,31–33 they were recently evaluated as a possible tool for mapping sensorimotor cortex. Breshears et al32 studied 8 subjects undergoing surgical treatment for intractable epilepsy. SCPs were recorded from the cortical surface while awake and under propofol anesthesia. To test brain-mapping utility, SCP networks were identified using data-driven (seed-independent) and anatomy-driven (seed-based) approaches. The sensitivity and specificity of these networks for identifying sensorimotor cortex (as determined by ECS) were calculated. The study demonstrated that these SCP-defined networks identified with a data-driven approach in patients under anesthesia and awake were 90% and 93% sensitive and 58% and 55% specific for sensorimotor cortex, respectively (example in Figure 3B). These results suggest that resting-state networks may be useful for tailoring stimulation mapping and could provide a means of identifying eloquent regions in patients while under anesthesia.
FUTURE DIRECTIONS
For these new brain mapping techniques to achieve a widespread adoption among the neurosurgical community, several scientific, technical, clinical, and commercial hurdles need to be addressed. First, whether it be task-based or resting-state mapping, there needs to be an improved understanding of the nature of these signals and their relationship to the underlying physiology of the cognitive operation. As an example, the best task for inducing a consistent cortical response that best represents essential cortical sites has yet to be determined. Furthermore, the current gold standard for comparison of the quality of these signals to map clinical brain function has been cortical stimulation. Although this is a well-studied and trusted method, it is still limited to very specific simple motor and speech tasks that may not fully capture cognitive operations that may be better measured with recording methodologies. Perhaps the most meaningful measurement of these techniques will be to assess their ability to alter clinical outcomes (eg, functional preservation and survival). This measure would provide a more direct evaluation of how these new techniques can be assessed and used in the future.
In addition to the science, the technology needs to be further refined. As an example, the optimal electrode spacing and size are currently not defined. To date, current electrode arrays are those used for implantation for extraoperative seizure monitoring. Although optimized to balance cortical coverage with adequate spatial resolution for seizure surgery, they may not be ideal for an awake craniotomy and localization of an eloquent cortical zone, which may be on the order of 1 cm.2 Additionally, the electrodes are made for transcutaneous passage of the wires. These are not, however, made for efficient and rapid connection to an amplifier and computer, which would be necessary in the operating room. The signal analysis methods and software also require substantial development. To date, these types of mapping techniques are being used only at tertiary centers with a very high level of technical capability that can perform sophisticated signal analysis and computer programming. For these methods to reach wider neurosurgical use, the signal analysis and software need to be automated and presented in a fashion that is more easily interpreted by the “nonexpert” neurosurgeon. Additionally, the incorporation of these techniques into stereotactic navigation will further improve anatomic-functional localization of a given eloquent site. These last several features, including ease of visualization, user factors, and integration with existing technology, will likely require industry interest and adoption.
CONCLUSION
The use of a patient’s cortical physiology, whether at rest or during a task, is an emerging resource of information to help guide and facilitate a neurosurgeon in identifying critical sites that need to be preserved. Going forward, more scientific, clinical, and technical research is necessary to understand how best to interpret these signals, to understand their optimal clinical use, and to design user-friendly software and hardware that will enable widespread adoption.
For related video content, please access the Supplemental Digital Content: http://www.youtube.com/watch?v=p25PK8Zv62Y
Acknowledgments
We would like to thank the generous support from the National Institute of Health (R21 CA159470-01A1 to Dr Leuthardt and Shimony) and the McDonnell Center for Higher Brain Function (Dr Leuthardt), which has made this research possible.
Footnotes
Disclosures
Dr Leuthardt holds stock in Neurolutions, General Sensing, and Osteovantage. The other authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES
- 1.Keles GE, Lundin DA, Lamborn KR, et al. Intraoperative subcortical stimulation mapping for hemispherical perirolandic gliomas located within or adjacent to the descending motor pathways: evaluation of morbidity and assessment of functional outcome in 294 patients. J Neurosurg. 2004;100 (3):369–375. [DOI] [PubMed] [Google Scholar]
- 2.Berger MS, Kincaid J, Ojemann GA, Lettich E. Brain mapping techniques to maximize resection, safety, and seizure control in children with brain tumors. Neurosurgery. 1989;25(5):786–792. [DOI] [PubMed] [Google Scholar]
- 3.Burchiel KJ, Clarke H, Ojemann GA, Dacey RG, Winn HR. Use of stimulation mapping and corticography in the excision of arteriovenous malformations in sensorimotor and language-related neocortex. Neurosurgery. 1989;24(3):322–327. [DOI] [PubMed] [Google Scholar]
- 4.Haglund MM, Berger MS, Shamseldin M, Lettich E, Ojemann GA. Cortical localization of temporal lobe language sites in patients with gliomas. Neurosurgery. 1994;34(4):567–576; discussion 576. [DOI] [PubMed] [Google Scholar]
- 5.Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358(1):18–27. [DOI] [PubMed] [Google Scholar]
- 6.Blume WT, Jones DC, Pathak P. Properties of after-discharges from cortical electrical stimulation in focal epilepsies. Clin Neurophysiol. 2004;115(4):982–989. [DOI] [PubMed] [Google Scholar]
- 7.Wood CC, Spencer DD, Allison T, et al. Localization of human sensorimotor cortex during surgery by cortical surface recording of somatosensory evoked potentials. J Neurosurg. 1988;68(1):99–111. [DOI] [PubMed] [Google Scholar]
- 8.Sloan TB. Anesthetic effects on electrophysiologic recordings. J Clin Neurophysiol. 1998;15(3):217–226. [DOI] [PubMed] [Google Scholar]
- 9.Boisseau N, Madany M, Staccini P, et al. Comparison of the effects of sevoflurane and propofol on cortical somatosensory evoked potentials. Br J Anaesth. 2002;88(6):785–789. [DOI] [PubMed] [Google Scholar]
- 10.Cedzich C, Taniguchi M, Schäfer S, Schramm J. Somatosensory evoked potential phase reversal and direct motor cortex stimulation during surgery in and around the central region. Neurosurgery. 1996; 38(5):962–970. [DOI] [PubMed] [Google Scholar]
- 11.Goldring S, Harding GW, Gregorie EM. Distinctive electrophysiological characteristics of functionally discrete brain areas: a tenable approach to functional localization. J Neurosurg. 1994;80(4):701–709. [DOI] [PubMed] [Google Scholar]
- 12.Pfurtscheller G, Graimann B, Huggins JE, Levine SP, Schuh LA. Spatiotemporal patterns of beta desynchronization and gamma synchronization in corticographic data during self-paced movement. Clin Neurophysiol. 2003; 114(7):1226–1236. [DOI] [PubMed] [Google Scholar]
- 13.Levine SP, Huggins JE, BeMent SL, et al. Identification of electrocorticogram patterns as the basis for a direct brain interface. J Clin Neurophysiol. 1999;16(5):439–447. [DOI] [PubMed] [Google Scholar]
- 14.Huggins JE, Levine SP, BeMent SL, et al. Detection of event-related potentials for development of a direct brain interface. J Clin Neurophysiol. 1999;16(5):448–455. [DOI] [PubMed] [Google Scholar]
- 15.Rohde MM, BeMent SL, Huggins JE, et al. Quality estimation of subdurally recorded, event-related potentials based on signal-to-noise ratio. IEEE Trans Biomed Eng. 2002;49(1):31–40. [DOI] [PubMed] [Google Scholar]
- 16.da Lopes FH Silva, G Pfurtscheller, eds. Event-Related Desynchronization: Handbook of Electroencephalography and Clinical Neurophysiology. Amsterdam, Netherlands: Elsevier; 1999. [Google Scholar]
- 17.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. [DOI] [PubMed] [Google Scholar]
- 18.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. [DOI] [PubMed] [Google Scholar]
- 19.Fox MD, Snyder AZ, Zacks JM, Raichle ME. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat Neurosci. 2006;9(1):23–25. [DOI] [PubMed] [Google Scholar]
- 20.Cordes D, Haughton VM, Arfanakis K, et al. Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am J Neuroradiol. 2000;21(9):1636–1644. [PMC free article] [PubMed] [Google Scholar]
- 21.Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp. 2002;15(4):247–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106(31):13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang D, Johnston JM, Fox MD, et al. Preoperative sensorimotor mappingin brain tumor patients using spontaneous fluctuations in neuronal activity imaged with functional magnetic resonance imaging: initial experience. Neurosurgery. 2009;65(suppl 6):226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He BJ, Snyder AZ, Zempel JM, Smyth MD, Raichle ME. Electrophysiological correlates of the brain’s intrinsic large-scale functional architecture. Proc Natl Acad Sci U S A. 2008;105(41):16039–16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birbaumer N, Elbert T, Canavan AG, Rockstroh B. Slow potentials of the cerebral cortex and behavior. Physiol Rev. 1990;70(1):1–41. [DOI] [PubMed] [Google Scholar]
- 26.Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev. 1985;65(1):37–100. [DOI] [PubMed] [Google Scholar]
- 27.Ko AL, Darvas F, Poliakov A, Ojemann J, Sorensen LB. Quasi-periodic fluctuations in default mode network electrophysiology. J Neurosci. 2011;31(32):11728–11732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baria AT, Baliki MN, Parrish T, Apkarian AV. Anatomical and functional assemblies of brain BOLD oscillations. J Neurosci. 2011;31(21): 7910–7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim W, Miller JW, Ojemann JG, Miller KJ. Ictal localization by invasive recording of infraslow activity with DC-coupled amplifiers. J Clin Neurophysiol. 2009;26(3):135–144. [DOI] [PubMed] [Google Scholar]
- 30.Schalk G, Miller KJ, Anderson NR, et al. Two-dimensional movement control using electrocorticographic signals in humans. J Neural Eng. 2008;5(1):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larson-Prior LJ, Zempel JM, Nolan TS, et al. Cortical network functional connectivity in the descent to sleep. Proc Natl Acad Sci U S A. 2009;106 (11):4489–4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breshears JD, Roland JL, Sharma M, et al. Stable and dynamic cortical electrophysiology of induction and emergence with propofol anesthesia. Proc Natl Acad Sci U S A. 2010;107(49):21170–21175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vincent JL, Patel GH, Fox MD, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–86. [DOI] [PubMed] [Google Scholar]
- 34.Crone NE, Boatman D, Gordon B, Hao L. Induced electrocorticographic gamma activity during auditory perception: Brazier Award-winning article, 2001. Clin Neurophysiol. 2001;112(4):565–582. [DOI] [PubMed] [Google Scholar]
- 35.Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis, II: event-related synchronization in the gamma band. Brain. 1998;121 (pt 12):2301–2315. [DOI] [PubMed] [Google Scholar]
- 36.Crone NE, Miglioretti DL, Gordon B, et al. Functional mapping of humansen sorimotor cortex with electrocorticographic spectral analysis, I: alpha and beta event-related desynchronization. Brain. 1998;121(pt 12):2271–2299. [DOI] [PubMed] [Google Scholar]
- 37.Wu M, Wisneski K, Schalk G, et al. Electrocorticographic frequency alteration mapping for extraoperative localization of speech cortex. Neurosurgery. 2010;66(2):E407–E409. [DOI] [PubMed] [Google Scholar]
- 38.Leuthardt EC, Miller K, Anderson NR, et al. Electrocorticographic frequency alteration mapping: a clinical technique for mapping the motor cortex. Neurosurgery. 2007;60(4 suppl 2):260–270; discussion 270–271. [DOI] [PubMed] [Google Scholar]
- 39.Sinai A, Bowers CW, Crainiceanu CM, et al. Electrocorticographic high gamma activity versus electrical cortical stimulation mapping of naming. Brain. 2005;128(pt 7):1556–1570. [DOI] [PubMed] [Google Scholar]
- 40.Crone NE, Sinai A, Korzeniewska A. High-frequency gamma oscillations and human brain mapping with electrocorticography. Prog Brain Res. 2006;159:275–295. [DOI] [PubMed] [Google Scholar]
- 41.Cervenka MC, Boatman-Reich DF, Ward J, Franaszczuk PJ, Crone NE. Language mapping in multilingual patients: electrocorticography and cortical stimulation during naming. Front Hum Neurosci. 2011;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schalk G, Leuthardt EC, Brunner P, et al. Real-time detection of event-related brain activity. Neuroimage. 2008;43(2):245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brunner P, Ritaccio AL, Lynch TM, et al. A practical procedure for real-time functional mapping of eloquent cortex using electrocorticographic signals in humans. Epilepsy Behav. 2009;15(3):278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roland J, Brunner P, Johnston J, Schalk G, Leuthardt EC. Passive real-time identification of speech and motor cortex during an awake craniotomy. Epilepsy Behav. 2010;18:123–128. [DOI] [PubMed] [Google Scholar]