Abstract
Although gamma aminobutyric acid (GABA) is of particular importance for efficient motor functioning, very little is known about the relationship between regional GABA levels and motor performance. Some studies suggest this relation to be subject to age-related differences even though literature is scarce. To clarify this matter, we employed a comprehensive approach and investigated GABA levels within young and older adults across multiple motor tasks as well as multiple brain regions. Specifically, 30 young and 30 older adults completed a task battery of three different bimanual tasks. Furthermore, GABA levels were obtained within bilateral primary sensorimotor cortex (SM1), bilateral dorsal premotor cortex, the supplementary motor area and bilateral dorsolateral prefrontal cortex (DLPFC) using magnetic resonance spectroscopy. Results indicated that older adults, as compared to their younger counterparts, performed worse on all bimanual tasks and exhibited lower GABA levels in bilateral SM1 only. Moreover, GABA levels across the motor network and DLPFC were differentially associated with performance in young as opposed to older adults on a manual dexterity and bimanual coordination task but not a finger tapping task. Specifically, whereas higher GABA levels related to better manual dexterity within older adults, higher GABA levels predicted poorer bimanual coordination performance in young adults. By determining a task-specific and age-dependent association between GABA levels across the cortical motor network and performance on distinct bimanual tasks, the current study advances insights in the role of GABA for motor performance in the context of aging.
Keywords: GABA, Aging, MRS, Motor performance
1. Introduction
As the population of older adults is rapidly increasing, optimizing motor performance to maintain quality of life has become a topic of profound interest. Considering that intact bimanual coordination is essential for achieving and maintaining functional independence, bimanual coordination serves as an ideal candidate to explore motor performance deficits in the context of healthy aging. Indeed, previous studies reported age-related differences in motor performance across a wide range of bimanual coordination tasks (for a review, see Maes et al., 2017). Due to the complex nature of these movements, an efficient interplay between multiple (motor) brain regions is essential to support motor performance (Swinnen and Gooijers, 2015). First, the role of the primary sensorimotor cortex (SM1) is self-evident as it is serves as the main cortical origin of motor output. Second, SMA is especially relevant for bimanual coordination as it plays a pivotal role in the internal generation of movements as well as the proper tuning between both cerebral hemispheres (Sadato et al., 1997; Swinnen and Wenderoth, 2004). Third, the dorsal premotor cortex (PMd) is crucially involved in the planning, execution and learning of motor tasks in both young and older adults (Hardwick et al., 2013; Kantak et al., 2012; Swinnen and Wenderoth, 2004). Fourth, the dorsolateral prefrontal cortex (DLPFC) is of particular importance for control of movement in the context of aging. Although DLPFC has been primarily related to executive functioning, older adults often recruit this brain region during performance of simple motor tasks, whereas both young and older adults activate this region during initial practice of unfamiliar complex motor tasks (Beets et al., 2015; Debaere et al., 2004; Goble et al., 2010; Heuninckx, 2005; Santos Monteiro et al., 2017). Functional connections between these regions, both within and across hemispheres, are known to be vital for bimanual coordination (Gerloff and Andres, 2002; Solesio-Jofre et al., 2018). These functional projections heavily rely upon intracortical and interhemispheric inhibitory processes which are known to decline with advancing age, thereby contributing to degraded motor performance (Bhandari et al., 2016; Fling and Seidler, 2012; Fujiyama et al., 2016; Levin et al., 2014; Verstraelen et al., 2020). This body of knowledge predominantly originated from transcranial magnetic stimulation (TMS) studies that provide information on synaptic inhibitory mechanisms mediated by gamma aminobutyric acid (GABA) receptors (Ziemann et al., 2015).
Surprisingly, much less is known about the importance of GABA levels within a specific brain region of interest as quantified by magnetic resonance spectroscopy (MRS). Whereas TMS is a spatially focused technique that is primarily used for to the investigation of inhibitory processes involving the primary motor cortex (M1), MRS provides the opportunity to examine inhibitory processes beyond M1. Specifically, regional MRS-derived GABA levels are thought to reflect the overall inhibitory tone of a certain brain region and have been linked to cognitive, perceptual and motor performance in young adults. For example, previous work in young adults suggested that higher SMA GABA/water levels were related to decreased cortical responsiveness (Boy et al., 2010) and higher SM1 GABA/Cr levels predicted poorer performance on a sequence learning task (Stagg et al., 2011). Whereas these studies seem to support the idea that higher GABA levels are related to poorer performance, other studies reported that higher GABA levels relate to an increased fine-tuning of neural activity, thereby supporting performance. Specifically, higher thalamic GABA/water levels were indicative of improved response selection (Dharmadhikari et al., 2015) and higher occipital GABA/Cr and SM1 GABA/water levels correlated with better visual and sensory discrimination, respectively (Kurcyus et al., 2018; Puts et al., 2011). The discrepancy in results seems to suggest that the direction of the association between GABA and performance is dependent on the brain region of interest as well as the specific task requirements. Nonetheless, as research so far mostly focused on GABA levels within a limited number of brain regions and/or performance on one specific motor task, this hypothesis is yet to be confirmed. As discussed above, bimanual coordination tasks provide great potential to study the role of GABA across distinct cortical motor network regions.
Notably, very little is known about the role of GABA levels in aging-induced motor performance deficits. This is striking as previous work reported a decline in GABA levels with advancing age across multiple brain regions (Gao et al., 2013; Hermans et al., 2018; Huang et al., 2017; Porges et al., 2020). So far, only a few studies explored the association between GABA and motor performance. Hermans and coworkers (2018) found that higher pre-SMA GABA/water levels were associated with better reactive inhibition on a stop-signal task in older but not young adults. Furthermore, higher SM1 GABA/water levels were found to benefit performance on a bimanual coordination task (Chalavi et al., 2018) and the composite score of a sensorimotor test battery (Cassady et al., 2019) in a group of older adults. Considering that age-related decreases in GABA levels seem to be at least partially explained by aging-induced gray matter atrophy (Maes et al., 2018; Porges et al., 2017b), it is important to note that these associations were observed after correcting the MRS-derived GABA levels for the tissue composition of the voxel of interest (Harris et al., 2015). Although these first studies suggest higher GABA levels to consistently relate to better motor performance, future work is required to confirm this association across different tasks and brain regions. The investigation of regional specificity is especially relevant in the context of aging as some studies suggest a non-uniform distribution of aging-induced decreases in GABA levels across the brain as well as hemispherical differences (Cuypers et al., 2020; Maes et al., 2018). Furthermore, as current research in young adults rather suggests a task-dependent association between GABA levels and motor performance, additional insights could emerge from the inclusion of multiple task paradigms. Indeed, GABA levels in young versus older adults may be differentially relevant for bimanual motor performance depending on task features such as speed and coordination requirements as well as the level of task complexity. Therefore, a comprehensive approach to identify age-related differences in the interplay between regional GABA levels and motor performance is indispensable.
Here, we investigated age-related differences in GABA levels across key cortical nodes of the motor network and bilateral dorsolateral prefrontal cortex (DLPFC). Furthermore, a set of tasks measuring different features of bimanual coordination was utilized to examine the link between GABA levels and motor performance in young as compared to older adults. GABA levels were expected to be lower in older relative to young adults. Moreover, higher GABA levels were hypothesized to invariably predict better motor performance in older adults, whereas the association in young adults is yet to be determined because it may be contingent upon the functionality of each region in relation to the specific task requirements.
2. Materials & methods
2.1. Participants
Thirty young adults (15 female, aged 19 – 35 years, mean ± SD = 24.5 ± 4.1) and 30 older adults (16 female, aged 61–79 years, mean ± SD = 67.8 ± 4.9) participated in this study. All participants were right-handed (Oldfield, 1971), in good self-reported physical and mental health and had no contraindications for MRI scanning. The Montreal Cognitive assessment (MoCA) was used as screening tool for cognitive impairment using a cut-off score of 23/30 (Carson et al., 2018). Due to a low MoCA score (21/30) as well as poor compliance with the experimental design, data of 1 young adult were excluded from further analyses. Age groups did not differ with respect to MoCA score (young adults: mean ± SD = 28.6 ± 1.3; older adults: mean ± SD = 28.4 ± 1.4; independent samples t-test: t = 0.33, p = 0.74) and handedness (Laterality quotient (LQ) young adults: mean ± SD = 93.4 ± 11.2; LQ older adults: mean ± SD = 95.6 ± 8.7; independent samples t-test: t = −0.83, p = 0.41). Informed consent was obtained from all participants prior to the experiment. The study protocol was in accordance with the declaration of Helsinki (1964) and was approved by the Ethics Committee Research of UZ/KU Leuven (study number S60428).
2.2. Experimental design
During a first session, participants filled in screening-related questionnaires and completed a battery of bimanual coordination tasks. During a second and third experimental session, GABA levels were acquired in multiple brain regions using MRS. The mean ± SD time window between sessions was 2.3 ± 2.4 days and 3.8 ± 5.7 days between the first and last two experimental sessions, respectively. Note that the third experimental session also included other measures that are not relevant for the current manuscript.
2.3. Bimanual task battery
Participants performed a bimanual task battery including a set of tasks that targeted different features of the motor repertoire. Specifically, the Purdue Pegboard test, finger tapping tasks as well as a bimanual tracking task were performed to assess manual dexterity, motor speed capacity and visuo-motor coordination abilities, respectively.
2.3.1. Purdue Pegboard test
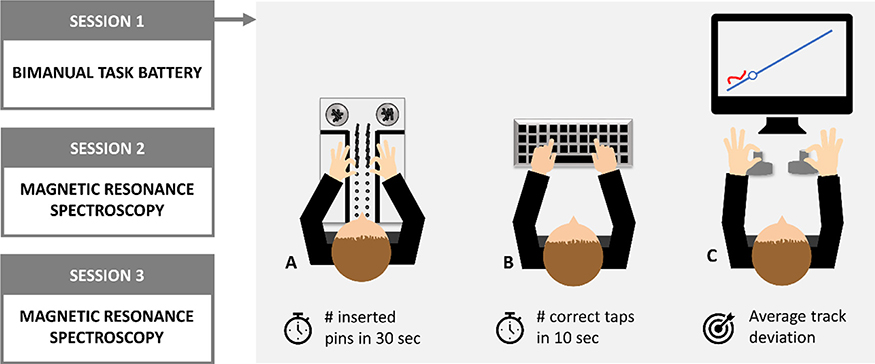
The Purdue Pegboard test (PPT) assesses manual dexterity. Here, participants are instructed to insert as many pins as possible in two vertical columns of pinholes on a board (Tiffin and Asher, 1948) (Fig. 1A). This task has been validated in an aging population and sensitivity to changes in performance has been demonstrated within the relevant age group (Desrosiers et al., 1995). In this study, only the bimanual subtask was performed in which participants were instructed to insert the pins with their left and right hand simultaneously. The number of pins inserted within a timeframe of 30 s was used as dependent variable.
Fig. 1. Overview of the experimental design and bimanual tasks.
A. Purdue Pegboard test. Participants were instructed to insert as many pins as possible with left and right hand simultaneously within a timeframe of 30 s. B. Finger tapping tasks. Participants were instructed to tap on a computer keyboard with both index fingers, either simultaneously or alternately, as many times as possible within a timeframe of 10 s. C. Bimanual tracking task. A simultaneous rotation of left and right dial was required to track a dot that was moving along a target template as accurately as possible in space and time. Visual online feedback was provided by a red cursor.
2.3.2. Finger tapping tasks
To assess basic motor speed capacity, two bimanual finger tapping tasks were performed (Fig. 1B). During these tasks, participants had to tap with their left and right index finger alternately or simultaneously as fast as possible. Tapping was performed on a regular computer keyboard and the number of correct taps within a timeframe of 10 s was used as dependent variable. To avoid fatigue effects, participants were allowed a short break (± 10 s) in between the two task variants. Computerized finger tapping tasks were previously proven to be valid and sensitive to age-related declines in tapping rate (Hubel et al., 2013).
2.3.3. Bimanual tracking task
An in-house developed visuomotor bimanual tracking task (BTT) was used to assess bimanual coordination abilities (Sisti et al., 2011) (Fig. 1C). Previous work of our group using a similar task design confirmed its sensitivity to age-related decrements in bimanual coordination performance (e.g. King et al., 2018; Levin et al., 2019; Pauwels et al., 2015; Santos Monteiro et al., 2017). In this task, participants tracked a white dot moving along a blue target template, visualized on a computer screen in front of them, by rotating two dials. Whereas the left dial corresponded to horizontal movement of the cursor (right or left for clockwise or counterclockwise rotation, respectively), rotation of the right dial resulted in a vertical movement of the cursor (up or down for clockwise or counterclockwise rotation, respectively). Trials consisted of a planning phase (1 s) and an execution phase (7 s) and were separated by an inter-trial interval of 2 s. During the planning phase, the target template was visualized and a green circle informed the participant on the starting location of that trial. The start of the execution phase was marked by the disappearance of the green circle as well as by the appearance of a white target dot that started moving along the target template. During the execution phase, participants were instructed to track the white target dot as accurately as possible in both space and time. Online visual feedback was provided by a red cursor that moved on the screen based on the participant’s dial rotation. During the inter-trial interval, the screen turned black. The target template consisted of a straight line at an angle of 45°, requiring a rotation of both dials at equal speed. This movement was performed in all four possible movement orientations (both hands clockwise/counterclockwise, left hand clockwise – right hand counterclockwise, left hand counterclockwise – right hand clockwise). Participants were instructed to always rotate both hands simultaneously. An experimenter was present during task performance to assure compliance with the prescribed movement instructions. After completing a familiarization run of 8 trials, participants performed 16 trials, i.e. 4 in each movement orientation. Performance was assessed by error scores that were defined as the average track deviation, i.e. by the perpendicular distance of the participant’s cursor to the white dot at each point in time. Data of the four movement orientations were collapsed and the average track deviation was calculated across trials. To account for the skewness present in the dataset, error scores were log(10)-transformed. Furthermore, to match interpretation of the independent variable with the other task variants (i.e. high scores reflecting better performance) negative values of the error scores were used in subsequent analysis.
2.4. Magnetic resonance spectroscopy
2.4.1. MRI data acquisition
Neuro-anatomical and spectroscopy data were acquired using a 3 Tesla Philips Achieva scanner with a 32 channel receiver head coil (University Hospital Leuven, Gasthuisberg). GABA levels of seven volumes of interest (VOIs) were measured: bilateral SM1, PMd and DLPFC as well as a single medial voxel covering left and right SMA. GABA levels within all VOIs were acquired during the first MRI session, except for GABA levels within the left SM1 that were always acquired during the second MRI session. During the first MRI session, MRS data of the different VOIs were acquired in a pre-defined order that was kept constant across all participants. If the quality of MRS data acquired during the first MRI session was unsatisfactory, the respective scans were repeated at the beginning of the second MRI session (for an overview, see supplementary Table S1).
At the beginning of each MRI session, a high-resolution T1-weighted anatomical image was acquired using a chemical shift three-dimension turbo field echo (3DTFE) (TE = 4.6, 1 × 1 × 1 mm voxel size, field of view (FOV) = 256 × 242 × 182 mm, 182 sagittal slices) to position the MRS VOI. In addition, 2 short T1 anatomical images (TE = 4.6, 1.5 × 1.5 × 1.5 mm voxel size, field of view (FOV) = 256 × 244 × 182 mm, 182 sagittal slices) were acquired after two to three consecutive MRS scans, to verify whether subsequent voxel positioning needed to be adjusted (i.e. in case of head movement). MRS data were acquired using the MEGA-PRESS sequence (Edden and Barker, 2007; Mescher et al., 1998) (TE = 68 ms, TR = 2 s, 2 kHz spectral width) using parameters similar to previous work of our group (Chalavi et al., 2018; Hermans et al., 2018; King et al., 2020). Considering the shape and dimensions of each region of interest and based on previous studies, voxel dimensions were 30 × 30 × 30 mm for the SMA and SM1 VOIs (Boy et al., 2010; Maes et al., 2018), whereas the dimensions of the DLPFC and PMd voxels were 40 × 25 × 25 mm (Greenhouse et al., 2016). For SMA, bilateral PMd and DLPFC voxels, 160 averages were acquired. As reported by Mikkelsen and coworkers (2018a) the number of averages could be reduced for the SM1 VOI without affecting data quality. Therefore, 112 averages were acquired for left and right SM1 VOIs. ON and OFF spectra were acquired in an interleaved fashion, corresponding to an editing pulse at 1.9 or 7.46 ppm, respectively. Prior to each MRS acquisition, an automatic shimming procedure was performed. For all MRS regions, 16 unsuppressed water averages were acquired within the same VOI using identical acquisition parameters. Although macromolecules will be co-edited (GABA + macromolecules), we refer to it as GABA for the sake of simplicity. In line with previous MRS research in aging (Chalavi et al., 2018; Cuypers et al., 2020; Hermans et al., 2018; Maes et al., 2018), water was used as a reference compound.
MRS VOIs were identified on a subject-to-subject basis using anatomical landmarks (Fig. 2). SM1 VOIs were placed over the hand knob of the motor cortex and in line with the cortical surface in the sagittal plane (Yousry et al., 1997). To correctly position the SMA VOI, a virtual line was drawn perpendicular to the line connecting the anterior and posterior commissure. The VOI was positioned posterior to this line and along the cortical surface in the sagittal plane (Kim et al., 2010). By placing the VOI at the midline of the brain, both left and right SMA were covered at once. PMd VOIs were located anterior to the hand knob with the medial side of the VOI along the longitudinal fissure in the axial plane (Greenhouse et al., 2016). For DLPFC VOIs, the center of the voxel was positioned in the axial slice above the superior margin of the lateral ventricles. In this slice, the VOI was placed at one third of the anterior-to-posterior distance of the brain, centered in between the lateral and medial wall of each hemisphere (O’Gorman et al., 2011). Notably, MRS requires the use of relatively large voxels to ensure an acceptable signal-to-noise ratio (SNR) (Mullins et al., 2014). As the VOIs in this study were localized in close vicinity of each other, some overlap was expected (Fig. 2B). This overlap was quantified for each participant (supplementary table S2). Importantly, the center of each VOI of interest was region-specific, ensuring that VOI overlap was minimal and only present at the outside borders of a VOI.
Fig. 2. MRS spectra and VOI positioning.
A. MRS VOIs and their respective spectra. The figure shows an example of placement of each VOI, co-registered to an MNI template, as well as a visual representation of the acquired spectra per VOI. For the SM1, PMd and DLPFC VOIs, spectra were acquired bilaterally by mirroring the position of the VOI to the other hemisphere. B. Three-dimensional visual representation of the positioning and overlap between the different VOIs of interest.
Color coding: SM1 VOIs in blue, PMd VOIs in red, DLPFC VOIs in gold and the medial SMA VOI in green.
2.4.2. MRS analysis
MRS data were analyzed using the GABA analysis toolkit ‘Gannet’ (version 3.1.4) (Edden et al., 2014). In a first step, data were frequency- and phase-corrected by applying spectral registration (Near et al., 2015). ON spectra were subtracted from the OFF spectra, and the resulting difference spectrum was fitted between 4.2 and 2.8 ppm using a three-Gaussian function. The water signal was fitted using a Gaussian-Lorentzian model and was used as reference metabolite. Subsequently, MRS voxels were co-registered to their corresponding anatomical image and tissue fractions (gray matter, white matter and cerebrospinal fluid) were calculated by segmenting the voxel using Statistical Parametric Mapping (SPM) version 12 software. To ensure high resolution of the to-be segmented anatomical data, the high-resolution anatomical image was co-registered to the short anatomical images that were used to verify optimal voxel positioning. These tissue fractions were used to correct the obtained GABA levels, assuming that GABA is absent in the cerebrospinal fluid and has a concentration twice as high in gray as compared to white matter (Harris et al., 2015, Equation 5). Finally, as the tissue composition of the VOIs differed across age groups (see supplementary Table S3), GABA levels were normalized to the average voxel composition of each group (Harris et al., 2015, Equation 6). For completeness, the equations mentioned above are reported in the supplementary material. Data quality was assessed by visual inspection of the Gannet output for lipid contamination and poor water suppression as well as by quantitative inspection of the fit error (cutoff < 7.5%) and SNR of the GABA signal (cutoff mean + 3*SD) (Mullins et al., 2014). GABA levels within left SM1 were not acquired for one younger participant as only the first two experimental sessions were completed. Furthermore, 14 out of the 412 acquired spectra were excluded from data analysis due to excessive lipid or insufficient data quality. An overview of excluded datasets, data quality of the included MRS data is provided in supplementary Table S1.
2.5. Statistical analyses
Statistical analyses were carried out using the ‘lme4’, ‘car’, and ‘parameters’ packages in R (Bates et al., 2015; Fox and Weisberg, 2019; Lüdecke et al., 2020) Statistical tests used to examine age-related differences in bimanual motor performance, GABA levels and the association between both are discussed separately below. To account for multiple comparisons, the Holm correction method was applied for all analyses (Holm, 1979).
2.5.1. Task battery
To assess age-related differences in performance on the PPT and the BTT, independent samples t tests were carried out. To examine finger tapping performance, a 2 (Task variant) x 2 (Age group) repeated measures ANOVA was performed. Greenhouse-Geisser correction was applied if the sphericity assumption was violated.
2.5.2. GABA levels
To investigate differences in GABA levels between age groups, brain regions and hemispheres and accounting for intra-individual variations, linear mixed effects (LME) modeling was performed (Pinheiro and Bates, 2000). We chose a two-step approach, to first investigate the hemispheric laterality of GABA level variations (inclusion of bilateral homologous regions only) and second, to investigate GABA level variations across brain regions (inclusion of all regions). For the first model, Age group (YA, OA), Brain region (SM1, PMd, DLPFC) and Hemisphere (Left, Right) were included as fixed factors. SMA data were not included in the first model because only a single medial voxel was tested. For the second model, Age group (YA, OA) and Brain region (left SM1, right SM1, left PMd, right PMd, left DLPFC, right DLPFC, SMA) were modelled as fixed effects. For both models, random intercepts were fitted on participant level using restricted maximum likelihood (REML) fit to estimate variances of random effects. Model fit was evaluated based on the distribution of the residuals. Pairwise comparisons for factor levels were based on standardized contrasts of the marginal means extracted directly from the LME model (Lenth, 2020). Conditional R2 and marginal R2 are reported to indicate the total explanatory power of the model and the explanatory power of the fixed effects part alone, respectively (Cohen, 1988).
2.5.3. GABA levels in relation to bimanual task performance
To examine whether age and/or GABA levels within the different ROIs of interest predicted performance, linear regression analyses were carried out. This was performed for the PPT, BTT and finger tapping tasks separately. With respect to finger tapping, an average score across simultaneous and alternated finger tapping was used. To avoid multicollinearity issues, GABA values were standardized and centered by means of a z-transformation (Gelman, 2008). Wald statistics using OA and SMA as reference categories for Age group and Brain region, respectively, are reported. Furthermore, main effects of Age, GABA and Brain region and their interaction terms are reported using the chi-square test. Significant Age x GABA interaction terms were followed up with subsequent linear regression analyses within age group to identify the direction of the association between GABA levels and bimanual motor performance.
3. Results
3.1. Bimanual task battery
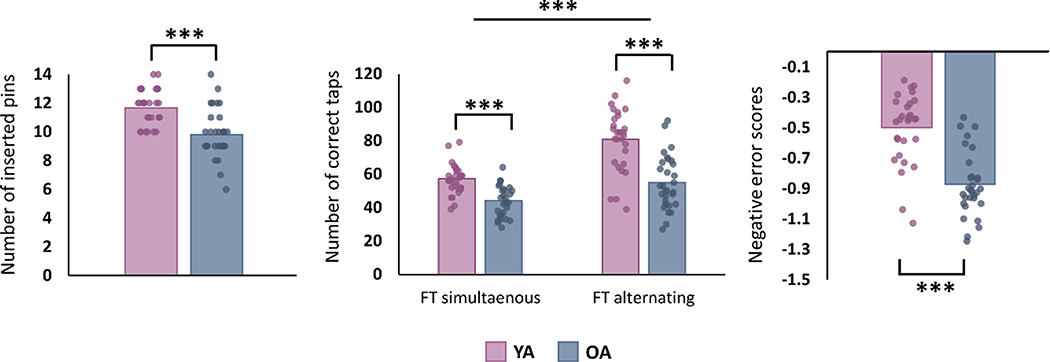
Results of the bimanual tasks are summarized in Table 1 and Fig. 3. For all bimanual tasks, analyses demonstrated a significantly poorer performance for older adults as compared to their younger counterparts (for all tasks, p < 0.001). Furthermore, with respect to finger tapping performance, results of the 2 (Task variant) x 2 (Age group) repeated measures ANOVA indicated a significant Task Variant x Age group interaction effect (F = 9.54, p = 0.003), indicating a more pronounced age-related decline in performance during alternated as compared to simultaneous index finger tapping.
Table 1.
Behavioral results.
| df | t/F | p | |
|---|---|---|---|
| Purdue Pegboard Test: independent samples t-test | |||
| Age group | 57 | −4.77 | < 0.001 |
| Bimanual Tracking task: independent samples t-test | |||
| Age group | 57 | 6.48 | < 0.001 |
| Finger Tapping tasks: 2 (Task variant) x 2 (Age group) repeated measures ANOVA | |||
| Age group | 1,57 | 43.31 | < 0.001 |
| Task variant | 1,57 | 68.04 | < 0.001 |
| Age group x Task variant | 1,57 | 9.54 | 0.003 |
Significant p-values are indicated in bold.
Fig. 3. Behavioral results.
Older adults performed poorer on all tasks. With respect to finger tapping, results demonstrated a significant Age x Task variant interaction effect, indicating that age-related differences in performance were more pronounced in the alternated as compared to the simultaneous finger tapping task variant. Bar plots show mean values with the corresponding individual datapoints superimposed. *** p < 0.001.
3.2. GABA levels
Results of the LME analyses are visualized in Fig. 4. The LME statistical results and the pairwise comparisons based on estimated marginal mean contrasts are summarized in Table 2 and supplementary table S4, respectively. In Model 1, hemispherical differences in GABA levels were examined in the context of aging by a LME including Age group (YA, OA), Brain region (SM1, PMd, DLPFC) and Hemisphere (Left, Right) as fixed factors and Participant as a random intercept. Results indicated a significant general effect of Brain region (χ2 = 305.61, p < 0.001), with overall lower GABA levels in DLPFC and PMd as compared to SM1 as indicated by the parameter estimates (see supplementary table S4 – model 1: Effect of brain region). Furthermore, the Brain region x Age group interaction effect was statistically significant (χ2 = 6.29, p = 0.043). Pairwise comparisons revealed that GABA levels were lower in older as compared to young adults in the SM1 VOI only (t = −2.67, p = 0.042) (for an overview, see supplementary table S4 – model 1: Effect of Age group per brain region). Finally, the Brain region x Hemisphere interaction effect was significant (χ2 = 29.54, p < 0.001). Moreover, pairwise comparisons indicated that GABA levels were higher in the dominant left as compared to the non-dominant right hemisphere for SM1 only (t = −6.56, p < 0.001) (for an overview, see supplementary table S4 – model 1: Effect of Hemisphere per Brain region). As GABA levels in left and right SM1 were acquired in 2 separate sessions, a secondary analysis confirmed that this lateralization effect was not explained by inter-session differences in quality metrics including GABA SNR, full-width half-maximum (FWHM) of the modeled NAA signal nor frequency drift. In Model 2, we investigated GABA level variation across all brain regions using a LME including Brain region (left SM1, right SM1, left PMd, right PMd, left DLPFC, right DLPFC, SMA) and Age group (YA, OA) as fixed factors and Participant as a random intercept. Results indicated a main effect of Brain region (χ2 = 732.15, p < 0.001), thereby extending results of the first model to SMA in which higher GABA levels were measured as compared to the other VOIs (see pairwise comparisons supplementary table S4 – model 2: Effect of Brain region). Together, LME modeling results indicated GABA levels to differ across brain regions. Furthermore, in the SM1 VOI only, GABA levels were lower in older as compared to young adults and lower in the non-dominant right as compared to the dominant left hemisphere, whereas no age and/or lateralization effects were identified in the other VOIs.
Fig. 4. Tissue-corrected GABA levels for each VOI in young and older adults.
GABA levels differed across brain regions. Furthermore, GABA levels were lower in older as compared to young adults and lower in the non-dominant as compared to the dominant hemisphere for the SM1 VOI only. Bar plots show mean values with the corresponding individual datapoints superimposed. * p < 0.05; *** p < 0.001.
Table 2.
Linear mixed effect modeling results on GABA levels.
| Predictors | Estimates | std. Error | CI | Statistic | p | df | Chi-square (df) | p |
|---|---|---|---|---|---|---|---|---|
| Model 1: Age group (YA, OA) x Hemisphere (L, R) x Brain region (SM1, PMd, DLPFC) | ||||||||
| Intercept | 2.26 | 0.05 | 2.18 – 2.35 | 49.89 | < 0.001 | 329 | ||
| Age group | 2.27 (1) | 0.13 | ||||||
| YA | Reference | |||||||
| OA | − 0.07 | 0.06 | − 0.19 – 0.06 | − 1.05 | 0.29 | 329 | ||
| Brain region | 305.61 (2) | < 0.001 | ||||||
| DLPFC | Reference | |||||||
| SM1 | 0.62 | 0.06 | 0.51 – 0.73 | 10.87 | < 0.001 | 329 | ||
| PMd | 0.21 | 0.06 | 0.10 – 0.32 | 3.76 | < 0.001 | 329 | ||
| Hemisphere | 17.01 (1) | < 0.001 | ||||||
| Left | Reference | |||||||
| Right | − 0.08 | 0.06 | − 0.19 – 0.03 | − 1.48 | 0.14 | 329 | ||
| Age group x Brain region | 6.29 (2) | 0.04 | ||||||
| Age group OA: Brain region SM1 | − 0.08 | 0.08 | − 0.24 – 0.07 | − 1.05 | 0.30 | 329 | ||
| Age group OA: Brain region PMd | 0.12 | 0.08 | − 0.03 – 0.27 | 1.58 | 0.11 | 329 | ||
| Age group x Hemisphere | 0.12 (1) | 0.07 | ||||||
| Age group OA: Hemisphere R | 0.04 | 0.08 | − 0.12 – 0.19 | 0.46 | 0.65 | 329 | ||
| Age group x Hemisphere x Brain region | 2.12 (2) | 0.35 | ||||||
| Age group OA: Hemisphere R: Brain region SM1 | − 0.01 | 0.11 | − 0.23 – 0.21 | − 0.07 | 0.94 | 329 | ||
| Age group OA: Hemisphere R: Brain region PMd | − 0.14 | 0.11 | − 0.35 – 0.07 | − 1.30 | 0.19 | 329 | ||
| Hemisphere x Brain region | 29.54 (2) | < 0.001 | ||||||
| Hemisphere R: Brain region SM1 | − 0.19 | 0.08 | − 0.35 – − 0.04 | − 2.44 | 0.02 | 329 | ||
| Hemisphere R: Brain region PMd | 0.17 | 0.08 | 0.02 – 0.32 | 2.19 | 0.03 | 329 | ||
| Random Effects | ||||||||
| σ2 | 0.04 | |||||||
| τ00 Participant | 0.01 | |||||||
| ICC | 0.26 | |||||||
| NParticipant | 59 | |||||||
| Observations | 343 | |||||||
| Marginal R2 / Conditional R2 | 0.45 / 0.59 | |||||||
| AIC | 29.13 | |||||||
| Model 2: Age group (YA, OA) x Brain region (R SM1, L SM1, R PMd, L PMd, R DLPFC, L DLPFC, SMA) | ||||||||
| Intercept | 3.13 | 0.05 | 3.03 – 3.22 | 66.92 | < 0.001 | 383 | ||
| Age group | 3.11 (1) | 0.08 | ||||||
| YA | Reference | |||||||
| OA | − 0.13 | 0.07 | − 0.25 – 0.00 | − 1.96 | 0.05 | 383 | ||
| Brain region | 732.15 (6) | < 0.001 | ||||||
| SMA | Reference | |||||||
| L DLPFC | − 0.86 | 0.06 | − 0.98 – − 0.75 | − 15.11 | < 0.001 | 383 | ||
| L SM1 | − 0.25 | 0.06 | − 0.36 – − 0.13 | − 4.23 | < 0.001 | 383 | ||
| L PMd | − 0.66 | 0.06 | − 0.77 – − 0.55 | − 11.60 | < 0.001 | 383 | ||
| R DLPFC | − 0.94 | 0.06 | − 1.06 – − 0.83 | − 16.70 | < 0.001 | 383 | ||
| R SM1 | − 0.52 | 0.06 | − 0.63 – − 0.41 | − 9.12 | < 0.001 | 383 | ||
| R PMd | − 0.57 | 0.06 | − 0.68 – − 0.46 | − 10.04 | < 0.001 | 383 | ||
| Age group x Brain region | 9.43 (6) | 0.15 | ||||||
| Age Group OA: Brain region L DLPFC | 0.06 | 0.08 | − 0.10 – 0.22 | 0.76 | 0.45 | 383 | ||
| Age Group OA: Brain region L SM1 | − 0.02 | 0.08 | − 0.18 – 0.14 | − 0.27 | 0.79 | 383 | ||
| Age Group OA: Brain region L PMd | 0.18 | 0.08 | 0.03 – 0.34 | 2.31 | 0.02 | 383 | ||
| Age Group OA: Brain region R DLPFC | 0.10 | 0.08 | − 0.06 – 0.25 | 1.21 | 0.23 | 383 | ||
| Age Group OA: Brain region R SM1 | 0.01 | 0.08 | − 0.15 – 0.16 | 0.07 | 0.94 | 383 | ||
| Age Group OA: Brain region R PMd | 0.08 | 0.08 | − 0.08 – 0.23 | 0.97 | 0.33 | 383 | ||
| Random Effects | ||||||||
| σ2 | 0.04 | |||||||
| τ00 Participant | 0.02 | |||||||
| ICC | 0.25 | |||||||
| NParticipant | 59 | |||||||
| Observations | 399 | |||||||
| Marginal R2 / Conditional R2 | 0.59 / 0.69 | |||||||
| AIC | 41.18 | |||||||
Significant p-values are indicated in bold. L: left, R: right, YA: young adults, OA: older adults, SM1: primary sensorimotor cortex, PMd: dorsal premotor cortex, SMA: supplementary motor area, DLPFC: dorsolateral prefrontal cortex.
3.3. Bimanual motor performance in association with GABA levels within the motor network and DLPFC
A linear model was fitted for PPT, finger tapping and BTT performance separately and results are summarized in Table 3 and Fig. 5. For completeness, graphs visualizing age-related differences in the association between GABA and task performance per VOI are included in the supplementary material.
Table 3.
| Predictors | Estimates | std. Error | CI | Statistic | p | Chi-square (df) | p |
|---|---|---|---|---|---|---|---|
| Purdue Pegboard test | |||||||
| (Intercept) | 11.97 | 0.73 | 10.53 – 13.41 | 16.34 | < 0.001 | ||
| Age Group | 141.91 (1) | < 0.001 | |||||
| YA | Reference | ||||||
| OA | − 2.49 | 0.92 | − 4.30 – − 0.69 | − 2.72 | 0.007 | ||
| GABA | − 0.19 | 0.43 | − 1.05 – 0.66 | − 0.44 | 0.66 | 4.65 (1) | 0.03 |
| Age Group x GABA | 6.52 (1) | 0.01 | |||||
| Age Group OA:GABA | 0.54 | 0.58 | − 0.61 – 1.69 | 0.92 | 0.36 | ||
| Brain Region | 3.23 (6) | 0.78 | |||||
| SMA | Reference | ||||||
| L DLPFC | − 0.01 | 0.57 | − 1.12 – 1.11 | − 0.01 | 0.99 | ||
| L SM1 | 0.21 | 0.65 | − 1.07 – 1.49 | 0.32 | 0.75 | ||
| L PMd | 0.17 | 0.67 | − 1.15 – 1.49 | 0.25 | 0.80 | ||
| R DLPFC | 0.42 | 0.58 | − 0.72 – 1.56 | 0.73 | 0.47 | ||
| R SM1 | 0.63 | 0.72 | − 0.78 – 2.03 | 0.88 | 0.38 | ||
| R PMd | − 0.26 | 0.68 | − 1.59 – 1.07 | − 0.39 | 0.70 | ||
| Age Group x Brain Region | 4.08 (6) | 0.67 | |||||
| Age Group OA: Brain Region L DLPFC | 0.73 | 0.81 | − 0.87 – 2.32 | 0.90 | 0.37 | ||
| Age Group OA: Brain Region L SM1 | 0.28 | 0.87 | − 1.43 – 1.98 | 0.32 | 0.75 | ||
| Age Group OA: Brain Region L PMd | 0.25 | 0.88 | − 1.47 – 1.98 | 0.29 | 0.77 | ||
| Age Group OA: Brain Region R DLPFC | − 0.30 | 0.85 | − 1.97 – 1.36 | − 0.36 | 0.72 | ||
| Age Group OA: Brain Region R SM1 | − 1.24 | 1.07 | − 3.33 – 0.86 | − 1.16 | 0.25 | ||
| Age Group OA: Brain Region R PMd | 0.04 | 0.87 | − 1.66 – 1.75 | 0.05 | 0.96 | ||
| Observations | 399 | ||||||
| R2 Nagelkerke | 0.65 | ||||||
| AIC | 1482.11 | ||||||
| Finger tapping (averaged across simultaneous and alternated finger tapping) | |||||||
| (Intercept) | 70.27 | 5.51 | 59.43 – 81.10 | 12.75 | < 0.001 | ||
| Age Group | 287.12 (1) | < 0.001 | |||||
| YA | Reference | ||||||
| OA | − 20.69 | 6.90 | − 34.26 – − 7.11 | − 3.00 | 0.003 | ||
| GABA | − 1.06 | 3.27 | − 7.48 – 5.37 | − 0.32 | 0.75 | 0.76 (1) | 0.39 |
| Age Group x GABA | 0.78 (1) | 0.38 | |||||
| Age Group OA: GABA | 1.02 | 4.40 | − 7.63 – 9.66 | 0.23 | 0.82 | ||
| Brain Region | 8.97 (6) | 0.18 | |||||
| SMA | Reference | ||||||
| L DLPFC | 0.88 | 4.25 | − 7.47 – 9.23 | 0.21 | 0.84 | ||
| L SM1 | − 4.41 | 4.89 | − 14.02 – 5.20 | − 0.90 | 0.37 | ||
| L PMd | 5.71 | 5.04 | − 4.21 – 15.63 | 1.13 | 0.26 | ||
| R DLPFC | 6.12 | 4.36 | − 2.45 – 14.68 | 1.40 | 0.16 | ||
| R SM1 | − 7.93 | 5.38 | − 18.51 – 2.65 | − 1.48 | 0.14 | ||
| R PMd | 2.94 | 5.09 | − 7.07 – 12.94 | 0.58 | 0.56 | ||
| Age x Brain Region | 5.47 (6) | 0.49 | |||||
| Age Group OA: Brain Region L DLPFC | − 0.75 | 6.11 | − 12.76 – 11.26 | − 0.12 | 0.90 | ||
| Age Group OA: Brain Region L SM1 | 3.99 | 6.53 | − 8.86 – 16.83 | 0.61 | 0.54 | ||
| Age Group OA: Brain Region L PMd | − 2.99 | 6.60 | − 15.96 – 9.98 | − 0.45 | 0.65 | ||
| Age Group OA: Brain Region R DLPFC | − 0.85 | 6.37 | − 13.37 – 11.67 | − 0.13 | 0.89 | ||
| Age Group OA: Brain Region R SM1 | 13.83 | 8.02 | − 1.94 – 29.60 | 1.72 | 0.09 | ||
| Age Group OA: Brain Region R PMd | − 2.17 | 6.51 | − 14.97 – 10.64 | − 0.33 | 0.74 | ||
| Observations | 399 | ||||||
| R2 Nagelkerke | 1.00 | ||||||
| AIC | 3092.44 | ||||||
| Bimanual Tracking Task | |||||||
| (Intercept) | − 0.48 | 0.11 | − 0.69 – − 0.26 | − 4.43 | < 0.001 | ||
| Age | 280.6 (1) | < 0.001 | |||||
| YA | Reference | ||||||
| OA | − 0.54 | 0.13 | − 0.81 – − 0.28 | − 4.03 | < 0.001 | ||
| GABA | − 0.02 | 0.06 | − 0.14 – 0.11 | − 0.3. | 0.77 | 2.09 (1) | 0.15 |
| Age x GABA | 9.03 (1) | 0.003 | |||||
| Age OA:GABA | 0.13 | 0.09 | − 0.04 – 0.29 | 1.48 | 0.14 | ||
| Brain Region | 6.40 (6) | 0.38 | |||||
| SMA | Reference | ||||||
| L DLPFC | − 0.04 | 0.08 | − 0.21 – 0.12 | − 0.52 | 0.60 | ||
| L SM1 | − 0.05 | 0.10 | − 0.24 – 0.14 | − 0.54 | 0.59 | ||
| L PMd | − 0.16 | 0.10 | − 0.35 – 0.03 | − 1.63 | 0.10 | ||
| R DLPFC | − 0.10 | 0.09 | − 0.26 – 0.07 | − 1.12 | 0.26 | ||
| R SM1 | 0.03 | 0.11 | − 0.18 – 0.23 | 0.26 | 0.79 | ||
| R PMd | − 0.10 | 0.10 | − 0.29 – 0.10 | − 0.98 | 0.33 | ||
| Age x Brain Region | 1.80 (6) | 0.94 | |||||
| Age Group OA: Brain Region L DLPFC | − 0.05 | 0.12 | − 0.28 – 0.19 | − 0.38 | 0.71 | ||
| Age Group OA: Brain Region L SM1 | − 0.04 | 0.13 | − 0.29 – 0.21 | − 0.30 | 0.76 | ||
| Age Group OA: Brain Region L PMd | 0.08 | 0.13 | − 0.17 – 0.33 | 0.63 | 0.53 | ||
| Age Group OA: Brain Region R DLPFC | − 0.07 | 0.12 | − 0.31 – 0.18 | − 0.54 | 0.59 | ||
| Age Group OA: Brain Region R SM1 | − 0.08 | 0.16 | − 0.39 – 0.22 | − 0.53 | 0.60 | ||
| Age Group OA: Brain Region R PMd | − 0.01 | 0.13 | − 0.26 – 0.24 | − 0.08 | 0.94 | ||
| Observations | 399 | ||||||
| R2 Nagelkerke | 0.47 | ||||||
| AIC | − 51.81 | ||||||
Fig. 5. Visualization of the association between tissue-corrected GABA levels and bimanual motor performance for young and older adults.
The black line represents the association of GABA levels across the motor network and DLPFC with motor performance on the PPT, finger tapping and BTT. Color coding is used to visualize the individual contribution of each ROI. For the Purdue Pegboard test and BTT, a significant Age x GABA interaction effect was observed, indicating that the association between overall GABA levels and motor performance was age-dependent. Post-hoc analyses within age groups confirmed that for the Purdue Pegboard Test, higher GABA levels in older adults predicted better performance whereas no significant association between GABA levels and performance was found in young adults. For the BTT, however, post-hoc analyses demonstrated higher GABA levels in young adults to relate to poorer performance, whereas no significant GABA – performance association was observed in older adults.
3.3.1. Purdue Pegboard test
Results indicated a main effect of Age (χ2 = 141.91, p < 0.001) and GABA (χ2 = 4.65, p = 0.03) as well as a significant Age x GABA interaction effect (χ2 = 6.52, p = 0.01). These results suggest that overall GABA levels across the motor network and DLPFC differentially predicted performance in young as compared to older adults. Post-hoc analyses within age groups revealed that higher GABA levels were indicative of better performance within older adults (χ2 = 7.97, p = 0.005), whereas no significant association between GABA and performance was observed within young adults (χ2 = 0.08, p = 0.78) (Fig. 5).
3.3.2. Finger tapping performance
With respect to finger tapping performance (averaged across the simultaneous and alternated finger tapping task variants), Age group was identified as the only significant predictor of performance (χ2 = 287.12, p < 0.001) such that older age was related to poorer finger tapping performance.
3.3.3. Bimanual tracking task
In line with results from the other tasks, Age group significantly contributed to performance (χ2 = 280.55, p < 0.001). Furthermore, a significant Age x GABA interaction effect was observed indicating that age affected the association between overall GABA levels and bimanual coordination performance. Post-hoc analyses within age groups revealed that this interaction effect was driven by the younger adults. Specifically, higher GABA levels were indicative of poorer performance within young adults (χ2 = 9.48, p = 0.002), whereas no significant association between overall GABA levels and BTT performance was observed within the older age group (χ2 = 1.22, p = 0.27) (Fig. 5).
4. Discussion
We investigated GABA levels across key nodes of the motor network and DLPFC and their relation to bimanual motor performance in a group of young and older adults. Results revealed that age-related declines in GABA levels were region-specific, with older adults demonstrating lower GABA levels in the SM1 only. Furthermore, the link between GABA levels and bimanual motor performance was found to be task-specific and age-dependent, although results did not confirm the hypothesized regional dependency of this association. Specifically, overall GABA levels across the motor network and bilateral DLPFC differentially predicted performance in young adults as compared to older adults on performance of the visually-guided Purdue pegboard task and BTT but not the speed-demanding finger tapping task.
4.1. Age-related decline in bimanual motor performance
As expected, our results indicated lower performance levels in older as compared to young adults across all bimanual tasks. This was confirmed by regression analyses in which age group explained a significant portion of performance measured on each bimanual task. Moreover, results of the finger tapping tasks revealed that the effect of age became more apparent as task complexity increased from simultaneous to alternated index finger tapping. Together, these results are in line with an abundancy of literature indicating a decline in bimanual motor performance with advancing age that interacts with task complexity (for a review, see Maes et al., 2017).
4.2. Age-related differences in GABA levels
Our results demonstrated that GABA levels differed across the different VOIs, with the highest GABA levels measured in SMA, followed by SM1, PMd and DLPFC. These results are in agreement with a large body of evidence indicating regional specificity of GABA levels across multiple brain regions (Chalavi et al., 2018; Gao et al., 2013; Greenhouse et al., 2016; Hermans et al., 2018; Maes et al., 2018; Porges et al., 2017). With respect to hemispherical differences, results are in accordance with a recent study that also revealed a lateralization effect within SM1, i.e. higher GABA/water levels within the dominant left as compared to the non-dominant right SM1 in right-handers, independent of age (Cuypers et al., 2020). Notably, considering the effect of age on GABA levels, our results indicated that, irrespective of hemisphere, GABA levels were lower in older as compared to young adults for the SM1 VOI only. This finding is supported by other studies which also demonstrated an age-related decline in tissue-corrected GABA/water levels within SM1 (Cassady et al., 2019; Chalavi et al., 2018; Cuypers et al., 2020).
The absence of an effect of age on GABA levels within other VOIs, however, is rather unexpected as previous studies reported an overall decrease in GABA levels across multiple VOIs (Gao et al., 2013; Hermans et al., 2018; E.C. Porges et al., 2017). As VOIs reported in these studies differed from those investigated in the present study, we suggest the effect of age to be non-uniformly distributed across the brain. With respect to the frontal lobe, for example, previous work demonstrated that GABA levels within frontal brain regions decreased with advancing age using either Cr and NAA or water as a reference (Gao et al., 2013; Porges et al., 2017). Importantly, whereas the present study investigated left and right DLPFC, the aforementioned studies acquired data in one medial frontal VOI. This may imply that age effects might even be evident at subregional level. Further research using a whole-brain approach such as magnetic resonance spectroscopic imaging (MRSI) is warranted to further clarify regional specificity of age-related differences in GABA levels. Moreover, the speed at which brain GABA levels decline with age might differ, with some brain regions demonstrating a steeper decline in GABA levels as compared to others. In this respect, it should be noted that our older cohort consisted of relatively young and healthy community dwelling older adults. Although the study by Hermans et al. (2018) demonstrated age-related differences in GABA levels using a similar cohort of young and older adults, other studies investigated a group of older adults at the higher end of older age (Cassady et al., 2019; E.C. Porges et al., 2017) and/or used a lifespan cross-sectional design rather than examining distinct age groups (Gao et al., 2013; Porges et al., 2017).
4.3. GABA levels in association with bimanual motor performance
With respect to the association between GABA levels and motor performance, the existing literature has been inconsistent so far. As previous studies were often limited to one specific brain region and/or task of interest, the present study aimed to provide a more comprehensive view by examining performance on several bimanual tasks in relation to GABA levels in multiple brain regions. Each bimanual task targeted divergent features of motor function: PPT, finger tapping and BTT targeted manual dexterity, motor speed capacity and visuo-motor coordination ability, respectively. Considering this diversity in types of bimanual tasks, the role of GABA in distinct brain regions was hypothesized to be task-dependent. Furthermore, based on previous work, age was hypothesized to affect the association such that higher GABA levels would invariably benefit motor performance in older adults whereas the direction of the association was hypothesized to be region- and task-dependent in young adults.
4.3.1. The association between GABA levels and performance is task-dependent
In general, our results confirmed that the contribution of GABA levels to task performance was dependent on the task of interest. Specifically, overall GABA levels across the motor network and DLPFC were related to task performance on the PPT and BTT but not the finger tapping task. Although all these tasks demand for proper bimanual coordination abilities, some differences in the required control mechanisms and motor output can be identified. Whereas the PPT and BTT required a fine dexterity pinch grip and a precise visually-guided dial rotation speed, respectively, finger tapping included the execution of a rather basic motor movement, albeit repeated at high speed. As such, our results seem to suggest that overall GABA levels are especially related to the level of manual precision required in bimanually coordinated movements. Indeed, previous work that established an association between GABA and motor performance also made use of tasks that require a paced and/or selective motor output including response selection tasks (Boy et al., 2010; Dharmadhikari et al., 2015), discrimination tasks (Kurcyus et al., 2018; Puts et al., 2011) as well as sequence learning (Stagg et al., 2011) and coordination tasks (Chalavi et al., 2018). Along the same lines, TMS studies have repeatedly linked GABAergic inhibition to regulate the efficiency of motor output (for a review, see Levin et al., 2014). By investigating different type of tasks, this study sheds light on the specificity of the role of GABA in relation to motor performance, highlighting that overall GABA levels within the motor network may be relevant for tailoring motor output to specific task requirements. Further research is required to validate this hypothesis.
4.3.2. The association between GABA levels and task performance is age-dependent
Importantly, for both the PPT and BTT, age affected the association between GABA levels and task performance such that GABA levels differentially predicted performance in young as compared to older adults. With respect to the BTT, a posteriori analyses within age groups demonstrated that higher GABA levels were indicative of poorer bimanual coordination performance in young but not older adults. In a previous study by our group employing a similar task design, higher SM1 GABA levels were also found to predict poorer initial performance, yet for the total group of participants (Chalavi et al., 2018). With respect to the PPT, however, results revealed that higher GABA levels across the motor network and DLPFC were indicative of better performance in older but not young adults. This is in line with previous work that consistently related higher GABA levels to better task performance in an older population (Cassady et al., 2019; Hermans et al., 2018). Interestingly, however, no association was observed within the younger age group. Together, these results suggest that age differentially affects the association of GABA levels and task performance in different task contexts. Future work is required to elucidate on the origin of these differences.
To obtain a deeper understanding of these results, it is meaningful to consider the known association between regional GABA levels and neural activity from multimodal imaging studies. In young adults, higher resting-state GABA levels in several brain regions (including SM1) have consistently been related to smaller stimulus-induced fMRI BOLD responses (for a review, see Duncan et al., 2014). Indeed, it has been suggested that baseline GABA levels may shape the responsiveness of a brain region, with higher GABA levels relating to a lower responsiveness (Boy et al., 2010; Kurcyus et al., 2018). Thereby, the tonic inhibitory level of a particular brain region, as quantified by baseline MRS-derived GABA levels, might be an important predictor of task-induced activity within that region, as measured using fMRI BOLD (Duncan et al., 2014). Applied to our own work, these results suggest that baseline GABA levels might possibly serve as a proxy of the responsiveness of the cortical motor network during task performance. Stated differently, a working hypothesis may be that higher baseline GABA levels are associated with a lower degree of motor network responsiveness which in turn relates to poorer bimanual coordination performance, at least in young adults. Indeed, previous work by our group confirmed all brain regions of interest in this study to be relevant to BTT performance (Beets et al., 2015; Maes et al., 2020; Santos Monteiro et al., 2017), As such, this study reflects on the importance of task features and their corresponding neural correlates in identifying the association between GABA and motor performance.
In older adults, however, it is proposed that GABA levels are related to the extent of aging-induced neural dedifferentiation. That is, lower GABA levels have been related to poorer neural distinctiveness (less selective activity patterns) and lower network segregation (higher inter-network connectivity and thus less selective functional connectivity) (Cassady et al., 2019; Lalwani et al., 2019). In turn, both distinctiveness and network segregation have been related to motor performance (Carp et al., 2011; Cassady et al., 2020; King et al., 2018). It is possible that baseline GABA levels influence behavior by controlling the overall inhibitory tone of a particular brain region, i.e. a critical process to maintain specificity of neural representations and to modulate activity synchrony between various brain regions (Duncan et al., 2014; Simmonite et al., 2019). Indeed, seminal work by Leventhal et al. (2003), using single cell recording in monkeys revealed that differences in GABA levels are at the origin of less differentiated neural representations at older age. Therefore, our results may provide another indirect indication for the broader notion of neural dedifferentiation within the aging brain, as previously implied for brain activity and network functioning (Bernard and Seidler, 2012; Cabeza, 2002; Heuninckx et al., 2005; Li and Lindenberger, 1999).
4.3.3. The association between GABA levels and task performance is not region-specific within the motor network
Whereas the association between GABA levels and motor performance was designated to a specific region of interest in previous studies (Boy et al., 2010; Chalavi et al., 2018; Hermans et al., 2018), results of the present study did not support our hypothesis of a region-dependent association between GABA levels and motor performance. Although visual inspection of the data seemed to indicate some differential association of GABA levels in distinct brain regions with performance (Fig. 5, also see supplementary material), no effect of Brain region nor an interaction of Age with GABA levels within a specific VOI was observed. As MRS quantifies GABA levels within a relatively large region, the technique might lack sensitivity to detect more subtle differences between brain regions that are part of the same network. Moreover, as we included a high number of VOIs, the present study might have lacked power to detect a regional effect. Furthermore, MRS-derived GABA levels are thought to reflect the level of tonic inhibition, whereas phasic inhibition at the level of the synapse might be more closely related to motor performance.
4.4. Limitations
The present study entails some limitations that need to be considered. First, it is important to bear in mind that the reported GABA signal is contaminated by macromolecules. As numerous studies reported higher macromolecules in older as opposed to young adults (Aufhaus et al., 2013; Marjańska et al., 2018; Noworolski et al., 1999), our results might underestimate the effect of age on pure GABA levels. Along the same line, macromolecular suppression might strengthen the association between MRS-derived GABA levels and motor performance (Mikkelsen et al., 2018b). Second, considering the multidimensionality of the present dataset (2 Age groups, 7 VOIs and 3 Task paradigms), power might be too low to reveal more subtle differences in GABA levels between age groups, their regional specificity and their association with bimanual performance. Nevertheless, we hope that our results add to the understanding of regional differences in GABA levels and their implications for motor performance in the context of aging and can serve as a first steppingstone for future work.
Conclusion
The present study demonstrated the effect of age on GABA to be non-uniformly distributed across the frontal motor network. Furthermore, the association of motor network GABA levels with motor performance appeared to be task- and age-dependent but not region-specific. Together, these results advance our insights into the association between GABA levels and motor performance in the context of aging.
Supplementary Material
Acknowledgments
This work was supported by the Research Fund KU Leuven (C16/15/070), the Research Foundation Flanders (FWO) grant (G089818N) and Excellence of Science grant (EOS 30446199, MEMODYN), and by the Hercules Fund I005018, awarded to SPS and coworkers. CM is funded by an FWO aspirant fellowship, JG is funded by an FWO Postdoctoral Fellowship. This work applies tools developed under NIH R01 EB016089 EB023693 and P41 EB015909, awarded to RAEE. The authors declare no competing financial interests. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors would like to thank René Clerckx for programming the tasks and for technical assistance.
Footnotes
Declaration of Competing Interest
None.
Data/code availability statement
Public access to the data online is not permitted without additional ethical approval on an individual user and purpose basis. Although such requests can be made directly to the Ethics Committee Research of UZ/KU Leuven, the authors are also happy to support such applications.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neuroimage.2021.117871.
References
- Aufhaus E, Weber-Fahr W, Sack M, Tunc-Skarka N, Oberthuer G, Hoerst M, Ende G, 2013. Absence of changes in GABA concentrations with age and gender in the human anterior cingulate cortex: a MEGA-PRESS study with symmetric editing pulse frequencies for macromolecule suppression. Magn. Reson. Med 69 (2), 317–320. doi: 10.1002/mrm.24257. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker BM, Walker SC, 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw 67 (1), 1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Beets IAM, Gooijers J, Boisgontier MP, Pauwels L, Coxon JP, Wittenberg G, Swinnen SP, 2015. Reduced neural differentiation between feedback conditions after bimanual coordination training with and without augmented visual feedback. Cereb. Cortex 25 (7), 1958–1969. doi: 10.1093/cercor/bhu005. [DOI] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD, 2012. Evidence for motor cortex dedifferentiation in older adults. Neurobiol. Aging 33 (9), 1890–1899. doi: 10.1016/j.neurobiolaging.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari A, Radhu N, Farzan F, Mulsant BH, Rajji TK, Daskalakis ZJ, Blumberger DM, 2016. A meta-analysis of the effects of aging on motor cortex neurophysiology assessed by transcranial magnetic stimulation. Clin. Neurophysiol 127 (8), 2834–2845. doi: 10.1016/j.clinph.2016.05.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boy F, Evans CJ, Edden RAE, Singh KD, Husain M, Sumner P, 2010. Individual differences in subconscious motor control predicted by GABA concentration in SMA. Curr. Biol 20 (19), 1779–1785. doi: 10.1016/j.cub.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, 2002. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol. Aging 17 (1), 85–100. doi: 10.1037/0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Carp J, Park J, Hebrank A, Park DC, Polk TA, 2011. Age-related neural dedifferentiation in the motor system. PLoS ONE 6 (12), e29411. doi: 10.1371/jour-nal.pone.0029411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson N, Leach L, Murphy KJ, 2018. A re-examination of montreal cognitive assessment (MoCA) cutoff scores. Int. J. Geriatr. Psychiatry 33, 379–388. doi: 10.1002/gps.4756. [DOI] [PubMed] [Google Scholar]
- Cassady K, Gagnon H, Freiburger E, Lalwani P, Simmonite M, Park DC, …, Polk TA, 2020. Network segregation varies with neural distinctiveness in sensorimotor cortex. Neuroimage 212, 116663. doi: 10.1016/j.neuroimage.2020.116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassady K, Gagnon H, Lalwani P, Simmonite M, Foerster B, Park D, Polk TA, 2019. Sensorimotor network segregation declines with age and is linked to GABA and to sensorimotor performance. Neuroimage 186, 234–244. doi: 10.1016/j.neuroimage.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalavi S, Pauwels L, Heise KF, Zivariadab H, Maes C, Puts NAJ, Swinnen SP, 2018. The neurochemical basis of the contextual interference effect. Neurobiol. Aging 66, 85–96. doi: 10.1016/j.neurobiolaging.2018.02.014. [DOI] [PubMed] [Google Scholar]
- Cohen J, 1988. Statistical Power Analysis for the Social Sciences, 2nd eti.
- Cuypers K, Verstraelen S, Maes C, Hermans L, Hehl M, Heise K, Chalavi S, 2020. Task-related measures of short-interval intracortical inhibition and GABA levels in healthy young and older adults : a multimodal TMS-MRS study. Neuroimage 208, 116470. doi: 10.1016/j.neuroimage.2019.116470, (August 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP, 2004. Cerebellar and premotor function in bimanual coordination: parametric neural responses to spatiotemporal complexity and cycling frequency. Neuroimage 21 (4), 1416–1427. doi: 10.1016/j.neuroimage.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Desrosiers J, Hebert R, Bravo G, Dutil E, 1995. The Purdue Pegboard test : normative data for people aged 60 and over. Disab. Rehabil 17 (5), 217–224. doi: 10.3109/09638289509166638. [DOI] [PubMed] [Google Scholar]
- Dharmadhikari S, Ma R, Yeh C−L, Stock A−K, Snyder S, Zauber SE, …, Beste C, 2015. Striatal and thalamic GABA level concentrations play differential roles for the modulation of response selection processes by proprioceptive information. Neuroimage 120, 36–42. doi: 10.1016/j.neuroimage.2015.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan NW, Wiebking C, Northoff G, 2014. Associations of regional GABA and glutamate with intrinsic and extrinsic neural activity in humans — A review of multimodal imaging studies. Neurosci. Biobehav. Rev 47, 36–52. doi: 10.1016/j.neubiorev.2014.07.016. [DOI] [PubMed] [Google Scholar]
- Edden RAE, Barker PB, 2007. Spatial effects in the detection of gamma-aminobutyric acid: improved sensitivity at high fields using inner volume saturation. Magn. Reson. Med 58, 1276–1282. doi: 10.1002/mrm.21383. [DOI] [PubMed] [Google Scholar]
- Edden RAE, Puts NAJ, Harris AD, Barker PB, Evans CJ, 2014. Gannet: a batch-processing tool for the quantitative analysis of gamma-aminobutyric acit-d-edited MR spectroscopy spectra. J. Magn. Reson. Imaging 40 (6), 1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling BW, Seidler RD, 2012. Task-dependent effects of interhemispheric inhibition on motor control. Behav. Brain Res 226 (1), 211–217. doi: 10.1016/j.bbr.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Weisberg S, 2019. An R Companion to Applied regression, 3rd Edit Sage, Thousand Oaks CA: Retrieved from https://socialsciences.mcmaster.ca/jfox/Books/Companion/. [Google Scholar]
- Fujiyama H, Van Soom J, Rens G, Gooijers J, Leunissen I, Levin O, Swinnen SP, 2016. Age-related changes in frontal network structural and functional connectivity in relation to bimanual movement control. J. Neurosci 36 (6), 1808–1822. doi: 10.1523/JNEUROSCI.3355-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Edden RAE, Li M, Puts NAJ, Wang G, Liu C, Barker PB, 2013. Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage 78, 75–82. doi: 10.1016/j.neuroimage.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A, 2008. Scaling regression inputs by dividing by two standard deviations. Stat. Med 27, 2865–2873. doi: 10.1002/sim.3107. [DOI] [PubMed] [Google Scholar]
- Gerloff C, Andres FG, 2002. Bimanual coordination and interhemispheric interaction. Acad. Radiol 110, 161–186. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Coxon JP, Van Impe A, De Vos J, Wenderoth N, Swinnen SP, 2010. The neural control of bimanual movements in the elderly: brain regions exhibiting age-related increases in activity, frequency-induced neural modulation, and task-specific compensatory recruitment. Hum. Brain Mapp 31 (8), 1281–1295. doi: 10.1002/hbm.20943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhouse I, Noah S, Maddock RJ, Ivry RB, 2016. Individual differences in GABA content are reliable but are not uniform across the human cortex. Neuroimage 139, 1–7. doi: 10.1016/j.neuroimage.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RM, Rottschy C, Miall RC, Eickhoff SB, 2013. A quantitative meta-analysis and review of motor learning in the human brain. Neuroimage 67, 283–297. doi: 10.1016/j.neuroimage.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AD, Puts NAJ, Edden RAE, 2015. Tissue correction for GABA-edited MRS: considerations of voxel composition, tissue segmentation, and tissue relaxations. J. Magn. Reson. Imaging 42 (5), 1431–1440. doi: 10.1002/jmri.24903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans L, Leunissen I, Pauwels L, Cuypers K, Peeters R, Puts NAJ, Swinnen SP, 2018a. Brain GABA levels are associated with inhibitory control deficits in older adults. J. Neurosci 38 (36), 7844–7851. doi: 10.1523/jneurosci.0760-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans L, Levin O, Maes C, van Ruitenbeek P, Heise KF, Edden RAE, …, Cuypers K, 2018b. GABA levels and measures of intracortical and interhemispheric excitability in healthy young and older adults: an MRS-TMS study. Neurobiol. Aging 65, 168–177. doi: 10.1016/j.neurobiolaging.2018.01.023. [DOI] [PubMed] [Google Scholar]
- Heuninckx S, 2005. Neural basis of aging: the penetration of cognition into action control. J. Neurosci 25 (29), 6787–6796. doi: 10.1523/JNEUROSCI.1263-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx Sofie, Wenderoth N, Debaere F, Peeters R, Swinnen SP, 2005. Neural basis of aging: the penetration of cognition into action control. J. Neurosci. : Off. J. Soc. Neurosci 25 (29), 6787–6796. doi: 10.1523/JNEUROSCI.1263-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S, 1979. A simple sequentially rejective multiple test procedure. Scand. J. Stat 6 (2), 65–70. Retrieved from https://www.jstor.org/stable/4615733. [Google Scholar]
- Huang D, Liu D, Yin J, Qian T, Shrestha S, Ni H, 2017. Glutamate-glutamine and GABA in brain of normal aged and patients with cognitive impairment. Eur. J. Radiol 27, 2698–2705. doi: 10.1007/s00330-016-4669-8. [DOI] [PubMed] [Google Scholar]
- Hubel KA, Reed B, Yund WE, Herron TJ, Woods DL, 2013. Computerized measures of finger tapping: effects of hand dominance, age, and sex. Percept. Motor Skills: Motor Skills Ergon 116 (3), 929–952. doi: 10.2466/25.29.PMS.116.3.929-952. [DOI] [PubMed] [Google Scholar]
- Kantak SS, Stinear JW, Buch ER, Cohen LG, 2012. Rewiring the brain: potential role of the premotor cortex in motor control, learning, and recovery of function following brain injury. Neurorehabil. Neural Repair 256 (3), 282–292. doi: 10.1177/1545968311420845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lee JM, Jo HJ, Kim SH, Lee JH, Kim ST, Saad ZS, 2010. Defining functional SMA and pre-SMA subregions in human MFC using resting state fMRI: functional connectivity-based parcellation method. Neuroimage 49 (3), 2375–2386. doi: 10.1016/j.neuroimage.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BR, Van Ruitenbeek P, Leunissen I, Cuypers K, Heise KF, Santos Monteiro T, Swinnen SP, 2018. Age-related declines in motor performance are associated with decreased segregation of large-scale resting state brain networks. Cereb. Cortex 28 (12), 4390–4402. doi: 10.1093/cercor/bhx297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King Bradley R, Verbaanderd JRE, Heise KF, Dolfen N, Sunaert S, Doyon J, Swinnen SP, 2020. Baseline sensorimotor GABA levels shape neuroplastic processes induced by motor learning in older adults. Hum. Brain Mapp 1–16. doi: 10.1002/hbm.25041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurcyus K, Annac E, Hanning NM, Harris AD, Oeltzschner G, Edden R, Riedl V, 2018. Opposite dynamics of GABA and glutamate levels in the occipital cortex during visual processing. J. Neurosci 38 (46), 9967–9976. doi: 10.1523/jneu-rosci.1214-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalwani P, Gagnon H, Cassady K, Simmonite M, Peltier S, Seidler RD, Polk TA, 2019. Neural distinctiveness declines with age in auditory cortex and is associated with auditory GABA levels. Neuroimage 201, 116033. doi: 10.1016/j.neuroimage.2019.116033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth R, 2020. EM means: Estimated Marginal Means, Aka Least-Squaes Means Retrieved from https://cran.r-project.org/package=emmeans.
- Leventhal AG, Wang Y, Pu M, Zhou Y, Ma Y, 2003. GABA and its agonists improved visual cortical function in senescent monkeys. Science 300 (5620), 812–815. 10.1126/science.1082874. [DOI] [PubMed] [Google Scholar]
- Levin O, Fujiyama H, Boisgontier MP, Swinnen SP, Summers JJ, 2014. Aging and motor inhibition: a converging perspective provided by brain stimulation and imaging approaches. Neurosci. Biobehav. Rev 43, 100–117. doi: 10.1016/j.neubiorev.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Levin O, Weerasekera A, King BR, Heise KF, Sima DM, Chalavi S, …, Swinnen SP, 2019. Sensorimotor cortex neurometabolite levels as correlate of motor performance in normal aging : evidence from a 1 H-MRS study. Neuroimage 202, 116050. doi: 10.1016/j.neuroimage.2019.116050, July. [DOI] [PubMed] [Google Scholar]
- Li SC, Lindenberger U, 1999. Cross-level unification: a computational exploration of the link between deterioration of neurotransmitter systems dedifferentation of cognitive abilities in old age. In: Cognitive Neuroscience of Memory. Hogrefe & Huber, Seattle, pp. 103–146. [Google Scholar]
- Lüdecke D, Ben-shachar MS, Patil I, Makowski D, 2020. Extracting, computing and exploring the parameters of statistical models using R. J. Open Source Softw 5 (53), 2445. doi: 10.21105/joss.02445. [DOI] [Google Scholar]
- Maes C, Gooijers J, Orban de Xivry J−J, Swinnen SP, Boisgontier MP, 2017. Two hands, one brain, and aging. Neurosci. Biobehav. Rev 75, 234–256. doi: 10.1016/j.neubiorev.2017.01.052. [DOI] [PubMed] [Google Scholar]
- Maes C, Hermans L, Pauwels L, Chalavi S, Leunissen I, Levin O, Swinnen SP, 2018. Age-related differences in GABA levels are driven by bulk tissue changes. Hum. Brain Mapp 39 (9), 3652–3662. doi: 10.1002/hbm.24201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes C, Swinnen SP, Albouy G, Sunaert S, Gooijers J, Chalavi S, Pauwels L, 2020. The role of the PMd in task complexity : functional connectivity is modulated by motor learning and age. Neurobiol. Aging 92, 12–27. doi: 10.1016/j.neurobiolaging.2020.03.016. [DOI] [PubMed] [Google Scholar]
- Marjańska M, Deelchand DK, Hodges JS, McCarten JR, Hemmy LS, Grant A, Terpstra M, 2018. Altered macromolecular pattern and content in the aging human brain. NMR Biomed. 31 (2), 1–8. doi: 10.1002/nbm.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R, 1998. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 11 (6), 266–272. doi: . [DOI] [PubMed] [Google Scholar]
- Mikkelsen M, Harris AD, Edden RAE, Puts NAJ, 2018a. Macromolecule-suppressed GABA measurements correlate more strongly with behavior than macromolecule-contaminated GABA + measurements. Brain Res. 1701, 204–211. doi: 10.1016/j.brainres.2018.09.021, (September). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen M, Loo RS, Puts NAJ, Edden RAE, Harris AD, 2018b. Designing GABA-edited magnetic resonance spectroscopy studies: considerations of scan duration, signal-to-noise ratio and sample size. J. Neurosci. Methods 303, 86–94. doi: 10.1016/j.jneumeth.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins PG, McGonigle DJ, O’Gorman RL, Puts NAJ, Vidyasagar R, Evans CJ, Wilson M, 2014. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage 86, 43–52. doi: 10.1016/j.neuroimage.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Near J, Edden R, Evans CJ, Paquin R, Harris A, Jezzard P, 2015. Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magn. Reson. Med 73 (1), 44–50. doi: 10.1002/mrm.25094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noworolski SM, Nelson SJ, Henry RG, Day MR, Wald LL, Star-Lack J, Vigneron DB, 1999. High spatial resolution 1H-MRSI and segmented MRI of cortical gray matter and subcortical white matter in three regions of the human brain. Magn. Reson. Med 41 (1), 21–29. doi: . [DOI] [PubMed] [Google Scholar]
- O’Gorman RL, Michels L, Edden RA, Murdoch JB, Martin E, 2011. In vivo detection of GABA and glutamate with MEGA-PRESS: reproducibility and gender effects. J. Magn. Reson. Imaging 33 (5), 1262–1267. doi: 10.1002/jmri.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC, 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pauwels L, Vancleef K, Swinnen SP, Anna I, Beets M, Beets I.a.M, 2015. Challenge to promote change: both young and older adults benefit from contextual interference. Front. Aging Neurosci 7, 1–12. doi: 10.3389/fnagi.2015.00157, (August). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM, 2000. Linear mixed-effects models: basic concepts and examples. In: Mixed-Effects Models in S and S-PLUS, pp. 3–56. [Google Scholar]
- Porges EC, Jensen G, Foster B, Edden RAE, Puts AJ, 2020. The Trajectory of Cortical GABA Levels Across the Lifespan : An individual Participant Data Meta-Analysis of Edited MRS Studies BioRxiv Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges EC, Woods AJ, Edden RAE, Puts NAJ, Harris AD, Chen H, Cohen RA, 2017a. Frontal GABA concentrations are associated with cognitive performance in older adults. Biol. Psychiatry: Cognit. Neurosci. Neuroimaging 2 (1), 38–44. doi: 10.1016/j.bpsc.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges EC, Woods AJ, Lamb DG, Williamson JB, Cohen RA, Edden RAE, Harris AD, 2017b. Impact of tissue correction strategy on GABA-edited MRS findings. Neuroimage 162, 249–256. doi: 10.1016/j.neuroimage.2017.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts NAJ, Edden RAE, Evans CJ, McGlone F, McGonigle DJ, 2011. Regionally specific human GABA concentration correlates with tactile discrimination thresholds. J. Neurosci 31 (46), 16556–16560. doi: 10.1523/jneurosci.4489-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadato N, Yonekura Y, Waki A, Yamada H, Ishii Y, 1997. Role of the supplementary motor area and the right premotor cortex in the coordination of bimanual finger movements. J. Neurosci 17 (24), 9667–9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro Santos, Beets T, M., I.A., Boisgontier MP, Gooijers J, Pauwels L, Chalavi S, Swinnen SP, 2017. Relative cortico-subcortical shift in brain activity but preserved training-induced neural modulation in older adults during bimanual motor learning. Neurobiol. Aging 58, 54–67. doi: 10.1016/j.neurobiolaging.2017.06.004. [DOI] [PubMed] [Google Scholar]
- Simmonite M, Carp J, Foerster BR, Ossher L, Petrou M, Weissman DH, Polk TA, 2019. Age-related declines in occipital GABA are associated with reduced fluid processing ability. Acad. Radiol 26 (8), 1053–1061. doi: 10.1016/j.acra.2018.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisti HM, Geurts M, Clerckx R, Gooijers J, Coxon JP, Heitger MH, Swinnen SP, 2011. Testing multiple coordination constraints with a novel bimanual visuomotor task. PLoS ONE 6 (8). doi: 10.1371/journal.pone.0023619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solesio-Jofre E, Beets IAM, Woolley DG, Pauwels L, Chalavi S, Mantini D, Swinnen SP, 2018. Age-dependent modulations of resting state connectivity following motor practice. Front. Aging Neurosci 10, 25. doi: 10.3389/fnagi.2018.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg Charlotte J., Bachtiar V, Johansen-Berg H, 2011. The role of GABA in human motor learning. Curr. Biol. 21 (6), 480–484. doi: 10.1016/j.cub.2011.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen SP, Gooijers J, 2015. Bimanual coordination. Brain Mapping: An Encyclopedic Reference, Vol. 2 Toga AW, Ed.. Elsevier Inc; doi: 10.1016/B978-0-12-397025-1.00030-0. [DOI] [Google Scholar]
- Swinnen SP, Wenderoth N, 2004. Two hands, one brain: cognitive neuroscience of bimanual skill. Trends Cogn. Sci 8 (1), 18–25. doi: 10.1016/j.tics.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Tiffin J, Asher EJ, 1948. The Purdue Pegboard: norms and studies of reliability and validity. Appl. Psychol 32 (3), 234–247. [DOI] [PubMed] [Google Scholar]
- Verstraelen S, Van Dun K, Duque J, Fujiyama H, Levin O, Swinnen SP, Meesen RLJ, 2020. Induced suppression of the left dorsolateral prefrontal cortex favorably changes interhemispheric communication during bimanual coordination in older adults – a neuronavigated rTMS study. Front. Aging Neurosci 12, 149. doi: 10.3389/fnagi.2020.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P, 1997. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 120 (1), 141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Reis J, Schwenkreis P, Rosanova M, Strafella A, Badawy R, Müller-dahlhaus F, 2015. Clinical neurophysiology TMS and drugs revisited 2014. Clin. Neurophysiol 126, 1847–1868. doi: 10.1016/j.clinph.2014.08.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.