Abstract
Purpose
Elderly people represent a growing stroke population with different pathophysiological states than younger. Whether intravenous thrombolysis (IVT) before mechanical thrombectomy (MT) is beneficial for elderly patients remains unclear. This study compared the efficacy and safety between elderly patients treated with MT alone and those treated with both IVT and MT.
Patients and Methods
Patients aged ≥65 years who were eligible for IVT within 4.5 h from symptom onset were selected from the ANGEL-ACT (Endovascular Treatment Key Technique and Emergency Work Flow Improvement of Acute Ischemic Stroke) registry, a prospective registry program for patients with endovascular treatment from 111 Chinese stroke centers. The primary efficacy outcome was the 90-day modified Rankin Scale score. We compared efficacy and safety outcomes using ordinal or binary logistic regression or a generalized linear model.
Results
In total, 482 elderly patients were included: 187 (38.8%) received IVT and MT (bridging MT) and 295 (61.2%) received MT alone (direct MT). There was no significant difference in the 90-day modified Rankin Scale score between the two groups (median: 4 vs 4 points, respectively; adjusted β=−0.048, P=0.822). The direct MT group had a shorter onset-to-puncture time (225 vs 255 min, respectively; adjusted β=−55.074, P=0.002) and a lower rate of parenchymal hemorrhage type 2 within 24 h (2.80% vs 6.63%, respectively; adjusted odds ratio [OR]=0.287, 95% confidence interval [CI]=0.096–0.856, P=0.025). In addition, the direct MT group showed a trend toward a lower incidence of sICH (5.67% vs 10.06%, adjusted OR=0.453, P=0.061), procedure-related complications (7.12% vs 12.30%, adjusted OR=0.499, P=0.052) and distal or new territorial embolization (4.07% vs 6.95%, adjusted OR=0.450, P=0.093).
Conclusion
Direct MT had similar efficacy to bridging MT in terms of the 90-day functional outcome in elderly patients, whereas bridging MT had a longer onset-to-puncture time and increased risk of hemorrhagic transformation and procedure-related complications.
Keywords: acute ischemic stroke, endovascular treatment, intravenous thrombolysis, elderly patients
Introduction
Mechanical thrombectomy (MT) is now the standard treatment for patients with acute ischemic stroke (AIS) caused by large vessel occlusion (LVO). Current guidelines recommend that patients eligible for intravenous thrombolysis (IVT) should receive IVT even if MT is being considered.1 However, there is some evidence that combining IVT with MT (bridging MT) may result in similar outcomes to MT alone (direct MT), including from observational studies,2–5 systematic reviews and meta-analyses,6,7 and two recently published randomized controlled trials (the DIRECT-MT8 and the DEVT9).
The progressive increase in life expectancy and aging of the population has resulted in a growing number of elderly patients with stroke. However, elderly patients have different pathophysiological states and more comorbidities than younger patients. Although the American Heart Association/American Stroke Association (AHA/ASA) guidelines show that IV alteplase treatment is recommended for patients aged >80 years under certain conditions,1 elderly patients have a higher risk of complications, including a higher risk of hemorrhagic transformation (HT) after IVT.10 Nevertheless, the efficacy of pretreatment with IVT prior to MT in elderly patients and whether this procedure increases the risk of HT remains unclear. Thus, the efficacy and safety of bridging and direct MT in elderly patients requires further study. Therefore, based on the data of the ANGEL-ACT (Endovascular Treatment Key Technique and Emergency Work Flow Improvement of Acute Ischemic Stroke; ClinicalTrial.gov ID: NCT03370939), a prospective, nationwide, multicenter registry in China, we explored the efficacy and safety of direct MT and bridging MT in elderly patients.
Patients and Methods
Study Population and Treatment
Our study population included patients registered in the ANGEL-ACT from November 2017 to March 2019. The ANGEL-ACT is a nationwide, multicenter, prospective registry program for patients who undergo endovascular treatment for acute LVO from 111 stroke centers in China.11 This research program was approved by the ethics committee of the Second Affiliated Hospital of Xi’an Jiaotong University and each participating site. Each participant or his/her representative gave written informed consent before being enrolled in the study. This study was conducted in accordance with the Declaration of Helsinki.
The inclusion criteria were (1) the time from onset-to-hospital admission <4.5 h; (2) computed tomography angiography, magnetic resonance angiography, or digital subtraction angiography confirmation of LVO, defined as occlusion of the internal carotid artery, vertebral artery, basilar artery, middle cerebral artery (M1/M2), anterior cerebral artery (A1/A2), and posterior cerebral artery (P1); (3) age of ≥65 years; and (4) treatment with MT. Patients with contraindications for IVT and missing primary efficacy outcomes were excluded. In total, 482 eligible patients were included in this study. The patients were divided into the bridging MT group (IVT+MT) and the direct MT group (MT without IVT) according to whether they received IVT.
Each patient or his/her legally authorized representative was fully informed of the purpose, cost and complications of IVT and MT. After full consideration, some patients chose to undergo direct MT even if they were transferred from the primary hospital to an advanced stroke center. The main reasons for not performing IVT included refusal by patients or their family members, fear of the side effect of IVT by physicians, and for the purpose of reducing financial burden.11 Each participant or his/her legally authorized representative provided written informed consent before IVT and MT treatment. IVT was administered according to the current AHA/ASA guidelines within 4.5 h from symptom onset in the majority of patients (alteplase 0.9 mg/kg, maximum 90 mg, over 1 h with 10% initial bolus1). The interventional neurologists of all centers had received standard training and were highly experienced in interventional neuroradiology. Stent retriever, aspiration, or a combination of both methods were included. The type and size of the equipment to be used in the operation, as well as other rescue therapies such as balloon angioplasty, stent implantation, or arterial thrombolysis, were determined based on the interventional neurologists’ experience.
Data Collection
Demographic data, vascular risk factors, imaging and laboratory data, stroke and surgery details, and 90-day functional outcomes were prospectively collected. The National Institutes of Health Stroke Scale (NIHSS) score and the modified Rankin Scale (mRS) score, which represent the severity of stroke and the recovery of neurological function, respectively, were recorded by trained and qualified professional investigators. The NIHSS scores were recorded at baseline and at 24 h and 7d after admission. Images were evaluated by an independent laboratory of 10 trained neuroradiologists who were blinded to the clinical data and outcomes. Two neuroradiologists independently evaluated each image; when their results were inconsistent, the final results were decided upon by a third physician.
Outcomes
Outcomes were divided into efficacy and safety outcomes. The primary efficacy outcome was the mRS score at 90 days, with an excellent functional outcome being defined as an mRS score of 0 or 1, a good functional outcome as mRS score of 0 to 2, and a favorable clinical outcome as mRS score of 0 to 3. The secondary efficacy outcomes were changes in the NIHSS score from baseline to 24 h and 7 days, successful recanalization, door-to-groin puncture time, onset-to-groin puncture time, puncture-to-recanalization time, number of thrombectomy passes, and total number of operations. Successful recanalization was defined as a modified Thrombolysis in Cerebral Infarction score of ≥2b. The puncture-to-recanalization time was defined as the duration of time from the moment of puncture to the first successful recanalization or the last contrast bolus (when the vessel was not successfully recanalized). The total number of operations was defined as the sum of all endovascular treatments including stent retriever, aspiration, balloon angioplasty, and stent implantation.
The main safety outcomes were symptomatic intracranial hemorrhage (sICH) within 24 h as defined by the criteria of the Heidelberg Bleeding Classification.12 The secondary safety outcomes were (1) any ICH within 24 h; (2) parenchymal hemorrhage type 2 (PH-2) within 24 h defined as blood clots in >30% of the infarcted area with a substantial space occupying effect;13 (3) mortality within 90 days; and (4) procedure-related complications, including distal or new territorial embolization, vascular perforation, dissection, and vasospasm.
Statistical Analysis
All continuous variables are presented as median with interquartile range (IQR), while categorical variables are presented as frequency and percentage. Differences in continuous variables were analyzed with the Mann–Whitney U-test, while categorical variables were analyzed with the χ2 test or Fisher’s exact test. The 90-day mRS score was treated as an ordinal and dichotomous variable and was analyzed with ordinal and binary logistic regression, respectively. Other binary outcomes were analyzed with logistic regression, while continuous outcomes were analyzed using a generalized linear model. A multivariate model was run to adjust for the use of IVT and variables with a P-value of <0.05 in the baseline characteristics. A second model was run to add the baseline NIHSS score, Alberta Stroke Program Early Computed Tomography Score (ASPECTS), location of the occluded vessels, and etiology as additional covariates. All statistical analyses were performed using statistical software (SAS v9.4; SAS Institute Inc, Cary, NC, USA). A P-value of <0.05 was considered statistically significant.
Results
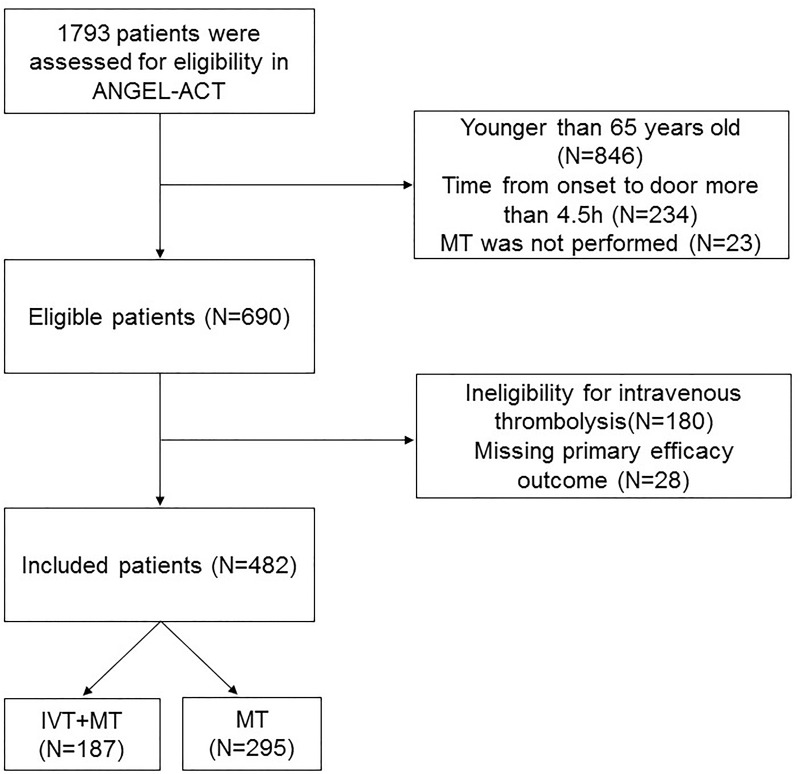
A total of 1793 patients were included in the ANGEL-ACT registry. 690 patients met the inclusion criteria, of whom 180 were ineligible for IVT and 28 were missing the main efficacy outcome. Finally, 482 patients with AIS treated with MT were included in the present study; 187 (38.8%) patients received IVT and MT (bridging MT) and 295 (61.2%) patients received MT alone (direct MT). A patient flowchart is shown in Figure 1.
Figure 1.
Flow chart of the patient inclusion steps.
The median patient age was 73 years (IQR, 69–78 years), and the median baseline NIHSS score was 17 (IQR, 13–22). Of the 482 enrolled patients, 259 (53.73%) were men and 392 (81.33%) had anterior circulation stroke. A total of 426 (88.38%) patients underwent stent thrombectomy, while 86 (17.84%) underwent aspiration. The median onset-to-door time was 90 min (IQR, 50–140 min). Most patients were directly transported to an endovascular-capable center, while 131 (27.18%) were transferred from the primary hospital to an advanced stroke center.
Baseline Characteristics
The baseline characteristics of patients who underwent direct MT and bridging MT are shown in Table 1. There was a higher proportion of men (58.31% vs 46.52%, respectively; P=0.012), use of preoperative anticoagulants (5.42% vs 0.53%, respectively; P=0.004), and use of intraoperative heparin (57.97% vs 32.09%, respectively; P<0.001) in the direct MT group than in the bridging MT group, while the proportion of patients undergoing aspiration catheterization was higher in the bridging MT group than in the direct MT group (25.13% vs 13.22%, respectively; P=0.001). There were no significant differences in any other factors between the two groups (P>0.05).
Table 1.
Comparison of Baseline Characteristics Between Direct Mechanical Thrombectomy (MT) and Bridging MT Patients
| Characteristics | Total (n=482) | BMT (n=187) | DMT (n=295) | P-value |
|---|---|---|---|---|
| Age, median (IQR), year | 73 (69–78) | 72 (68-78) | 73 (69-78) | 0.791 |
| Male (n, %) | 259 (53.73) | 87 (46.52) | 172 (58.31) | 0.012 |
| Medical history (n, %) | ||||
| Hypertension | 309 (64.11) | 115 (61.50) | 194 (65.76) | 0.342 |
| Diabetes mellitus | 97 (20.12) | 36 (19.25) | 61 (20.68) | 0.703 |
| Dyslipidemia | 34 (7.05) | 18 (9.63) | 16 (5.42) | 0.079 |
| Coronary heart disease | 108 (22.41) | 46 (24.60) | 62 (21.02) | 0.358 |
| Atrial fibrillation | 221 (45.85) | 85 (45.45) | 136 (46.10) | 0.890 |
| Prior stroke | 106 (21.99) | 33 (17.65) | 73 (24.75) | 0.067 |
| Current smoking | 348/391 (89.00) | 138/155 (89.03) | 210/236 (88.98) | 0.988 |
| SBP, median (IQR), mmHg | 147 (133–161) | 146 (133–165) | 148 (132–160) | 0.980 |
| NIHSS score, median (IQR) | 17 (13–22) | 17 (13–21) | 18 (13–23) | 0.084 |
| Serum glucose, median (IQR) | 7.0 (5.9–9.2) | 7.1 (6.0–9.1) | 7.0 (5.8–9.3) | 0.864 |
| WBC count, median (IQR) | 7.7 (6.3–9.8) | 7.5 (6.4–9.9) | 7.8 (6.3- 9.7) | 0.932 |
| Platelet count, median (IQR) | 190 (152–232) | 191 (156–232) | 190 (152–232) | 0.525 |
| INR, median (IQR) | 1.02 (0.96–1.10) | 1.01 (0.95–1.10) | 1.03 (0.97–1.10) | 0.224 |
| Serum creatinine, median (IQR) | 71.0 (60.4–83.3) | 72.0 (60.0–84.5) | 71.0 (60.6–83.0) | 0.996 |
| ASPECTS, median (IQR) | 10 (7–10) | 10 (7–10) | 10 (7–10) | 0.213 |
| Occlusion site (n, %) | ||||
| Anterior circulation | 392 (81.33) | 158 (84.49) | 234 (79.32) | 0.156 |
| Posterior circulation | 90 (18.67) | 29 (15.51) | 61 (20.68) | |
| Good collaterals (n, %) | 109/206 (52.91) | 37/75 (49.33) | 72/131 (54.96) | 0.436 |
| TOAST classification (n, %) | ||||
| Large artery atherosclerosis | 198/480 (41.25) | 75/186 (40.32) | 123/294 (41.84) | 0.551 |
| Cardioembolism | 217/480 (45.21) | 89/186 (47.85) | 128/294 (43.54) | |
| Other or unknown etiology | 65/480 (13.54) | 22/186 (11.83) | 43/294 (14.63) | |
| Treatment (n, %) | ||||
| Preoperative antiplatelet agents | 81 (16.80) | 26 (13.90) | 55 (18.64) | 0.175 |
| Preoperative anticoagulants | 17 (3.53) | 1 (0.53) | 16 (5.42) | 0.004* |
| General anesthesia | 184 (38.17) | 74 (39.57) | 110 (37.29) | 0.615 |
| Stent retriever | 426 (88.38) | 162 (86.63) | 264 (89.49) | 0.340 |
| Aspiration catheter | 86 (17.84) | 47 (25.13) | 39 (13.22) | 0.001 |
| Intra-arterial thrombolysis | 44 (9.13) | 14 (7.49) | 30 (10.17) | 0.319 |
| Balloon angioplasty | 79 (16.39) | 35 (18.72) | 44 (14.92) | 0.272 |
| Stenting | 63 (13.07) | 25 (13.37) | 38 (12.88) | 0.877 |
| Intraoperative heparin | 231 (47.93) | 60 (32.09) | 171 (57.97) | <0.001 |
| Infusion of GP IIb/IIIa inhibitor | 205 (42.53) | 74 (39.57) | 131 (44.41) | 0.296 |
| OTD time, median (IQR), min | 90 (50–140) | 90 (53–140) | 90 (48–144) | 0.583 |
| Drip and ship (n, %) | 131 (27.18) | 51 (27.27) | 80 (27.12) | 0.970 |
Notes: Good collaterals: American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology≥2; *Represents Fisher’s exact test.
Abbreviations: DMT, direct mechanical thrombectomy; BMT, bridging mechanical thrombectomy; SBP, systolic blood pressure; NIHSS, National Institutes of Health Stroke Scale; WBC, white blood cell count; INR, international normalized ratio; ASPECTS, Alberta Stroke Program Early CT Score; OTD, symptom onset to door.
Efficacy Outcomes
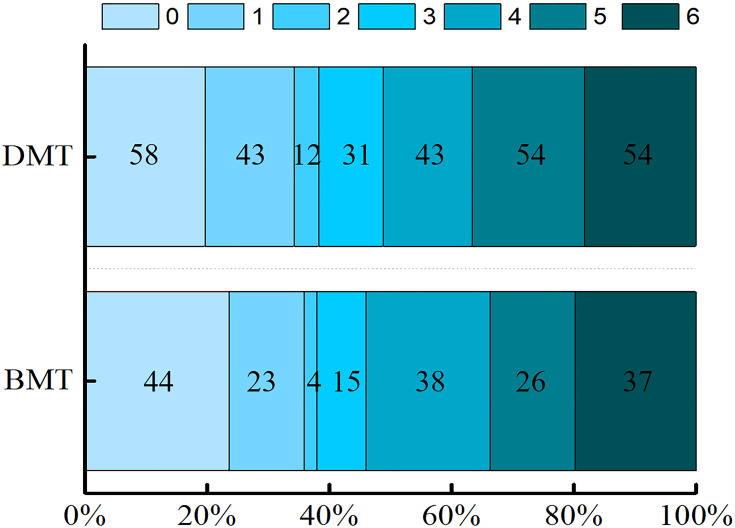
The median mRS at 90 days was 4 both in the direct MT group and the bridging MT group, and there was no significant difference between the two groups (P=0.858; Table 2). The distribution of mRS scores of 0–6 at 90 days is shown in Figure 2. There were no significant differences in the proportions of an excellent clinical outcome (35.83% vs 34.24%, respectively; P=0.721; Table 2), good functional outcome (37.97% vs 38.31%, respectively; P=0.941; Table 2), or favorable clinical outcome (45.99% vs 48.81%, respectively; P=0.546; Table 2) between the two groups. Ordinal logistic regression and binary logistic regression based on the classification of 0–1, 0–2, and 0–3 showed no significant difference in the 90-day mRS score (P>0.05 for each; Table 3). In total, 88 (18.26%) patients were ≥80 years old. A subgroup analysis stratified by age (65–79 vs ≥80 years) showed no significant difference in the ratio of a 90-day mRS score of 0–1, 0–2, and 0–3 (Table 4).
Table 2.
Univariate Analysis of Outcomes in the Direct Mechanical Thrombectomy (MT) and Bridging MT Patients
| Outcomes | BMT | DMT | OR/β (95% CI) | P-value |
|---|---|---|---|---|
| Efficacy outcome | ||||
| mRS at 90 d, median (IQR) | 4 (1–5) | 4 (1–5) | 0.03738 | 0.858 |
| mRS 0–1 at 90 d | 67 (35.83) | 101 (34.24) | 0.932 (0.635–1.368) | 0.721 |
| mRS 0–2 at 90 d | 71 (37.97) | 113 (38.31) | 1.014 (0.696–1.479) | 0.941 |
| mRS 0–3 at 90 d | 86 (45.99) | 144 (48.81) | 1.120 (0.776–1.617) | 0.546 |
| Change in NIHSS score at 24 h, median (IQR) | −2 (−6, 0) | −2 (−7, 0) | −1.56836 | 0.045 |
| Change in NIHSS score at 7 d, median (IQR) | −9 (−14, −3) | −9 (−14, −4) | −1.12063 | 0.275 |
| Successful recanalization | 163 (87.17) | 258 (87.46) | 1.027 (0.592–1.779) | 0.925 |
| OTP time, median (IQR), min | 255 (199–322) | 225 (175–273) | −61.53800 | <0.001 |
| DTP, median (IQR), min | 144 (100–207) | 109 (76–158) | −45.91377 | 0.060 |
| PTR time, median (IQR), min | 87 (53–123) | 83 (50–123) | 0.99663 | 0.857 |
| Number of MT passes, median (IQR) | 2 (1–3) | 1 (1–2) | −0.36955 | 0.009 |
| Total number of operation, median (IQR) | 2 (1–3) | 2 (1–3) | −0.36790 | 0.009 |
| Safety outcomes | ||||
| Symptomatic ICH within 24 h | 18/179 (10.06) | 16/282 (5.67) | 0.538 (0.267–1.085) | 0.083 |
| Any ICH within 24 h | 51/181 (28.18) | 62/286 (21.68) | 0.706 (0.459–1.083) | 0.111 |
| PH-2 within 24 h | 12/181 (6.63) | 8/286 (2.80) | 0.405 (0.162–1.012) | 0.053 |
| Death within 90 d | 37/143 (25.87) | 54/237 (22.78) | 0.845 (0.522–1.369) | 0.494 |
| Distal or new territorial embolization | 13 (6.95) | 12 (4.07) | 0.568 (0.253–1.272) | 0.169 |
| Arterial perforation | 5 (2.67) | 8 (2.71) | 1.015 (0.327–3.149) | 0.980 |
| Arterial dissection | 2 (1.07) | 0 (0) | – | 0.957 |
| Arterial vasospasm | 4 (2.14) | 1 (0.34) | 0.156 (0.017–1.403) | 0.097 |
| Total complications | 23 (12.30) | 21 (7.12) | 0.546 (0.293–1.018) | 0.057 |
Abbreviations: OTP time, time from symptom onset to puncture; DTP time, time from door to puncture; PTR time, time from puncture to recanalization.
Figure 2.
Distribution of the modified Rankin Scale (mRS) scores at 90 days.
Abbreviations: BMT, bridging mechanical thrombectomy; DMT, direct mechanical thrombectomy.
Table 3.
Multivariate Analysis of Outcomes in Elderly Patients
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Adjusted OR/β (95% CI) | P-value | Adjusted OR/β (95% CI) | P-value | |
| Efficacy outcome | ||||
| mRS at 90 d, median (IQR) | 0.02080 | 0.925 | −0.04827 | 0.822 |
| mRS 0–1 at 90 d | 0.945 (0.629–1.418) | 0.784 | 0.976 (0.634–1.503) | 0.914 |
| mRS 0–2 at 90 d | 1.003 (0.672–1.496) | 0.990 | 1.050 (0.687–1.604) | 0.822 |
| mRS 0–3 at 90 d | 1.185 (0.802–1.752) | 0.394 | 1.278 (0.846–1.930) | 0.244 |
| Change in NIHSS score at 24 h, median (IQR) | −1.81529 | 0.029 | −1.41694 | 0.075 |
| Change in NIHSS score at 7 d, median (IQR) | −1.51778 | 0.165 | −0.74295 | 0.436 |
| Successful recanalization | 1.158 (0.649–2.066) | 0.620 | 1.158 (0.641–2.089) | 0.627 |
| OTP time, median (IQR), min | −59.94518 | 0.001 | −55.07449 | 0.002 |
| DTP time, median (IQR), min | −49.80116 | 0.053 | −44.82975 | 0.085 |
| PTR time, median (IQR), min | 2.78556 | 0.634 | 2.97991 | 0.612 |
| Pass number of thrombectomy, median (IQR) | −0.12366 | 0.354 | −0.07015 | 0.599 |
| Total number of operation, median (IQR) | −0.16219 | 0.243 | −0.10225 | 0.466 |
| Safety outcomes | ||||
| Symptomatic ICH within 24 h | 0.522 (0.244–1.116) | 0.093 | 0.453 (0.198–1.036) | 0.061 |
| Any ICH within 24 h | 0.816 (0.515–1.291) | 0.385 | 0.839 (0.513–1.374) | 0.486 |
| PH-2 within 24 h | 0.318 (0.114–0.890) | 0.029 | 0.287 (0.096–0.856) | 0.025 |
| Death within 90 d | 0.813 (0.484–1.365) | 0.433 | 0.775 (0.450–1.335) | 0.359 |
| Distal or new territorial embolization | 0.403 (0.164–0.990) | 0.047 | 0.450 (0.178–1.141) | 0.093 |
| Total complications | 0.463 (0.234–0.916) | 0.027 | 0.499 (0.248–1.004) | 0.052 |
Notes: Model 1 was adjusted for men, preoperative anticoagulants, aspiration catheter, intraoperative heparin, and the use of IVT. Model 2 was additional adjusted for baseline NIHSS, ASPECTS, location of the occluded vessels, and TOAST classification.
Table 4.
The 90-Day mRS Between Bridging and Direct MT in Patients of Aged 65–79 and ≥80 Years Old
| 65–79 | ≥80 | |||||
|---|---|---|---|---|---|---|
| BMT (N=148) | DMT (N=246) | P-value | BMT (N=39) | DMT (N=49) | P-value | |
| mRS 0–1 | 53 (35.81) | 88 (35.77) | 0.994 | 14 (35.90) | 13 (26.53) | 0.344 |
| mRS 2–6 | 95 (64.19) | 158 (64.23) | 25 (64.10) | 36 (73.47) | ||
| mRS 0–2 | 56 (37.84) | 95 (38.62) | 0.877 | 15 (38.46) | 18 (36.73) | 0.868 |
| mRS 3–6 | 92 (62.16) | 151 (61.38) | 24 (61.54) | 31 (63.27) | ||
| mRS 0–3 | 68 (45.95) | 122 (49.59) | 0.483 | 18 (46.15) | 22 (44.90) | 0.906 |
| mRS 4–6 | 80 (54.05) | 124 (50.41) | 21 (53.85) | 27 (55.10) | ||
The direct MT group had a shorter onset-to-puncture time than the bridging MT group (225 vs 255 min, respectively; P<0.001; Table 2). This finding remained unchanged after controlling for other factors by multiple linear regression (model 1: adjusted β=−59.94518, P=0.001; model 2: adjusted β=−55.07449, P=0.002; Table 3). Although there were significant differences in the 24-h NIHSS score change (P=0.045; Table 2), number of thrombectomy passes (P=0.009; Table 2), and total number of operations (P=0.009; Table 2), the multivariate analysis showed no significant differences after adjusting for other factors (P>0.05 for each; Table 3).
Safety Outcomes
Univariate analysis showed a trend toward a lower incidence of PH-2 within 24 h in the direct MT group than in the bridging MT group (2.80% vs 6.63%, respectively; P=0.053; Table 2). Further, after adjusting for other factors, multivariate logistic regression analysis confirmed that the incidence of PH-2 was lower in the direct MT group (model 1: OR=0.318, 95% CI=0.114–0.890, P=0.029; model 2: OR=0.287, 95% CI=0.096–0.856, P=0.025; Table 3). The direct MT group also showed a trend toward a lower incidence of sICH (5.67% vs 10.06%, respectively; model 1: OR=0.522, P=0.093; model 2: OR=0.453, P=0.061; Table 3), procedure-related complications (7.12% vs 12.30%, respectively; model 1: OR=0.463, P=0.027; model 2: OR=0.499, P=0.052; Table 3) and distal or new territorial embolizations (4.07% vs 6.95%, respectively; model 1: OR=0.403, P=0.047; model 2: OR=0.450, P=0.093; Table 3). There were no differences in other safety outcomes, including any ICH within 24 h and death within 90 days, between the two groups.
Discussion
As a prospective registry study of 111 centers, the present study reflects the real-world situation of endovascular treatment for AIS in China. By comparing the efficacy and safety of MT with or without pre-treatment IVT in elderly patients, we found that direct MT had a similar 90-day mRS score to that for bridging MT.
Two recently published randomized controlled trials (the DIRECT-MT trial8 and the DEVT trial9) proved that endovascular thrombectomy alone was noninferior to bridging MT in terms of functional outcome. Later, the SKIP trial14 showed that endovascular treatment alone and bridging MT had similarly favorable outcomes, although the trial failed to demonstrate noninferiority. Our findings are consistent with those trials. The patients in the above trial were >18 years old, and only the SKIP trial performed a subgroup analysis for ages of <70 and ≥70 years; however, it showed no significant heterogeneity of effect on the mRS score. Previous data on the effects of direct MT and bridging MT in elderly patients predominantly comes from observational studies. For example, in a study examining the factors associated with a favorable functional outcome after MT in elderly patients, pre-treatment with IVT was found to be related to a better functional outcome.15 In contrast, some studies have revealed no association of IVT pre-treatment prior to MT with prognosis in elderly patients.16–18 It is important to note that those studies were not designed to compare the effects of direct MT and bridging MT. Furthermore, some of those studies were not multicenter studies. The present study makes up for these confounders. Given some studies defined elderly patients as ≥80 years old, we performed an exploratory subgroup analysis stratified by age (65–79 vs ≥80 years), but found no difference in the 90-day mRS of patients between these subgroups.
In terms of safety, we found bridging MT increased the incidence of PH-2 and showed a trend toward a higher incidence of sICH (10.06% vs 5.67%, respectively; adjusted P=0.061). The SKIP trial and two multicenter retrospective studies also showed that bridging thrombectomy had a higher risk of ICH,11,14,19 which support our result to a certain extent. This suggests that IVT increases the risk of ICH in elderly patients. In fact, some studies have also shown that IVT may be associated with an increased risk of sICH in elderly patients,20,21 and that Asian patients may have a higher risk of sICH after IVT.22 Therefore, we believe that bridging MT is probably associated with a higher risk of sICH. The relatively small sample size might explain the lack of a statistically significant difference in the incidence of sICH. Further studies with larger cohorts are needed to explore this issue. Furthermore, the bridging MT group showed a trend toward more procedure-related complications, especially distal or new territorial embolization, which is consistent with the DEVT trial.9 This may be related to thrombus migration and resolution caused by intravenous alteplase.23 Bridging thrombolysis has also been found to be a risk factor for periprocedural thrombus fragmentation and subsequent downstream embolism,24 which further supports our conclusion. Although bridging MT was associated with more HT and periprocedural complications, we found no difference in the 90-day mRS score between the two treatments. This may be related to the finding that there were no differences in the main factors that could affect the prognosis, such as the NIHSS score and ASPECTS, between the two groups.
In the present study, the time from onset to groin puncture was longer in the bridging MT group. Similar findings were reported in the multicenter registry MR CLEAN (Multicenter Randomized Clinical Trial of Endovascular Treatment of Acute Ischemic Stroke),25 which supports our conclusion. Because the time from symptom onset to door was similar between the two groups (90 [53–140] vs 90 [48–144] min, respectively; P=0.583), the difference in the onset-to-puncture time mainly resulted from the door-to-groin puncture time. The time from door to groin puncture did showed a trend toward being longer in the bridging MT group (144 [100–207] vs 109 [76–158] min, respectively; P=0.060]). Another multicenter study from China also showed that bridging MT had a longer door-to-groin puncture time,19 which supports our conclusion. Overall, these data suggest that bridging MT might delay the time for endovascular treatment. In the DIRECT-MT trial, the median time from hospital admission to groin puncture in the bridging MT group was only 1.5 min longer than that in the direct MT group (85.5 [70–115] vs 84 [67–105] min, respectively), which is markedly less than that in the present study (144 [100–207] vs 109 [76–158] min, respectively). To some extent, this also suggests that some flaws remain in the emergency management of AIS. For example, some patients may not receive MT immediately after IVT, although guidelines recommend that observation after IVT to assess for a clinical response should not be performed.1 Furthermore, patients and their relatives are required to be fully informed and provide written consent before IVT in China, which can also somewhat delay the initiation of treatment.
As a nationwide, multicenter prospective registry program, the advantage of the present study is its high external validity. Further, we provide a reference for elderly patients to help with the selection of direct or bridging MT. However, our study has some limitations, and our findings must be interpreted with caution. First, this was not a randomized controlled trial; unknown confounders affecting the results cannot be completely ruled out, although we adjusted for possible confounding factors. Second, patients treated with IVT and recanalized successfully to avoid MT were not enrolled, which may underestimate the efficacy of IVT. Third, because most patients were treated with alteplase, the effects of different thrombolytic drugs were not analyzed. Fourth, the sample size was relatively small. Finally, there are some missing data in this study. Thus, although relevant statistical methods were used to fill in these missing data, there is the potential for bias.
Conclusion
Our findings suggest that direct MT has similar efficacy to bridging MT in terms of the 90-day functional outcome in elderly patients with LVO, while bridging MT has a longer onset-to-puncture time and increased risk of HT and procedure-related complications.
Acknowledgments
We thank all the ANGEL-ACT study groups for providing the data: Zhongrong Miao, MD; Liqiang Gui, MD; Cunfeng Song, MD; Ya Peng, MD; Jin Wu, MD; Shijun Zhao, MD; Junfeng Zhao, MD; Zhiming Zhou, MD; Yongli Li, MD; Ping Jing, MD; Lei Yang, MD; Yajie Liu, MD; Qingshi Zhao, MD; Yan Liu, MD; Xiaoxiang Peng, MD; Qingchun Gao, MD; Zaiyu Guo, MD; Wenhuo Chen, MD; Weirong Li, MD; Xiaojiang Cheng, MD; Yun Xu, MD; Yongqiang Zhang, MD; Guilian Zhang, MD; Yijiu Lu, MD; Xinyu Lu, MD; Dengxiang Wang, MD; Yan Wang, MD; Hao Li, MD; Yang Hua, MD; Deqin Geng, MD; Haicheng Yuan, MD; Hongwei Wang, MD; Haihua Yang, MD; Zengwu Wang, MD; Liping Wei, MD; Xuancong Liufu, MD; Xiangqun Shi, MD; Juntao Li, MD; Wenwu Yang, MD; Wenji Jing, MD; Xiang Yong, MD; Leyuan Wang, MD; Chunlei Li, MD; Yibin Cao, MD; Qingfeng Zhu, MD; Peng Zhang, MD; Xiang Luo, MD; Shengli Chen, MD; WenWu Peng, MD; Lixin Wang, MD; Xue Wen, MD; Shugui Shi, MD; Wanming Wang, MD; Wang Bo, MD; Pu Yuan, MD; Dong Wang, MD; Haitao Guan, MD; Wenbao Liang, MD; Daliang Ma, MD; Long Chen, MD; Yan Xiao, MD; Xiangdong Xie, MD; Zhonghua Shi, MD; Xiangjun Zeng, MD; Fanfan Su, MD; MingZe Chang, MD; Jijun Yin, MD; Hongxia Sun, MD; Chong Li, MD; Yong Bi, MD; Gang Xie, MD; Yuwu Zhao, MD; Chao Wang, MD; Peng Zhang, MD; Xianjun Wang, MD; Dongqun Li, MD; Hui Liang, MD; Zhonglun Chen, MD; Yan Wang, MD; Yu Xin, Wang, MD; Lin Yin, MD; HongKai Qiu, MD; Jun Wei, MD; Yaxuan Sun, MD; Xiaoya Feng, MD; Weihua Wu, MD; Lianbo Gao, MD; Zhibing Ai, MD; Tan Lan, MD; Li Ding, MD; Qilong Liang, MD; Zhimin Wang, MD; Jianwen Yang, MD; Ping Xu, MD; Wei Dong, MD; Quanle Zheng, MD; Zhenyun Zhu, MD; Liyue Zhao, MD; Qingbo Meng, MD; Yuqing Wei, MD; Xianglin Chen, MD; Wei Wang, MD; Dong Sun, MD; Yongxing Yan, MD; Guangxiong Yuan, MD; Yadong Yang, MD; Jianfeng Zhou, MD; Zhi Yang, MD; Zhenzhong Zhang, MD; Ning Guan, MD; Huihong Wang, MD.
We thank Justin Dean and Angela Morben from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of the manuscript.
Funding Statement
This study is supported by grants from the National Key Research and Development Program of China (2016YFC1301500, 2018YFC1312801), the National Natural Science Foundation of China (81971116), the Shaanxi Provicial Key Research and Development Project of China (2019ZDLSF01–04), and a Postdoctoral Science Foundation (2019M650773).
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author Zhongrong Miao (zhongrongm@163.com) upon reasonable request.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: a Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 2.Kass-Hout T, Kass-Hout O, Mokin M, et al. Is bridging with intravenous thrombolysis of any benefit in endovascular therapy for acute ischemic stroke? World Neurosurg. 2014;82:e453–8. doi: 10.1016/j.wneu.2013.01.097 [DOI] [PubMed] [Google Scholar]
- 3.Rai AT, Boo S, Buseman C, et al. Intravenous thrombolysis before endovascular therapy for large vessel strokes can lead to significantly higher hospital costs without improving outcomes. J Neurointerv Surg. 2018;10(1):17–21. doi: 10.1136/neurintsurg-2016-012830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sallustio F, Koch G, Alemseged F, et al. Effect of mechanical thrombectomy alone or in combination with intravenous thrombolysis for acute ischemic stroke. J Neurol. 2018;265(12):2875–2880. doi: 10.1007/s00415-018-9073-7 [DOI] [PubMed] [Google Scholar]
- 5.Guimarães Rocha M, Carvalho A, Rodrigues M, et al. Primary thrombectomy versus combined mechanical thrombectomy and intravenous thrombolysis in large vessel occlusion acute ischemic stroke. J Stroke Cerebrovasc Dis. 2019;28(3):627–631. doi: 10.1016/j.jstrokecerebrovasdis.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 6.Phan K, Dmytriw AA, Lloyd D, et al. Direct endovascular thrombectomy and bridging strategies for acute ischemic stroke: a network meta-analysis. J Neurointerv Surg. 2019;11(5):443–449. doi: 10.1136/neurintsurg-2018-014260 [DOI] [PubMed] [Google Scholar]
- 7.Tsivgoulis G, Katsanos AH, Mavridis D, Magoufis G, Arthur A, Alexandrov AV. Mechanical thrombectomy improves functional outcomes independent of pretreatment with intravenous thrombolysis. Stroke. 2016;47(6):1661–1664. doi: 10.1161/STROKEAHA.116.013097 [DOI] [PubMed] [Google Scholar]
- 8.Yang P, Zhang Y, Zhang L, et al. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020;382(21):1981–1993. doi: 10.1056/NEJMoa2001123 [DOI] [PubMed] [Google Scholar]
- 9.Zi W, Qiu Z, Li F, et al. Effect of endovascular treatment alone vs intravenous alteplase plus endovascular treatment on functional independence in patients with acute ischemic stroke: the DEVT Randomized Clinical Trial. JAMA. 2021;325(3):234–243. doi: 10.1001/jama.2020.23523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whiteley WN, Slot KB, Fernandes P, Sandercock P, Wardlaw J. Risk factors for intracranial hemorrhage in acute ischemic stroke patients treated with recombinant tissue plasminogen activator: a systematic review and meta-analysis of 55 studies. Stroke. 2012;43(11):2904–2909. doi: 10.1161/STROKEAHA.112.665331 [DOI] [PubMed] [Google Scholar]
- 11.Tong X, Wang Y, Fiehler J, et al. Thrombectomy versus combined thrombolysis and thrombectomy in patients with acute stroke: a Matched-Control Study. Stroke. 2021;52:1589–1600. doi: 10.1161/STROKEAHA.120.031599 [DOI] [PubMed] [Google Scholar]
- 12.von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46(10):2981–2986. doi: 10.1161/STROKEAHA.115.010049 [DOI] [PubMed] [Google Scholar]
- 13.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352(9136):1245–1251. doi: 10.1016/s0140-6736(98)08020-9 [DOI] [PubMed] [Google Scholar]
- 14.Suzuki K, Matsumaru Y, Takeuchi M, et al. Effect of mechanical thrombectomy without vs with intravenous thrombolysis on functional outcome among patients with acute ischemic stroke: the SKIP Randomized Clinical Trial. JAMA. 2021;325(3):244–253. doi: 10.1001/jama.2020.23522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barral M, Lassalle L, Dargazanli C, et al. Predictors of favorable outcome after mechanical thrombectomy for anterior circulation acute ischemic stroke in octogenarians. J Neuroradiol. 2018;45(4):211–216. doi: 10.1016/j.neurad.2018.01.055 [DOI] [PubMed] [Google Scholar]
- 16.Meyer L, Alexandrou M, Flottmann F, et al. Endovascular treatment of very elderly patients aged ≥90 with acute ischemic stroke. J Am Heart Assoc. 2020;9(5):e014447. doi: 10.1161/JAHA.119.014447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alawieh A, Starke RM, Chatterjee AR, et al. Outcomes of endovascular thrombectomy in the elderly: a ‘real-world’ multicenter study. J Neurointerv Surg. 2019;11(6):545–553. doi: 10.1136/neurintsurg-2018-014289 [DOI] [PubMed] [Google Scholar]
- 18.Meyer L, Alexandrou M, Leischner H, et al. Mechanical thrombectomy in nonagenarians with acute ischemic stroke. J Neurointerv Surg. 2019;11(11):1091–1094. doi: 10.1136/neurintsurg-2019-014785 [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Zi W, Hao Y, et al. Direct endovascular treatment: an alternative for bridging therapy in anterior circulation large-vessel occlusion stroke. Eur J Neurol. 2017;24(7):935–943. doi: 10.1111/ene.13311 [DOI] [PubMed] [Google Scholar]
- 20.Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet. 2012;379(9834):2364–2372. doi: 10.1016/S0140-6736(12)60738-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929–1935. doi: 10.1016/S0140-6736(14)60584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menon BK, Saver JL, Prabhakaran S, et al. Risk score for intracranial hemorrhage in patients with acute ischemic stroke treated with intravenous tissue-type plasminogen activator. Stroke. 2012;43(9):2293–2299. doi: 10.1161/STROKEAHA.112.660415 [DOI] [PubMed] [Google Scholar]
- 23.Alves HC, Treurniet KM, Jansen IGH, et al. Thrombus migration paradox in patients with acute ischemic stroke. Stroke. 2019;50(11):3156–3163. doi: 10.1161/STROKEAHA.119.026107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaesmacher J, Boeckh-Behrens T, Simon S, et al. Risk of thrombus fragmentation during endovascular stroke treatment. AJNR Am J Neuroradiol. 2017;38(5):991–998. doi: 10.3174/ajnr.A5105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chalos V, LeCouffe NE, Uyttenboogaart M, et al. Endovascular treatment with or without prior intravenous alteplase for acute ischemic stroke. J Am Heart Assoc. 2019;8(11):e011592. doi: 10.1161/JAHA.118.011592 [DOI] [PMC free article] [PubMed] [Google Scholar]