Abstract
The sense of smell allows animals to discriminate a large number of volatile environmental chemicals. Such chemical signaling modulates the behavior of several species that depend on odorant compounds to locate food, recognize territory, predators, and toxic compounds. Olfaction also plays a role in mate choice, mother-infant recognition, and social interaction among members of a group. A key assay to assess the ability to smell odorants is the buried food-seeking test, which checks whether the food-deprived mice can find the food pellet hidden beneath the bedding in the animal’s cage. The main parameter observed in this test is the latency to uncover a small piece of chow, cookie, or other pleasant food, hidden beneath a layer of cage bedding, within a limited amount of time. It is understood that food-restricted mice which fail to use odor cues to locate food within a given time period are likely to have deficits in olfactory abilities. Investigators who used the buried food test, or versions of the buried food test, demonstrated that it is possible to evaluate olfactory deficits in different models of murine studies (Alberts and Galef, 1971; Belluscio et al., 1998 ; Luo et al., 2002 ; Li et al., 2013 ). We have recently used this assay to demonstrate that olfactory-specific Ric-8B knock-out mice (a guanine nucleotide exchange factor that interacts with olfactory-specific G-protein) show an impaired sense of smell ( Machado et al., 2017 ). Here we describe the protocol of the buried food-seeking test, as adopted in our assays.
Keywords: Buried food-seeking test, Ric-8B knock-out mice, Olfactory behavior tests, Olfactory impairment, Olfactory sensory neuron
Background
The buried food-seeking test was first described in 1971 (Alberts and Galef, 1971). Since then, additional versions of the test have been described. This test has been used to investigate the consequences of olfactory impairment in a variety of situations, such as: analysis of the effects of olfactory function on the performance of female mice in social behavior towards male conspecifics ( Yamada et al., 2001 ), the discrimination of the participation of both the main olfactory system and the vomeronasal organ in behavior (Del Punta et al., 2002 ) or in animal models of hyposulphataemia, a disturbance in sulphate metabolism ( Dawson et al., 2005 ). It was also used to assess sociability and cognitive function in neuronal cell adhesion molecule (Nrcam) null mice ( Moy et al., 2009 ), to study the effects of the selective non-imidazole histamine H3 receptor antagonist in anxiety and depression-like disorders ( Bahi et al., 2014 ), to analyze the role of endocannabinoids in olfactory sensory neurons ( Hutch et al., 2015 ), and others. There are variations in the buried food test methods used in these studies. For example, some authors used pre-test acclimation in the testing cage to reduce novelty-induced exploratory activity during the olfaction test, while others did not. It is important to note that this acclimation can help in decreasing response variability within groups. In our previous work, we used the buried food test, in association with other motivational, behavioral, and cellular tests, to determinate whether the sense of smell is impaired in olfactory-specific Ric-8B knock-out mice ( Machado et al., 2017 ). The present manuscript describes the protocol of the buried food-seeking test as adopted in our previous assays, in order to observe aspects of olfactory deficits in mice.
Materials and Reagents
-
Mice should be at least 8-week-old
Notes:
We used 8-week-old C57BL/6J background mice of both sexes, it is important that the animals have the same age.
It is possible to use this protocol with other strains of mice. It might be applicable to rats as well, but needs standardization of procedures, such as: size of the cage, depth of the bedding, size of the chow pellet, as well as food deprivation time.
In females, estrus can effect olfactory discrimination. Considering this, we initially did statistical analysis in separating genders: wild-type (4 males and 5 females), heterozygote (3 males and 3 females) and conditional knock-out mice (4 males and 5 females). However, we observed no differences between genders, so in our final results, we used both genders in all groups. We recommend evaluating the differences between genders before deciding to use mixed-gender groups or not.
Filtered and autoclaved water
-
Chow pellets (Food stimulus)
Notes:
We used a 2 g pellet of the same chow the animals were regularly fed with.
We used AIN-93G chow (for use during rapid growth, pregnancy and lactation, from Rhoster, described in Reeves et al. (1993), because it was the chow type regularly fed to our animals.
Other types of pleasant foods have been previously used as stimulus, such as: Oreo cookie, Kellog’s Fruit Loops, chow covered in peanut butter and other. We found, however, that Kellog’s Fruit Loops did not stimulate foraging through olfactory cues in the mice. In our experiments, regular food chow pellets showed better results in foraging behavior than the Fruit Loops. This may be due to novelty induced hypophagia, so we recommend the use of food stimulus that the animals are accustomed to in order to avoid novelty induced hypophagia.
Equipment
-
Experimental animal room
Note: An adequate procedure room is essential for the successful development of the behavior test, since it is sensitive to external distractions. This requirement should be carefully considered during the planning stages. An adequate procedure room consists of an isolated and silent experimentation room, where it is possible to avoid the entrance of people during the test.
-
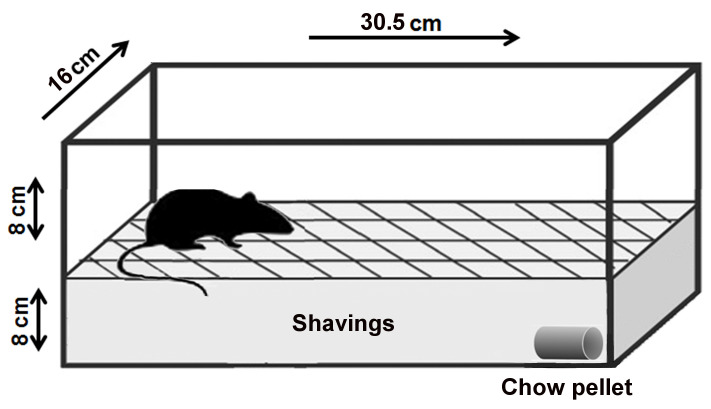
Clean mouse cage of a regular size (30.5 cm length x 16 cm width x 16 cm height or similar) (Figure 1) (We used the M.I.C.E.® Animal Care Systems cage [Animal Care Systems, catalog number: M/P 79010])
Note: Do not use regular cage lids. The stainless-steel top which holds food and water interferes with observation and should not be included in the setup. If needed, it is best to use acrylic lids.
-
Fresh cage bedding to create an 8 cm layer in each cage
Notes:
For each subject, a clean cage washed and dried by the animal care cage washing facility and clean bedding should be used. Do not re-use cages or bedding.
Cage lining: we use Premium Hygienic Animal Bedding LIGNOCEL® FS-14 to bury the pellet.
-
Portable digital scale (Denver Instrument, model: TR-403, or equivalent scale)
Note: To weigh the pellet chow, we used a digital scale (Denver Instrument, TR-403, Max = 410 g, d = 0.001g).
-
Digital stopwatch
Note: The stopwatch is used to monitor the test length.
-
Digital video camera (optional, we used Sony Cyber-shot 14.1 mega pixels) (Sony, model: DSC-W330)
Notes:
The presence of a human observer may influence animal behavior. The use of a video camera may help reduce human interference during the test.
All videos were recorded in .avi format and viewed using VLC media player.
Figure 1. Layout of the buried food-seeking test.
The purpose of this experimental test is to measure the animal's ability to use olfactory cues for foraging. The main parameter measured in this test is the latency to find the hidden food. Latency is defined as the time between when the mouse was placed in the cage and when the mouse uncovered the food pellet. For the test, 8-week-old mice were deprived of food for 24 h but received water ad libitum. Next day, a 2 g pellet of regular chow was buried 8 cm beneath the surface of the fresh bedding in one end of a clean test cage. The site of animal placement and the site at which the pellet was buried remained constant.
Procedure
-
Deprive 8-week-old mice of food for 24 h: 24 h before the test, remove all chow pellets from the home cage. Check inside the cage to remove any pellet fragments that may be scattered within the cage. Do not remove the water bottle.
Note: During standardization, we tried using 6-week-old mice and had ambiguous results. 8-week-old mice or older should be used for the behavioral tests because 6-week-old mice are still considered juvenile and show a larger variability in behaviors.
On day 2, it is advisable to acclimate the mouse to the room in the testing cage in the same conditions of the test, before the insertion of the pellet. Acclimation should last as long as the test will last and the animal should be taken out of the cage before the insertion of the pellet.
Initiate the test by burying a 2 g pellet of regular chow 8 cm beneath the surface of the fresh bedding in one of the corners of the test cage. Transfer the mouse from its home cage back into the test cage, in the opposite corner in regard to the pellet. The time from its introduction into the test cage until it finds the food pellet is recorded as the latency (Video 1). The digital stopwatch should be halted as soon as the mouse touches the pellet of chow. If an animal is not able to find the food pellet within 10 min, the test is terminated and the latency is recorded as 600 sec. Importantly, for each subject, a clean cage, clean bedding, and new pellet of chow should be used.
-
It is important to control for motivation in food-seeking behavior. This can be done with either the unburied food-seeking test ( Li et al., 2013 ) or other motivation and locomotion tests ( Machado et al., 2017 ). The unburied food-seeking test is the simplest way to control for motivation. It can be done using either parallel control groups (groups that undergo the unburied food-seeking test at the same time that the test groups undergo the buried food-seeking test) or the same animals used in the buried food-seeking test, after one week. The unburied food seeking test is the same as the buried food-seeking test, with exception to the pellet being on the surface of the bedding, instead of buried. Therefore, this test does not depend on olfactory cues and controls for motivation level among groups.
Notes:
In the eventuality of an animal escaping the cage during or immediately before the test, exclude this animal from the experiment.
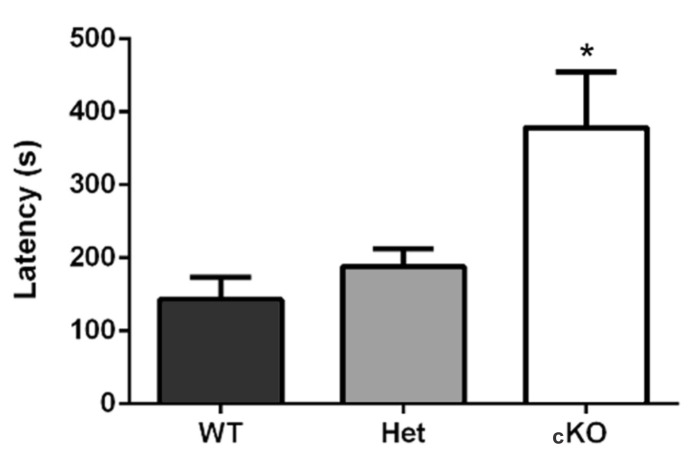
In Machado et al. (2017), while testing for behavioral differences through olfactory impairment, three groups were used: the test-group (conditional knock-out, n = 9) and two control groups (wild-type, n = 9; and heterozygote mice, n = 6). The test-group showed longer latency in retrieving the food pellet, in comparison to the control-groups (WT 143.1 ± 30.2 sec; Het 188.3 ± 24.5 sec; cKO 378.5 ± 76.3 sec; p = 0.02, F(2,15) = 4.54, one-way ANOVA and Dunnett post-test). It is important to note that in this article, we acclimatized the mice to the experimentation room, but not to the cage. It is possible that further acclimation to the cage may render more intensely different latencies between groups (Figure 2). Additionally, in this study we did not perform the unburied food-seeking test because we evaluated motivation and locomotion through the open-field, light/dark, elevated plus-maze, and tail suspension tests.
In order to evaluate possible differences between male and female mice, the three groups used in Machado et al. (2017) were initially divided in genders: wild-type (4 males and 5 females), heterozygote (3 males and 3 females) and conditional knock-out mice (4 males and 5 females). However, after initial statistical analysis, we observed no differences between genders, so the final results contain both genders in each group.
Video 1. Example of a wild-type mouse foraging during the test.

The chow pellet is located in the right corner. The digital stopwatch is halted as soon as the mouse touches the pellet.
Figure 2. Buried food-seeking test results.
Ric-8b conditional knock-out (cKO) mice required significantly longer times to find the food. WT (n = 9), Het (n = 6), and cKO (n = 9) (extracted from Machado et al., 2017 ).
Data analysis
For experimental design, at least three animals of each group test should be used.
The time necessary for the animal to retrieve the pellet (latency) was measured in seconds up to a maximum of 10 min (600 sec was the maximum score).
Any preferred statistical program can be used for statistical analysis. To compare two data sets, use Student’s t-test. To compare three data sets or more, use One-way ANOVA and post-hoc Dunnett or similar. For not normal distribution of data or small groups, use Mann-Whitney test to replace the Student’s t-test and Kruskal-Wallis in replace for one-way ANOVA. Differences are considered significant at P ≤ 0.05.
Notes
The score on a behavioral test can be influenced by many factors, such as lab temperature, noise and the animal’s circadian rhythm. In this way, the experiments should always be done at the same time of day, always respecting the animal's circadian cycle, in an isolated and silent experimentation room, avoiding the entrance of people during the test. It is highly recommended to acclimate the animals in a separate room dedicated specifically for acclimation; this room should be similar to the testing room.
A single experimenter should complete an experiment in its entirety, to reduce variability. Importantly, if multiple cohorts of mice are used, it is preferable that the same experimenter conduct testing on all the mice in all the cohorts of the study to reduce variability.
We recommend double-blind tests. The experimenter that does the video analysis should be blind to the experimental groups in order to avoid bias.
Acknowledgments
This work was supported by Grants from Fundação de Amparo à Pesquisa do Estado de São Paulo [FAPESP; 2016/24471-0 (B.M.), and 2012/24640-6 (C.F.M.)]. We thank Silvânia S. P. Neves, Renata Spalutto Fontes, and Flávia de Moura Prates Ong for technical assistance in our animal facility. This protocol was adapted from previous works (Alberts and Galef, 1971; Li et al., 2013 ). The authors declare no competing financial interests.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Alberts J. R. and Galef B. G., Jr (1971). Acute anosmia in the rat: a behavioral test of a peripherally-induced olfactory deficit. Physiol Behav 6(5): 619-621. [DOI] [PubMed] [Google Scholar]
- 2. Bahi A., Schwed J. S., Walter M., Stark H. and Sadek B.(2014). Anxiolytic and antidepressant-like activities of the novel and potent non-imidazole histamine H3 receptor antagonist ST -1283. Drug Des Devel Ther 8: 627-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Belluscio L., Gold G. H., Nemes A. and Axel R.(1998). Mice deficient in Golf are anosmic . Neuron 20(1): 69-81. [DOI] [PubMed] [Google Scholar]
- 4. Dawson P. A., Steane S. E. and Markovich D.(2005). Impaired memory and olfactory performance in NaSi-1 sulphate transporter deficient mice . Behav Brain Res 159(1): 15-20. [DOI] [PubMed] [Google Scholar]
- 5. Del Punta K., Leinders-Zufall T., Rodriguez I., Jukam D., Wysocki C. J., Ogawa S., Zufall F. and Mombaerts P.(2002). Deficient pheromone responses in mice lacking a cluster of vomeronasal receptor genes. Nature 419(6902): 70-74. [DOI] [PubMed] [Google Scholar]
- 6. Hutch C. R., Hillard C. J., Jia C. and Hegg C. C.(2015). An endocannabinoid system is present in the mouse olfactory epithelium but does not modulate olfaction. Neuroscience 300: 539-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li F., Ponissery-Saidu S., Yee K. K., Wang H., Chen M. L., Iguchi N., Zhang G., Jiang P., Reisert J. and Huang L.(2013). Heterotrimeric G protein subunit Gγ13 is critical to olfaction. J Neurosci 33(18): 7975-7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luo A. H., Cannon E. H., Wekesa K. S., Lyman R. F., Vandenbergh J. G. and Anholt R. R.(2002). Impaired olfactory behavior in mice deficient in the α subunit of Go . Brain Res 941(1-2): 62-71. [DOI] [PubMed] [Google Scholar]
- 9. Machado C. F., Nagai M. H., Lyra C. S., Reis-Silva T. M., Xavier A. M., Glezer I., Felicio L. F. and Malnic B.(2017). Conditional deletion of Ric-8b in olfactory sensory neurons leads to olfactory impairment. J Neurosci 37(50): 12202-12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moy S. S., Nonneman R. J., Young N. B., Demyanenko G. P. and Maness P. F.(2009). Impaired sociability and cognitive function in Nrcam-null mice . Behav Brain Res 205(1): 123-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reeves P. G., Nielsen F. H. and Fahey G. C., Jr (1993). AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123(11): 1939-1951. [DOI] [PubMed] [Google Scholar]
- 12. Yamada K., Wada E. and Wada K.(2001). Female gastrin-releasing peptide receptor(GRP-R)-deficient mice exhibit altered social preference for male conspecifics: implications for GRP/GRP-R modulation of GABAergic function. Brain Res 894(2): 281-287. [DOI] [PubMed] [Google Scholar]