Abstract
Corn stalk rot caused by Fusarium spp., a genus of soil-borne fungal pathogens, has become a major concern of maize production. This disease normally causes significant reduction of maize yield and quality worldwide. The field assay for identifying stalk rot resistance using adult plants is largely relying on large population, yet time-consuming, labor costs, and often influenced by environmental conditions. Therefore, a rapid and reliable assay for investigating maize stalk rot caused by Fusarium spp. is required for screening the resistant lines and functional study of maize resistance to this pathogen. We have developed a seedling assay to rapidly screen the resistant lines using 12-day to 2-week-old seedlings. The entire assay can be completed within approximately 16-18 days post seed germination, with inexpensive labor cost and high repeatability. This simple, rapid and reliable assay can be widely used for identifying the maize resistance to stalk rot caused by Fusarium spp. and other similar fungal pathogens.
Keywords: Corn stalk rot, Disease resistance, Fusarium spp. , Seedling assay
Background
Maize is one of the most important staple food crops and energy plants worldwide. It has been becoming the No.1 crop species in China and worldwide regarding both yield and planting areas since 2012. However, corn stalk rot has become one of the most destructive diseases, which normally lead to significant yield loss and quality reduction of maize extensively (Oerke, 2006). The pathogens causing corn stalk rot mainly include the soil-borne fungal species, such as Fusarium graminearum, F. verticillioides, Colletotrichum graminicola, Pythium aphanidermatum, and Pectobacterium chrysanthemi. Infection of maize by F. graminearum (teleomorph Gibberella zeae Schw. Petch) normally causes Gibberella stalk rot with reddish-pink discoloration inside the stalk, and Gibberella ear rot with carcinogenic mycotoxins deoxynivalenol (DON) and zearalenone produced in mature corn kernels that are harmful to human and animals ( Mesterhazy et al., 2012 ). Moreover, F. graminearum is also the major causal agent infecting other small grain cereal crops, such as wheat, leading to the well-known Fusarium head blight (FHB), which is one of the most serious diseases impacting wheat production in China and is endemic in many wheat-producing countries ( Walter et al., 2010 ; Ma et al., 2017 ). F. verticillioides [=F. moniliforme J. Sheld.(sexual stage: G. moniliformis Wineland)], on the other hand, predominantly infect maize, resulting in Fusarium stalk rot and Fusarium ear rot, and producing Fumonisins, another type of mycotoxins in maize kernels ( Presello et al., 2008 ). Thus, the increasing prevalence of mycotoxins produced by Fusarium spp., along with their direct impact on yield losses in many cereal-growing regions worldwide, has become one of the major concerns of much research.
In recent years, due to the rapid development and application of mechanization, the harvesting technology of corn grain by machines puts forward a high requirement of disease resistance to corn stalk rot. Currently, the field assay for identifying stalk rot resistance using adult plants is largely relying on large population, yet time-consuming, labor costs, and often influenced by environmental conditions. Therefore, a rapid, reliable and large-scale method for screening disease resistant lines and identifying the resistance to corn stalk rot is extremely in urgent, which can provide a great convenience for the researchers to rapidly excavate the elite genetic resources of maize. We have previously deployed a seedling assay to identify the resistance of maize lines to stalk rot caused by F. verticillioides ( Gao et al., 2007 ). Here, we describe the detailed procedures of modified protocol of a seedling assay, which can be applied to evaluate the stalk rot not only caused by Fusarium spp., but also other fungal pathogens, such as C. graminicola.
Materials and Reagents
1.5 ml centrifuge tubes (Nanjing Qingke Biological Company)
50 ml centrifuge tubes (Nanjing Qingke Biological Company)
Long pot (20 cm tall x 5 cm diameter), hand-made with PVC tubes with a holed bottom cover
Parafilm (Bemis, catalog number: PM996)
Press-in Saran Wrap (GLADR, Pressin Seal, 21.6 m x 30 cm), a type of sealing saran wrap with stickiness that can be pressed on the surface to seal tightly the object
Conical bottle (200 ml)
Paper towel (230 x 225 mm, Yong Li Yu Investment Company Limited, May Flower, catalog number: A18250S)
Cheese cloth (40S Warp x 40S Weft; 5 m x 1.2 m), made by combed cotton
Soil (Nanjing Shoude Company, nutrient soil: vermiculite = 2:1)
Syringe needle (Luer-Lok 20G, BD, catalog number: 309634)
Plastic square trays (Purchased from market) (100 cm length x 80 cm width x 10 cm height)
Maize seeds (Inbred line: B73; select the similar size seeds with germinating rate > 90%)
Fungal strains: Fusarium graminearum (isolate: F0609); F. verticillioides (isolate: 7600)
Sterile ddH2O
Glycerin
PDA (Potato dextrose agar) (Qingdao Hope Bio-Technology, catalog number: HB0233)
Tween-20 (SunShine Bio, catalog number: T0014)
Mung Bean (Purchased from market)
PDA media (see Recipes)
Mung bean soup (see Recipes)
Equipment
Pipettes (Eppendorf, Research Plus, catalog numbers: 3120000020, 3120000046, 3120000062)
Hemacytometer (0.100 mm, Hausser Scientific, catalog number: 3110)
Water bath (Changzhou Nuoji Instrument, catalog number: HHS-11-1)
Clean bench (AIRTECH, model: SW-CJ-1FD, catalog number: A16116317)
Constant temperature incubator (Ningbo Jiangnan Instrument Factory, catalog number: DNP-9162)
Growth chamber (Ningbo Jiangnan Instrument Factory, model: RXM-508C-3, catalog number: 17091559)
Centrifuge (Eppendorf, model: 5424, catalog number: 5424FL570190)
Microscope (Olympus, catalog number: BX41)
Autoclave
Procedure
-
Growing maize Seedlings
Sow the seeds of different maize inbred lines in the soil in long pots made with PVC cylinders (5 cm in diameter and 20 cm in depth), with 4 seeds per cylinder, covered with a layer of soil in 2 cm depth. To prove the stalk rot phenotype, a resistant line 1145 and a susceptible line Y331 to Gibberella stalk rot, described by Yang et al. (2010) can be included as controls.
Place the pots in a controlled growth chamber (light cycle and temperature: 14 light/10 dark at 26 ± 2 °C; light intensity: 600 Lux) to allow the seedling growth.
Select the seedlings in similar size at approximately 12-d to 2-week-old stage and transfer to a culture room for infection assay.
-
Preparation of fungal spore suspension
-
Fungal strain culture
Fungal isolates (F. graminearum or F. verticillioides) are initially stored in 20% glycerin at -80 °C. Inoculate the fungal isolate on a PDA plate and incubate it in an incubator (25 °C) in the dark for at least 10 days.
-
Stimulating sporulation
Cut several agar blocks (5 x 5 mm) aseptically and place them in sterilized mung bean soup, and incubate in an incubator at 200 rpm, 28 °C for 2-3 d (Figure 1).
-
Spore enumeration and preparation of inoculum
Filter the spore suspension in mung bean soup through two layers of cheese cloth, followed by centrifugation at 3,800 × g for 5 min. Discard the supernatant, re-suspend and wash the concentrated conidia twice with sterile water before diluting it to a concentration of 1.0 x 106/ml in 0.001% Tween-20.
-
-
Inoculation of seedling stem with fungal spores
-
Preparation of maize seedlings
Keep the plastic square tray (100 x 80 x 10 cm) in a controlled incubation room (light cycle and temperature: 14 light/10 dark at 24 ± 2 °C; light intensity: 200 Lux). To maintain a high humidity (~75%) inside the tray, add 250 ml sterile water to a layer of paper towel on the bottom of the tray, until the towel is wetted completely. Discard the extra water if needed. Line-up the pots with seedlings horizontally in the tray (Figure 2).
-
Artificial inoculation process
To facilitate the infection of fungal spores, punch a tiny hole in approximately 1 mm depth in the middle position of first internode of the seedling stem using a syringe needle (Figure 3, Video 1), then drop 20 μl of spore suspensions in 0.001% Tween-20 to the wounded point carefully using a pipette (Figure 4, Video 2). Seal the plastic square tray with Press-in saran wrap to maintain the moisture. To allow the air exchange, make 6-8 uniform strip holes (in approximately 2 cm length) on the wrap. Upon infection, maintain the seedlings under a condition with 14 h light/10 h dark at 24 ± 2 °C for 3 d without moving (Figure 5). Check the humidity daily to ensure a high humidity at approximately 75%. Add the water if needed.
-
Figure 1. Mung bean soup with F. graminearum cultured for 2 days .
Figure 2. The arrangement of pre-inoculation environment.
Maize seeds are planted and grown in a PVC cylinder in a control room for 12-14 days. The pots with seedlings are then lined up horizontally in a tray with a layer of paper towel completely wetted on the bottom of the tray.
Figure 3. Punching a hole on the stem of maize seedling.
Video 1. Punch a hole on the stem of maize seedling.
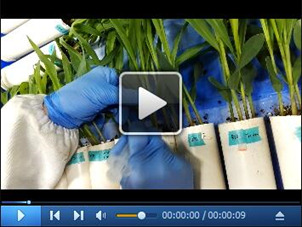
. Use a syringe needle to carefully make a tiny hole in approximately 1 mm depth on the first internode of maize seedling.
Figure 4. Inoculation of maize stem with spore suspensions.
20 μl of spore suspensions in 0.001% Tween-20 is dropped to the wounded site on the seedling stem.
Video 2. Inoculate maize seedling stem with Fusarium spp. spore suspensions .
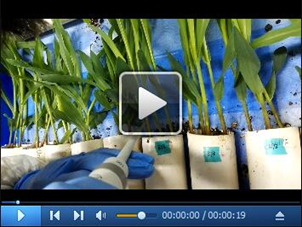
. Drop 20 μl of spore suspensions in 0.001% Tween-20 to the wounded point carefully using a pipette.
Figure 5. Maintainance of seedlings after inoculation.
The plastic square tray is sealed with Press-in saran wrap to maintain a moisture > 75%. Several uniform holes are made on the cover surface. The tray is kept steadily in a controlled condition with 14 h light/10 h dark at 24 ± 2 °C.
Data analysis
-
Rating scales of disease severity
Score the stalk rot symptoms 3 days after artificial inoculation, with at least three scorings (15-20 seedlings per scoring per maize line). Based on the data collected from three or more scorings, estimate the disease severity for each individual plant using a 5-point classification scale ranging from 1 (highest resistance) to 5 (highest susceptibility) (Figure 6). Rating standards of corn stalk rot symptom are described in Table 1.
-
Calculation of disease severity index (DSI)
Calculate the DSI according to the formula described by Ma et al. (2017) with some modifications:
DSI (%) = ∑(ranking value x number of plants with that value) x 100/(1 x total number of plants). All of the statistical analyses were performed using SPSS (14.0) software for ANOVA and the Student’s t-test, with the level of significance set at P < 0.05.
Figure 6. Phenotypic characteristics of seedlings by a 5-point classification scale.
CK: non-inoculated control; 1, the most resistant; 5, the most susceptible.
Table 1. Scales and scoring standards of Gibberella stalk rot of maize seedling.
| Scale | Phenotypic features |
|---|---|
| 1 | No obvious hyphae observed; no color change on the seedling. |
| 2 | Few and scattered hyphae appear around the injection site; tissues around infection site start to decay. |
| 3 | Obvious hyphae appear around the injection site; injection site becomes brown; Obvious decay and softening occur on infection site; the stem still strong and upright. |
| 4 | Hyphae expanded and observed 2-3 cm beyond infection site; inoculation site got rotten with obvious water soak surrounding infection site. |
| 5 | Obviously massive and white hyphae appear and expand upward and downward from the inoculation site; rotten part becomes dark brown; stem easily bent if lifted up. |
Notes
Select the healthy, non-stressed and uniform seedlings for infection assay.
If the inoculum drops immediately from the infection site after inoculation, redo the infection.
Maintain the appropriate humidity and temperature is the key for successful development and penetration of fungal pathogens.
Recipes
-
PDA media (200 ml)
9.2 g Potato glucose agar powder dissolved in 200 ml ddH2O
Sterilize with autoclave for 15 min at 120 °C
-
Mung bean soup (1 L)
Boil 40 g Mung bean in 250 ml ddH2O in a 85 °C water bath for 1 h
Then filter through a layer of gauze, and dilute in autoclaved ddH2O to 1 L
Sterilize the soup at 120 °C for 15 min
Acknowledgments
This protocol is partially adapted from our previous work ( Gao et al., 2007 ), with some modifications. This work was supported by The National Key Research and Development Program of China (No. 2016YFD0101002), the NSFC (No. 31471508, No. 31670702), and from the Technology Foundation for Selected Overseas Chinese Scholar, Ministry of Personnel of China (No. G0101500090) and the Innovation Team Program for Jiangsu Universities (2014). The authors declare no conflicts of interest or competing interests.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Gao X., Shim W. B., Gobel C., Kunze S., Feussner I., Meeley R., Balint-Kurti P. and Kolomiets M.(2007). Disruption of a maize 9-lipoxygenase results in increased resistance to fungal pathogens and reduced levels of contamination with mycotoxin fumonisin. Mol Plant Microbe Interact 20(8): 922-933. [DOI] [PubMed] [Google Scholar]
- 2. Ma C., Ma X., Yao L., Liu Y., Du F., Yang X. and Xu M.(2017). qRfg3, a novel quantitative resistance locus against Gibberella stalk rot in maize . Theor Appl Genet 130(8): 1723-1734. [DOI] [PubMed] [Google Scholar]
- 3. Mesterhazy A., Lemmens M. and Reid L. M.(2012). Breeding for resistance to ear rots caused by Fusarium spp. in maize– a review . Plant Breeding 131: 1-19. [Google Scholar]
- 4. Oerke E. C.(2006). Crop losses to pests. Journal of Agricultural Science 144: 31-43. [Google Scholar]
- 5. Presello D. A., Botta G., Iglesias J., Eytherabide G. H.(2008). Effect of disease severity on yield and grain 591 fumonisin concentration of maize hybrids inoculated with Fusarium verticillioides . Crop Protection 5): 572-576. [Google Scholar]
- 6. Walter S., Nicholson P. and Doohan F. M.(2010). Action and reaction of host and pathogen during Fusarium head blight disease . New Phytol 185(1): 54-66. [DOI] [PubMed] [Google Scholar]
- 7. Yang Q., Yin G., Guo Y., Zhang D., Chen S. and Xu M.(2010). A major QTL for resistance to Gibberella stalk rot in maize. Theor Appl Genet 121: 673-687. [DOI] [PubMed] [Google Scholar]