Abstract
Viruses infect their host cells to produce progeny virus particles through the sequential steps of the viral life cycle, such as viral attachment, entry, penetration and post-entry events. This protocol describes time-of-addition and temperature-shift assays that are employed to explore which step(s) in the viral life cycle is blocked by an antiviral substance(s).
Keywords: Viral life cycle, Time-of-addition assay, Pretreatment, Co-treatment, Post-entry treatment, Temperature-shift assay, Attachment, Penetration
Background
Viruses are obligate intracellular parasites that hijack host cell machineries to replicate their own genome. The viral life cycle proceeds through the attachment (binding) of an infectious viral particle (virion) to the surface of the host cell and its penetration (internalization, fusion) into intracellular compartments, where virion uncoating (disassembly) takes place, followed by viral genome transcription/replication, viral protein synthesis and virion assembly, which eventually results in the production and release of progeny virions from the infected cell (Scheel and Rice, 2013).
To explore which step(s) of the viral lifecycle is blocked by an antiviral substance, time-of-drug addition experiments are performed. In brief, an antiviral substance is added to the virus and/or host cells at different time points relative to viral inoculation to the cells ( Chen et al., 2017 ): (1) Pre-treatment of the cells with an antiviral substance before viral inoculation examines whether the substance could block the viral receptor to inhibit viral attachment to the host cells or if it could induce production of antiviral host factors, such as interferon. (2) Pre-treatment of virus followed by inoculation of the treated virus to the cells examines the virucidal or neutralizing activity of the antiviral substance. (3) Co-treatment of cells and virus during virus inoculation examines the antiviral effect on the virus entry steps including virucidal (neutralizing) activity and blockade of viral attachment and penetration to the cells. (4) Treatment of virus-infected cells during the entire post-inoculation period examines the antiviral effect during the post-entry steps, such as genome translation and replication, virion assembly and virion release from the cells. In addition, temperature-shift assay can differentiate between (5) antiviral activity against attachment that occurs at both 37 °C and 4 °C and (6) antiviral activity against penetration (internalization and/or fusion) that occurs only at 37 °C. An interesting example is that secreted phospholipase A2 obtained from bee venom inhibits penetration of human immunodeficiency virus (HIV) virion without inhibiting virion attachment to the cell surface ( Fenard et al., 1999 ).
In this article, we describe procedures of time-of-addition and temperature-shift assays for the mechanistic analyses of antiviral substances using a fluorescent antibody (FA) method, which have been reported elsewhere ( Wahyuni et al., 2013 ; Adianti et al., 2014 ; Ratnoglik et al., 2014 ; El- Bitar et al., 2015 ; Apriyanto et al., 2016 ; Chen et al., 2017 ). Alternative titration methods, such as plaque assay, 50% tissue culture infectious dose (TCID50) assay and quantitative PCR (qPCR and qRT-PCR), are also used to determine viral titers as described elsewhere.
Materials and Reagents
-
Disposable tips
10 μl capacity (Thermo Fisher Scientific, Molecular BioProducts, catalog number: 3510-05)
200 μl capacity (Thermo Fisher Scientific, Molecular BioProducts, catalog number: 3900)
1 ml capacity (FUKAEKASEI and WATSON, catalog number: 110-502C)
100 mm culture dish (Corning, Falcon®, catalog number: 353003)
24-well culture plate (Corning, Falcon®, catalog number: 353047)
Cover slip (13 mm in diameter; Matsunami Glass, catalog number: C013001)
1.5 ml Microcentrifuge tube (FUKAEKASEI and WATSON, catalog number: 131-715C)
-
Disposable serological pipette
1 ml capacity (Corning, Falcon®, catalog number: 356521)
5 ml capacity (Iwaki, catalog number: 7153-005)
10 ml capacity (Iwaki, catalog number: 7154-010)
Huh7it-1 cells ( Apriyanto et al., 2016 )
-
Viruses ( Chen et al., 2017 ):
Hepatitis C virus (HCV; J6/JFH-1 strain)
Dengue virus type 2 (DENV-2; Trinidad 1751 strain)
Japanese encephalitis virus (JEV; Nakayama strain)
Influenza A virus (FLUAV; A/Udorn/307/72[H3N2])
Sendai virus (SeV; Fushimi strain)
Herpes simplex virus type 1 (HSV-1; CHR3 strain)
Coxsackievirus B3 (CV-B3; Nancy strain)
Crushed ice
4% paraformaldehyde (Wako Pure Chemical Industries, catalog number: 163-20145)
Triton X-100 (Wako Pure Chemical Industries, catalog number: 169-21105)
Bovine serum albumin (BSA; Wako Pure Chemical Industries, catalog number: 015-21274)
Primary antibodies ( Chen et al., 2017 ): Antibodies against viruses, such as HCV, DENV-2, JEV, FLUAV, SeV, HSV-1 and CV-B3
-
Secondary antibodies:
FITC-conjugated goat anti-human IgG (MEDICAL & BIOLOGICAL LABORATORIES, catalog number: 104AG)
Alexa Flour488-conjugated goat anti-mouse IgG (Thermo Fisher Scientific, Invitrogen, catalog number: A-11001)
Alexa Flour488-conjugated goat anti-rabbit IgG (Thermo Fisher Scientific, Invitrogen, catalog number: A-11008)
Hoechst 33342 (Thermo Fisher Scientific, catalog number: H3570)
Vectashield solution (Vector Laboratories, catalog number: H-1000)
Trypsin-EDTA solution (Wako Pure Chemical Industries, catalog number: 209-16941)
High glucose Dulbecco’s modified Eagle’s medium (Wako Pure Chemical Industries, catalog number: 044-29765)
MEM with non-essential amino acids (Thermo Fisher Scientific, GibcoTM, catalog number: 10370-021)
Fetal bovine serum (FBS; Biowest, catalog number: S1820)
Penicillin-Streptomycin solution (Wako Pure Chemical Industries, catalog number: 168-23191)
Sodium chloride (NaCl; Wako Pure Chemical Industries, catalog number: 191-01665)
Potassium chloride (KCl; Wako Pure Chemical Industries, catalog number: 163-03545)
Sodium phosphate dibasic dodecahydrate (Na2HPO4·12H2O; Sigma-Aldrich, catalog number: 71649)
Potassium dihydrigen phosphate (KH2PO4; Wako Pure Chemical Industries, catalog number: 169-04245)
Sodium citrate dehydrate (Sigma-Aldrich, catalog number: W302600)
Citric acid (Wako Pure Chemical Industries, catalog number: 030-05525)
Culture medium (see Recipes)
10x phosphate-buffered saline without Ca2+ and Mg2+ (PBS[-]) and 1x PBS(-) (see Recipes)
Citrate buffer (see Recipes)
Equipment
Biosafety cabinet (e.g., Panasonic, model: MHE-S1301A2)
CO2 incubator (e.g., Panasonic, model: MCO-20AIC)
Autoclave (e.g., TOMY SEIKO, model: SX-500)
Refrigerated tabletop centrifuge (e.g., Eppendorf, model: 5424)
Micropipette (Gilson, P20, P200, P1000)
Hemocytometer (e.g., Erma, catalog number: 03-303-1)
-80 °C freezer (e.g., PHC, model: MDF-384)
Inverted microscope (e.g., Olympus, model: CKX53)
Fluorescent microscope (e.g., Carl Zeiss, model: LSM700)
Procedure
Figures 1 and 2 show the whole procedures of this protocol. In this protocol, FA method is used for virus titration.
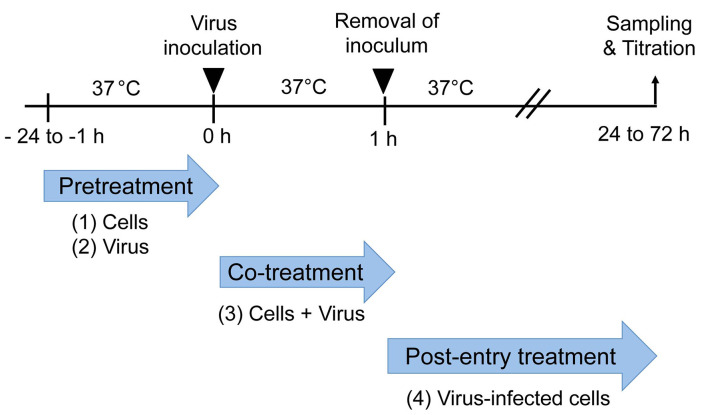
Figure 1. Time-of-addition experiment.
Pretreatment: Cells (1) or virus (2) are treated with an antiviral substance before virus inoculation to the cells. Co-treatment: Virus mixed with an antiviral substance (3) is inoculated to the cells. Post-entry treatment: Virus-infected cells (4) are treated with an antiviral substance. A representative result of a time-of-addition experiment is shown in Figure 3.
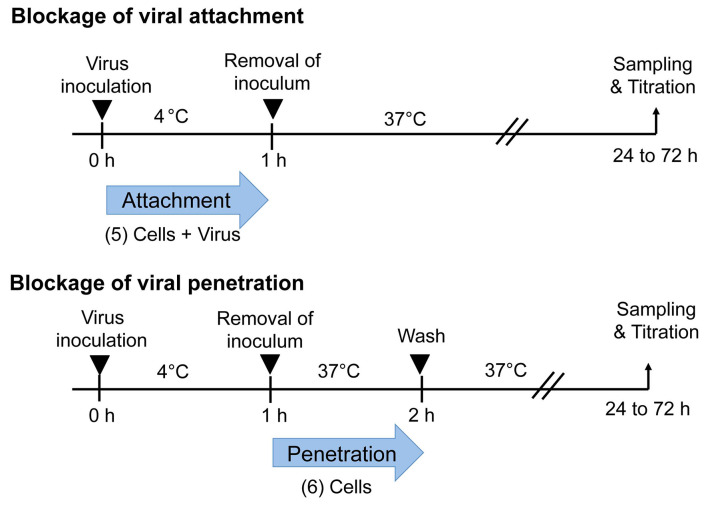
Figure 2. Temperature-shift assay to differentiate viral attachment and penetration.
Blockade of attachment: Cells and viral inoculum are treated with an antiviral substance at 4 °C (5). Blockade of penetration: Cells inoculated with virus at 4 °C are treated with an antiviral substance at 37 °C (6).
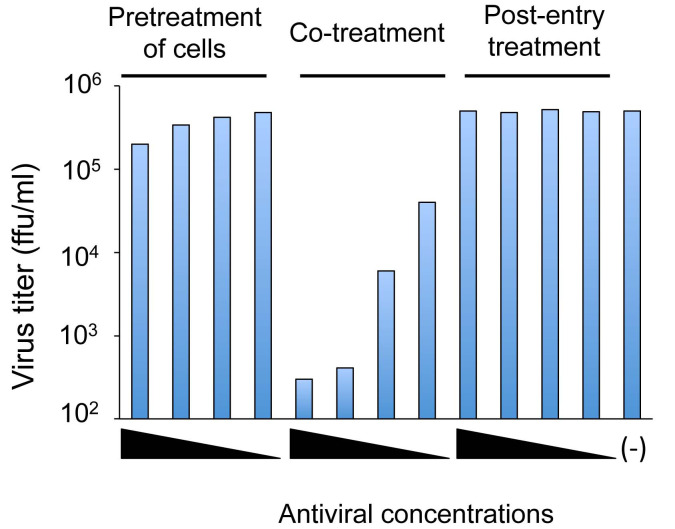
Figure 3. A representative result of a time-of-addition experiment.
Pretreatment of cells: Cells are treated with serial dilutions of an antiviral substance before virus inoculation to the cells. Co-treatment: Virus and cells are treated with serial dilutions of an antiviral substance. Post-entry treatment: Virus-infected cells are treated with serial dilutions of an antiviral substance. (-): mock-treated control. The result shows a dose-dependent inhibition of viral infection under the co-treatment condition, but not under the pretreatment of cells or post-entry treatment conditions.
-
Pretreatment of cells before virus infection (see Figure 1)
Day 1
Place a glass coverslip into each well of a 24-well plate and seed Huh7it-1 cells (2 x 105 cells) to each well (500 μl/well).
-
Incubate the cells at 37 °C in a 5% CO2 incubator overnight.
Note: The cells become 80-90% confluent after overnight culture.
Day 2
Remove culture medium from each well.
Add 200 μl of culture medium containing serial dilutions of an antiviral substance (e.g., 1,000, 100, 10 and 1 ng/ml).
Incubate for 1 h to 24 h at 37 °C in a 5% CO2 incubator.
To prepare virus solution, dilute the virus stock in an appropriate volume of culture medium (e.g., 2,000 focus-forming unit [FFU] in 200 μl) in a 1.5 ml tube and vortex gently.
Remove the medium containing the antiviral substance and rinse the cells twice with culture medium.
-
Inoculate virus onto drug-pretreated cells (multiplicity of infection [MOI] = 0.01 to 0.1 FFU/cell) (200 μl/well).
Note: Virus is inoculated to cells in the absence of the antiviral substance.
Incubate for 1 h at 37 °C in a 5% CO2 incubator.
Remove the inoculum and rinse the cells three times with culture medium.
Add culture medium (500 μl/well) without the antiviral substance.
Incubate for 24 h at 37 °C in a 5% CO2 incubator.
Day 3
Remove culture medium from each well and rinse the cells twice with PBS(-).
-
To fix infected cells, carefully add 4% paraformaldehyde to a coverslip into each well (200 μl/well) and leave for 20 min.
Note: More detailed instruction of cell fixation has been described in Bio-protocol “Immunofluorescent staining of mouse intestinal stem cells” (O’Rourke et al., 2016).
After washing the cells three times with PBS(-) (200 μl/well), add 0.1% Triton X-100 in PBS(-) (200 μl/well) to permeabilize cells for 15 min at room temperature.
Wash the cells three times with PBS(-) (200 μl/well).
Incubate the cells with 1% BSA in PBS(-) (200 μl/well) for 1 h to avoid nonspecific FA staining.
-
Incubate the cells with an appropriate concentration of antiviral antibody (primary antibody) in PBS(-) (200 μl/well) for 1 h at room temperature.
Note: As primary antibody, we used rabbit anti-DENV PrM (1:500 dilution), mouse anti-DENV (1:500 dilution), anti-HCV human serum (1:500 dilution), rabbit anti-CV-B3 (1:100 dilution), rabbit anti-FLUAV (1:1,000 dilution), rabbit anti-SeV (1:1,000 dilution) and rabbit anti-HSV-1 (1:1,000 dilution).
Wash the cells three times with PBS(-) (200 μl/well) to remove the unbound antibody.
Incubate the cells with FITC or Alexa488-conjugated secondary antibody (1:800 dilution, 200 μl/well) against IgG of appropriate origin (human, mouse, rabbit, etc.) for 1 h at room temperature.
Wash the cells three times with PBS(-) (200 μl/well) to remove the unbound antibody.
Counterstain the cells with Hoechst 33342 diluted with PBS(-) (1:1,000 dilution) for 5 min and rinse three times with PBS(-) (200 μl/well).
-
Put a small amount of Vectashield solution on a slide glass. Hold a coverslip with tweezers and touch the edge to a paper towel to remove excess PBS(-). Turn the coverslip upside-down and sandwich the coverslip and slide glass together, avoiding bubbles. After air-drying at room temperature in the dark, observe under a fluorescence microscope.
Note: Infectivity of vesicular stomatitis New Jersey virus (VSNJV), Sindbis virus (SINV) and encephalomyocarditis virus (EMCV) can be determined by plaque assay and/or 50% tissue culture infectious dose (TCID50) assay, as described elsewhere. Also, amounts of viral proteins and viral nucleic acids (DNA and/or RNA) in the infected cells can be determined by Western blotting and quantitative PCR (qPCR and qRT-PCR) analyses, respectively.
-
Pretreatment of virus before inoculation to the cells (see Figure 1)
Day 1
See Day 1 of Procedure A.
Day 2
Prepare serial dilutions of an antiviral substance (2,000, 200, 20 and 2 ng/ml) in culture medium in a 1.5 ml tube.
To prepare virus solution, dilute the virus stock in an appropriate volume of culture medium (2,000 FFU in 100 μl) in a 1.5 ml tube and vortex gently.
-
Add 100 μl of an antiviral substance solution and 100 μl of virus in a 1.5 ml tube.
Note: The final concentrations of the antiviral substance are 1,000, 100, 10 and 1 ng/ml.
Vortex gently.
-
Incubate for 1 h at 37 °C.
Note: Virus is pretreated with an antiviral substance for 1 h before inoculation to the cells.
Remove culture medium from each well.
Inoculate 200 μl of virus-antiviral substance mixture onto the cells (MOI = 0.01 to 0.1).
Incubate for 1 h at 37 °C in a 5% CO2 incubator.
Remove the inoculum and rinse the cells three times with culture medium.
Add culture medium (500 μl/well) without the antiviral substance.
Incubate the cells for 24 h at 37 °C in a 5% CO2 incubator.
Day 3
See Day 3 of Procedure A as described above.
-
Co-treatment of cells and virus (see Figure 1)
Day 1
See Day 1 of Procedure A.
Day 2
Prepare serial dilutions of an antiviral substance (2,000, 200, 20 and 2 ng/ml) in culture medium in a 1.5 ml tube.
To prepare virus solution, dilute the virus stock in the appropriate volume of culture medium (2,000 FFU in 100 μl) in a 1.5 ml tube and vortex gently.
-
Add 100 μl of antiviral substance solution and 100 μl of virus in a 1.5 ml tube.
Note: The final concentrations of antiviral substance are 1,000, 100, 10 and 1 ng/ml.
Vortex gently.
Remove culture medium from each well.
-
Inoculate 200 μl of virus-antiviral substance mixture onto the cells (MOI = 0.01 to 0.1).
Note: After the virus is mixed with an antiviral substance, immediately inoculate to the cells without incubation time.
Incubate for 1 h at 37 °C in a 5% CO2 incubator.
Remove the inoculum and rinse the cells three times with culture medium.
Add culture medium (500 μl/well) without the antiviral substance.
Incubate for 24 h at 37 °C in a 5% CO2 incubator.
Day 3
See Day 3 of Procedure A.
-
Post-entry treatment of virus-infected cells (see Figure 1)
Day 1
See Day 1 of Procedure A.
Day 2
To prepare virus solution, dilute the virus stock in the appropriate volume of culture medium (2,000 FFU in 100 μl) in a 1.5 ml tube and vortex gently.
Remove the culture medium from each well.
Inoculate 200 μl of the virus to the cells (MOI = 0.01 to 0.1).
Incubate for 1 h at 37 °C in a 5% CO2 incubator.
Prepare serial dilutions of an antiviral substance (1,000, 100, 10 and 1 ng/ml) in the culture medium.
Remove inoculated virus and rinse the cells three times with culture medium.
Add culture medium containing serial dilutions of the antiviral substance (500 μl/well).
Incubate for 24 h at 37 °C in a 5% CO2 incubator.
Day 3
See Day 3 of Procedure A.
-
Blockade of viral attachment (Temperature-shift assay) (see Figure 2)
Day 1
See Day 1 of Procedure A.
Day 2
Place the 24-well plate containing the cells at 4 °C for 1 h.
Prepare serial dilutions of an antiviral substance (2,000, 200, 20 and 2 ng/ml) in culture medium at 4 °C.
To prepare virus solution, dilute the virus stock in the appropriate volume of culture medium (2,000 FFU in 100 μl) in a 1.5 ml tube at 4 °C.
-
Mix 100 μl of antiviral substance solution and 100 μl of virus in a 1.5 ml tube at 4 °C.
Note: The final concentrations of antiviral substance are 1,000, 100, 10 and 1 ng/ml.
Vortex gently.
Remove the culture medium from each well.
-
Inoculate 200 μl of virus-antiviral substance mixture to the pre-chilled cells at 4 °C. (MOI = 0.1 to 0.01)
Note: Virus-antiviral substance mixture is inoculated to the pre-chilled cells immediately after the virus is mixed with the antiviral substance.
Incubate for 1 h at 4 °C.
Remove the inoculum and rinse the cells three times with chilled culture medium.
Add culture medium without the antiviral substance (500 μl /well).
Incubate for 24 h at 37 °C in a 5% CO2 incubator.
Day 3
See Day 3 of Procedure A.
-
Blockade of viral penetration (Temperature-shift assay) (see Figure 2)
Day 1
See Day 1 of Procedure A.
Day 2
Place the 24-well plate containing the cells at 4 °C for 1 h.
To prepare virus solution, dilute the virus stock in the appropriate volume of culture medium (2,000 FFU in 100 μl) in a 1.5 ml tube at 4 °C.
Inoculate 200 μl of the virus to the pre-chilled cells at 4 °C (MOI = 0.01 to 0.1).
Incubate for 1 h at 4 °C.
Prepare serial dilutions of an antiviral substance (1,000, 100, 10 and 1 ng/ml) in culture medium at 4 °C.
Remove virus inoculum and rinse the cells three times with chilled culture medium.
Add culture medium containing serial dilutions of the antiviral substance (200 μl/well).
Incubate for 1 h at 37 °C in a 5% CO2 incubator.
Remove culture medium containing the antiviral substance.
-
Remove non-internalized virus by washing with either a citrate buffer or PBS(-).
Note: Some viruses such as HCV, DENV, JEV and West Nile virus penetrate into the cells via a pH-mediated entry pathway and are sensitive to acidic treatment. When virus-inoculated cells are exposed to citrate buffer (pH of 3.0), virus particles bound on the cell surface, but not those that have already penetrated into the cells, are inactivated.
Add culture medium without the antiviral substance (500 μl /well).
Incubate for 24 h at 37 °C in a 5% CO2 incubator.
Day 3
See Day 3 of Procedure A.
Data analysis
-
All experiments should be repeated at least three times independently.
The 50% inhibitory concentration (IC50) for each sample is estimated by four-parameter logistic equation:
[y = D + (A - D)/1 + (x/C) x B;
y = response, x = concentration, A = minimum asymptote, B = slope factor, C = concentration corresponding to the response midway between A and D, and D = maximum asymptote] ( Sebaugh et al., 2011 ). The detailed procedure is described in Bio-protocol “Virucidal and neutralizing activity tests for antiviral substances and antibodies” (Aoki-Utsubo et al., in press).
Alternatively, the data for each sample are plotted in a graph of a sigmoid curve and the IC50 is determined from a graph as described in Bio-protocol “Virucidal and neutralizing activity tests for antiviral substances and antibodies” (Aoki-Utsubo et al., in press).
Notes
-
Based on the immunofluorescence observation results, virus titers (FFU/ml) can be calculated as follows.
Randomly select 10 fields of the glass slip under a microscope and count the number of virus-antigen positive cells for each field.
Calculate the average number of virus-antigen positive cells (called focus).
-
FFU/ml is the average number of the virus-antigen positive cells x area conversion factor x dilution factor x volume factor (ml).
Note: Area conversion factor is based on the area of the field through the microscope against the area of a well in the 24-well plate.
In each assay, prepare positive and negative controls either treated with the antiviral substance or mock-treated. As a positive control, cells can be treated with an inhibitor(s) that blocks a particular step of the virus life cycle (e.g., heparin as an HCV entry inhibitor and cyclosporine as an HCV replication inhibitor). The mock-treated negative control sample is the infected cells treated with DMSO-containing culture medium or diluent.
Percent inhibition of viral infection by test samples can be calculated by comparing with the data obtained with mock-treated control, respectively.
Recipes
-
Culture medium
Dulbecco’s modified Eagle’s medium (DMEM)
1x non-essential amino acids
10% fetal bovine serum (FBS; heat-inactivated at 56 °C for 30 min)
100 U/ml penicillin and streptomycin
-
10x phosphate-buffered saline without Ca2+ and Mg2+ (PBS[-]) and 1x PBS(-)
Dissolve NaCl (80 g), KCl (2 g), Na2HPO4·12H2O (28.8 g) and KH2PO4 (2.4 g) in 800 ml dH2O
Adjust volume to 1 L with dH2O
Autoclave at 121 °C for 20 min
Dilute to 1x with distilled water
-
Citrate buffer (50 mM sodium citrate, 4 mM KCl, pH 3.0)
Prepare 100 mM citrate acid monohydrate (21 g/L: citric acid)
Dissolve sodium citrate dehydrate (1.47 g) and KCl (29.82 mg) in an appropriate volume of dH2O (< 100 ml)
Adjust pH using 100 mM citric acid
Fill-up to 100 ml with dH2O
Autoclave at 121 °C for 20 min and store at room temperature
Acknowledgments
This work was supported in part by grants-in-aid for Research on Viral Hepatitis from the Ministry of Health, Labour and Welfare, Japan, and from the Japan Agency for Medical Research and Development (AMED). This work was also supported in part by a grant-in-aid for Special Research on Dengue Vaccine Development from Tokyo Metropolitan Government. This protocol was adapted from procedures published in Chen et al. (2017). The authors do not have any conflicts of interest or competing interests to declare.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Adianti M., Aoki C., Komoto M., Deng L., Shoji I., Wahyuni T. S., Lusida M. I., Soetjipto, Fuchino H., Kawahara N. and Hotta H.(2014). Anti-hepatitis C virus compounds obtained from Glycyrrhiza uralensis and other Glycyrrhiza species . Microbiol Immunol 58(3): 180-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aoki-Utsubo, C., Chen, M. and Hotta, H. Virucidal and neutralizing activity tests. Bio-protocol.(in press). [DOI] [PMC free article] [PubMed]
- 3. Apriyanto D. R., Aoki C., Hartati S., Hanafi M., Kardono L. B., Arsianti A., Louisa M., Sudiro T. M., Dewi B. E., Sudarmono P., Soebandrio A. and Hotta H.(2016). Anti-hepatitis C virus activity of a crude extract from Longan(Dimocarpus longan Lour.) leaves . Jpn J Infect Dis 69(3): 213-220. [DOI] [PubMed] [Google Scholar]
- 4. Chen M., Aoki-Utsubo C., Kameoka M., Deng L., Terada Y., Kamitani W., Sato K., Koyanagi Y., Hijikata M., Shindo K., Noda T., Kohara M. and Hotta H.(2017). Broad-spectrum antiviral agents: secreted phospholipase A2 targets viral envelope lipid bilayers derived from the endoplasmic reticulum membrane . Sci Rep 7(1): 15931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. El-Bitar A. M., Sarhan M. M., Aoki C., Takahara Y., Komoto M., Deng L., Moustafa M. A. and Hotta H.(2015). Virocidal activity of Egyptian scorpion venoms against hepatitis C virus. Virol J 12: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fenard D., Lambeau G., Valentin E., Lefebvre J. C., Lazdunski M. and Doglio A.(1999). Secreted phospholipases A2, a new class of HIV inhibitors that block virus entry into host cells . J Clin Invest 104(5): 611-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O’Rourke K. P., Dow L. E. and Lowe S. W.(2016). Immunofluorescent staining of mouse intestinal stem cells. Bio-protocol 6(4): e1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ratnoglik S. L., Aoki C., Sudarmono P., Komoto M., Deng L., Shoji I., Fuchino H., Kawahara N. and Hotta H.(2014). Antiviral activity of extracts from Morinda citrifolia leaves and chlorophyll catabolites, pheophorbide a and pyropheophorbide a, against hepatitis C virus. Microbiol Immunol 58(3): 188-194. [DOI] [PubMed] [Google Scholar]
- 9. Scheel T. K. and Rice C. M.(2013). Understanding the hepatitis C virus life cycle paves the way for highly effective therapies. Nat Med 19(7): 837-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sebaugh J. L.(2011). Guidelines for accurate EC50/IC50 estimation . Pharm Stat 10(2): 128-134. [DOI] [PubMed] [Google Scholar]
- 11. Wahyuni T. S., Tumewu L., Permanasari A. A., Apriani E., Adianti M., Rahman A., Widyawaruyanti A., Lusida M. I., Fuad A., Soetjipto Nasronudin, Fuchino H., Kawahara N., Shoji I., Deng L., Aoki C. and Hotta H.(2013). Antiviral activities of Indonesian medicinal plants in the East Java region against hepatitis C virus. Virol J 10: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]