Abstract
Studying lipid metabolism in cultured cells is complicated by the fact that cells are typically cultured in the presence of animal serum, which contains a wide, variable, and undefined variety of lipid species. Lipid metabolism can impact cell physiology, signaling, and proliferation, and the ability to culture cells in the absence of exogenous lipids can reveal the importance of lipid biosynthesis pathways and facilitate the generation of media with defined lipid species. We have adapted a protocol to remove lipids from serum without eliminating its ability to support the proliferation of cells in culture. This method requires di-isopropyl ether and butanol and can be used to generate small batches of lipid-stripped serum in four days. The resulting serum supports proliferation of many cell lines in culture and can be used to compare the metabolism of cells in lipid replete and depleted conditions.
Keywords: Delipidation, Lipid-depleted media, Cell culture conditions, Lipid-free serum, Metabolism, Proliferation
Background
Lipids are the major constituents of cell membranes that delineate biological compartments, and also play an important role in signaling pathways and energy storage ( Baenke et al., 2013 ). Lipid metabolism is dysregulated in a variety of diseases, and recent studies have suggested that disrupting lipid biosynthesis may be an approach to inhibit tumor growth (Svensson and Shaw, 2016). Consequently, there is interest in studying the metabolic pathways that produce cellular lipid species. Culturing cells in media deprived of specific metabolic components can help provide a better understanding of how metabolic pathways support cell function. Standard culture media for most mammalian cells includes animal serum as a source of protein and growth factors (most commonly fetal bovine serum [FBS]). Animal serum contains a variety of lipid species within lipoprotein complexes and bound to serum albumin. The composition of animal serum is not identical across lots, and the nutrients levels in serum are not well defined in most experiments. Cells are able to obtain lipids from both de novo synthesis and from exogenous sources ( Kamphorst et al., 2013 ; Hosios et al., 2016 ; Balaban et al., 2017 ), so the presence of serum lipids can complicate studies of de novo lipid metabolism. For example, although inhibitors of fatty acid synthase can inhibit proliferation, this effect is strengthened in lipid-depleted serum ( Svensson et al., 2016 ). Microenvironments within the body likely vary in their lipid composition (Tourtellotte, 1959; Nanjee et al., 2000 ), suggesting that there are physiological cases where cells may be deprived of lipids.
In addition to serving as a source of lipids, serum also provides growth factors and other components that support the cell proliferation, and most cells cannot be studied for long periods of time in the absence of serum. Serum-free media formulations and lipoprotein-depleted sera are commercially available, but these are often expensive, are not optimized to support the growth of all cell lines, and lack a corresponding lipid-replete serum to serve as a control. To overcome these challenges, we have employed a bi-phasic extraction to remove lipids from FBS without denaturing serum proteins (Cham and Knowles, 1976). This method has been previously described on LipidomicNet, and we modified this approach to generate lipid-depleted serum and a corresponding lipid-replete serum from the same original FBS lot ( Hosios et al., 2016 ). In our hands, some cells can be cultured in this serum for several passages without a substantial change in their proliferation rate, while other cells are more sensitive to the absence of lipids. We have also demonstrated increased de novo lipid synthesis in cells cultured with this lipid-depleted serum, indicating the expected metabolic response to this condition. This protocol provides an efficient way to remove lipids from a range of volumes of FBS, and generates both lipid-depleted serum and dialyzed, lipid-replete control serum for use in cell culture experiments.
Materials and Reagents
50 ml conical tubes (e.g., Corning, catalog number: 430829)
0.2 µm low-protein binding filters (e.g., Thermo Fisher Scientific, catalog number: 566-0020)
Slide-A-Lyzer dialysis cassettes (ThermoFisher), molecular weight cut-off ≤ 10 kDa
Plastic wrap (e.g., Saran wrap)
Glass serological pipettes
Fetal bovine serum (FBS)
Di-isopropyl ether (Sigma-Aldrich, catalog number: 296856)
N-Butanol (Sigma-Aldrich, catalog number: 34867)
Sodium chloride (Sigma-Aldrich, catalog number: 793566)
Protein concentration assay (e.g., Bio-Rad Protein Assay, Bio-Rad Laboratories, catalog number: 5000006)
Cholesterol assay (e.g., Sigma-Aldrich, catalog number: MAK043)
Triglycerides assay (e.g., Thermo Fisher Scientific, catalog number: TR22421)
Tissue culture media (e.g., DMEM and RPMI)
Saline solution at 4 °C (9 g/L sodium chloride, 154 mM) (see Recipes)
Equipment
Pipettes
Glass beakers
Glass graduated cylinders
Separating funnel (if preparing large volumes of serum)
Magnetic stir-plate
Chemical fume hood
Biological safety cabinet (level 2)
Tabletop centrifuge (capable of spinning 50 ml conical tubes at 4,000 × g)
Nitrogen gas source (industrial grade)
Spectrophotometer capable of measuring absorbance at the wavelength appropriate to the protein assay (e.g., 595 nm for Bio-Rad Protein Assay)
Procedure
Note: All steps involving open tubes or bottles containing di-isopropyl ether should be conducted in a chemical fume hood.
Thaw fetal bovine serum (FBS) at 4 °C. We have carried out this protocol with volumes of serum ranging from 50 to 500 ml. Thaw two aliquots: one for de-lipidation and one to use as a lipid-replete control. Reserve the serum at 4 °C to be used as the lipid-replete control until Step 8. De-lipidation is accomplished in Steps 2-7.
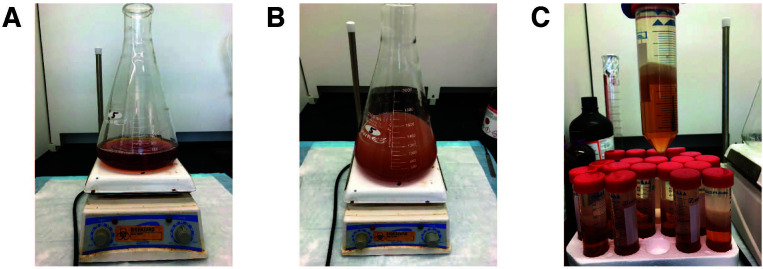
Stir thawed FBS at room temperature on a magnetic stir plate in a beaker able to accommodate at least twice the volume of FBS used. Add 0.8 vol di-isopropyl ether and 0.2 vol n-butanol and continue stirring for 30 min at room temperature (e.g., for 100 ml of serum, use 80 ml of di-isopropyl ether, and 20 ml of n-butanol). Separate phases should not be observed at this point (Figures 1A and 1B). If the phases do separate, increase the stirring speed (300-400 rpm is typically sufficient). Solvents should be measured with glass graduated cylinders or glass serological pipettes.
Centrifuge at 4,000 × g for 15 min at 4 °C to separate the phases. The mixture can be aliquoted into 50 ml conical tubes during this step (Figure 1C).
Using a glass pipette, push through the viscous upper phase and interphase, and transfer the lower phase into a fresh beaker. Discard the upper phase. Care should be taken to minimize the amount of upper phase that is carried over, even if this reduces the yield of the lower phase.
Mix the lower phase with a volume of di-isopropyl ether equal to the original volume of serum used. Stir on a magnetic plate for 30 min at room temperature. As before, separate phases should not be visible during mixing.
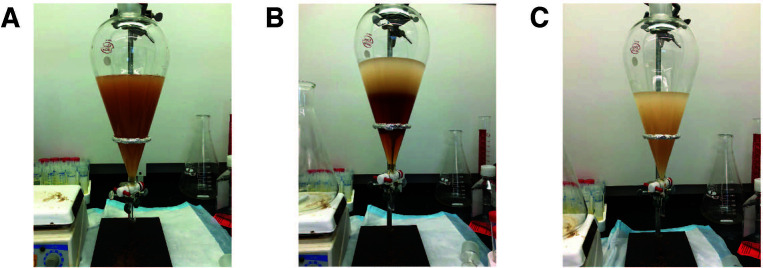
If a small volume of FBS was used, centrifuge as before. Remove the upper phase with a pipette, and collect the lower phase. If a larger volume of FBS was used, transfer the mixture to a separating funnel, and allow the phases to separate (Figures 2A and 2B). If a separating funnel is not available, the mixture can be aliquoted into conical tubes and centrifuged as above. Remove upper phase completely, using either the separating funnel or, if centrifuging, by pipetting and discarding the upper phase (Figure 2C).
Transfer the lower phase to a new beaker. Stir on a magnetic plate at a low speed while allowing a slow stream of nitrogen gas to blow over the surface of the serum. The flow rate should be low, so as to prevent significant evaporation of water from the serum, while allowing volatile organics to evaporate. Mixing for 2 h at room temperature is sufficient to remove most contaminating solvents. Minor water evaporation (which would concentrate the protein) will be corrected in Step 9.
-
Removal of any remaining solvent contaminants by dialysis
Pre-wet appropriately sized dialysis cassettes in cold saline, and transfer both the lipid-stripped (from Step 7) and lipid-replete (from Step 1) sera to the cassettes.
Dialyze overnight against 4 L of saline (9 g/L sodium chloride) at 4 °C, gently stirring the mixture in one beaker covered with plastic wrap.
The next day, transfer cassettes to a new 4 L of saline, and repeat. Dialyze in fresh saline for a third day. It is important to dialyze both the lipid-stripped and lipid-replete sera in the same beaker. If dialyzing more than 400 ml total, use a larger volume of saline.
Measure the concentration of protein in both sera (e.g., by Bradford or BCA Assay). It may be necessary to dilute the sera so that the protein concentrations are in the linear range of the assay method used. If the concentrations are unequal (owing to volume loss, particularly in Step 7), dilute the more concentrated serum with saline to ensure that their concentrations are equal.
Sterilize the sera by filtering them through a 0.2 µm filter in a biological safety cabinet.
Aliquot, and store at -20 °C. Both sera can be used in place of normal FBS for standard cell culture assays.
Figure 1. Initial lipid extraction from fetal bovine serum (FBS).
(Steps 2-3). A. FBS before extraction. B. FBS mixing with di-isopropyl ether and n-butanol. Mixing speed should be sufficient to prevent phase separation. C. Following initial centrifugation, lipids are in the upper phase and interphase, while serum proteins are retained in the aqueous, lower phase.
Figure 2. Removal of serum proteins using a separating funnel (Step 6) following the second organic extraction.
A. FBS mixed with di-isopropyl ether in Step 5 is transferred to a separating funnel. B. Separate phases form after the mixture is allowed to stand. C. The lower phase is removed and transferred to a new beaker for the subsequent steps. The organic, upper phase is retained in the separating funnel and then discarded.
Data analysis
A variety of approaches can be used to confirm the success of this method. We have measured the abundance of individual lipid species in serum by gas chromatography coupled to mass spectrometry (GC/MS) to analyze fatty acid methyl ester (FAME) derivatives of Folch extracts (2:1 chloroform:methanol) from the serum ( Hosios et al., 2016 ). A variety of commercial enzymatic assays may also be used to confirm depletion of specific lipid classes. These include assays for cholesterol or triglycerides. We have also demonstrated that de novo lipogenesis is increased in cells cultured with lipid-depleted serum, which is the expected response to lipid deprivation ( Hosios et al., 2016 ). This can be measured by incorporation of carbon-14 acetate into Folch-extractable cellular material.
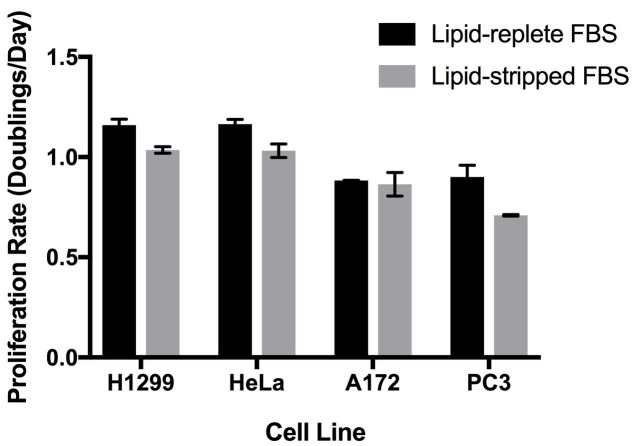
We have cultured a variety of cell lines in DMEM and RPMI media containing 10% (by volume) dialyzed de-lipidated or lipid-replete serum ( Hosios et al., 2016 ). Some cell lines proliferate similarly in both sera and can be maintained for several passages in lipid-free conditions, but other cell lines proliferate more slowly with lipid-depleted serum (Figure 3). To ensure that any reduction in proliferation rate observed is the result of lipid starvation rather than incomplete removal of the organic chemicals, we recommend returning individual lipid species or a mixture of lipids to the serum to demonstrate rescue of cell proliferation. We have had success with bovine serum albumin conjugated to palmitate ( Oliveira et al., 2015 ) as well as commercially available lipid supplements. Importantly, serum contains a large number of lipid species, and the growth of individual cell lines may be rescued by supplementation of de-lipidated serum with different lipid species. We have also used a water-soluble cholesterol conjugate to supplement this lipid to cells in culture.
Figure 3. Proliferation rates of cell lines in culture medium containing lipid-replete or lipid-stripped FBS.
Cells were seeded sparsely one day before washing with PBS and exposure to the experimental media. Cell counts were obtained at that point and four days later, and these values were used to calculate the proliferation rate of the cells by fitting to an exponential growth equation. Each bar represents the average of n = 3 replicates ± standard deviation.
Notes
Use the same lot of serum to produce both the dialyzed lipid-replete serum and the dialyzed lipid-depleted serum. The resulting batches of sera are used in experiments when the comparison between conditions with and without lipids is necessary. Ideally, lipid-depleted and replete sera produced at the same time should be used for experiments comparing the two conditions.
Di-isopropyl ether is volatile and should be used in a chemical fume hood.
We prefer to dialyze the sera against saline, rather than phosphate buffered saline, to avoid substantially increasing the concentration of phosphate when the sera are added to cell culture media.
Recipes
-
Cold saline solution
Prepare a 5 M sodium chloride solution by dissolving 292.2 g of sodium chloride in 700 ml of deionized water. The solution may need to be warmed while mixing on a magnetic stir plate. Bring the volume up to 1 L with additional water. This solution can be stored at room temperature.
Mix 123.2 ml of 5 M sodium chloride with 3876.8 ml of deionized water. Store in a beaker covered with plastic wrap at 4 °C for one day to cool before use.
Acknowledgments
This protocol has been adapted from one published on LipidomicNet by the Thiele Lab. The authors acknowledge support by grants from the National Cancer Institute, the Lustgarten Foundation, and Stand Up To Cancer. M.G.V.H. is supported in part by a faculty scholar award from the Howard Hughes Medical Institute. We are also indebted to members of the Vander Heiden lab for helpful discussions about the use of de-lipidated serum to study lipid metabolism.
The authors are not aware of any conflict of interest in writing this protocol.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Baenke F., Peck B., Miess H. and Schulze A.(2013). Hooked on fat: the role of lipid synthesis in cancer metabolism and tumour development. Dis Model Mech 6(6): 1353-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balaban S., Shearer R. F., Lee L. S., van Geldermalsen M., Schreuder M., Shtein H. C., Cairns R., Thomas K. C., Fazakerley D. J., Grewal T., Holst J., Saunders D. N. and Hoy A. J.(2017). Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab 5: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cham B. E. and Knowles B. R.(1976). A solvent system for delipidation of plasma or serum without protein precipitation. J Lipid Res 17(2): 176-181. [PubMed] [Google Scholar]
- 4. Hosios A. M., Hecht V. C., Danai L. V., Johnson M. O., Rathmell J. C., Steinhauser M. L., Manalis S. R. and Vander Heiden M. G.(2016). Amino acids rather than glucose account for the majority of cell mass in proliferating mammalian cells. Dev Cell 36(5): 540-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kamphorst J. J., Cross J. R., Fan J., de Stanchina E., Mathew R., White E. P., Thompson C. B. and Rabinowitz J. D.(2013). Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc Natl Acad Sci U S A 110(22): 8882-8887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nanjee M. N., Cooke C. J., Olszewski W. L. and Miller N. E.(2000). Lipid and apolipoprotein concentrations in prenodal leg lymph of fasted humans. Associations with plasma concentrations in normal subjects, lipoprotein lipase deficiency, and LCAT deficiency. J Lipid Res 41(8): 1317-1327. [PubMed] [Google Scholar]
- 7. Oliveira A. F., Cunha D. A., Ladriere L., Igoillo-Esteve M., Bugliani M., Marchetti P. and Cnop M.(2015). In vitro use of free fatty acids bound to albumin: A comparison of protocols . Biotechniques 58(5): 228-233. [DOI] [PubMed] [Google Scholar]
- 8. Svensson R. U. and Shaw R. J.(2016). Lipid synthesis is a metabolic liability of non-small cell lung cancer. Cold Spring Harb Symp Quant Biol 81: 93-103. [DOI] [PubMed] [Google Scholar]
- 9. Svensson R. U., Parker S. J., Eichner L. J., Kolar M. J., Wallace M., Brun S. N., Lombardo P. S., Van Nostrand J. L., Hutchins A., Vera L., Gerken L., Greenwood J., Bhat S., Harriman G., Westlin W. F., Harwood H. J. Jr. Saghatelian A., Kapeller R., Metallo C. M. and Shaw R. J.(2016). Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumor growth of non-small-cell lung cancer in preclinical models. Nat Med 22(10): 1108-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tourtellotte W. W.(1959). Study of lipids in cerebrospinal fluid. VI. The normal lipid profile. Neurology 9(6): 375-383. [DOI] [PubMed] [Google Scholar]