Abstract
Lipid peroxidation is a physiological indicator of both biotic and abiotic stress responses, hence is often used as a biomarker to assess stress-induced cell damage or death. Here we demonstrate an easy, quick and cheap staining method to assess lipid peroxidation in plant tissues. In this methodology, Schiff’s reagent, is used to assay for membrane degradation. Histochemical detection of lipid peroxidation is performed in this protocol. In brief, Schiff’s reagent detects aldehydes that originate from lipid peroxides in stressful condition. Schiff’s reagent is prepared and applied to plants tissue. After the reaction, plant tissue samples are rinsed with a sulfite solution to retain the staining color. From this analysis, qualitative visualization of lipid peroxidation in plant tissue is observed in the form of magenta coloration. This reagent is useful for visualization of stress induced lipid peroxidation in plants. In this protocol, Indica rice root, Assam tea root and Indian mustard seedlings are used for demonstration.
Keywords: Schiff’s reagent , Rice , Tea , Mustard , Membrane degradation , Lipid peroxidation
Background
Oxidative stress induced by both biotic and abiotic stress factors leads to lipid peroxidation. The peroxidation of unsaturated lipids in membrane is the most apparent symptom of oxidative stress. Lipid peroxidation is a deleterious process in plants, which affects membrane properties, causes protein degradation and limits the capacity of ionic transport, ultimately triggering the cell death process ( Yamamoto et al., 2001 ). Malondialdehyde (MDA) content, which is a byproduct of lipid peroxidation process was found to be enhanced in rice ( Ma et al., 2012 ; Awasthi et al., 2017 ), Salvinia ( Mandal et al., 2013 ), pea ( Yamamoto et al., 2001 ), Soyabean (Cakmak and Horst, 1991; Du et al., 2010 ), Indian mustard ( Saha et al., 2016 ) on exposure to stress. The reactive oxygen species (ROS) associated with oxidative stress acts on membrane lipids to decrease membrane stability. Thus making the study of lipid peroxidation an important parameter and our protocol offers a very rapid method for the study. Schiff’s reagent reacts with aldehyde functional group of MDA to give the magenta coloration, thus acting as a key determinant of lipid peroxidation in plant tissues.
Materials and Reagents
Glass Petri plates of 10 cm diameter (Scott Duran)
Absorbent cotton
Whatman 1 filter paper
Razor blade (Glassvan, No. 23, Scalpel blade)
15 ml disposable centrifuge tubes (Tarsons)
Slide
Needle (Dispovan, sterile needle, 0.45 x 13 mm)
Brush (Camel, Size 0)
Gas mask
Plant tissue (Leaf, root or whole seedling) collected freshly after stress treatment
Distilled water
Mercuric chloride (HgCl2) (SRL Sisco Research Laboratories, catalog number: 25699)
Schiff’s reagents (HiMedia Laboratories, catalog number: S074-500ML)
Calcium nitrate tetrahydrate (Ca(NO3)2·4H2O) (HiMedia Laboratories, catalog number: GRM3903-500G)
Potassium nitrate (KNO3) (HiMedia Laboratories, catalog number: GRM1401-500G)
Potassium phosphate monobasic (KH2PO4) (HiMedia Laboratories, catalog number: RM3943-500G)
Magnesium sulfate heptahydrate (MgSO4·7H2O) (HiMedia Laboratories, catalog number: RM684-5KG)
Boric acid (H3BO3) (HiMedia Laboratories, catalog number: MB007-1KG)
Manganese(II) chloride tetrahydrate (MnCl2·4H2O) (HiMedia Laboratories, catalog number: RM685-500G)
Zinc sulfate heptahydrate (ZnSO4·7H2O) (HiMedia Laboratories, catalog number: PCT0118-1KG)
Sodium molybdate dihydrate (Na2MoO4·2H2O) (HiMedia Laboratories, catalog number: GRM415-100G)
Copper(II) sulfate pentahydrate (CuSO4·5H2O) (HiMedia Laboratories, catalog number: RM630-500G)
Ferric chloride (FeCl3) (HiMedia Laboratories, catalog number: RM1178-1KG)
EDTA (HiMedia Laboratories, catalog number: RM678-100G)
Ammonium sulfate ((NH4)2SO4) (HiMedia Laboratories, catalog number: MB004-250G)
Potassium sulfate (K2SO4) (HiMedia Laboratories, catalog number: GRM404-500G)
Manganese sulfate monohydrate (MnSO4·H2O) (SRL Sisco Research Laboratories, catalog number: 1347151, 500G)
Sodium Meta-bisulphate (Na2S2O5) (Sigma-Aldrich, catalog number: 255556-100G)
Calcium chloride dihydrate (CaCl2·2H2O) (HiMedia Laboratories, catalog number: PCT0004-500G)
Aluminum chloride (AlCl3) (Merck, catalog number: 8010810100)
Cadmium chloride (CdCl2) (Thermo Fisher Scientific, catalog number: Q17584)
Zinc chloride (ZnCl2) (Thermo Fisher Scientific, catalog number: Q28785)
Ethanol (Diluent for DNA Extraction, HiMedia Laboratories, catalog number: MB228-500ML)
Glycerol (Sigma-Aldrich, catalog number: V800196-500ML)
Acetic acid (Thermo Fisher Scientific, catalog number: Q21057)
Hydrochloric acid (HCl) (HiMedia Laboratories, catalog number: AS004-2.5L)
Schiff’s reagent staining solution (see Recipe 1)
Hoagland solution (see Recipe 2)
Modified nutrient solution (see Recipe 3)
Treatment solutions (AlCl3, CdCl2, ZnCl2) (see Recipe 4)
Sulfite solution (see Recipe 5)
Bleaching solution (see Recipe 6)
Storage solution (see Recipe 7)
Equipment
Pipettes (Gilson, PIPETMAN, 2-20,20-200 and 100-1,000 µl)
pH meter (pH Tutor, Eutech Instruments)
Magnetic stirrer cum hot plate (Tarsons)
Weighing balance (Sartorius, 0.1 mg-220 g)
Water bath (Equitron unstirred water bath, Medica Instruments)
Light microscope (Olympus, 10x objective)
Digital camera (Nikon, model: COOLPIX P100, 10.3 megapixel, 26x zoom)
Procedure
Prepare Schiff’s reagent solution (Recipe 1).
-
Grow plants hydroponically.
-
Indica rice: Germinate surface sterilized (with 0.01% mercuric chloride) rice seeds (undehusked) in Petri plates with cotton bed (absorbent cotton covered with Whatman 1 filter paper) soaked in Hoagland nutrient solution (Recipe 2) at 28 ± 2 °C in dark condition. Grow the germinated seedlings hydroponically on Hoagland nutrient solution (Recipe 2) at 26 ± 2 °C over a photoperiod of 14 h with a photon flux density of 220 μmol m-2 sec-1 (PAR) for 5 days and then pretreat with 500 µM CaCl2 solution for 24 h. Expose the pretreated seedlings to 100 µM AlCl3 solution (dilute from the stock solution, Recipe 4) containing 500 µM CaCl2 at pH 4.5 for 48 h and harvest root tips for histochemical assay.
Note: Because of toxic nature of mercuric chloride (HgCl2), use of precautionary clothing is highly recommended.
Assam tea: Plant the cuttings procured from local tea gardens, after pulling out the polythene sleeves and then acclimatize for 30-35 days in soil at 26 ± 2 °C over a photoperiod of 14 h with photon flux density of 220 μmol m -2 sec-1 (PAR). After acclimatization, grow tea plantlets hydroponically in pots with the nutrient solution (Recipe 3). Pre-culture the plants for a week in the nutrient solution (unsterile) before the application of Al3+ in the form of AlCl3, at a concentration of 250 µM. The pH is maintained at 4.2. After 3 days, harvest the root segments for histochemical assay.
-
Indian mustard: Surface sterilize healthy seeds of Brassica juncea (L.) Czern & Coss with 0.01% HgCl2 and set for germination. Impart 1 mM CdCl2 and 2.5 mM ZnCl2 treatments (dilute from the stock solutions, Recipe 4) to seeds by placing in Petri plates with cotton bed soaked in the stress solutions when kept for germination. After 7th day, harvest the seedlings for histochemical study.
Note: Since no carbohydrate source is added to the nutrient solution, so no bacterial contamination occurs.
-
In another set of experiments, keep control samples apart from stress and allow to grow under normal unstressed condition (plants not treated with any of the stress factors).
-
Excise (using a razor blade) root tissues for Indica rice and Assam tea. Transfer root tissues and whole seedlings of Indian mustard to 15 ml centrifuge tubes and add 5 ml of Schiff’s reagent solution to each tube.
Note: Tissues should be completely immersed in the solution.
-
Vacuum infiltrate the tissue samples dipped in Schiff’s reagent for 1-2 h and then rinsed with a sulfite solution for 10 min (Recipe 5).
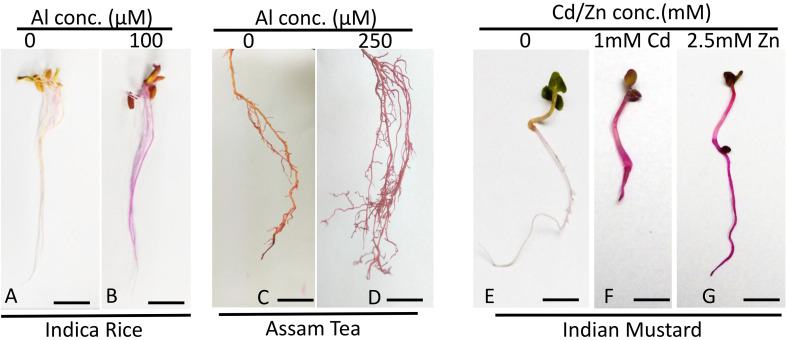
Note: The time for vacuum infiltration varies with tissue and plant age, should be kept until and unless a contrasting visual difference in Magenta coloration is obtained. A representative image that shows the extent of color change indicative of sufficient infiltration has been provided (Figure 1).
-
Wash thoroughly with distilled water to remove the extra stain and then bleach the tissue samples with bleaching solution (Recipe 6) for 5-10 min at 100 °C in a water bath.
Note: Chlorophyll should be completely removed from tissue samples.
-
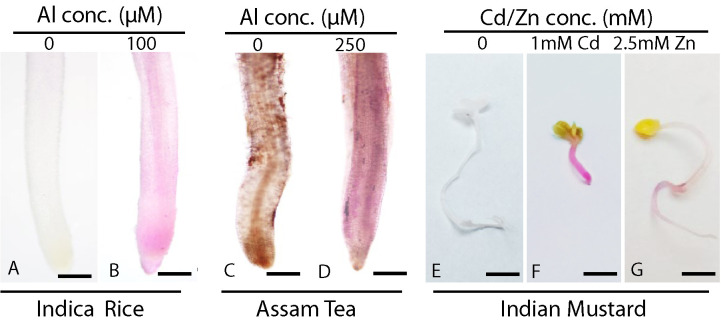
After complete bleaching is achieved, carefully mount samples on slide (for rice and tea roots using needle and brush) and take photographs under a microscope at 10x magnification (Figure 2). Whereas for mustard, take direct photographs with a digital camera for seedlings (Figure 2). If required, store the tissue samples by completely dipping in storage solution at 4 °C (Recipe 7) for future photography sessions.
Note: Root in Figure 2C looks brownish because of various phenolic compounds secreted by tea plant.
Figure 1. Representative image that shows the color change indicative of sufficient infiltration/treatment.
Schiff’s reagent in Indica rice root (A) control, (B) stressed, scale bar = 1 cm; Assam tea roots (C) control, scale bar = 1 cm, (D) stressed, scale bar = 1 cm; and in Indian mustard seedling (E) control, scale bar = 1 cm, (F) 1 mM Cd treated seedling, scale bar = 1 cm, (G) 2.5 mM Zn treated seedling, scale bar = 1 cm.
Figure 2. Histochemical determination of lipid peroxidation in different plant species by Schiff’s reagent.
Detection of lipid peroxidation using Schiff’s reagents in Indica rice root (A) control, scale bar = 100 μm; (B) stressed, scale bar = 100 μm; Assam tea roots (C) control, scale bar = 100 μm, (D) stressed, scale bar = 100 μm; and in Indian mustard seedling (E) control, scale bar = 5 mm, (F) 1 mM Cd treated seedling, scale bar = 5 mm, (G) 2.5 mM Zn treated seedling, scale bar = 5 mm.
Data analysis
Since this is a qualitative assay, no data analysis is performed. The stained plant tissue samples are simply documented by taking photographs with a digital camera. A similar analysis is presented in the following paper: Awasthi et al. (2017) .
Recipes
-
Schiff’s reagent staining solution (Jensen, 1962)
Prepare 10% (v/v) Schiff’s reagent solution in distilled water and mixed well
Note: Schiff’s reagent commercially available is used. Schiff’s reagent staining solution is prepared freshly each time.
-
Hoagland solution (Table 1)
Prepare stock solutions separately and mix in appropriate proportions to obtain working solution. Finally adjust the pH to 6.0-6.2 with 1 N NaOH
-
Modified nutrient solution ( Ghanati et al., 2005 ) (Table 2)
Prepare stock solutions separately and mix in appropriate proportions to obtain a working solution. Finally adequate nutrient solution and adjust the pH to 6.0-6.2 with 1 N NaOH
-
Treatment solutions
AlCl3, CdCl2, ZnCl2: Prepare 10 mM stock and store separately at 4 °C for future use
Note: When preparing AlCl3 solution noxious fumes of HCl are formed. So it should be prepared with proper precaution like in a fume hood, use of gas mask etc. CdCl2 is highly carcinogenic, so use of precautionary clothing is highly recommended.
-
Sulfite solution
0.5% (w/v) Na2S2O5 in 0.05 M HCl
-
Bleaching solution
Acetic acid:glycerol:ethanol (1:1:3, v/v/v) solution
-
Storage solution
Glycerol:ethanol (1:4, v/v) solution
Table 1. Composition of Hoagland solution .
| Stock | Chemical constituents | Stock preparation | Required concentration | Required volume for the preparation of 1 L for full strength |
|---|---|---|---|---|
| A | Ca(NO3)·4H2O | 826.35 g/L | 3.5 M | 2 ml |
| B | KNO3 | 252.75 g/L | 2.5 M | 2 ml |
| C | KH2PO4 | 136.1 g/L | 1 M | 2 ml |
| D | MgSO4·7H2O | 246.5 g/L | 1 M | 2 ml |
| E |
H3BO3 MnCl2·4H2O ZnSO4·7H2O NaMoO4 CuSO4·5H2O |
2.8 g/L 1.8 g/L 0.2 g/L 0.025 g/L 0.1 g/L |
45.2 mM 9.09 mM 695.5 µM 171.1 µM 400 µM |
2 ml |
| F |
FeCl3 EDTA |
5 g/L 5 g/L |
30.82 mM 17.09 mM |
2 ml |
Table 2. Composition of modified nutrient solution .
| Stock | Chemical constituents | Stock preparation | Required concentration | Required volume for the preparation of 1 L for full strength |
|---|---|---|---|---|
| A | (NH4)2SO4 | 0.094 g/L | 0.713 mM | 1 ml |
| B | KNO3 | 0.074 g/L | 0.73 mM | 1 ml |
| C | KH2PO4 | 0.063 g/L | 0.46 mM | 1 ml |
| D | MgSO4·7H2O | 0.101 g/L | 0.41 mM | 1 ml |
| E |
H3BO3 CaCl2 ZnSO4·7H2O NaMoO4 CuSO4·5H2O |
0.003 g/L 0.055 g/L 0.003 g/L 0.001 g/L 0.001 g/L |
0.046 mM 0.5 mM 0.0091 mM 0.0026 mM 0.003 mM |
1 ml |
| F |
FeCl3 EDTA |
4.99 g/L 4.99 g/L |
30.82 mM 17.09 mM |
1 ml |
| G | K2SO4 | 0.008 g/L | 0.46 mM | 1 ml |
| H | MnSO4 | 0.014 g/L | 0.09 mM | 1 ml |
Acknowledgments
This protocol is adapted from Pompella et al. (1987) and Yamamoto et al. (2001). We sincerely acknowledge the financial support from UGC, India (UGC NON-NET fellowship). Authors declare no conflicts of interest or competing interests.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Awasthi J. P., Saha B., Regon P., Sahoo S., Chowra U., Pradhan A., Roy A. and Panda S. K.(2017). Morpho-physiological analysis of tolerance to aluminum toxicity in rice varieties of North East India. PLoS One 12(4): e0176357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cakmak I. and Horst W. J.(1991). Effect of aluminum on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean(Glycine max) . Physiologia Plantarum 83: 463-468. [Google Scholar]
- 3. Du B., Nian H., Zhang Z. and Yang C.(2010). Effects of aluminum on superoxide dismutase and peroxidase activities, and lipid peroxidation in the roots and calluses of soybeans differing in aluminum tolerance. Acta Physiologiae Plantarum 32: 883-890. [Google Scholar]
- 4. Ghanati F., Morita A. and Yokota H.(2005). Effects of aluminum on the growth of tea plant and activation of antioxidant system. Plant Soil 276: 133-141. [Google Scholar]
- 5. Jensen W. A.(1962). Botanical Histochemistry: Principles and Practice. W. H. Freeman and Company, San Francisco. [Google Scholar]
- 6. Ma B., Gao L., Zhang H., Cui J. and Shen Z.(2012). Aluminum-induced oxidative stress and changes in antioxidant defenses in the roots of rice varieties differing in Al tolerance. Plant Cell Rep 31(4): 687-696. [DOI] [PubMed] [Google Scholar]
- 7. Mandal C., Ghosh N., Maiti S., Das K., Gupta S., Dey N. and Adak M. K.(2013). Antioxidative responses of Salvinia(Salvinia natans Linn.) to aluminium stress and it's modulation by polyamine . Physiol Mol Biol Plants 19(1): 91-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pompella A., Maellaro E., Casini A. F. and Comporti M.(1987). Histochemical detection of lipid peroxidation in the liver of bromobenzene-poisoned mice. Am J Pathol 129(2): 295-301. [PMC free article] [PubMed] [Google Scholar]
- 9. Saha B., Mishra S., Awasthi J. P., Sahoo L., and Panda S. K.(2016). Enhanced drought and salinity tolerance in transgenic mustard[Brassica juncea(L.) Czern& Coss.] overexpressing Arabidopsis group 4 late embryogenesis abundant gene(AtLEA4-1) . Environ Exp Bot 128: 99-111. [Google Scholar]
- 10. Yamamoto Y., Kobayashi Y. and Matsumoto H.(2001). Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol 125(1): 199-208. [DOI] [PMC free article] [PubMed] [Google Scholar]