Abstract
The Rapid Alkalinization Factor (RALF) is a plant hormone peptide that inhibits proton transport causing alkalinization of the extracellular media. To detect the alkalinization response elicited by RALF peptides in root cells, Arabidopsis seedlings are carefully transferred to a gel containing the pH-sensitive indicator bromocresol purple, treated with the peptide and photographed after 30 min. Herein the protocol is optimized for evaluation of exogenous treatment, described in detail and expected results are presented.
Keywords: pH indicator, Alkalinization, RALF, Bromocresol purple
Background
Proton transport is induced by a myriad of signals and is used by plants to coordinate growth, defense and development. Some plant hormone peptides can affect proton transport, causing a strong alkalinization of the extracellular medium (Felix and Boller, 1995; Pearce et al., 2001a ). The 5 kDa peptide hormone Rapid Alkalinization Factor (RALF), after being secreted, binds to its receptor FERONIA, and causes the phosphorylation of the plasma membrane H+-Adenosine triphosphatase 2, inhibiting proton transport and alkalinizing the extracellular media ( Pearce et al., 2001b ; Haruta et al., 2014 ).
Growth media containing the pH indicator bromocresol purple is an effective method to visualize alkalinization or acidification in the media around the roots. This method has been used previously to show that NaRALF is required for regulating root hair extracellular pH ( Wu et al., 2007 ), and that roots of plants overexpressing AtRALF23 have reduced capacity to acidify the rhizosphere ( Srivastava et al., 2009 ). Growth medium with the pH indicator bromocresol purple was also used by Masachis et al. (2016) to demonstrate that RALF homologs produced by fungal pathogens induced alkalinization of media around the roots of tomato plants.
We have optimized the pH indicator bromocresol purple protocol and using the improved protocol we were able to visualize the alkalinization effect around Arabidopsis roots after AtRALF1 treatment in wild type and mutant seedlings ( Dressano et al., 2017 ). Here we demonstrate how this assay can provide qualitative information on the extracellular pH that surrounds Arabidopsis roots.
Materials and Reagents
-
Biological material
Arabidopsis thaliana seeds and seedlings (Ecotype Columbia, Col-0)
In-house produced 6xHis AtRALF1 recombinant peptide
-
Chemicals and materials for seed sterilization and growth
Square Petri dish with Grid 100 mm W x 15 mm H, sterile (Electron Microscopy Sciences, catalog number: 70691)
Clear plastic wrap
Graduated cylinder 1,000, 100 and 10 ml (Uniglas)
1,000 µl filter tips (NEST Scientific, catalog number: NPT1000-B-B)
200 µl filter tips (NEST Scientific, catalog number: NPT0200-B-Y)
10 µl filter tips (NEST Scientific, catalog number: 301001)
Gellan Gum Powder (Culture Gel TM Type I- BioTech Grade) (PhytoTechnology Laboratories, catalog number: G434)
Murashige & Skoog Basal Salt Mixture (PhytoTechnology Laboratories, catalog number: M524)
Sterile distilled water
Sodium hypochlorite solution
Sodium hypochlorite (NaClO) solution 50% (v/v) (see Recipes)
-
Chemicals and materials for gel containing the pH-sensitive indicator bromocresol purple
Graduated cylinder 100 ml (Uniglas)
Petri dish 150 x 20 mm sterile
Bromocresol purple free acid reagent Grade (C2H16Br2O5S, AMRESCO, catalog number: 0531-25G)
Calcium sulfate dihydrate (CaSO4·2H2O, Merck, catalog number: 102161)
Potassium hydroxide (KOH)
Hydrochloric acid (HCl)
Agarose RA (Biotechnology Grade, AMRESCO, catalog number: N605-500G)
Sterile distilled water
Equipment
Weighing balance (RADWAG Wagi Elektroniczne, model: AS 220/C/2)
Magnetic stirrer (Fisher Scientific)
pH meter (Thermo Orion, PerpHecT LongR meter, model: 350)
Pipettes (Nichipet EX, P1000, P200, P10)
Beaker 1,000 ml (PHOX, Boro 3.3)
Beaker 250 ml (APRX, Boro 3.3)
Autoclave (Sercon, model: HS 1-0101)
Laminar flow hood (Pachane, model: PA 440)
Growth chamber/Controlled environment room
Erlenmeyer (PIREX)
Microwave (BRASTEMP, catalog number: BMJ38ARANA)
Tweezers
Digital camera (Cyber-shot, Sony, model: DSC-H300)
Procedure
-
Seed sterilization and germination
To analyze the root media alkalinization, seedlings were grown on plates containing half-strength MS medium (see Recipes) for 9 days.
Surface sterilize A. thaliana seeds in Eppendorf tubes using 1 ml sodium hypochlorite solution 50% (v/v) for 10 min followed by 5 washes using 1 ml sterile Milli-Q water each. Seeds are washed with the aid of a micropipette (1,000 µl) (Figures 1A).
Stratify the sterilized seeds for 72 h at 4 °C in water and then drop in a line using a micropipette (10 µl) on a square plate dish containing half-strength MS medium (Figures 1B-1D).
Seal the plates containing the seeds with clear plastic wrap.
Incubate the plates vertically in a controlled environmental growth chamber at 22 ± 2 °C, 16 h light (150 μmol m-2 sec-1) and 8 h dark for 9 days (Figure 1E).
-
Gel containing the pH-sensitive indicator bromocresol purple
-
Prepare gel containing the pH-sensitive indicator bromocresol purple
Add 0.006% (w/v) of bromocresol purple free acid reagent grade (60 g L-1); 1 mM of CaSO4 (172 g L-1) in sterile distilled water. Adjust pH to 5.7 using KOH or HCl. Pour the solution into an Erlenmeyer.
Add agarose (15 g L-1) into the Erlenmeyer and heat in the microwave until completely dissolved.
Pour the melted gel into Petri dishes (150 x 20 mm), 50 ml per dish (approx. 0.4 cm thick), with the aid of a graduated cylinder (100 ml). The agarose gel is allowed to cool until solidified. (Figure 2).
Gently transfer groups of seedlings (6-8) at once onto each Petri dish containing the pH indicator bromocresol purple (pH 5.7) with tweezers (Figures 3A-3B; see Video 1).
Once placed in the gel pH-sensitive indicator bromocresol purple, only the roots of the seedling are treated with 10 µl AtRALF1 (10 μM solution) or water (10 µl as a control) (see Video 2).
Capture images with a digital camera (Sony, Cyber-shot) 30 min after treatment.
-
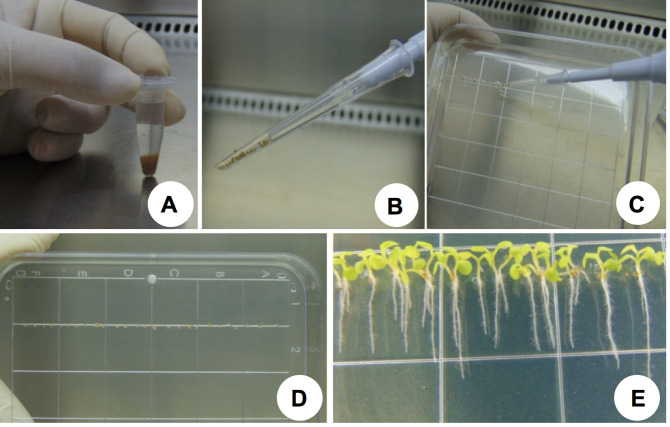
Figure 1. Seed sterilization and seedling growth.
A. A. thaliana seeds during the surface sterilization procedure with sodium hypochlorite solution 50% (v/v); B. Pipette tip (which has been cut and then autoclaved) containing stratified seeds; C. Stratified seeds being disposed in half-strength MS medium; D. Seeds aligned on a square Petri dish containing half-strength MS medium; F. Arabidopsis seedlings (9 days old) grown in the growth chamber.
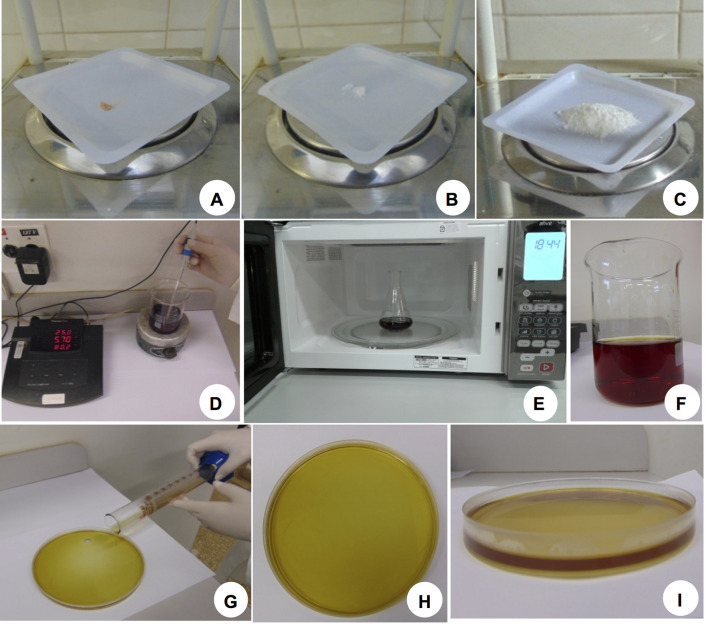
Figure 2. pH-Sensitive indicator bromocresol purple in gel.
A. Bromocresol purple; B. Calcium sulfate (CaSO4); C. Agarose; D. Bromocresol purple solution in sterile distilled water pH 5.7; E. The solution is heated in a microwave until agarose is completely dissolved; F. Gel containing bromocresol purple completely dissolved and ready to be poured. G. Gel being poured in a Petri dish. H. Plate containing the solidified gel and ready to receive the seedlings (top view). I. Lateral view of the plate shown in H. Gel should be about 0.4 cm thick and a dark yellow color is expected to allow detection of pH differences.
Figure 3. Evaluation of root pH change on a gel containing bromocresol purple.
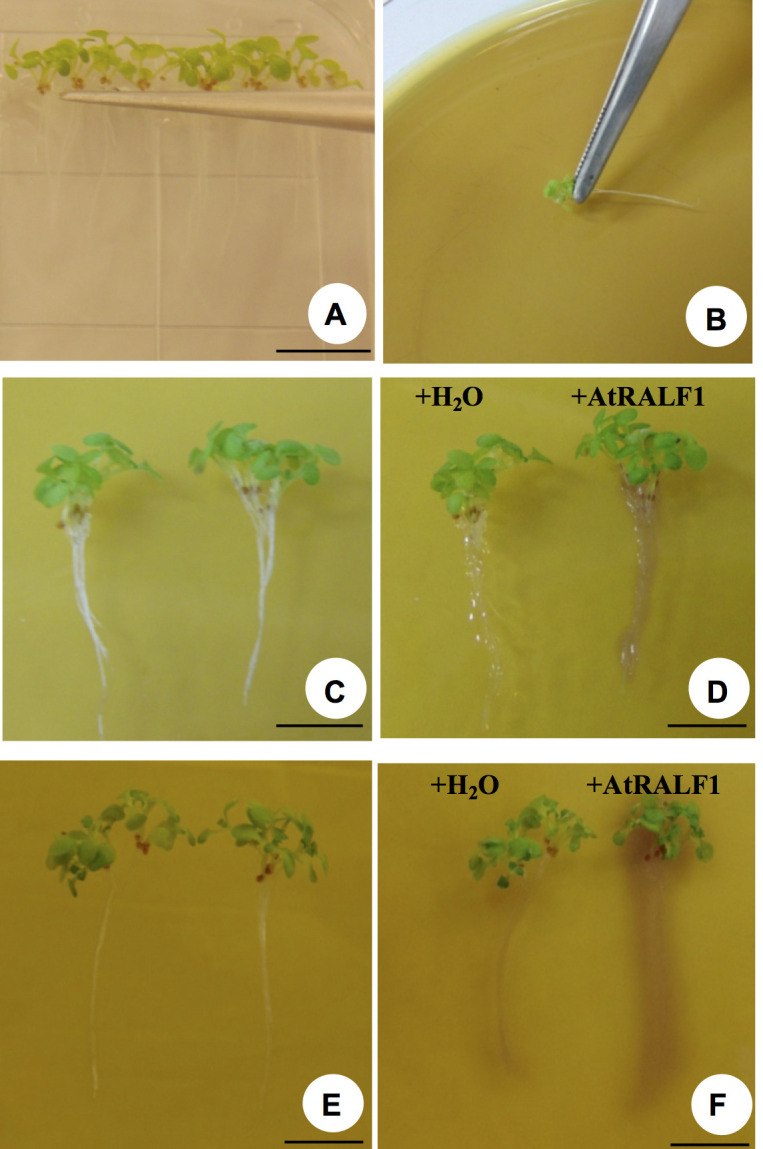
A. Arabidopsis seedlings being selected to be transferred onto a gel containing the pH indicator. B. Seedlings being placed on top of the gel. C. Pair of 6-8 seedlings before AtRALF1 or water treatment. D. The same pair shown in C, 30 min after treatment. E. Pair of 6-8 seedlings before AtRALF1 or water treatment. F. The same pair shown in E, 30 min after treatment. Image was taken after reducing the distance between the plate and the white background. Scale bars = 1 cm.
Video 1. Seedlings being transferred to Petri dish containing the pH indicator bromocresol purple.
Video 2. Roots of Arabidopsis seedlings treated with AtRALF1 solution .
Data analysis
The data produced is qualitative, highly reproducible but seedlings have to be manipulated carefully. Wounds that may be unintentionally inflicted by handling itself are a signal that causes proton fluxes and may cause alkalinization.
After 30 min, AtRALF1-treated seedlings show a purple color around the roots while water-treated control seedlings develop no color (Figures 3C-3D).
Photographic record: the best results that we have obtained are using a white background and a source of white light from behind the plate. Good photographic records are the result of good equipment, photographer skills and light setup. Reducing the distance between the plate and the white background increases the detection of the color change in the gel. An example of this effect is shown in Figure 3F.
Notes
In-house produced 6xHis AtRALF1 recombinant peptide was obtained as described by do Canto et al. (2014) .
In our experience, the most critical points for reproducibility are: the thickness of the gel (0.4 cm) that facilitates imaging; the time of incubation with the peptide (30 min); maintaining intact (roots should not be touched/injured with forceps or in any way during handling); and a small volume of peptide solution for root treatment (10 μl).
Recombinant AtRALF1 (7,631 g/mol) is dissolved in distilled water (7.631 μg/100 μl). The final concentration of the solution is 10 μM.
Recipes
-
Half-strength MS medium
Note: Weigh all the chemicals used in weighing balance (± 0.0001). Adjust the pH of MS medium using a magnetic stirrer and a pH meter.
Add 2.215 g L-1 MS salts without sucrose and vitamins (PhytoTechnology Laboratories), 10.0 g sucrose to 800 ml ddH2O, adjust pH to 5.7 using KOH
Add Gellan Gum Powder (6 g L-1, PhytoTechnology Laboratories) and add up to 1 L with dH2O
Autoclave for 15 min at 121 °C
After autoclaving, pour the half-strength MS medium in square Petri dishes, 30 ml per plate, with the aid of a graduated cylinder (100 ml) in a laminar flow hood
-
Gel containing bromocresol purple (pH 5.7)
0.006% (w/v) bromocresol purple (free acid reagent grade) (60 g L-1)
1 mM of CaSO4 (172 g L-1)
Sterile distilled water
Agarose (15 g L-1)
-
Sodium hypochlorite (NaClO) solution 50% (v/v)
50 ml of sodium hypochlorite solution
50 ml of sterile distilled water
Acknowledgments
This research was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). DSM is a research fellow of CNPq. This protocol was adapted from Wu et al. (2007).
The authors declare that they do not have any conflicts of interest or competing interests.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Do Canto A. M., Ceciliato P. H. O., Ribeiro B., Ortiz Morea F. A., Franco Garcia A. A., Silva-Filho M. C. and Moura D. S.(2014). Biological activity of nine recombinant AtRALF peptides: implications for their perception and function in Arabidopsis . Plant Physiol Biochem 75: 45-54. [DOI] [PubMed] [Google Scholar]
- 2. Dressano K, Ceciliato P. H. O., Silva A. L., Guerrero-Abad J. C., Bergonci T., Ortiz-Morea F. A., Bürger M., Silva-Filho M. C. and Moura D. S.(2017). BAK1 is involved in AtRALF1-induced inhibition of root cell expansion. PLoS Genet 13(10): e1007053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Felix G. and Boller T.(1995). Systemin induces rapid ion fluxes and ethylene biosynthesis in Lycopersicon peruvianum cells . Plant J 7: 381-389. [Google Scholar]
- 4. Haruta M., Sabat G., Stecker K., Minkoff B. B. and Sussman M. R.(2014). A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 343(6169): 408-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Masachis S., Segorbe D., Turra D., Leon-Ruiz M., Furst U., El Ghalid M., Leonard G., Lopez-Berges M. S., Richards T. A., Felix G. and Di Pietro A.(2016). A fungal pathogen secretes plant alkalinizing peptides to increase infection. Nat Microbiol 1(6): 16043. [DOI] [PubMed] [Google Scholar]
- 6. Pearce G., Moura D. S., Stratmann J. and Ryan C. A.(2001). Production of multiple plant hormones from a single polyprotein precursor. Nature 411(6839): 817-820. [DOI] [PubMed] [Google Scholar]
- 7. Pearce G., Moura D. S., Stratmann J. and Ryan C. A., Jr (2001). RALF, a 5-kDa ubiquitous polypeptide in plants, arrests root growth and development. Proc Natl Acad Sci U S A 98(22): 12843-12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Srivastava R., Liu J. X., Guo H., Yin Y. and Howell S. H.(2009). Regulation and processing of a plant peptide hormone, AtRALF23, in Arabidopsis . Plant J 59(6): 930-939. [DOI] [PubMed] [Google Scholar]
- 9. Wu J., Kurten E. L., Monshausen G., Hummel G. M., Gilroy S. and Baldwin I. T.(2007). NaRALF, a peptide signal essential for the regulation of root hair tip apoplastic pH in Nicotiana attenuata, is required for root hair development and plant growth in native soils . Plant J 52(5): 877-890. [DOI] [PubMed] [Google Scholar]