Abstract
The non-medical (i.e., recreational) misuse of synthetic cannabinoids (SCs) is a worldwide public health problem. When compared to cannabis, the misuse of SCs is associated with a higher incidence of serious adverse effects, suggesting the possible involvement of non-cannabinoid sites of action. Here, we find that, unlike the phytocannabinoid Δ9-tetrahydrocannabinol, the indole-moiety containing SCs, AM2201 and JWH-018, act as positive allosteric modulators (PAMs) at the 5-HT1A receptor (5-HT1AR). This suggests that some biological effects of SCs might involve allosteric interactions with 5-HT1ARs. To test this hypothesis, we examined effects of AM2201 on 5-HT1AR agonist-activated G protein-coupled inwardly-rectifying potassium channel currents in neurons in vitro, and on the hypothermic response to 5-HT1AR stimulation in mice lacking the cannabinoid receptor 1. We found that both 5-HT1AR effects were potentiated by AM2201, suggesting that PAM activity at 5-HT1AR may represent a novel non-cannabinoid receptor mechanism underlying the complex profile of effects for certain SCs.
Keywords: synthetic cannabinoids, CB1 receptor, 5-HT1A receptor, positive allosteric modulation
Graphical Abstract
Positive allosteric modulation of the 5-HT1A receptor by indole-based synthetic cannabinoids abused by humans. Hideaki Yano, Pramisha Adhikari, Sett Naing, Alexander F. Hoffman, Michael H. Baumann, Carl R. Lupica, Lei Shi
INTRODUCTION
Many synthetic cannabinoids (SCs) in recreational drug markets are structurally unlike the phytocannabinoid, Δ9-tetrahydrocannabinol (Δ9-THC), in that they possess an indole ring moiety in their chemical structures. The misuse of such indole-based SCs has been associated with serious medical complications including psychosis,1, 2 hypertension,3-7 hypothermia,3 and catatonia8 that are rarely reported with cannabis smoking.9, 10 Like Δ9-THC, SCs are agonists at both cannabinoid receptors 1 and 2 (CB1R and CB2R),11, 12 However, the observations that some in vivo effects of SCs are not reversed by cannabinoid antagonists likely indicate involvement of non-cannabinoid receptor sites of action.13 Indeed, two of the aminoalkylindole-containing SCs commonly confiscated by law enforcement in the early 2010s, namely AM2201 and JWH-018, possess low-affinity antagonism for the serotonin 5-HT2B receptor (5-HT2BR).14 Here, we test the hypothesis that SCs act at non-cannabinoid receptor sites to perhaps contribute to their complex biological profile observed in vivo.
RESULTS
AM2201 and JWH-018 allosterically modulate G protein coupling at the 5-HT1A receptor
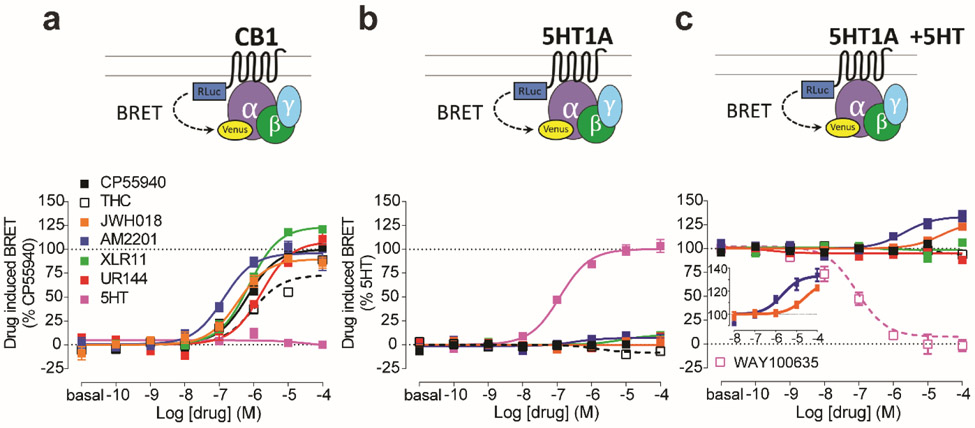
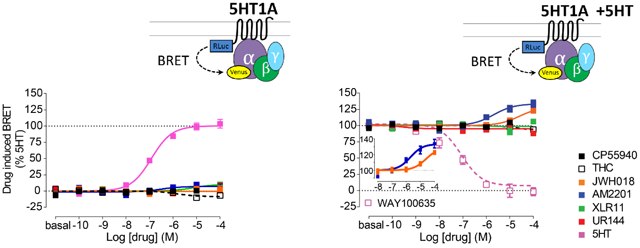
We first assessed the functional properties of several aminoalkylindole SCs at CB1Rs using a Bioluminescence Resonance Energy Transfer (BRET)-based CB1R/Gi protein engagement assay. As expected, the aminoalkylindoles demonstrated higher efficacies and potencies compared to the partial agonist, Δ9-THC, at CB1Rs (Fig. 1a). Moreover, consistent with previous reports,15-17 we found functional differences among these compounds. Specifically, the fluorine-substituted SCs AM2201 and XLR-11 showed higher efficacy and potency compared to their structural analogs, JWH-018 and UR-144, respectively (Fig. 1 and Supplementary Table 1).
Figure 1.
SCs allosterically modulate 5-HT1AR activity. a. The engagement BRET changes between CB1R-Rluc and Gi1-Venus in response to CP55940 (black filled), Δ9-THC (black open), JWH-018 (orange), AM2201 (blue), XLR-11 (green), UR-144 (red), 5-HT (magenta). b. The engagement BRET changes between 5-HT1AR-Rluc and Gi-Venus in response to the same set of compounds as in a. c. The engagement BRET changes between 5-HT1AR-Rluc and Gi-Venus in response to the same set of compounds as in a and WAY100635 (magenta open), in the presence of 1 μM 5-HT. Inset demonstrates the effects of JWH-018 and AM2201 curves on a magnified y-axis. The error bars represent S.E.M. of 3 experiments performed in triplicate (see Supplementary Table 1 for statistical comparisons).
Using similar engagement assays, we next investigated potential “off-target” interactions of the aminoalkylindole SCs with other G protein-coupled receptors that are hypothesized to play roles in producing symptoms observed in human SC users. Thus, we evaluated dopamine D2 receptors (D2R), serotonin 1A receptors (5-HT1AR), 5-HT2ARs, 5-HT2BRs, and adrenergic α1 and α2 receptors (α1AR and α2AR), due to their involvement in psychosis, hypothermia, catatonia, and cardiovascular effects. We found that the aminoalkylindole SCs did not interact with D2Rs, 5-HT2ARs, α1ARs, or α2ARs in either an orthosteric (Supplementary Fig. 1) or an allosteric way (Supplementary Fig. 2). However, these compounds did demonstrate significant antagonism of 5-HT2BRs (Supplementary Figs. 1d and 2d, also see ref 14). Importantly, it should be noted that 5-HT2BR antagonist activity is likely to be cardioprotective rather than cardiotoxic,18, 19 suggesting that the cardiovascular side-effects of SCs may not be explained by their interactions with 5-HT2BRs.
Although all tested aminoalkylindoles lacked orthosteric activity at 5-HT1AR (Fig. 1b), we found two compounds that allosterically modulated this receptor. Thus, in the presence of the endogenous agonist, 5-HT (1 μM), both AM2201 and JWH-018 produced a significant > 28% enhancement of the maximal effect of 5-HT (Emax) in activating Gi (Fig. 1c and Supplementary Table 1). Using a fixed concentration (10 μM) of AM2201 or JWH-018, the enhancement of efficacy was 20.8% and 11.6% respectively (Supplementary Fig. 3a). Moreover, a similar degree of enhancement was observed when the 5-HT1AR-selective agonist, 8-OH-DPAT, was used to activate the receptor (18.3% and 11.0% enhancement with 10 μM AM2201 and JWH-018, respectively; Supplementary Fig. 3b).
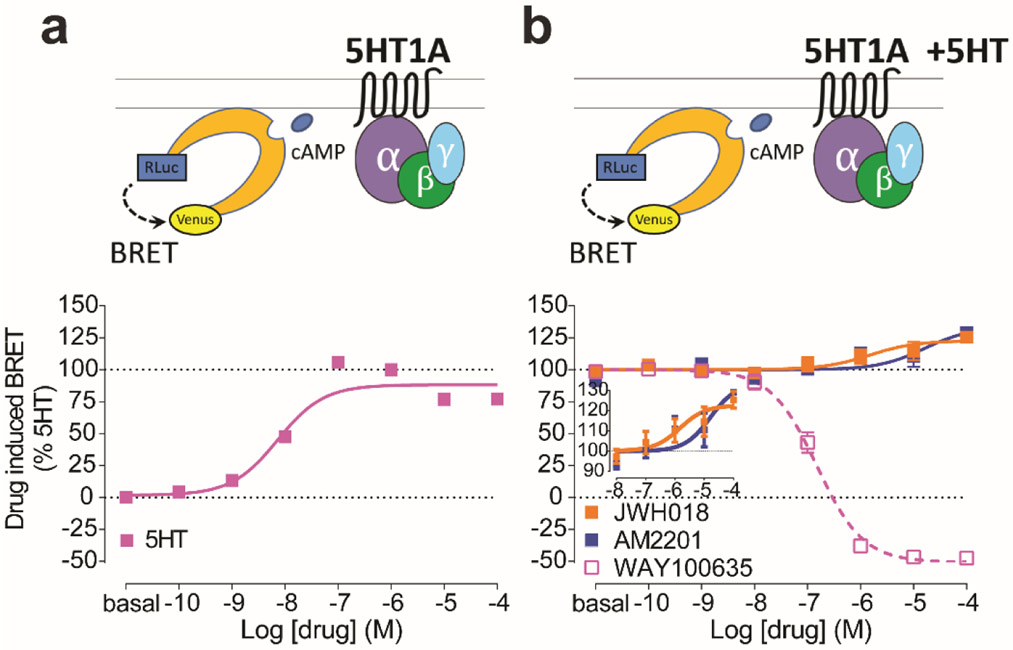
Activation of 5-HT1ARs with 5-HT would also lead to inhibition of cAMP accumulation. By measuring cAMP with a BRET-based biosensor, we revealed that AM2201 and JWH-018 both significantly potentiated such inhibition (Fig. 2). Taken together, the augmented functional effects of 5-HT1AR agonists by AM2201 or JWH-018, in the absence of orthosteric effects, suggest that SCs can act as positive allosteric modulators (PAMs) at 5-HT1ARs.
Figure 2.
SCs allosterically modulate cAMP inhibition via 5-HT1AR. a-b. Dose response curves for the cAMP inhibition via 5-HT1AR using CAMYEL biosensor by (a) 5-HT alone or (b) WAY100635 (magenta open), AM2201 (blue), or JWH-018 (orange) in the presence of 10−6 M 5-HT. Inset demonstrates effects of JWH-018 and AM2201 on a magnified y-axis. The error bars represent S.E.M. of 3 experiments performed in triplicate (see Supplementary Table 1 for statistical comparisons). The efficacy value obtained using 10 μM 5-HT in panel a was used to establish 100% baseline in panel b.
AM2201 allosterically modulates agonist binding at 5-HT1AR
To further characterize the nature of the interaction of AM2201 with 5-HT1AR, we conducted in vitro radioligand binding analyses. In a competition binding assay, AM2201 enhanced the displacement of the 5-HT1AR-selective antagonist [3H]WAY100635 by 5-HT. Specifically, at 30 μM, AM2201 significantly increased the 5-HT-mediated displacement of [3H]WAY100635 by 11.2% compared to vehicle control (Supplementary Fig. 4a and Supplementary Table 2). However, in the absence of 5-HT, AM2201 at the same concentration had negligible effects on [3H]WAY100635 binding (Supplementary Fig. 4b). This suggests that AM2201 acts at 5-HT1ARs via binding in an allosteric, rather than an orthosteric, site.
AM2201 and JWH-018 enhance 5-HT-mediated neuronal activity in CB1R-knockout mice
The 5-HT1AR is widely expressed on serotonergic neurons of the dorsal raphe nucleus (DRN) where these receptors can serve as autoreceptors to reduce 5-HT release by limiting somatic excitability.20, 21 This occurs via 5-HT1AR activation of Gi proteins, leading to opening of G protein-coupled inwardly-rectifying potassium (GIRK) channels, and resultant neuronal hyperpolarization.22, 23 To evaluate the PAM activity of AM2201 and JWH-018 at 5-HT1ARs in native tissue, we performed in vitro whole-cell voltage clamp experiments in DRN cells and measured 5-HT-generated GIRK channel currents. Tetrodotoxin (TTX) was included in all experiments to eliminate action potentials and any presynaptic drug actions. In wildtype mice, 30 μM 5-HT generated inhibitory outward currents, whereas 3 μM had no effect in DRN neurons (Supplementary Fig. 5a). In addition, the 5-HT1AR-selective antagonist, WAY100635 (300 nM), inhibited the 5-HT-elicited currents (Supplementary Fig. 5b).
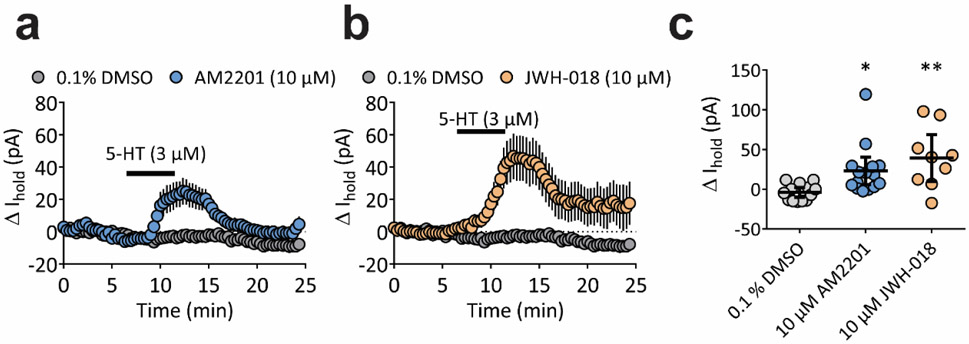
To evaluate potential PAM effects of SCs on 5-HT responses in DRN neurons in the absence of CB1R-mediated effects,24 we used CB1R-knockout (CNR1−/−) mice. Neither AM2201 nor JWH-018 (each 10 μM) alone had effects on DRN neurons from CNR1−/− mice (Supplementary Fig. 6). However, in the presence of these SCs, a concentration of 5-HT (3 μM) that was subthreshold for GIRK activation, now elicited a significant outward current, consistent with PAM activity of these SCs at 5-HT1ARs (Fig. 3; one way ANOVA, F(2,34) = 6.903, p = 0.003).
Figure 3.
AM2201 and JWH-018 enhance 5-HT1A-mediated outward currents in DRN neurons obtained from CNR1−/− mice. a and b, Average time course (mean ± SEM) of 5-HT-induced change in holding current (pA) during perfusion with 3 μM 5-HT following 30 min pre-incubation with 10 μM AM2201, 10 μM JWH-018, or 0.1% DMSO vehicle. c, Peak change in holding current (pA) for individual cells for a and b. The mean and 95% confidence intervals are shown. (F(2,34) = 6.903, p = 0.003, one-way ANOVA; *p <0.05, **p<0.01, Dunnett’s post-hoc test vs. 0.1% DMSO).
AM2201 augments 5-HT1A receptor-induced hypothermia in CB1R-knockout mice
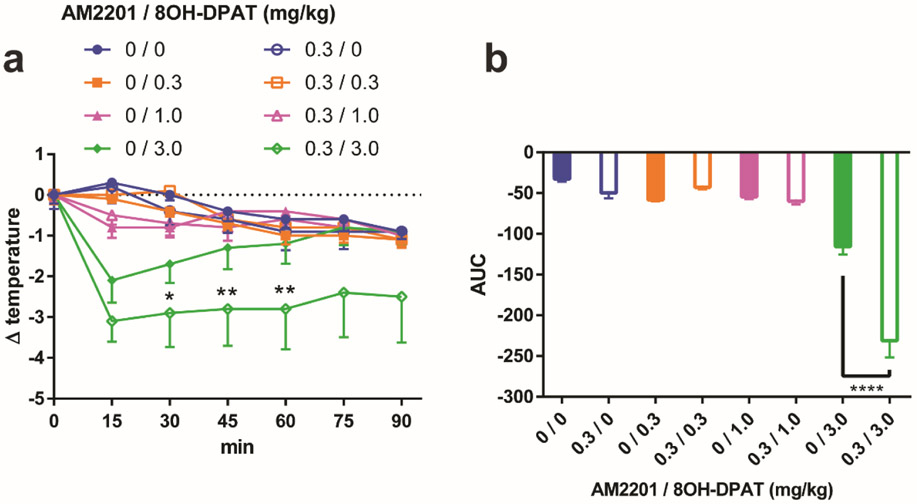
A well-characterized effect of 5-HT1AR activation in rodents is hypothermia.21, 25 To further evaluate the physiological relevance of the PAM activity of AM2201 at 5-HT1AR, we examined the effects of AM2201 on 5-HT1AR-mediated hypothermia in CNR1−/− mice. We found that a 3.0 mg/kg dose of the selective 5-HT1AR agonist 8-OH-DPAT significantly reduced core temperature in mice, which peaked at 15 min post-injection and tapered off by 60 min (Fig. 4a). The hypothermic response to 8-OH-DPAT was also reversed by the highly selective 5-HT1AR antagonist, WAY100635 (10.0 mg/kg) (Supplementary Fig. 7). When 8-OH-DPAT (3.0 mg/kg) was injected together with AM2201 (0.3 mg/kg), both the extent and duration of hypothermia were enhanced, whereas this dose of AM2201 alone had no effect on mean body temperature in these CNR1−/− mice (Fig. 4).
Figure 4.
AM2201 enhances 5-HT1AR-induced hypothermia in CB1R KO mice. a, Core temperature measurement after acute i.p. injections of 0 (blue), 0.3 (orange), 1.0 (magenta), or 3.0 (green) mg/kg 8-OH-DPAT with vehicle (filled symbol) or 0.3 mg/kg AM2201 (open symbol). b, Total area under the curve (AUC) for each dose condition in (a) is plotted. The error bars represent S.E.M. n = 8 mice per group. Two-way analysis of variance followed by post hoc Newman-Keuls test was used for a (F(6,392) = 7.223, 0/3.0 vs. 0.3/3.0 at 30 min *p < 0.05, 45, 60 min **p < 0.01). One-way analysis of variance followed by post hoc Tukey test was used for b (F(7,56) = 39.75, ****p < 0.0001). Compared to the hypothermic effect elicited by 8-OH-DPAT alone, the additional hypothermic effect by AM2201 is significant both in the comparison of the temperature drops at 30, 45, and 60 min (a) and in the comparison of the areas under the curves (b).
DISCUSSION AND CONCLUSIONS
The increased propensity for SCs to produce serious adverse clinical effects in humans, when compared to effects of Δ9-THC, could be related to their high potency and efficacy at brain CB1Rs.26, 27 However, we and others have postulated that SCs may also act at “off-site” non-cannabinoid targets to exert their complex in vivo effects.13, 14 For example, we have demonstrated that AM2201 and JWH-018 produce acute increases in mean arterial pressure in rats that are not reversed by the CB1R antagonist rimonabant, suggesting involvement of non-cannabinoid mechanisms.13 Here, we used in vitro and in vivo methods to provide converging evidence that certain indole-based SCs exhibit PAM activity at 5-HT1ARs. In particular, AM2201 enhanced 5-HT1AR-mediated 5-HT binding, G protein coupling, and activation of GIRK currents in native tissue. It is noteworthy that the effects of AM2201 on 5-HT1ARs were modest (i.e., 10-30% enhancement) in both the radioligand binding and BRET assays. Nevertheless, in the DRN slice experiments, AM2201 converted an ineffective dose of 5-HT into a substantial activator of GIRK-mediated hyperpolarization. To explore the possibility that SCs might exert PAM activity at 5-HT1ARs in vivo, we examined the effects of AM2201 administration on temperature responses to 8-OH-DPAT. It was found that AM2201 enhanced the magnitude and duration of 5-HT1AR-mediated hypothermia.
Although our collective findings are consistent with SC-mediated PAM activities at 5-HT1ARs, there are potential alternative interpretations. For example, AM2201 might enhance 5-HT elicited currents28 via the inhibition of 5-HT uptake. If AM2201 and other SCs bind to serotonin transporters (SERT) to block 5-HT uptake, this could elevate extracellular concentrations of 5-HT. However, previous studies show that the SCs tested in this study do not exhibit detectable binding at SERT14, suggesting that this is not a viable explanation for the effects that we observed. Additionally, with regard to our in vivo studies, we cannot exclude the possibility that AM2201 might impair the metabolism or clearance of 8-OH-DPAT, thereby enhancing the observed pharmacodynamic effects of 8-OH-DPAT in CNR1−/− mice.
In the present study, we utilized genetically-modified CNR1−/− mice to reveal the ability of AM2201 to enhance 5-HT1AR-mediated hyperpolarizing currents and hypothermic responses in vivo. Importantly, our findings in CNR1−/− mice are consistent with recent reports using wildtype rats in which repeated injections of JWH-018 were accompanied by enhancement of 5-HT1AR-mediated hypothermia.29 Thus, PAM activities at 5-HT1ARs could represent a key non-cannabinoid mechanism of action for certain indole-based SCs that contributes to effects observed in both rodents and humans, especially after high dose exposure to SCs.
In contrast to the PAM effects we observed with JWH-018 and AM2201, the indole-based SCs UR-144 and XLR-11 did not alter 5-HT1AR function. We speculate that this difference may be due to structural differences among these SCs. Thus, whereas both UR-144 and XLR-11 contain a tetramethylcyclopropyl head groups, JWH-018 and AM2201 have naphthyl moieties at this molecular position (Supplementary Fig. 8). Therefore, these structural head group differences may be important to permit the distinct pharmacological properties of different SCs.
Although there are clear limitations in extrapolating the present findings to effects of SCs observed in humans, our results provide evidence that there may be important non-cannabinoid effects of certain classes of SCs that could be involved in the untoward clinical effects of these drugs. Therefore, our study suggests that non-cannabinoid, off-site effects of these drugs should be considered for newly-emerging SCs as they appear in recreational drug markets.
Supplementary Material
ACKNOWLEDGMENT
We thank Dr. Sergi Ferre for sharing instrumentation for tissue culture and BRET studies, Dr. Alessandro Bonifazi for technical suggestions for radioligand binding, Mr. Joshua Elmore for technical training and assistance for core body temperature measurement assay, and the NIDA-IRP Transgenic Core facility for their assistance in maintaining the CNR1−/− colony.
Funding Sources
Support for this research was provided by the National Institute on Drug Abuse–Intramural Research Program.
REFERENCES
- 1.Zawilska JB; Wojcieszak J, Spice/K2 drugs--more than innocent substitutes for marijuana. Int J Neuropsychopharmacol 2014, 17 (3), 509–25. [DOI] [PubMed] [Google Scholar]
- 2.Fattore L, Synthetic Cannabinoids-Further Evidence Supporting the Relationship Between Cannabinoids and Psychosis. Biol Psychiatry 2016, 79 (7), 539–48. [DOI] [PubMed] [Google Scholar]
- 3.Hermanns-Clausen M; Kneisel S; Szabo B; Auwarter V, Acute toxicity due to the confirmed consumption of synthetic cannabinoids: clinical and laboratory findings. Addiction 2013, 108 (3), 534–44. [DOI] [PubMed] [Google Scholar]
- 4.Schneir AB; Cullen J; Ly BT, "Spice" girls: synthetic cannabinoid intoxication. J Emerg Med 2011, 40 (3), 296–9. [DOI] [PubMed] [Google Scholar]
- 5.Clark BC; Georgekutty J; Berul CI, Myocardial Ischemia Secondary to Synthetic Cannabinoid (K2) Use in Pediatric Patients. J Pediatr 2015, 167 (3), 757–61 e1. [DOI] [PubMed] [Google Scholar]
- 6.Young AC; Schwarz E; Medina G; Obafemi A; Feng SY; Kane C; Kleinschmidt K, Cardiotoxicity associated with the synthetic cannabinoid, K9, with laboratory confirmation. Am J Emerg Med 2012, 30 (7), 1320 e5–7. [DOI] [PubMed] [Google Scholar]
- 7.Pacher P; Steffens S; Hasko G; Schindler TH; Kunos G, Cardiovascular effects of marijuana and synthetic cannabinoids: the good, the bad, and the ugly. Nat Rev Cardiol 2018, 15 (3), 151–166. [DOI] [PubMed] [Google Scholar]
- 8.Haro G; Ripoll C; Ibanez M; Orengo T; Liano VM; Meneu E; Hernandez F; Traver F, Could spice drugs induce psychosis with abnormal movements similar to catatonia? Psychiatry 2014, 77 (2), 206–8. [DOI] [PubMed] [Google Scholar]
- 9.Winstock AR; Barratt MJ, Synthetic cannabis: a comparison of patterns of use and effect profile with natural cannabis in a large global sample. Drug Alcohol Depend 2013, 131 (1-2), 106–11. [DOI] [PubMed] [Google Scholar]
- 10.Ford BM; Tai S; Fantegrossi WE; Prather PL, Synthetic Pot: Not Your Grandfather's Marijuana. Trends Pharmacol Sci 2017, 38 (3), 257–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banister SD; Connor M, The Chemistry and Pharmacology of Synthetic Cannabinoid Receptor Agonists as New Psychoactive Substances: Origins. Handbook of experimental pharmacology 2018, 252, 165–190. [DOI] [PubMed] [Google Scholar]
- 12.Wiley JL; Marusich JA; Thomas BF, Combination Chemistry: Structure-Activity Relationships of Novel Psychoactive Cannabinoids. Curr Top Behav Neurosci 2017, 32, 231–248. [DOI] [PubMed] [Google Scholar]
- 13.Schindler CW; Gramling BR; Justinova Z; Thorndike EB; Baumann MH, Synthetic cannabinoids found in "spice" products alter body temperature and cardiovascular parameters in conscious male rats. Drug Alcohol Depend 2017, 179, 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiley JL; Lefever TW; Marusich JA; Grabenauer M; Moore KN; Huffman JW; Thomas BF, Evaluation of first generation synthetic cannabinoids on binding at non-cannabinoid receptors and in a battery of in vivo assays in mice. Neuropharmacology 2016, 110 (Pt A), 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman AF; Lycas MD; Kaczmarzyk JR; Spivak CE; Baumann MH; Lupica CR, Disruption of hippocampal synaptic transmission and long-term potentiation by psychoactive synthetic cannabinoid 'Spice' compounds: comparison with Delta(9) - tetrahydrocannabinol. Addict Biol 2017, 22 (2), 390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brents LK; Reichard EE; Zimmerman SM; Moran JH; Fantegrossi WE; Prather PL, Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS One 2011, 6 (7), e21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banister SD; Stuart J; Kevin RC; Edington A; Longworth M; Wilkinson SM; Beinat C; Buchanan AS; Hibbs DE; Glass M; Connor M; McGregor IS; Kassiou M, Effects of bioisosteric fluorine in synthetic cannabinoid designer drugs JWH-018, AM-2201, UR-144, XLR-11, PB-22, 5F-PB-22, APICA, and STS-135. ACS chemical neuroscience 2015, 6 (8), 1445–58. [DOI] [PubMed] [Google Scholar]
- 18.Berger M; Gray JA; Roth BL, The expanded biology of serotonin. Annu Rev Med 2009, 60, 355–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothman RB; Baumann MH, Serotonergic drugs and valvular heart disease. Expert Opin Drug Saf 2009, 8 (3), 317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Celada P; Bortolozzi A; Artigas F, Serotonin 5-HT1A receptors as targets for agents to treat psychiatric disorders: rationale and current status of research. CNS Drugs 2013, 27 (9), 703–16. [DOI] [PubMed] [Google Scholar]
- 21.Savitz J; Lucki I; Drevets WC, 5-HT(1A) receptor function in major depressive disorder. Prog Neurobiol 2009, 88 (1), 17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams JT; Colmers WF; Pan ZZ, Voltage- and ligand-activated inwardly rectifying currents in dorsal raphe neurons in vitro. J Neurosci 1988, 8 (9), 3499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Penington NJ; Kelly JS; Fox AP, Whole-cell recordings of inwardly rectifying K+ currents activated by 5-HT1A receptors on dorsal raphe neurones of the adult rat. J Physiol 1993, 469, 387–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haring M; Marsicano G; Lutz B; Monory K, Identification of the cannabinoid receptor type 1 in serotonergic cells of raphe nuclei in mice. Neuroscience 2007, 146 (3), 1212–9. [DOI] [PubMed] [Google Scholar]
- 25.Haberzettl R; Bert B; Fink H; Fox MA, Animal models of the serotonin syndrome: a systematic review. Behav Brain Res 2013, 256, 328–45. [DOI] [PubMed] [Google Scholar]
- 26.Davidson C; Opacka-Juffry J; Arevalo-Martin A; Garcia-Ovejero D; Molina-Holgado E; Molina-Holgado F, Spicing Up Pharmacology: A Review of Synthetic Cannabinoids From Structure to Adverse Events. Adv Pharmacol 2017, 80, 135–168. [DOI] [PubMed] [Google Scholar]
- 27.Weinstein AM; Rosca P; Fattore L; London ED, Synthetic Cathinone and Cannabinoid Designer Drugs Pose a Major Risk for Public Health. Front Psychiatry 2017, 8, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi T; Washiyama K; Ikeda K, Inhibition of G protein-activated inwardly rectifying K+ channels by various antidepressant drugs. Neuropsychopharmacology 2004, 29 (10), 1841–51. [DOI] [PubMed] [Google Scholar]
- 29.Elmore JS; Baumann MH, Repeated Exposure to the "Spice" Cannabinoid JWH-018 Induces Tolerance and Enhances Responsiveness to 5-HT1A Receptor Stimulation in Male Rats. Front Psychiatry 2018, 9, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.