Abstract
Background
An abdominal aortic aneurysm (AAA) is an abnormal dilation in the diameter of the abdominal aorta of 50% or more of the normal diameter or greater than 3 cm in total. The risk of rupture increases with the diameter of the aneurysm, particularly above a diameter of approximately 5.5 cm. Perioperative and postoperative morbidity is common following elective repair in people with AAA. Prehabilitation or preoperative exercise is the process of enhancing an individual’s functional capacity before surgery to improve postoperative outcomes. Studies have evaluated exercise interventions for people waiting for AAA repair, but the results of these studies are conflicting.
Objectives
To assess the effects of exercise programmes on perioperative and postoperative morbidity and mortality associated with elective abdominal aortic aneurysm repair.
Search methods
We searched the Cochrane Vascular Specialised register, Cochrane Central Register of Controlled Trials, MEDLINE, Embase, CINAHL (Cumulative Index to Nursing and Allied Health Literature), and Physiotherapy Evidence Database (PEDro) databases, and the World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov trials registers to 6 July 2020. We also examined the included study reports' bibliographies to identify other relevant articles.
Selection criteria
We considered randomised controlled trials (RCTs) examining exercise interventions compared with usual care (no exercise; participants maintained normal physical activity) for people waiting for AAA repair.
Data collection and analysis
Two review authors independently selected studies for inclusion, assessed the included studies, extracted data and resolved disagreements by discussion. We assessed the methodological quality of studies using the Cochrane risk of bias tool and collected results related to the outcomes of interest: post‐AAA repair mortality; perioperative and postoperative complications; length of intensive care unit (ICU) stay; length of hospital stay; number of days on a ventilator; change in aneurysm size pre‐ and post‐exercise; and quality of life. We used GRADE to evaluate certainty of the evidence. For dichotomous outcomes, we calculated the risk ratio (RR) with the corresponding 95% confidence interval (CI).
Main results
This review identified four RCTs with a total of 232 participants with clinically diagnosed AAA deemed suitable for elective intervention, comparing prehabilitation exercise therapy with usual care (no exercise). The prehabilitation exercise therapy was supervised and hospital‐based in three of the four included trials, and in the remaining trial the first session was supervised in hospital, but subsequent sessions were completed unsupervised in the participants’ homes. The dose and schedule of the prehabilitation exercise therapy varied across the trials with three to six sessions per week and a duration of one hour per session for a period of one to six weeks. The types of exercise therapy included circuit training, moderate‐intensity continuous exercise and high‐intensity interval training.
All trials were at a high risk of bias. The certainty of the evidence for each of our outcomes was low to very low. We downgraded the certainty of the evidence because of risk of bias and imprecision (small sample sizes). Overall, we are uncertain whether prehabilitation exercise compared to usual care (no exercise) reduces the occurrence of 30‐day (or longer if reported) mortality post‐AAA repair (RR 1.33, 95% CI 0.31 to 5.77; 3 trials, 192 participants; very low‐certainty evidence). Compared to usual care (no exercise), prehabilitation exercise may decrease the occurrence of cardiac complications (RR 0.36, 95% CI 0.14 to 0.92; 1 trial, 124 participants; low‐certainty evidence) and the occurrence of renal complications (RR 0.31, 95% CI 0.11 to 0.88; 1 trial, 124 participants; low‐certainty evidence). We are uncertain whether prehabilitation exercise, compared to usual care (no exercise), decreases the occurrence of pulmonary complications (RR 0.49, 95% 0.26 to 0.92; 2 trials, 144 participants; very low‐certainty evidence), decreases the need for re‐intervention (RR 1.29, 95% 0.33 to 4.96; 2 trials, 144 participants; very low‐certainty evidence) or decreases postoperative bleeding (RR 0.57, 95% CI 0.18 to 1.80; 1 trial, 124 participants; very low‐certainty evidence). There was little or no difference between the exercise and usual care (no exercise) groups in length of ICU stay, length of hospital stay and quality of life.
None of the studies reported data for the number of days on a ventilator and change in aneurysm size pre‐ and post‐exercise outcomes.
Authors' conclusions
Due to very low‐certainty evidence, we are uncertain whether prehabilitation exercise therapy reduces 30‐day mortality, pulmonary complications, need for re‐intervention or postoperative bleeding. Prehabilitation exercise therapy might slightly reduce cardiac and renal complications compared with usual care (no exercise). More RCTs of high methodological quality, with large sample sizes and long‐term follow‐up, are needed. Important questions should include the type and cost‐effectiveness of exercise programmes, the minimum number of sessions and programme duration needed to effect clinically important benefits, and which groups of participants and types of repair benefit most.
Plain language summary
Exercise before planned surgery for abdominal aortic aneurysm
Background
The abdominal aorta is a major blood vessel in the body that carries blood from the heart to the major organs in the chest and abdomen. An abdominal aortic aneurysm (AAA) is a balloon‐like bulge of the aorta. If an AAA grows to over 5.5 cm in diameter (the length from one side to the other), the chance of the AAA rupturing (bursting) is increased. Ruptured AAAs cause death unless surgery is carried out soon after the event to repair the rupture. Surgery is recommended for people with AAAs bigger than 5.5 cm in diameter or who have pain due to the AAA, to decrease the risk of rupture and death. Complications following planned surgery for AAA are common. Exercise before surgery for AAA could help people make a better recovery from surgery. At the moment we do not know if exercise before surgery will help people make a better recovery after AAA surgery. We found only a few trials which looked at whether exercise before AAA surgery helps people make a better recovery, so more trials are needed before we can be certain the exercise helps.
Study characteristics and key results
We searched the literature on 6 July 2020, and we found four trials that included 232 participants with AAA who were on a waiting list for AAA surgery. The trials randomly assigned participants into two groups, one with exercise before surgery and another with usual care (no exercise before surgery, participants maintained normal physical activity). The types of exercise included circuit training, moderate‐intensity continuous exercise and high‐intensity interval training. In three of the four trials, the participants in the exercise group were supervised by healthcare professionals in hospital when they did their exercise sessions. In the other trial, the first exercise session was supervised in hospital, and the following sessions were completed by the participants on their own in their own homes. The number and length of the exercise sessions was different in the trials. Some exercise sessions took place three times a week and some took place six times a week. In some trials participants exercised for one week and some trials' participants exercised for six weeks before their surgery.
Limited information from a small number of trials showed that exercise before AAA surgery might slightly reduce heart and kidney complications after surgery, compared to no exercise (usual care) before AAA surgery. We are uncertain whether exercise before AAA surgery reduces death within 30 days of AAA surgery, lung complications, the need for further treatment or bleeding after surgery, compared to no exercise before AAA surgery. There was little or no difference between the exercise and usual care groups in length of intensive care unit stay, length of hospital stay and quality of life. None of the studies reported information for the number of days participants were on a ventilator and change in AAA size before and after exercise.
Certainty of the evidence
The certainty of the evidence is low or very low because of the way the studies were designed (risk of bias), and small number of people in the trials. Larger, well‐designed trials are needed in order to increase our confidence in any benefits of exercising before AAA surgery for reducing complications.
Summary of findings
Summary of findings 1. Exercise compared to no exercise for adults with clinically diagnosed AAA deemed suitable for elective repair.
| Exercise compared to no exercise for adults with clinically diagnosed AAA deemed suitable for elective repair | ||||||
| Patient or population: adults with clinically diagnosed AAA deemed suitable for elective repair Setting: hospital Intervention: exercise Comparison: usual care (no exercise) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care (no exercise) | Risk with exercise | |||||
| 30‐day mortality Follow‐up: 30 days |
Study population | RR 1.33 (0.31 to 5.77) | 192 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW a,b | ||
| 21 per 1000 | 28 per 1000 (6 to 120) | |||||
| Perioperative and postoperative complications: cardiac complications Follow‐up: 3 months |
Study population | RR 0.36 (0.14 to 0.92) | 124 (1 RCT) | ⊕⊕⊝⊝ LOW c,d | ||
| 226 per 1000 | 81 per 1000 (32 to 208) | |||||
| Perioperative and postoperative complications: pulmonary complications Follow‐up: 7 days ‐ 3 months |
Study population | RR 0.49 (0.26 to 0.92) | 144 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW d,e | ||
| 292 per 1000 | 143 per 1000 (76 to 268) | |||||
| Perioperative and postoperative complications: renal complications Follow‐up: 3 months |
Study population | RR 0.31 (0.11 to 0.88) | 124 (1 RCT) | ⊕⊕⊝⊝ LOW c,d | ||
| 210 per 1000 | 65 per 1000 (23 to 185) | |||||
| Perioperative and postoperative: need for re‐intervention Follow‐up: 3 months |
Study population | RR 1.29 (0.33 to 4.96) | 144 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW a,e | ||
| 42 per 1000 | 54 per 1000 (14 to 207) | |||||
| Perioperative and postoperative complications: postoperative bleeding Follow‐up: 72 hours |
Study population | RR 0.57 (0.18 to 1.80) | 124 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a,c | ||
| 113 per 1000 | 64 per 1000 (20 to 203) | |||||
| Length of ICU stay (days) | See comments | ‐ | 147 (2 RCTs) |
⊕⊝⊝⊝ VERY LOW f,g | Two studies reported on length of ICU stay, but we could not evaluate this in a meta‐analysis. Neither of the studies found a clear difference between the exercise and usual care groups in length of ICU stay. | |
| Length of hospital stay (days) | See comments | ‐ | 212 (3 RCTs) |
⊕⊝⊝⊝ VERY LOWg,h | Three studies reported on length of hospital stay, but we could not evaluate this in a meta‐analysis. One study reported shorter hospital stay for the exercise group and two studies reported no clear difference between the exercise and usual care groups. | |
| Number of days on a ventilator | See comments | ‐ | ‐ | ‐ | No studies reported number of days on a ventilator | |
| QoL Follow‐up: 12 weeks |
See comments | ‐ | 53 (1 RCT) |
⊕⊕⊝⊝ LOWi | One study reported QoL. The study found little or no difference between the exercise and usual care group participants. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AAA: abdominal aortic aneurysm;CI: confidence interval; ICU: intensive care unit; QoL: quality of life; RCT: randomised controlled trial;RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aThe 95% CI includes no effect, and includes default values for appreciable harm (i.e. CI > 1.25), appreciable benefit (i.e. CI < 0.75), or both; the optimal information size was not met (i.e. sample size < 2000 participants); therefore, we downgraded the certainty of evidence by 2 levels for imprecision. bHigh overall risk of bias due to lack of blinding of outcome assessors, selective reporting, selection bias, attrition bias and/or other bias (Barakat 2016; Dronkers 2008; Tew 2017); therefore, we downgraded the certainty of evidence by 2 levels for methodological limitations. cStudy did not state whether outcome assessors were blinded, outcomes reported in protocol were not reported in study (risk of reporting bias) (Barakat 2016); therefore, we downgraded the certainty of evidence by 1 level for methodological limitations. dThe optimal information size was not met (i.e. sample size < 2000); therefore, we downgraded the certainty of evidence by 1 level for imprecision. eHigh overall risk of bias due to lack of blinding of outcome assessors, selective reporting, selection bias, attrition bias and/or other bias (Barakat 2016; Dronkers 2008); therefore, we downgraded the certainty of evidence by 2 levels for methodological limitations. fHigh overall risk of bias due to lack of blinding of outcome assessors, selective reporting, selection bias, and/or attrition bias (Barakat 2016; Richardson 2014); therefore, we downgraded the certainty of evidence by 2 levels for methodological limitations. gUnable to assess imprecision due to the way the studies report the outcome; therefore, we downgraded the certainty of evidence by 1 level. hHigh overall risk of bias due to lack of blinding of outcome assessors, selective reporting, selection bias, attrition bias and/or other bias (Barakat 2016; Richardson 2014; Tew 2017); therefore, we downgraded the certainty of evidence by 2 levels for methodological limitations. iHigh overall risk of bias due to selective reporting, attrition bias and other bias (Tew 2017); therefore, we downgraded the certainty of evidence by 2 levels for methodological limitations.
Background
Description of the condition
An abdominal aortic aneurysm (AAA) is defined as an abnormal dilation in the diameter of the abdominal aorta of 50% or more of the normal diameter or greater than 3 cm in total (NICE 2020). Most AAAs are asymptomatic and are frequently discovered incidentally during imaging or clinical examination for other conditions (Brown 2012). As well as having many risk factors in common with atherosclerosis (including tobacco smoking, advanced age, male sex, and hypertension), genetic factors and family history are likely to influence the development of abdominal aneurysms (Blanchard 2000; Larsson 2009; Lederle 1997).
The natural history of AAA is expansion (which in some cases causes the aneurysm to become symptomatic) and eventually, acute rupture. In the case of acute rupture, the classical presentation is the triad of sudden, severe abdominal or back pain (or both), a pulsatile abdominal mass and haemodynamic collapse. Mortality among people presenting with a ruptured aneurysm is high (particularly if the rupture occurs out of hospital), and even for those who do make it to hospital and undergo emergency surgery, mortality is approximately 35% (Gunnarsson 2016; Schermerhorn 2012; Sweeting 2015).
The average annual progression in diameter of small aneurysms (≤ 5.5 cm) is estimated to be between 2.0 and 3.0 mm/year, while progression is greater for aneurysms with a larger initial diameter (Bown 2013; Moll 2011). The risk of rupture increases with the diameter of the aneurysm, particularly above a diameter of approximately 5.5 cm (Powell 2008; Powell 2011).
Previously, the prevalence of AAA has been reported to range from 1.3% in women aged 65 to 80 years to between 4% and 7.7% in men aged 65 to 80 years (Ashton 2002; Ashton 2007; Lindholt 2005; Nordon 2011; Norman 2004; Scott 2002). The annual incidence of AAA in Western populations has been estimated at between 0.4% and 0.67% (Forsdahl 2009; Lederle 2002; Nordon 2011; Vardulaki 1999), but may be lower for Asian populations (Spark 2001). More recent evidence suggests that AAA incidence is decreasing, most likely because of a reduction in tobacco smoking and improvement in cardiovascular disease risk factor management (Anjum 2012). The current prevalence rates are closer to 1.5% for men aged 65 and 0.7% for women over 60 years old (Jacomelli 2016; Svensjö 2014; Ulug 2016). There has also been discussion on the importance of the 'subaneurysmal' aorta (diameter 2.5 cm to 2.9 cm), since two‐thirds of these will become aneurysmal over a period of five years (Wild 2013).
In asymptomatic people in whom AAA is suspected clinically, a definite diagnosis can be made using abdominal ultrasound to measure the diameter of the aneurysm (Moll 2011). More detailed information regarding the anatomy and relation to renal and visceral vessels can be obtained from computerised tomography (CT) scanning, if required. In the case of aneurysmal rupture, emergency CT scanning is widely used to confirm the diagnosis and enable the planning of aneurysm repair. Following trials of ultrasound screening, screening programmes to reduce male mortality from AAA have been recommended (Cosford 2007; LeFevre 2014). An example is the UK screening programme in which an ultrasound is offered to all men in their 65th year. A very similar programme is effective in Sweden, whereas screening is focused on older male smokers in the USA.
Because the risk of rupture is low in small AAA (≤ 5.5 cm), management is usually non‐surgical, using regular ultrasound monitoring to screen for expansion of the aneurysm as well as modifying general cardiovascular risk factors, in particular smoking cessation (Bown 2013; Brewster 2003; Filardo 2015; Hirsch 2006; Moll 2011). National guidelines from the European Society of Vascular Surgery (ESVS) and from the American College of Cardiology (ACC) and American Heart Association (AHA) recommend that: when an AAA reaches a diameter of ≥ 5.5 cm (men) or ≥ 5.2 cm (women), demonstrates rapid expansion, or becomes symptomatic (regardless of size), the risk of rupture exceeds the risk of surgical repair and the individual should be referred to a vascular surgeon for consideration of surgical intervention (Hirsch 2006; Moll 2011). Medical therapies to reduce aneurysm growth rates remain unproven and are not widely used in clinical practice (Rughani 2012). There are two main options for surgical intervention: open surgical repair (OSR) and endovascular aneurysm repair (EVAR). OSR involves replacement of the affected section of the aorta with a graft that is sutured in place. EVAR involves the insertion of an intraluminal stent, via a catheter introduced in a distal artery (e.g. femoral artery). Although OSR has a higher 30‐day mortality than endovascular stenting (3.0% versus 0.6%, respectively) (Waton 2018), EVAR is prone to endoleak (some blood flow still remaining in the aneurysm cavity) in the long term, which requires regular follow‐up to detect and possible further surgery to treat (Greenhalgh 2010; Paravastu 2014; Patel 2016; Prinssen 2004). Complications of AAA repair include cardiac complications, respiratory complications, limb ischaemia and renal failure. People undergoing OSR are more susceptible to these complications than those undergoing EVAR (Waton 2018). The choice of which surgical intervention to undertake is usually made on an individual basis, taking into account perioperative comorbidities (in particular, cardiac and respiratory conditions) and the individual risk of rupture. The anatomy of the aneurysm is also important because EVAR grafts are only suitable for particular anatomical configurations.
Description of the intervention
The majority of people with indications for elective AAA repair are older adults (Forsdahl 2009; Howard 2015; Kent 2010; Li 2013), who often present with multiple comorbidities (Mousa 2016). In addition to a common history of smoking (Jahangir 2015; Salzler 2015), and a sedentary lifestyle, these people tend to have lower fitness levels compared to their age‐matched controls (Myers 2014). Significant perioperative metabolic and cardiopulmonary challenges are associated with AAA repair (OSR or EVAR), which requires the individual undergoing the procedure to have a good level of fitness to withstand the stress. There is evidence that level of fitness is associated with important postoperative morbidity and mortality rates in people undergoing AAA repair (Moran 2016). For instance, Grant and colleagues reported a 1.4 x higher three‐year (86.4% vs 59.9%) post‐AAA repair survival for people with zero or one sub‐threshold cardiopulmonary exercise test value compared with those with three sub‐threshold test values (Grant 2015).
Exercise therapy is a prescribed and planned physical activity that aims to improve, maintain, or decrease the rate of decline of physical capacity and function, as well as overall health and well‐being. In people with cardiovascular disease who are not undergoing surgery, exercise therapy has been shown to be beneficial in improving fitness and reducing morbidity and mortality risks (Boden 2014). Evidence also supports the use of preoperative or prehabilitation exercise therapy to improve recovery, as well as to reduce postoperative complications and length of hospital stay following cardiovascular surgeries (Hoogeboom 2014). This includes interventions for vascular conditions (Aherne 2015). Exercise therapy for cardiovascular conditions is safe, with the rate of adverse events ranging from one per 49,565 patient‐hours of exercise training in cardiac patients (Pavy 2006), to one per 10,340 patient‐hours in peripheral arterial disease (Gommans 2015). Few data are available regarding exercise testing in people with AAA disease. Myers 2011 found that people with AAA had a slightly higher incidence of hyper‐ and hypotensive responses to exercise than age‐matched referrals, but no serious events related to the cardiopulmonary exercise tests occurred during the study period.
How the intervention might work
Undergoing surgery promotes an inflammatory response, which increases the demand for oxygen consumption (Barakat 2015). Exercise improves cardiorespiratory fitness, which improves oxygen delivery to local tissue (Smith 2009), and is also associated with anti‐inflammatory mechanisms (Petersen 2005). Older 2013 hypothesised that increased lactate production due to lower levels of cardiorespiratory fitness may contribute to postoperative complications, as the body has a reduced ability to metabolise lactate postoperatively.
Optimal fitness potentially provides people with the ability to withstand the metabolic and cardiopulmonary stress associated with surgery. Improved cardiovascular and respiratory fitness, and the potential benefit of improved response to surgery‐related stress, may benefit people undergoing AAA repair (Grant 2015; Prentis 2012; Thompson 2011).
Why it is important to do this review
Perioperative and postoperative complications are common following elective repair in people with AAA. For instance, the estimated prevalence of morbidity is 28% following open AAA repair and 12% following EVAR (Giles 2010). There is a growing interest in the role of prehabilitation or preoperative exercise therapy for people with AAA undergoing elective repair. Three previous reviews have been conducted on the impact of exercise in people with AAA (Kato 2019; Pouwels 2015; Wee 2019). However, these reviews focused on heterogeneous populations with or without indications for surgery. The outcomes of prehabilitation or preoperative exercise therapy for people undergoing AAA repair is unclear from these reviews. If prehabilitation exercise decreases complications and the length of hospital stay, there are benefits for participants in terms of increased quality of life and reduced re‐intervention, as well as potential cost savings. We performed a systematic review to synthesise evidence about the impact of exercise therapy prior to repair on mortality and morbidity in individuals with AAA. We also evaluated the impact of different forms of exercise therapy, and investigated whether the effect of exercise therapy is influenced by the subsequent type of repair. The findings of this review will provide evidence to help aid decision making and inform practice, with the aim of reducing the perioperative and postoperative complications reported after OSR AAA repair.
Objectives
To assess the effects of exercise programmes on perioperative and postoperative morbidity and mortality associated with elective abdominal aortic aneurysm repair.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) that compared exercise therapy with usual care (no exercise) before elective abdominal aortic aneurysm (AAA) repair.
Types of participants
We included participants aged 18 years and older, of either sex, with clinically diagnosed AAA deemed suitable for elective intervention (open surgical repair (OSR) or endovascular aneurysm repair (EVAR)). We included all types of AAA: infrarenal; juxtarenal; and suprarenal. We did not apply restrictions on the size of the aneurysm. We excluded studies that only involved participants undergoing emergency repair. If a study included both elective and emergency participants, we extracted data for the elective participants only, if the trial reported these separately.
Types of interventions
We included any prehabilitation exercise before elective AAA repair, provided that the trial compared it against usual care (no exercise therapy). The exercise therapy could be in hospital, community or home‐based settings. We included, but were not limited to, variations of exercise therapy, such as circuit training, moderate‐intensity continuous exercise, high‐intensity interval training, and inspiratory muscle training. We included upper limb and lower limb exercises, as well as both aerobic and strength training programmes. We included studies that combined exercise with other interventions (e.g. psychological counselling, structured education or behaviour change interventions), if both the exercise and no exercise study arms received the same additional interventions. We included multi‐arm studies that compared exercise with no exercise and other interventions if data were available for the exercise versus no exercise comparison.
We included both supervised and unsupervised exercise, and did not limit exercise to any frequency, duration, or intensity, but did take these variations into account in the meta‐analysis. This review also considered performing subgroup analysis of supervised versus unsupervised exercise if data were available.
We defined a supervised exercise therapy group as one in which participants underwent a programme of exercise delivered and formally supervised by a trained health professional. We defined an unsupervised exercise therapy group as one in which participants received advice to exercise without supervision (with or without a predetermined exercise regimen or logbook), or received advice to exercise on their own, with regular contact and exercise support from trained personnel (structural home‐based exercise programme). We defined a no exercise group as one in which the participants maintained normal physical activity. We aimed to analyse supervised and unsupervised therapy where possible.
Types of outcome measures
Primary outcomes
30‐day (or longer if reported) mortality post‐AAA repair
Perioperative and postoperative complications (cardiac, pulmonary, renal, infection, re‐intervention, postoperative bleeding). We defined perioperative complications as those occurring after enrolment, including preoperative events, whilst postoperative complications were defined as those occurring within one to 30 days (or longer if reported) post‐AAA repair.
Secondary outcomes
Length of intensive care unit (ICU) stay
Length of hospital stay
Number of days on a ventilator
Change in aneurysm size pre‐ and post‐exercise
Quality of life (QoL), assessed using validated physical summary score scales such as Short Form 12 (SF‐12) Health Survey (Ware 1996), Medical Outcomes Study (MOS) 36‐Item Short‐Form Health Survey (SF‐36) (Ware 1992), and Assessment of Quality of Life (AQoL) instruments (AQoL‐8D, 7D, 6D or 4D) (Hawthorne 1999).
We reported these outcomes at the last follow‐up presented by the included studies. We also aimed to report on adherence to exercise, if the included studies presented this.
Search methods for identification of studies
Electronic searches
We conducted systematic searches of the following databases for randomised controlled trials and controlled clinical trials without language, publication year or publication status restrictions:
the Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web) (searched 6 July 2020);
the Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 6) via the Cochrane Register of Studies Online (CRSO);
MEDLINE (Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE) (1946 onwards; searched 6 July 2020);
Embase Ovid (from 1974 onwards; searched 6 July 2020);
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; from 1982 onwards searched 6 July 2020);
PEDro (Physiotherapy Evidence Database), University of Sydney (searched 6 July 2020).
We modelled search strategies for other databases on the search strategy designed for MEDLINE. Where appropriate, we combined these with adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6, Lefebvre 2011). Search strategies for major databases are provided in Appendix 1.
We also searched the following trials registries on 6 July 2020:
ClinicalTrials.gov (clinicaltrials.gov);
World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch).
Searching other resources
We examined the included study reports' bibliographies to identify other relevant articles.
Data collection and analysis
Selection of studies
We identified and excluded duplicates and collated multiple reports of the same study. Three of the review authors (CF, UA, AT) independently screened the titles and abstracts from the search results, identifying those to be retrieved for full‐text review. Two of the review authors (UA, AT) independently screened the full texts and identified studies for inclusion. We resolved any disagreement by discussion until we reached a consensus. Where necessary, we consulted a fourth review author (JM). We illustrated the study selection process in a PRISMA flow diagram (Figure 1) (Liberati 2009). We listed all articles excluded after full‐text assessment in the 'Characteristics of excluded studies' table, and provided the reasons for their exclusion.
1.
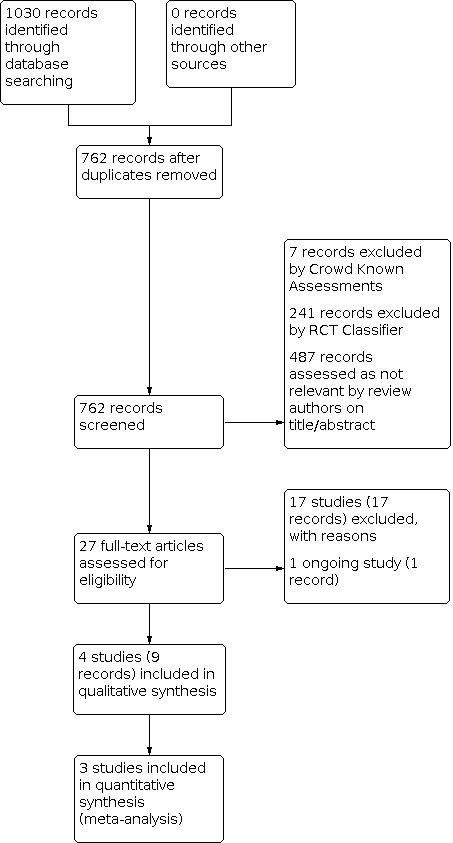
PRISMA flow diagram
We used Cochrane’s Screen4Me workflow to help assess the search results. We used two Screen4Me components: known assessments (a service that matches records in the search results to records that have already been screened in Cochrane Crowd and been labelled as 'an RCT' or as 'Not an RCT') and the RCT classifier (a machine learning model that distinguishes RCTs from non‐RCTs). The Screen4Me process is shown in Figure 2.
2.
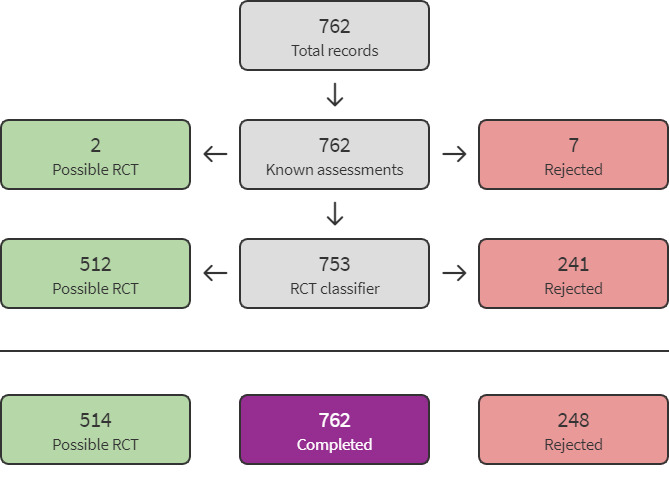
Screen4Me summary diagram
Data extraction and management
Three review authors (CF, UA, AT) independently extracted relevant population and intervention characteristics, outcome data, and risk of bias components from the included studies using a standard data extraction form, which we piloted on one study in the review. We entered data into Review Manager 5 (Review Manager 2020). We resolved any disagreement about data extraction by discussion, and consulted a fourth review author (JM) when necessary.
Assessment of risk of bias in included studies
Two review authors (UA, AT) assessed the risk of bias for all included studies, using the Cochrane risk of bias tool, described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We judged the risk of bias in the following seven domains to be low, high or unclear.
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective outcome reporting (reporting bias)
Other sources of bias
We judged the overall risk of bias of a study to be ‘high', if we judged trials to be ’unclear’ or ‘high risk’ in one or more risk of bias domains.
Measures of treatment effect
Dichotomous outcomes
We calculated risk ratios (RR) for dichotomous data, with 95% confidence intervals (CI).
Continuous outcomes
If studies measured continuous outcomes on the same scale, we planned to compare the mean difference (MD) in change scores. If studies used different scales to measure the same continuous outcomes, we planned to calculate the standardised mean difference (SMD). We used 95% CIs for all continuous data.
We planned to narratively describe skewed data reported as medians and interquartile ranges.
Unit of analysis issues
We considered each participant as the unit of analysis in the randomised trials. In RCTs with a parallel design, we took multiple treatment arms into account, when relevant, to avoid double counting. For trials that considered multiple interventions in the same group, we analysed only the partial data of interest.
Dealing with missing data
We analysed the available data and contacted trial authors to request missing data (such as the number of screened or randomised participants, lack of data regarding intention‐to‐treat (ITT) analyses, or data on as‐treated or per‐protocol analyses) in order to perform our analyses as thoroughly as possible. We reported dropout rates in the Characteristics of included studies table, and used ITT analysis. Where possible, we planned to use the Review Manager 5 calculator to calculate missing standard deviations (SD) using other data from the trial, such as CIs. Where this was not possible, and we considered the missing data to introduce serious bias, we planned to use a sensitivity analysis to explore the impact of including such studies in the overall assessment of results.
Assessment of heterogeneity
We inspected forest plots visually to consider the direction and magnitude of effects, and the degree of overlap between CIs. We quantified inconsistency among the pooled estimates using the I2 statistic (I2 = ((Q ‐ df)/Q) × 100%, where Q is the Chi2 statistic and 'df' represents the degree of freedom) (Higgins 2021). This illustrates the percentage of the variability in effect estimates that results from heterogeneity rather than sampling error (Higgins 2021). If we identified substantial heterogeneity (I2 > 50%), we reported it and explored possible causes by prespecified subgroup analysis.
Assessment of reporting biases
We planned to assess the presence of publication bias and other reporting bias using funnel plots, if we identified sufficient studies (more than 10) for inclusion in the meta‐analysis (Higgins 2021).
Data synthesis
We performed statistical analysis using RevMan 5 software (Review Manager 2020). We undertook meta‐analyses where it was meaningful to do so, i.e. if the included studies' treatments, participants, and underlying clinical questions were similar enough for pooling to make sense. We summarised the data for each study in a forest plot, and presented 95% CI for all summary estimates. We planned to report data narratively if it was not appropriate to combine data in a meta‐analysis.
We performed meta‐analyses according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). We considered a fixed‐effect model where we found no substantial heterogeneity (I2 < 50%). We planned to use a random‐effects model if we found substantial heterogeneity (I2 > 50%).
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses to investigate possible reasons for heterogeneity. Where data were available, we planned to carry out subgroup analyses based on:
participants age (≤ 80 versus > 80 years) as the over 80s are known to have higher rates of complications (Sonesson 2018);
type of repair (OSR versus EVAR);
type of exercise therapy (e.g. aerobic versus isometric; supervised versus unsupervised).
Sensitivity analysis
We aimed to conduct sensitivity analyses to establish whether findings were robust by limiting the analyses to studies with low risk of bias in the selection bias domain, the detection bias domain or both. Additionally, where missing data were thought to introduce serious bias, we aimed to explore the impact of including such studies in the overall assessment of results. However, due to the limited data available this was not possible.
Summary of findings and assessment of the certainty of the evidence
We created Table 1 to provide the key information presented in the review for the exercise versus no exercise comparison, using GRADEpro software (GRADEpro GDT). We included the following outcomes, which are of most clinical relevance:
30‐day (or longer if reported) mortality post‐AAA repair;
perioperative and postoperative complications (cardiac, pulmonary, renal, infection, re‐intervention, and postoperative bleeding);
length of ICU stay;
length of hospital stay;
number of days on a ventilator;
QoL.
We assessed the certainty of the evidence for each outcome as high, moderate, low or very low, based on the five GRADE considerations of risk of bias, inconsistency, indirectness, imprecision, and publication bias, using the GRADE approach (Atkins 2004). We based the tables on methods described in Chapters 11 and 12 of the Cochrane Handbook for Systematic Reviews of Interventions, and will justify any departures from the standard methods (Atkins 2004; Higgins 2021). Two review authors (UA, AT) independently judged the certainty of the evidence and, if required, resolved any disagreements by consensus or discussion with a third review author (CF). We justified all decisions to downgrade the evidence using footnotes and we made comments to aid the reader's understanding of the review where necessary.
Results
Description of studies
Results of the search
The search identified a total of 1030 search results, which was reduced to 762 after removing duplicates (Figure 1). In assessing the studies, we used Cochrane’s Screen4Me workflow to help identify potential reports of randomised trials. The results of the Screen4Me assessment process are shown in Figure 2. The Screen4Me assessment process excluded seven records by Crowd Known Assessments and 241 records by RCT Classifier. Of the remaining 514 records, we assessed 487 records as not relevant based on title/abstract screening.
We assessed 27 full‐text articles for eligibility; we included four studies (nine records), excluded 17 studies (17 records) with reasons and identified one ongoing study.
Included studies
See Characteristics of included studies.
We included four trials with a total number of 232 participants (Barakat 2016; Dronkers 2008; Richardson 2014; Tew 2017).
Two trials included fewer than 50 participants (Dronkers 2008; Richardson 2014), one had 53 participants (Tew 2017), and another included 136 participants (Barakat 2016). Richardson 2014 did not specify the number of participants per study arm.
Inclusion and exclusion criteria varied between the included studies, but trials typically excluded people with severe disabling disorders limiting mobility, contraindications to exercise testing or training, BMI < 20 or > 40 kg/m2, serious comorbidities that would compromise an exercise programme or make it impractical; people whose AAA was not infrarenal; people under 18 or over 80 years old; and people requiring expedited repair. No trials took a multimodal approach.
All four trials compared exercise versus usual care, but one trial did not describe the components of the usual care implemented (Richardson 2014). In the remaining three trials, usual care components varied. One trial described usual care as a ‘standard treatment’ in which participants were "clearly instructed to continue with their normal lifestyle, and avoid any additional, unsupervised exercises" (Barakat 2016). Another trial described usual care as an evidence‐based medical optimisation, without providing further details (Tew 2017). Lastly, Dronkers 2008 reported usual care as a programme of diaphragmatic breathing, deep breathing inspirations with the aid of incentive spirometer, and coughing and ‘forced expiratory technique' (FET) done one day before surgery.
Exercise regimens implemented in the included trials also varied, although most studies implemented at least two sessions weekly for a minimum of two weeks prior to surgery (Barakat 2016; Dronkers 2008; Tew 2017). However, one trial implemented a regimen of a submaximal cycling exercise at a moderate intensity implemented for three consecutive days, with the last session completed 48 hours before surgery (Richardson 2014). Types of exercise included circuit training, moderate‐intensity continuous exercise and high‐intensity interval training. Similarly, exercise intensity in included trials comprised a range of lower, moderate and high intensity programmes. Three trials specified complete supervision of exercise (Barakat 2016; Richardson 2014; Tew 2017), but in the trial by Dronkers 2008, one session per week was supervised, while the remaining five sessions per week were unsupervised. Programme duration of treatment generally fell within three days to six weeks. More details of the exercise regimens are provided in the Characteristics of included studies table.
Richardson 2014 included participants who underwent OSR. Barakat 2016 and Tew 2017 included participants who underwent either EVAR or OSR. One trial did not document the type of repair participants received (Dronkers 2008).
The included trials assessed a range of outcomes using varied outcome measures. Three trials assessed post‐repair mortality and documented mortality within 30 days (Barakat 2016; Tew 2017), or 35 days (Dronkers 2008), post‐repair. One trial additionally assessed mortality at 12 weeks post‐repair (Tew 2017). All four trials assessed at least one postoperative complication, but the range of postoperative complications and methods of assessment reported in individual trial results showed considerable variation. One trial reported data on postoperative cardiac complications, pulmonary complications and renal complications (Barakat 2016). Barakat 2016 also reported postoperative complications as a composite endpoint of cardiac, pulmonary, composite and renal complications. One trial reported on atelectasis as a postoperative pulmonary complication (Dronkers 2008). Richardson 2014 and Tew 2017 reported the use the postoperative morbidity survey (POMS) to report postoperative complications. Three trials assessed length of hospital stay (Barakat 2016; Richardson 2014; Tew 2017), whilst two trials assessed length of critical care stay (Barakat 2016; Richardson 2014), and need for intervention (Barakat 2016; Dronkers 2008). One trial each assessed postoperative bleeding or transfusion of more than four units (Barakat 2016), exercise‐related adverse events, health‐related QoL, and adherence to exercise (Tew 2017).
Excluded studies
We excluded a total of 17 studies from this review, based on full‐text assessment (Bailey 2018; Barakat 2014; Gunasekera 2014; Hayashi 2016; Lo Sapio 2014; Myers 2010; Myers 2014; NCT00349947; NCT01234610; NCT02097186; NCT02292927; NCT02767518; NCT02997618; NCT03985202; Takeuchi 2016; Tew 2012; UMIN000028237). The reasons for exclusion included:
studies investigated participants with small aneurysm without indication for repair (Bailey 2018; Gunasekera 2014; Myers 2010; Myers 2014; NCT00349947; NCT01234610; NCT02997618; Tew 2012);
studies were not RCTs (Hayashi 2016; NCT02292927; NCT03985202; UMIN000028237);
studies implemented an intervention not of relevance to this review (Lo Sapio 2014; NCT02097186);
studies focused on participants with thoracic aneurysm (NCT02767518; Takeuchi 2016);
study focused on outcomes of fitness before surgery (Barakat 2014).
Details of all excluded studies are given in the Characteristics of excluded studies table.
Ongoing studies
We identified one study as ongoing (NCT04169217). This study is detailed within the Characteristics of ongoing studies table.
Risk of bias in included studies
An overall summary of bias present within each of the included studies is presented in Figure 3 and Figure 4 (see also the Characteristics of included studies table).
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All studies were RCTs. In assigning risk of bias judgement, we considered any trial described as 'randomised' with no explanation as to how this was done as unclear risk of bias. Two trials described adequate random sequence generation and allocation concealment and were at low risk of selection bias (Barakat 2016; Tew 2017). The remaining two did not provide details on random sequence generation and allocation concealment (Dronkers 2008; Richardson 2014), other than stating that a sealed and numbered envelope was used (Dronkers 2008).
Blinding
We considered blinding of participants not practically possible as the nature of exercise‐based studies involved an activity versus usual care. To standardise our approach, we scored all trials as having low risk of bias secondary to participant blinding.
Included trials may have an additional risk of bias as outcome assessors may not be blinded to the group to which a participant was randomised. One trial indicated that an investigator blinded to group allocation assessed the outcomes, so we judged this to be at low risk of detection bias (Tew 2017). Dronkers 2008 reported that a blinded radiologist assessed the main study outcome (postoperative pulmonary complications; atelectasis). The remaining two trials did not report whether outcome assessors were blinded; we deemed these to be at unclear risk of detection bias (Barakat 2016; Richardson 2014).
Incomplete outcome data
One trial reported that there were no participants lost to follow‐up (Barakat 2016). We judged one trial to be at unclear risk of bias because only a few participants were assessed on day one and two, and the study report did not explain the reason for this (Dronkers 2008). We judged one trial to be at high risk of bias because the study abstract stated that 23 participants were enrolled, but the clinical trial registry (posted after the trial was completed) stated that 21 participants were enrolled (Richardson 2014). In addition, Richardson 2014 did not report how many participants were allocated to each study arm. The remaining trial had an attrition rate of over 20%, had a small sample size and did not implement ITT analysis; we judged this to be associated with a high risk of bias (Tew 2017).
Selective reporting
The trial by Dronkers 2008 reported all outcomes, so we judged this trial to be at low risk of reporting bias. We deemed two studies to be at high risk of bias. In the first study, the trial protocol stated that the participants’ destination would be recorded (i.e. ward or critical care), but the final study report did not present this information (Tew 2017). Similarly, the study report did not include the duration of critical care stay (Tew 2017). In the second study, the trial protocol indicated that they would measure quality of life scores, but the study paper did not report this. The trial also reported Acute Physiology and Chronic Health Evaluation II (APACHE II) scores, reoperation, and postoperative bleeding, which were not outcomes listed in the protocol (Barakat 2016). One study had an unclear risk of reporting bias, as the trial protocol was registered on clinicaltrials.gov after the study was complete (Richardson 2014).
Other potential sources of bias
Two studies were at low risk of other bias (Barakat 2016; Richardson 2014). We labelled the other two studies as having a high risk of bias, as participants in the intervention group were significantly older than the participants in the control group (70 ± 6 years versus 59 ± 6 years, respectively; P = 0.001) (Dronkers 2008), or the study was not powered to detect the effect size or clinically important difference (Tew 2017).
Effects of interventions
See: Table 1
30‐day (or longer if reported) mortality post‐AAA repair
Three trials with 192 participants reported on the occurrence of 30‐day (or longer if reported) mortality post‐AAA repair (Barakat 2016; Dronkers 2008; Tew 2017). There was no statistical heterogeneity between studies (I2 = 0%, P = 0.55), therefore we used a fixed‐effect model. Overall, we are uncertain whether prehabilitation exercise reduces the occurrence of 30‐day (or longer if reported) mortality post‐AAA repair (RR 1.33, 95% CI 0.31 to 5.77; 3 trials, 192 participants; very low‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: Exercise versus usual care (no exercise), Outcome 1: 30‐day mortality
We investigated different types of repair (OSR, EVAR and any AAA surgery) (see Analysis 1.1 and Table 2). These are summarised below. No differences were detected by the test for subgroup differences (P = 0.55).
1. Summary of findings for subgroups.
| Exercise compared to no exercise for adults with clinically diagnosed AAA deemed suitable for elective repair | ||||||
|
Patient or population: adults with clinically diagnosed AAA deemed suitable for elective repair Setting: hospital Intervention: exercise Comparison: usual care (no exercise) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care (no exercise) | Risk with exercise | |||||
| 30‐day mortality Follow‐up: 30 days |
Open surgical repair | RR 0.50 (0.05 to 5.29) | 78 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a,b | ||
| 51 per 1000 | 26 per 1000 (3 to 271) | |||||
| Endovascular aneurysm repair | RR 3.00 (0.13 to 70.02) | 46 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a,b | There were no deaths in the usual care (no exercise) group. | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Any AAA repair | RR 3.00 (0.14 to 65.90) |
68 (2 RCTs) |
⊕⊝⊝⊝ VERY LOW b c | There were no deaths in the usual care (no exercise) group. | ||
| 0 per 1000 | 0 per 1000 (0 to 0) |
|||||
| Perioperative and postoperative complications: cardiac complications Follow‐up: 3 months |
Open surgical repair | RR 0.36 (0.13 to 1.04) | 78 (1 RCT) | ⊕⊕⊝⊝ LOW a,d | ||
| 282 per 1000 | 102 per 1000 (37 to 293) | |||||
| Endovascular aneurysm repair | RR 0.33 (0.04 to 2.97) | 46 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a,b | |||
| 130 per 1000 | 43 per 1000 (5 to 387) | |||||
| Perioperative and postoperative complications: pulmonary complications Follow‐up: 3 months |
Open surgical repair | RR 0.78 (0.32 to 1.88) | 78 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a,b | ||
| 231 per 1000 | 180 per 1000 (74 to 434) | |||||
| Endovascular aneurysm repair | RR 0.11 (0.01 to 1.95) | 46 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a,b | |||
| 174 per 1000 | 19 per 1000 (2 to 339) | |||||
| Any AAA repair | RR 0.38 (0.14 to 1.02) |
20 (1 RCT) |
⊕⊝⊝⊝ VERY LOW b e | |||
| 800 per 1000 | 304 per 1000 (112 to 816) |
|||||
| Perioperative and postoperative complications: renal complications Follow‐up: 3 months |
Open surgical repair | RR 0.25 (0.08 to 0.82) | 78 (1 RCT) | ⊕⊕⊝⊝ LOW a,d | ||
| 308 per 1000 | 77 per 1000 (25 to 252) | |||||
| Endovascular aneurysm repair | RR 1.00 (0.07 to 15.04) | 46 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a,b | |||
| 43 per 1000 | 43 per 1000 (3 to 654) | |||||
| Perioperative and postoperative complications: need for re‐intervention Follow‐up: 3 months |
Open surgical repair | RR 0.67 (0.12 to 3.77) | 78 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a,b | ||
| 77 per 1000 | 52 per 1000 (9 to 290) | |||||
| Endovascular aneurysm repair | not estimable | 46 (1 RCT) |
⊕⊕⊝⊝ LOW a,d | There were no events in either of the arms. | ||
| See comments | ||||||
| Any AAA repair | RR 5.00 (0.27 to 92.62) |
20 (1 RCT) |
⊕⊝⊝⊝ VERY LOW b e | |||
| 0 per 1000 | 0 per 1000 (0 to 0) |
|||||
| Perioperative and postoperative complications: postoperative bleeding Follow‐up: 72 hours |
Open surgical repair | RR 0.57 (0.18 to 1.80) | 78 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a,b | ||
| 179 per 1000 | 102 per 1000 (32 to 323) | |||||
| Endovascular aneurysm repair | not estimable | 46 (1 RCT) | ⊕⊕⊝⊝ LOW a,d | There were no events in either of the arms. | ||
| See comments | ||||||
| Length of ICU stay (days) | Open surgical repair | ‐ | 101 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW f g | Two studies reported on length of ICU stay in OSR participants, but we could not evaluate this in a meta‐analysis. Neither of the studies found a clear difference between the exercise and usual care groups in length of ICU stay. | |
| See comments | ||||||
| Endovascular aneurysm repair | ‐ | 46 (1 RCT) |
⊕⊕⊝⊝ LOW a,d | One study reported no clear difference between the exercise and usual care group in EVAR participants (P = 0.21). | ||
| See comments | ||||||
| Length of hospital stay (days) | Open surgical repair | ‐ | 101 (2 RCTs) |
⊕⊝⊝⊝ VERY LOW f g | Two studies reported no clear difference in length of hospital stay between exercise and usual care groups. | |
| See comments | ||||||
| Endovascular aneurysm repair | ‐ | 46 (1 RCT) |
⊕⊝⊝⊝ VERY LOW a d | One study reported shorter hospital stay for the exercise group compared with the usual care group for EVAR participants (P = 0.013) | ||
| See comments | ||||||
| Any AAA repair | ‐ | 48 (1 RCT) |
⊕⊕⊝⊝ LOW h | One study reported no clear difference between exercise and usual care groups. | ||
| See comments | ||||||
| Number of days on a ventilator | See comments | ‐ | ‐ | ‐ | No studies reported number of days on a ventilator. | |
| QoL Follow‐up: 12 weeks |
Any AAA repair | ‐ | 53 (1 RCT) | ⊕⊕⊝⊝ LOWh | One study reported QoL. The study found little or no difference between the exercise and usual care group participants. | |
| See comments | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AAA: abdominal aortic aneurysm;CI: confidence interval; ICU: intensive care unit; OSR: open surgical repair; QoL: quality of life; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
a Study did not state whether outcome assessors were blinded; outcomes reported in protocol were not reported in study (risk of reporting bias) (Barakat 2016); therefore, we downgraded the certainty of evidence by 1 level for methodological limitations. b The 95% CI includes no effect, and includes default values for appreciable harm (i.e. CI > 1.25), appreciable benefit (i.e. CI < 0.75), or both; the optimal information size was not met (i.e. sample size < 2000 participants); therefore, we downgraded the certainty of evidence by 2 levels for imprecision. c High overall risk of bias due to selective reporting, selection bias, attrition bias and/or other bias (Dronkers 2008; Tew 2017); therefore, we downgraded the certainty of evidence by 2 levels for methodological limitations. d The optimal information size was not met (i.e. sample size < 2000); therefore, we downgraded the certainty of evidence by 1 level for imprecision. e Risk of bias due to selection bias, attrition bias and other bias (Dronkers 2008); therefore, we downgraded the certainty of evidence by 2 levels for methodological limitations. f High overall risk of bias due to lack of blinding of outcome assessors, selective reporting, selection bias, and/or attrition bias (Barakat 2016; Richardson 2014); therefore, we downgraded the certainty of evidence by 2 levels for methodological limitations. g Unable to assess imprecision due to the way the studies report the outcome; therefore, we downgraded the certainty of evidence by 1 level. h High overall risk of bias due to selective reporting, attrition bias and other bias (Tew 2017); therefore, we downgraded the certainty of evidence by 2 levels for methodological limitations.
Richardson 2014 reported four deaths 30 days post‐OSR in the usual care group and no deaths in the exercise group, but did not specify the number of participants in each study arm.
Open surgical repair
One study reported on participants who underwent OSR (Barakat 2016). We are uncertain whether prehabilitation exercises reduces the occurrence of 30‐day (or longer if reported) mortality post‐AAA repair compared to usual care (RR 0.50, 95% CI 0.05 to 5.29; 1 trial, 78 participants; very low‐certainty evidence; Analysis 1.1).
Endovascular aneurysm repair
One study reported on participants who underwent EVAR (Barakat 2016). We are uncertain whether prehabilitation exercises reduces the occurrence of 30‐day (or longer if reported) mortality post‐AAA repair compared to usual care (RR 3.00, 95% CI 0.13 to 70.02; 1 trial, 46 participants; very low‐certainty evidence; Analysis 1.1).
Any AAA repair
Two studies reported on participants who underwent AAA repair which was not specified (Dronkers 2008; Tew 2017). We are uncertain whether prehabilitation exercises reduces the occurrence of 30‐day (or longer if reported) mortality post‐AAA repair compared to usual care (RR 3.00, 95% CI 0.14 to 65.90; 2 trials, 68 participants; very low‐certainty evidence; Analysis 1.1).
Perioperative and postoperative complications: cardiac complications
One trial with 124 participants reported on the occurrence of cardiac complications (Barakat 2016). Overall, prehabilitation exercise may decrease the occurrence of cardiac complications compared to usual care (RR 0.36, 95% CI 0.14 to 0.92; 1 trial, 124 participants; low‐certainty evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: Exercise versus usual care (no exercise), Outcome 2: Cardiac complications
We investigated different types of repair (OSR and EVAR) (see Analysis 1.2 and Table 2). These are summarised below. No differences were detected by the test for subgroup differences (P = 0.94).
Open surgical repair
One study reported on participants who underwent OSR (Barakat 2016). Prehabilitation exercise may have little or no difference in the occurrence of cardiac complications compared to usual care (RR 0.36, 95% 0.13 to 1.04; 1 trial, 78 participants; low‐certainty evidence. Analysis 1.2).
Endovascular aneurysm repair
One study reported on participants who underwent EVAR (Barakat 2016). We are uncertain whether prehabilitation exercises reduces the occurrence of cardiac complications compared to usual care (RR 0.33, 95% CI 0.04 to 2.97; 1 trial, 46 participants; very low‐certainty evidence; Analysis 1.2).
Perioperative and postoperative complications: pulmonary complications
Two trials with 144 participants reported on the occurrence of pulmonary complications (Barakat 2016; Dronkers 2008). Moderate statistical heterogeneity (I2 = 15%, P = 0.31) was detected, but this did not meet the predetermined threshold requiring a random‐effects model (50%), so we used a fixed‐effect model. Overall, we are uncertain whether prehabilitation exercise decreases the occurrence of pulmonary complications compared to usual care (RR 0.49, 95% 0.26 to 0.92; 2 trials, 144 participants; very low‐certainty evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1: Exercise versus usual care (no exercise), Outcome 3: Pulmonary complications
We investigated different types of repair (OSR, EVAR, and any AAA surgery) (see Analysis 1.3 and Table 2). These are summarised below. No differences were detected by the test for subgroup differences (P = 0.31).
Open surgical repair
One study reported on participants who underwent OSR (Barakat 2016). No clear difference in the occurrence of pulmonary complications was detected between the exercise and usual care groups (RR 0.78, 95% 0.32 to 1.88; 1 trial, 78 participants; very low‐certainty evidence; Analysis 1.3).
Endovascular aneurysm repair
One study reported on participants who underwent EVAR (Barakat 2016). We are uncertain whether prehabilitation exercises reduces the occurrence of pulmonary complications compared to usual care (RR 0.11, 95% 0.01 to 1.95; 1 trial, 46 participants; very low‐certainty evidence; Analysis 1.3).
Any AAA repair
One study reported on participants who underwent AAA repair which was not specified (Dronkers 2008). We are uncertain whether prehabilitation exercises reduces the occurrence of pulmonary complications post‐AAA repair compared to usual care (RR 0.38, 95% CI 0.14 to 1.02; 1 trial, 20 participants; very low‐certainty evidence; Analysis 1.3).
Perioperative and postoperative complications: renal complications
One study with 124 participants reported on the occurrence of renal complications (Barakat 2016). Overall, prehabilitation exercise may reduce the risk of the occurrence of renal complications compared to usual care (RR 0.31, 95% CI 0.11 to 0.88; 1 trial, 124 participants; low‐certainty evidence; Analysis 1.4).
1.4. Analysis.

Comparison 1: Exercise versus usual care (no exercise), Outcome 4: Renal complications
We investigated different types of repair (OSR and EVAR) (see Analysis 1.4 and Table 2). These are summarised below. No differences were detected by the test for subgroup differences (P = 0.36).
Open surgical repair
One study reported on participants who underwent OSR (Barakat 2016). Prehabilitation exercise may have little or no difference in the occurrence of renal complications compared to usual care (RR 0.25, 95% 0.08 to 0.82; 1 trial, 78 participants; low‐certainty evidence; Analysis 1.4).
Endovascular aneurysm repair
One study reported on participants who underwent EVAR (Barakat 2016). We are uncertain whether prehabilitation exercises reduces the occurrence of renal complications compared to usual care (RR 1.0, 95% CI 0.07 to 15.04; 1 trial, 46 participants; very low‐certainty evidence; Analysis 1.4).
Perioperative and postoperative complications: need for re‐intervention
Two trials reported on the need for re‐intervention (Barakat 2016; Dronkers 2008). There was minimal statistical heterogeneity between the studies (I2 = 28%, P = 0.24), so we used a fixed‐effect model. We are uncertain whether prehabilitation exercise reduces the need for re‐intervention compared to usual care (RR 1.29, 95% 0.33 to 4.96; 2 trials, 144 participants; very low‐certainty evidence; Analysis 1.5).
1.5. Analysis.

Comparison 1: Exercise versus usual care (no exercise), Outcome 5: Need for re‐intervention
We investigated different types of repair (OSR, EVAR, and any AAA surgery) (see Analysis 1.5 and Table 2). These are summarised below. No differences were detected by the test for subgroup differences (P = 0.24).
Open surgical repair
One study reported on participants who underwent OSR (Barakat 2016). We are uncertain whether prehabilitation exercises reduces the need for re‐intervention compared to usual care (RR 0.67, 95%, CI 0.12 to 3.77; 1 trial, 78 participants; very low‐certainty evidence; Analysis 1.5).
Endovascular aneurysm repair
One study reported on participants who underwent EVAR (Barakat 2016). There were no events in either of the arms (46 participants; low‐certainty evidence; Analysis 1.5).
Any AAA repair
One study reported on participants who underwent AAA repair which was not specified (Dronkers 2008). We are uncertain whether prehabilitation exercises reduces the need for re‐intervention post‐AAA repair compared to usual care (RR 5.00, 95% CI 0.27 to 92.62; 1 trial, 20 participants; very low‐certainty evidence; Analysis 1.5).
Perioperative and postoperative complications: postoperative bleeding
One trial with 124 participants reported on the occurrence of postoperative bleeding requiring transfusion (Barakat 2016). Overall, we are uncertain whether prehabilitation exercises reduces the occurrence of postoperative bleeding compared to usual care (RR 0.57, 95% CI 0.18 to 1.80; 1 trial, 124 participants; very low‐certainty evidence; Analysis 1.6).
1.6. Analysis.

Comparison 1: Exercise versus usual care (no exercise), Outcome 6: Postoperative bleeding
We investigated different types of repair (OSR and EVAR) (see Analysis 1.6 and Table 2). These are summarised below.
Open surgical repair
One study reported on participants who underwent OSR (Barakat 2016). We are uncertain whether prehabilitation exercises reduces the occurrence of postoperative bleeding compared to usual care (RR 0.57, 95% CI 0.18 to 1.80; 1 trial, 78 participants; very low‐certainty evidence; Analysis 1.6).
Endovascular aneurysm repair
One study reported on participants who underwent EVAR (Barakat 2016). There were no events in either of the arms (46 participants; low‐certainty evidence; Analysis 1.6).
Length of intensive care unit (ICU) stay
Two studies reported on length of critical care stay (Barakat 2016; Richardson 2014).
Barakat 2016 reported length of critical care stay as the median number of days, with the interquartile range (IQR). For exercise group participants, length of critical care stay was 1.0 days (IQR 1.0 to 2.0) compared to 2.0 days (IQR 1.0 to 2.0) for usual care group participants (P = 0.85). For EVAR participants in the exercise group, the median length of critical care stay was not reported. The study paper gave the IQR as 1.0 to 1.0 days for the EVAR exercise group participants. For the EVAR participants in the usual care group, the median length of critical care stay was 1.0 (IQR 1.0 to 1.0). Barakat 2016 reported no clear differences between the exercise and usual care groups (P = 0.21) for participants undergoing EVAR. For OSR exercise group participants the length of critical care stay was 2.0 days (IQR 1.0 to 3.0), and for the OSR usual care group participants the length of critical care stay was 2.0 days (IQR 1.0 to 2.3). Barakat 2016 reported no clear differences between the exercise and usual care groups (P = 0.74).
Richardson 2014 reported length of stay in the intensive care unit (ICU) for OSR participants as six days in the usual care group compared with five days in the exercise group. For the for high dependency unit (HDU), the length of stay was three days for the usual care group and two days for the exercise group, with no clear differences between the groups. Richardson 2014 did not report number of participants per study arm.
Length of hospital stay
Three studies reported on length of stay in hospital (Barakat 2016; Richardson 2014; Tew 2017).
Barakat 2016 reported length of hospital stay as a median number of days with the IQR and P values for differences between the exercise and usual care groups. For exercise group participants, length of hospital stay was 7.0 days (IQR 5.0 to 9.0), compared to 8.0 days (IQR 6.0 to 12.3) for usual care group participants (P = 0.025). For EVAR participants, the length of hospital stay was 4.0 days (IQR 3.0 to 6.0) compared to 5.0 days (IQR 4.0 to 9.0) in the EVAR usual care group participants (P = 0.013). For OSR exercise group participants, the length of hospital stay was 8.5 days (IQR 7.0 to 10.0) compared to 9.0 days (IQR 7.5 to 13.5) for OSR control group participants (P = 0.14).
Tew 2017 reported that "The unadjusted median duration of hospital stay was 7 (IQR 4.5–8.5) days in the exercise group and 6 (IQR 4–8) days in the control group (48 participants)."
Richardson 2014 reported that the total length of stay in hospital for participants in the usual care group was 13 days, and for the exercise group it was 11 days (P > 0.05). However, they did not report the number of participants per study arm.
Number of days on a ventilator
No studies reported on number of days on a ventilator.
Change in aneurysm size pre‐ and post‐exercise
No studies reported on change in aneurysm size.
Quality of life
One study (Tew 2017), reported quality of life (QoL) and used the EQ‐5D, EQ‐VAS and SF‐36 measures. The EQ‐5D measure comprises five dimensions: mobility; self‐care; usual activities; pain/discomfort; and anxiety/depression (scores 0 to 1, 0 being as bad as dead, 1 being full health). The EQ‐VAS score records the participant’s self‐rated health on a vertical visual analogue scale, (scored 0 to 100, 0 'The worst health you can imagine’, 100 ‘The best health you can imagine’). The 36‐Item Short Form Survey (SF‐36) measure consists of eight scores covering physical and mental health, (scored 0‐100, 0 equivalent to maximum disability, 100 equivalent to no disability). The SF‐36 PH and SF‐36 MH are the physical function (PF) and mental health (MH) subscales of the SF‐36 scale.
After five weeks, the mean EQ‐5D utility score was 0.864 for the exercise group and 0.796 for the usual care group (difference 0.068, 95% CI 0.00 to 0.14). The mean EQ‐VAS score was 81.9 for the exercise group and 75.8 for the usual care group (difference 6.1, 95% CI ‐0.3 to 12.6). The mean SF‐36 PF score for the exercise group was 49.6, and for the usual care group it was 49.9 (difference ‐0.3, 95% CI ‐2.7 to 2.1). The mean SF‐36 MH score was 54.6 for the exercise group and 55.1 for the usual group (difference ‐0.5, 95% CI ‐3.3 to 2.3).
After 12 weeks, the mean EQ‐5D utility score was 0.84 for the exercise group and 0.76 for the usual group (difference 0.08, 95% CI 0.00 to 0.15). The mean EQ‐VAS score for the exercise group was 79.6, and it was 74.4 for the usual care group (difference 5.2, 95% CI ‐1.7 to 12.0). The mean SF‐36 PF score was 49.4 for the exercise group and 46.5 for the usual care group (difference 2.9, 95% CI 0.4 to 5.4). The mean SF‐36 MH score was 55.6 for the exercise group and 55.0 for the usual care group (difference 0.6, 95% CI ‐2.4 to 3.6).
Adherence to exercise
Tew 2017 defined participants as adherent if they completed at least 75% of the main‐phase sessions (at least nine of 12 sessions), plus all weekly maintenance sessions if surgery was delayed. Tew 2017 reported that 17/27 participants randomised to exercise achieved the adherence criterion (63%, 95% CI 35% to 81%).
Discussion
Summary of main results
This review identified four RCTs with a total of 232 participants who had clinically diagnosed AAA deemed suitable for elective intervention. The RCTs compared prehabilitation exercise therapy with usual care (no exercise). We deemed all trials to be at high overall risk of bias. The certainty of the evidence for our outcomes was low to very low.
The prehabilitation exercise therapy was supervised and hospital‐based in three of the included trials (Barakat 2016; Richardson 2014; Tew 2017). In the other trial (Dronkers 2008), the first session was supervised in hospital but subsequent sessions were completed unsupervised in the participants’ homes. The dose and schedule of the prehabilitation exercise therapy varied across the trials, with three to six sessions per week and a duration of one hour per session for a period of one to six weeks. The types of exercise therapy included circuit training, moderate‐intensity continuous exercise and high‐intensity interval training. The trials had different approaches to their control groups. Barakat 2016 advised those in the control group to "continue with their normal lifestyle, and avoid any additional, unsupervised exercises", Dronkers 2008 provided the control group with instruction on breathing techniques one day prior to surgery, and two trials did not provide details for the control group (Richardson 2014; Tew 2017).
Due to very low‐certainty evidence, we are uncertain whether prehabilitation exercise therapy reduces 30‐day mortality, pulmonary complications, need for re‐intervention or postoperative bleeding. Prehabilitation exercise therapy might slightly reduce cardiac and renal complications compared with no exercise. These results are summarised in Table 1. We deemed all trials to be at high overall risk of bias, so it is highly likely that our results overestimate benefit and underestimate harm.
None of the included trials reported data for the secondary outcomes that could be analysed in a meta‐analysis. However, we have reported evidence narratively for length of ICU stay, length of hospital stay, and quality of life. None of the studies reported data for the number of days on a ventilator, or change in aneurysm size pre‐ and post‐exercise. One study reported adherence to exercise outcomes.
There were insufficient data to perform subgroup analyses based on participants’ age or type of exercise therapy. Tests for subgroup differences showed no evidence of a difference between groups based on the type of AAA repair.
Our main results are summarised in Table 1. The results of the subgroup analyses are summarised in Table 2.
Overall completeness and applicability of evidence
We searched for RCTs irrespective of language, publication year, publication type and publication status. We also searched ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform to identify ongoing trials or any that had not yet been published.
There was an insufficient number of trials to assess reporting bias using funnel plots for any of the stated outcomes. None of the included trials reported data for the secondary outcomes that could be analysed in a meta‐analysis. Data for length of ICU stay, length of hospital stay, and quality of life were reported narratively. None of the studies reported data for the number of days on a ventilator, and change in aneurysm size pre‐ and post‐exercise. The minimal data for secondary outcomes combined with the low and very low certainty of the outcomes means that the findings should be interpreted with caution.
This review assessed clinically‐relevant postoperative outcomes, such as mortality and perioperative/postoperative complications, and did not consider postintervention evaluation to assess the health benefit of prehabilitation exercise therapy and how this may have affected postoperative outcomes.
The conclusions of this review are based on a limited number of RCTs. There is a need for high quality RCTs to provide more conclusive evidence on the effectiveness of prehabilitation exercise therapy before AAA repair. Additionally, future studies should investigate the influence of prehabilitation exercise therapy on the secondary outcomes described previously.
Quality of the evidence
We used the GRADE approach to assess the certainty of evidence of each predefined outcome (Atkins 2004). The GRADE assessments showed that the evidence ranged from very low certainty to low certainty. Accordingly, there is a high risk that future trials may overturn the results of the current review. The reasons for the GRADE assessments are described below and in the footnotes of Table 1 for the included studies' results at longest available follow‐up.
The lack and quality of the reporting of the methods in the majority of the included trials made it difficult to assess their risk of bias. We judged the overall risk of bias in all the trials to be ‘high risk’, as we judged these trials to be ’unclear’ or ‘high risk’ in one or more risk of bias domains. These studies had limitations including lack of reporting of random sequence generation, lack of blinding of outcome assessors, selective reporting, attrition bias, or other bias. For risk of bias, we downgraded by one level if 50% or less of the included trials had a high overall risk of bias, and by two levels if more than 50% of the included trials had a high overall risk of bias. However, for outcomes where Barakat 2016 was the only study contributing data, we only downgraded by one level as the main methodological limitation for this study was lack of reporting on whether outcome assessors were blinded, and we did not deem this significant enough to downgrade by two levels. For a summary of risk of bias, see Figure 4.
The degree of variability between three trials included in the meta‐analyses was never greater than 50%, which suggests that substantial heterogeneity was not a concern. Therefore, we assessed the risk of inconsistency as not serious.
We assessed the degree of imprecision in the results and downgraded by one level if the number of events was too low to calculate a precise effect estimate, or if the 95% CIs included both no effect and appreciable harm and appreciable benefit. This was evident in all of the results, and therefore we downgraded results for serious concerns (one level) or very serious concerns (two levels).
There was no risk of indirectness for any of the included studies, so we did not downgrade any of the outcomes for this domain. We did not detect a risk of publication bias, so did not downgrade any of the outcomes for this.
Potential biases in the review process
Strengths
The review was conducted according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). We followed the peer‐reviewed published protocol (Fenton 2020), which predefined participants, interventions, comparisons, and outcomes, with the intention of avoiding biases during review preparation. We performed a comprehensive literature search to identify published and unpublished studies according to our prespecified inclusion and exclusion criteria. We located full‐text publications of all included trials and, where possible, conducted meta‐analysis using available data from these trials. We assessed outcomes at last follow‐up presented by the included studies. We thoroughly assessed risk of bias for each trial to assess the risks of systematic errors (’bias’) (Higgins 2021), and assessed the certainty of the evidence according to GRADE (Atkins 2004; Higgins 2021).
Limitations
Our review has some limitations. Although we contacted authors for missing data or trial information, we obtained a poor response. Any literature searches hold the risk of missing items, e.g. limitations by number of databases, and limits due to search terms and filters.
Agreements and disagreements with other studies or reviews
This is the first Cochrane Review on prehabilitation exercise for postoperative outcomes in AAA. The results are consistent with other previous non‐Cochrane reviews (Barakat 2014; Pouwels 2015; Wee 2019), although these reviews reported on heterogeneous populations with or without indications for surgery and did not employ RoB and GRADE. This review adds to the body of literature, which has highlighted the dearth of good‐quality evidence supporting prehabilitation exercise for postoperative outcomes in AAA. The evidence is also in line with a recent National Institute for Health and Care Excellence (NICE) guideline (NICE 2020), and the European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto‐iliac artery aneurysms (Wanhainen 2019). These guidelines considered that the evidence on preoperative exercise interventions was not robust enough to support a recommendation on prehabilitation exercise for postoperative outcomes in individual with AAA undergoing elective surgical repair.
Authors' conclusions
Implications for practice.
We are uncertain whether prehabilitation exercise therapy reduces 30‐day mortality, pulmonary complications, need for re‐intervention or postoperative bleeding, due to very low‐certainty evidence from this review. Although there was evidence that prehabilitation exercise therapy might slightly reduce cardiac and renal complications compared with no exercise, all trials were at high overall risk of bias so it is likely that our results overestimate benefit and underestimate harm. The quantity of randomised controlled trials (RCTs) was limited, the overall sample size was relatively small, and the methodological limitations and imprecision of the included RCTs meant that we judged the certainty of this evidence to be low to very low. Therefore, this review could not find sufficient evidence of the benefit of prehabilitation exercise on postoperative outcomes for people with unruptured large‐size abdominal aortic aneurysm (AAA) in whom surgery is planned. The overall evidence from available trials was insufficient for us to draw conclusions.
Implications for research.
We were only able to include four studies in this review, with a small overall sample size. More RCTs of high methodological quality and with large sample sizes are needed to provide sufficient evidence for the benefit of prehabilitation exercise on postoperative outcomes in people with large AAA planned for repair. The body of evidence is small and the certainty of evidence is low. However, some NHS hospitals provide prehabilitation exercise programmes for people with AAA undergoing elective repair as part of research projects. Therefore, research into the effectiveness of these programmes is needed to inform funding decisions. Important questions should include the type of exercise programmes, the minimum number of sessions and the programme duration needed to effect clinically important benefits, and which groups of people and types of repair benefit most. It will also be important to understand cost‐effectiveness of prehabilitation, including preoperative exercise programmes, for improving outcomes for people who are having repair of an AAA.
Previous research is limited to short‐term outcomes, and trials with long‐term follow‐up are required to understand both the short‐term and longer‐term benefits. Both perioperative morbidity and mortality outcomes, postoperative complications, need for intervention, cardiovascular events, quality of life, and adverse effects are important outcomes and should be reported. Future trials should also report other outcomes, including length of ICU stay, length of hospital stay, number of days on a ventilator, quality of life, and adherence to exercise. Finally, outcomes should be standardised and reporting should be done in a manner that is analysable, as reporting composite outcomes makes it difficult to establish specific benefits (or harms) associated with the intervention.
History
Protocol first published: Issue 6, 2020
Acknowledgements
We would like to acknowledge the support of the Cochrane Vascular Group Editorial Base.
The review authors, and the Cochrane Vascular Editorial Base, are grateful to the following peer reviewers for their time and comments: Mr Adam Haque, University of Manchester, UK; Dr Aoife Kiernan, St Vincent's University Hospital, Dublin, Ireland; Dr Jonathan Moran, Saint James’s Hospital, Dublin, Ireland.
Appendices
Appendix 1. Search strategies
| Source | Search strategy | Hits retrieved |
| VASCULAR REGISTER IN CRS (Search date 6 July 2020) |
#1 Aortic Aneurysm AND INREGISTER #2 Aneurysm, Ruptured AND INREGISTER #3 Aorta, Abdominal AND INREGISTER #4 AAA AND INREGISTER #5 aneurysm* adj4 (abdom* or thoracoabdom* or thoraco‐abdom* or aort*) AND INREGISTER #6 aort* adj3 (balloon* or dilat* or bulg*) AND INREGISTER #7 abdom* adj3 (balloon* or dilat* or bulg*) AND INREGISTER #8 aneurism* adj4 (abdom* or thoracoabdom* or thoraco‐abdom* or aort*) AND INREGISTER #9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 #10 Exercise AND INREGISTER #11 Preoperative Care AND INREGISTER #12 home based train* AND INREGISTER #13 Interval Train* AND INREGISTER #14 Physical activit* AND INREGISTER #15 Physical train* AND INREGISTER #16 Physical Therap* AND INREGISTER #17 physiotherapy AND INREGISTER #18 prehabilitat* AND INREGISTER #19 pre‐habilitation AND INREGISTER #20 physical fitness AND INREGISTER #21 pre‐habilitation AND INREGISTER #22 #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #9 AND #22 |
6 July 2020: 52 |
| CENTRAL via CRSO (Search date 6 July 2020) |
#1 MESH DESCRIPTOR Aortic Aneurysm EXPLODE ALL TREES 766 #2 MESH DESCRIPTOR Aneurysm, Ruptured EXPLODE ALL TREES 187 #3 MESH DESCRIPTOR Aorta, Abdominal EXPLODE ALL TREES 330 #4 AAA*:TI,AB,KY 1108 #5 (aneurysm* adj4 (abdom* or thoracoabdom* or thoraco‐abdom* or aort*)):TI,AB,KY 956 #6 (aort* adj3 (balloon* or dilat* or bulg*)):TI,AB,KY 653 #7 (abdom* adj3 (balloon* or dilat* or bulg*)):TI,AB,KY 46 #8 (aneurism* adj4 (abdom* or thoracoabdom* or thoraco‐abdom* or aort*)):TI,AB,KY 3 #9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 2820 #10 MESH DESCRIPTOR Exercise EXPLODE ALL TREES 23631 #11 MESH DESCRIPTOR Exercise Therapy EXPLODE ALL TREES 13112 #12 MESH DESCRIPTOR Preoperative Care EXPLODE ALL TREES 5817 #13 (home based train*):TI,AB,KY 207 #14 (Interval Train*):TI,AB,KY 2280 #15 (Physical activit*):TI,AB,KY 28514 #16 (Physical train*):TI,AB,KY 1758 #17 (Physical Therap*):TI,AB,KY 9739 #18 Exercis*:TI,AB,KY 97206 #19 physiotherapy:TI,AB,KY 11138 #20 prehabilitat*:TI,AB,KY 288 #21 pre‐habilitation:TI,AB,KY 32 #22 (physical fitness):TI,AB,KY 4634 #23 pre‐habilitation:TI,AB,KY 32 #24 #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 131201 #25 #9 AND #24 160 |
6 July 2020: 160 |
| Clinicaltrials.gov (Search date 6 July 2020) |
Aortic Aneurysm OR Aneurysm, Ruptured OR Aorta, Abdominal OR AAA | Exercise OR Exercise Therapy OR Preoperative Care OR physical activity OR physiotherapy OR physical fitness OR prehabilitation | 6 July 2020: 48 |
| ICTRP Search Portal (Database not available 6 July 2020) |
Aortic Aneurysm OR Aneurysm, Ruptured OR Aorta, Abdominal OR AAA | Exercise OR Exercise Therapy OR Preoperative Care OR physical activity OR physiotherapy OR physical fitness OR prehabilitation | |
| Medline (Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE) 1946 to present (Search date 6 July 2020) |
1 exp Aortic Aneurysm/ 2 exp Aneurysm, Ruptured/ 3 exp Aorta, Abdominal/ 4 AAA*.ti,ab. 5 (aneurysm* adj4 (abdom* or thoracoabdom* or thoraco‐abdom* or aort*)).ti,ab. 6 (aort* adj3 (balloon* or dilat* or bulg*)).ti,ab. 7 (abdom* adj3 (balloon* or dilat* or bulg*)).ti,ab. 8 (aneurism* adj4 (abdom* or thoracoabdom* or thoraco‐abdom* or aort*)).ti,ab. 9 or/1‐8 10 exp Exercise/ 11 exp Exercise Therapy/ 12 exp Preoperative Care/ 13 "home based train*".ti,ab. 14 "Interval Train*".ti,ab. 15 "Physical activit*".ti,ab. 16 "Physical train*".ti,ab. 17 "Physical Therap*".ti,ab. 18 Exercis*.ti,ab. 19 physiotherapy.ti,ab. 20 prehabilitat*.ti,ab. 21 pre‐habilitation.ti,ab. 22 "physical fitness".ti,ab. 23 pre‐habilitation.ti,ab. 24 or/10‐23 25 9 and 24 26 randomized controlled trial.pt. 27 controlled clinical trial.pt. 28 randomized.ab. 29 placebo.ab. 30 drug therapy.fs. 31 randomly.ab. 32 trial.ab. 33 groups.ab. 34 or/26‐33 35 exp animals/ not humans.sh. 36 34 not 35 37 25 and 36 |
6 July 2020: 327 |
| EMBASE via OVID (Search date 6 July 2020) |
1 exp aortic aneurysm/ 2 exp aneurysm rupture/ 3 exp abdominal aorta/ 4 AAA*.ti,ab. 5 (aneurysm* adj4 (abdom* or thoracoabdom* or thoraco‐abdom* or aort*)).ti,ab. 6 (aort* adj3 (balloon* or dilat* or bulg*)).ti,ab. 7 (abdom* adj3 (balloon* or dilat* or bulg*)).ti,ab. 8 (aneurism* adj4 (abdom* or thoracoabdom* or thoraco‐abdom* or aort*)).ti,ab. 9 or/1‐8 10 exp exercise/ 11 exp kinesiotherapy/ 12 exp preoperative care/ 13 "home based train*".ti,ab. 14 "Interval Train*".ti,ab. 15 "Physical activit*".ti,ab. 16 "Physical train*".ti,ab. 17 "Physical Therap*".ti,ab. 18 Exercis*.ti,ab. 19 physiotherapy.ti,ab. 20 prehabilitat*.ti,ab. 21 pre‐habilitation.ti,ab. 22 "physical fitness".ti,ab. 23 pre‐habilitation.ti,ab. 24 or/10‐23 25 9 and 24 26 randomized controlled trial/ 27 controlled clinical trial/ 28 random$.ti,ab. 29 randomization/ 30 intermethod comparison/ 31 placebo.ti,ab. 32 (compare or compared or comparison).ti. 33 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 34 (open adj label).ti,ab. 35 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 36 double blind procedure/ 37 parallel group$1.ti,ab. 38 (crossover or cross over).ti,ab. 39 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 40 (assigned or allocated).ti,ab. 41 (controlled adj7 (study or design or trial)).ti,ab. 42 (volunteer or volunteers).ti,ab. 43 trial.ti. 44 or/26‐43 45 25 and 44 |
6 July 2020: 387 |
| CINAHL via EBSCO (Search date 6 July 2020) |
S39 S24 AND S38 S38 S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 S37 MH "Random Assignment" S36 MH "Triple‐Blind Studies" S35 MH "Double‐Blind Studies" S34 MH "Single‐Blind Studies" S33 MH "Crossover Design" S32 MH "Factorial Design" S31 MH "Placebos" S30 MH "Clinical Trials" S29 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" S28 TX crossover OR "cross‐over" S27 TX random* S26 TX trial* S25 TX "latin square" S24 S8 AND S23 S23 S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 S22 TX pre‐habilitation S21 TX "physical fitness" S20 TX pre‐habilitation S19 TX prehabilitat* S18 TX physiotherapy S17 TX Exercis* S16 TX "Physical Therap*" S15 TX "Physical train*" S14 TX "Physical activit*" S13 TX "Interval Train*" S12 TX "home based train*" S11 (MH "Preoperative Care+") S10 (MH "Therapeutic Exercise+") S9 (MH "Exercise+") S8 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 S7 TX (aneurism* N4 (abdom* or thoracoabdom* or thoraco‐abdom* or aort*)) S6 TX (abdom* N3 (balloon* or dilat* or bulg*)) S5 TX (aort* N3 (balloon* or dilat* or bulg*)) S4 TX (aneurysm* N4 (abdom* or thoracoabdom* or thoraco‐abdom* or aort*)) S3 TX AAA* S2 (MH "Aorta, Abdominal") S1 (MH "Aortic Aneurysm+") |
6 July 2020: 33 |
| PEDro (Search date 6 July 2020) |
Aortic Aneurysm OR Aneurysm, Ruptured OR Aorta, Abdominal OR AAA | 6 July 2020: 23 |
Data and analyses
Comparison 1. Exercise versus usual care (no exercise).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 30‐day mortality | 3 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.77] |
| 1.1.1 Open surgical repair | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.29] |
| 1.1.2 Endovascular aneurysm repair | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.13, 70.02] |
| 1.1.3 Any AAA repair | 2 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.14, 65.90] |
| 1.2 Cardiac complications | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.14, 0.92] |
| 1.2.1 Open surgical repair | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.13, 1.04] |
| 1.2.2 Endovascular aneurysm repair | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.97] |
| 1.3 Pulmonary complications | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.26, 0.92] |
| 1.3.1 Open surgical repair | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.32, 1.88] |
| 1.3.2 Endovascular aneurysm repair | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.95] |
| 1.3.3 Any AAA repair | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.14, 1.02] |
| 1.4 Renal complications | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.11, 0.88] |
| 1.4.1 Open surgical repair | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.08, 0.82] |
| 1.4.2 Endovascular aneurysm repair | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.07, 15.04] |
| 1.5 Need for re‐intervention | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.33, 4.96] |
| 1.5.1 Open surgical repair | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.77] |
| 1.5.2 Endovascular aneurysm repair | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.5.3 Any AAA repair | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.00 [0.27, 92.62] |
| 1.6 Postoperative bleeding | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.18, 1.80] |
| 1.6.1 Open surgical repair | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.18, 1.80] |
| 1.6.2 Endovascular aneurysm repair | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barakat 2016.
| Study characteristics | ||
| Methods | Study design: single‐centre, prospective RCT Number of participants: 136 people were randomised Exclusions post‐randomisation: 12 participants (6 from each group) withdrew from the study before operative interventions as their procedures were cancelled or postponed Losses to follow‐up: no participants were lost to follow‐up Intention‐to‐treat analysis: study authors report ITT analysis was performed but that 12 participants (6 in each group) withdrew because their procedures were cancelled or postponed. Duration/dates study conducted: September 2009 to January 2014 |
|
| Participants | Country: UK Setting: tertiary vascular surgical centre Age: 73.4 (SD 7.2); intervention = 73.8 (6.5), control = 72.9 (7.9) Sex: 111 men and 13 women Inclusion criteria: people scheduled for elective open or endovascular repair; older than 18 years; AAA ≥ 5.5 cm in maximum diameter; able to give informed written consent Exclusion criteria: presence of factors that may limit exercise participation, such as; severe musculoskeletal disorders; those requiring expedited or urgent aneurysm repair; thoracic aortic aneurysms |
|
| Interventions | Intervention group (and sample size): n = 62 Supervised exercise group. Hospital‐based exercise classes, carried out 3 times a week, for 1‐hour duration, in the physiotherapy gym, for 6 consecutive weeks. The scheduled exercise program was for a total of 6 consecutive weeks immediately preceding the intended operation date. Each exercise class consisted of the following: 5‐minute warm up and stretching, cycle ergometer against moderate resistance for 2 minutes, heel‐raise repetitions for 2 minutes, knee extensions against resistance repetitions for 2 minutes, dumbbells biceps/arm curls repetitions for 2 minutes, step‐up lunges repetitions for 2 minutes, knee bends (bodyweight) repetitions for 2 minutes, and 5 minutes for cool down and stretching. Between each of the exercise stations, participants either walked around the gym or on a treadmill, or rested for 2 minutes before moving on to the next exercise. Control group (and sample size): n = 62 Standard treatment, i.e. "Patients allocated to the control group were clearly instructed to continue with their normal lifestyle, and avoid any additional, unsupervised exercises" |
|
| Outcomes | Primary outcome: composite endpoint of cardiac, pulmonary, and renal complications Secondary outcome: 30‐day mortality; lengths of hospital and critical care stay; Acute Physiology and Chronic Health Evaluation II (APACHE II) scores; reoperation; postoperative bleeding |
|
| Notes | The authors did not receive any funding. Declaration of interest study authors: "No competing interests have been declared." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence prepared by an independent professional. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed using opaque, sealed, identical envelopes containing the treatment allocation, according to a computer‐generated sequence prepared by an independent professional. Participants were randomised into one of the two groups: the exercise (intervention) group or the standard treatment (control) group. The randomisation process was witnessed by an independent research professional and was carried out during the initial visit after obtaining informed consent, but before preoperative assessments and interventions. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Clinicians including consultant surgeons, anaesthetists, department’s medical and nursing staff, and interventional radiologists were blinded to participant group allocation. Due to the nature of the intervention it is not possible to blind participants and this score is for personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study reported that personnel were blinded, but did not state whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Protocol stated that they would measure quality of life scores, but this was not reported in the paper. The paper reported APACHE II scores, reoperation, and postoperative bleeding, which were not outcomes listed in the protocol. |
| Other bias | Low risk | No evidence of other bias. |
Dronkers 2008.
| Study characteristics | ||
| Methods | Study design: single‐blind randomised controlled trial Number of participants: 20 people were randomised Exclusions post‐randomisation: there were no exclusions post‐randomisation Losses to follow‐up: intervention = 2 (could not be followed up during the first 7 days after surgery because of acute reoperation for blood vessel occlusion in the leg); control = 2 (dropped out because they were not registered at the department of physical therapy when they were admitted to hospital and thus follow‐up was not possible) Intention‐to‐treat analysis: intervention = 10, control = 10 Duration/dates study conducted: not reported |
|
| Participants | Country: The Netherlands Setting: Gelderse Vallei Hospital Age, years (SD): intervention = 70 (6), control = 59 (6) Sex: intervention, women/men = 8/2, control, women/men= 7/3 Inclusion criteria: elective surgery for aneurysm of the abdominal aorta with a scheduled delay until surgery of at least two weeks (type of surgery not specified); at least one of the following risk factors: age > 65 years; smoking less than two months before surgery; COPD; and overweight (BMI > 27 kg/m2); proficient in Dutch; able to perform a valid spirometry test. Exclusion criteria: cerebrovascular disorders; immunosuppressive treatment < 30 days before the operation neuromuscular diseases; lung surgery in the medical history; cardiovascular instability; treatment by a physical therapist within 8 weeks before elective abdominal aortic aneurysm surgery. |
|
| Interventions | Intervention group (and sample size): n = 10 The intervention group took part in a training programme (6 sessions, 6 days a week for at least 2 weeks before surgery) designed to increase the strength and endurance of the inspiratory muscles. Each session consisted of 15 minutes of inspiratory muscle training; 1 session/week was supervised by the same physical therapist and the other 5 sessions were unsupervised. The participants were instructed to keep a daily diary during the study and were trained to use an inspiratory threshold‐loading device. The intervention group received care as usual 2 to 3 weeks before surgery, consisting of instruction in (a) diaphragmatic breathing, (b) deep inspirations with the aid of incentive spirometer, and (c) coughing and ‘forced expiration techniques’ (FET). Control group (and sample size): n = 10 Care as usual, consisting of instruction in (a) diaphragmatic breathing, (b) deep inspirations with the aid of incentive spirometer, and (c) coughing and FET. The control group received this usual care one day before surgery. |
|
| Outcomes | Primary outcome: postoperative pulmonary complications (atelectasis); feasibility (occurrence of adverse events during testing or training and participant satisfaction) Secondary outcomes: postoperative respiratory function (MIP, inspiratory muscle endurance, inspiratory vital capacity); inspiratory muscle strength (MIP at residual volume) |
|
| Notes | Funding source not reported. No conflict of interest declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Sealed and numbered envelope. However, it was not mentioned if envelope was opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Due to the nature of the intervention it is not possible to blind participants and this score is for personnel. The main postoperative outcome was atelectasis as diagnosed at the base of X‐rays by a blinded radiologist. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Main outcome assessed by blinded radiologist. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Two participants in control arm dropped out and 2 participants in intervention arm could not be followed up for the first 7 days after surgery. Methods stated ITT analysis was used. Data missing for several participants for each outcome. |
| Selective reporting (reporting bias) | Low risk | Reported mortality, but was not listed as one of the measured outcomes "The main outcome measure was postoperative pulmonary complications, operationalized as atelectasis," Secondary outcome measures were postoperative respiratory function determined by MIP, and the inspiratory vital capacity. All outcomes reported |
| Other bias | High risk | "Despite randomization, patients in the intervention group were significantly older than the patients in the control group (70 plus/minus 6 years versus 59 plus/minus 6 years, respectively; P = 0.001)." |
Richardson 2014.
| Study characteristics | ||
| Methods | Study design: single‐blinded randomised control trial Number of participants: 23 people were randomised Exclusions post‐randomisation: 0 Losses to follow‐up: abstract stated that 23 people were enrolled, but clinical trial registry posted after trial was complete stated that 21 people were enrolled Intention‐to‐treat analysis: NR Duration/dates study conducted: September 2011 to May 2015 |
|
| Participants | Country: UK Setting: Medway Maritime Hospital Age: NR Sex: NR Inclusion criteria: people aged > 18 years; able to give informed consent; able to comply with the study protocol; undergoing open surgical repair for an asymptomatic perirenal and infrarenal abdominal aortic aneurysm. Exclusion criteria: people with severe disabling disorders limiting mobility, e.g. severe osteoarthritis; people undergoing thoracoabdominal aneurysm surgery; people physically unable or unwilling to undertake maximal cardiopulmonary exercise testing and the other fitness tests; people younger than 18 years of age or older than 80 years of age |
|
| Interventions | Intervention group (and sample size): number of participants not reported. Usual care plus preoperative exercise. Cycled for 60 min at 60% VO2 peak (submaximal cycling exercise at a moderate exercise intensity) on three consecutive days immediately prior to surgery. During the 60 min of exercise, participants were provided with three equally spaced 3 min rest periods. The last exercise session was completed no more than 48 h prior to the operation. Control group (and sample size): usual care; number of participants not reported |
|
| Outcomes | Postoperative mortality (60‐day mortality); length of hospital stay (through study completion, on average up to 60 days postsurgery); postoperative complications as assessed by the POMS (on average up to 5 days postsurgery) ‐ pulmonary, infectious, renal, gastrointestinal, cardiovascular, neurological, wound, haematological and pain. | |
| Notes | Funding source and declaration of interests not recorded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Care providers blinded. Due to nature of intervention, participants could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Abstract stated that 23 people were enrolled, but the clinical trial registry posted after the trial was complete stated that 21 people were enrolled. No information on the numbers of participants in each study arm. |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was registered on clinicaltrials.gov after the study was completed. |
| Other bias | Low risk | No evidence of other bias. |
Tew 2017.
| Study characteristics | ||
| Methods | Study design: multi‐centre, parallel‐group, two‐arm randomised controlled feasibility trial Number of participants: 53 people were randomised Exclusions post‐randomisation: 3 people in the intervention group did not receive the intervention (declined surgery, n = 2; expedited surgery, n = 1) Losses to follow‐up: 3 people in the intervention group lost to follow‐up, 2 people in the control group lost to follow‐up Intention‐to‐treat analysis: intention‐to‐treat analysis not used, people excluded from analysis in every outcome. Duration/dates study conducted: September 2013 to January 2016 |
|
| Participants | Country: UK Setting: three teaching hospitals in England Age, years (SD): exercise group: 74.6 (5.5), control group: 74.9 (6.4) Sex: 50 men, 3 women Inclusion criteria: people aged at least 18 years who had been listed, following routine clinical assessment and vascular multidisciplinary team consideration, for an open or endovascular repair of an infrarenal AAA with a diameter of 5.5 cm to 7.0 cm. Exclusion criteria: refusal or inability to provide informed consent, AAA managed non‐operatively, not an infrarenal aneurysm (juxtarenal, suprarenal or thoracic), infrarenal AAA diameter exceeding 7.0 cm, emergency AAA repair, contraindication to exercise testing or training, specialist referral required (for example to cardiology) and BMI below 20 or above 40 kg/m2 |
|
| Interventions | Intervention group (and sample size): exercise, n = 27 Three hospital‐based exercise sessions per week, for the 4 consecutive weeks (weeks 1 to 4; main phase) immediately preceding their intended operation date (in week 5). Participants whose operation was delayed beyond week 5 (e.g. owing to lack of availability of a hospital bed) also received a maintenance phase of training (1 exercise session per week). All exercise was undertaken on a cycle ergometer. Each of the first 3 sessions comprised a 10‐min warm‐up of unloaded cycling, eight 2‐min intervals of high‐intensity cycling interspersed with 2‐min rest periods of unloaded cycling, and then a 5‐min cool‐down of unloaded cycling. In all subsequent sessions, participants had the choice of performing eight 2‐min or four 4‐min ‘work’ intervals for the main body of the workout. Control group (and sample size): usual care, n = 26 Evidence‐based medical optimisation |
|
| Outcomes | Organ specific morbidity (POMS) (postoperative complications); mortality; duration of critical care; length of hospital stay; HQoL (SF‐36 and EQ‐5D); adverse events. Exercise group only: adherence. |
|
| Notes | This study was funded by National Institute for Health Research under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number PB‐PG‐1111‐26068). No competing interests. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After baseline assessment, patients will be randomly allocated 1:1 to exercise or usual care control (no supervised exercise), using minimisation to ensure balance across trial arms for important prognostic factors." Protocol, page 5 paragraph 2 |
| Allocation concealment (selection bias) | Low risk | "After baseline assessment, patients will be randomly allocated 1:1 to exercise or usual care control (no supervised exercise), using minimisation to ensure balance across trial arms for important prognostic factors. We do not list these factors here, to avoid any risk of the staff recruiting patients being able to decipher the allocation sequence." Protocol, page 5 paragraph 2 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All peri‐operative care will be at the discretion of the vascular teams (as per usual practice) who will be blinded to group allocation." Protocol, page 5 paragraph 2 Due to the nature of the intervention, participants cannot be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators were blinded to allocation. Study report page 104, paragraph 3‐4 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data/participants excluded in every outcome Although relevant missing outcomes were fairly distributed across groups and many outcomes had > 20% attrition, the small sample meant that even a small amount of attrition might cause significant attrition bias. ITT analysis was not implemented to reduce the attrition bias. Study report page 104, figure 1 |
| Selective reporting (reporting bias) | High risk | Protocol stated that the participants’ destination would be recorded, i.e. ward or critical care, but this was not given in the study report. Duration of critical care stay was also not presented in the study report. Protocol, page 7 paragraph 4 |
| Other bias | High risk | Small sample size. Study not powered to detect effect size or clinically important difference. |
AAA: aortic abdominal aneurysm APACHE II: Acute Physiology and Chronic Health Evaluation II BMI: body mass index COPD: chronic obstructive pulmonary disease EQ‐5D: EuroQol quality of life questionnaire FET: forced expiration techniques HQoL: health‐related quality of life ITT: intention‐to‐treat MIP: maximal inspiratory pressure NR: not reported POMS: Post‐Operative Morbidity Survey RCT: randomised controlled trial SD: standard deviation SF‐36: short form 36 quality of life questionnaire VO2: oxygen consumption
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bailey 2018 | Compares people with AAA versus healthy adults. |
| Barakat 2014 | No control group; therefore single arm and not eligible. |
| Gunasekera 2014 | Describes participant recruitment and experiences. |
| Hayashi 2016 | Not an RCT. |
| Lo Sapio 2014 | Compares the effects of two different preoperative cardiac work‐up strategies. |
| Myers 2010 | Outcomes pre‐surgery. |
| Myers 2014 | Outcomes pre‐surgery. |
| NCT00349947 | Outcome is growth rate of AAAs. |
| NCT01234610 | Outcomes measured pre‐surgery. |
| NCT02097186 | Intervention is ischaemic preconditioning. |
| NCT02292927 | Not an RCT. |
| NCT02767518 | People with thoracic aneurysm. |
| NCT02997618 | Peak VO2 as measured by CPET. |
| NCT03985202 | Not an RCT. |
| Takeuchi 2016 | Thoracic aortic disease. |
| Tew 2012 | Outcomes measured pre‐surgery. |
| UMIN000028237 | Not an RCT. |
AAA: abdominal aortic aneurysm CPET: cardiopulmonary exercise testing RCT: randomised controlled trial Peak VO2: peak oxygen uptake
Characteristics of ongoing studies [ordered by study ID]
NCT04169217.
| Study name | POWER: PrehabilitatiOn Workshop and Mentored Exercise Programme in Patients Having Elective Aortic Aneurysm Repair (POWER) |
| Methods | Randomised control trial |
| Participants | 45 |
| Interventions | Group 1: this arm will be subject to a one‐off prehabilitation workshop and provided with a prehab booklet. Group 2: this arm will be subject to a one‐off workshop and provided with a prehab booklet and additional mentoring by means of:
|
| Outcomes |
|
| Starting date | 15 November 2019 |
| Contact information | Heena Bidd heena.bidd@gstt.nhs.uk |
| Notes |
prehab: prehabilitation
Differences between protocol and review
There are no differences between the protocol and the review.
Contributions of authors
CF: designing and drafting the protocol, designing literature searches, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review drafting and future review updates UA: designing and drafting the protocol, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review drafting and future review updates AT: designing and drafting the protocol, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review drafting and future review updates JM: designing and drafting the protocol, data interpretation, review drafting and future review updates
Sources of support
Internal sources
No sources of support provided
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
CF: none known UA: none known AT: none known JM: has declared that he has received travel support and meeting fees from Medtronic, Gore, Abbott, Cook and Terumo aortic to attend various meetings. He has received honoraria for lectures from Acelity KCI, Abbott and Gore. He is council member and treasurer of the British Society of Endovascular Therapy (BSET), for which he receives no payment. He is a cofounder of UK Endovascular TraineeS (UKETS), a trainee initiative which receives funding through sponsorship from endovascular technology and simulation companies. The majority of this is non‐financial (the companies supply trainers on the courses or allow use of their simulators), although Abbott, Cook, Terumo Aortic, Medtronic, Vascular Perspectives, Merit, and Boston Scientific give some direct financial input to UKETS that is used to run events. JM derives no personal profit from this initiative.
New
References
References to studies included in this review
Barakat 2016 {published data only}
- Barakat HM, Shahin Y, Khan JA, McCollum PT, Chetter IC. Preoperative supervised exercise improves outcomes after elective abdominal aortic aneurysm repair: a randomized controlled trial. Annals of Surgery 2016;264(1):47-53. [DOI] [PubMed] [Google Scholar]
- NCT01062594. Effect of pre-operative exercise in abdominal aortic aneurysms (AAA) patients. clinicaltrials.gov/ct2/show/NCT01062594 (first received 4 February 2010).
Dronkers 2008 {published data only}
- Dronkers J, Veldman A, Hoberg E, Waal C, Meeteren N. Prevention of pulmonary complications after upper abdominal surgery by preoperative intensive inspiratory muscle training: a randomized controlled pilot study. Clinical rehabilitation 2008;22(2):134-42. [DOI] [PubMed] [Google Scholar]
Richardson 2014 {published data only}
- NCT02845167. Effect of preoperative exercise on postoperative outcome in AAA patients: pilot study. clinicaltrials.gov/ct2/show/NCT02845167 (first received 27 July 2016).
- Richardson K, Sanders G, Hayden P, Marcora S, Hopker J. The effect of preoperative exercise on postoperative outcome in abdominal aortic aneurysm (AAA) patients: pilot study. Intensive Care Medicine 2014;40(1 Suppl 1):S136. [Google Scholar]
Tew 2017 {published data only}
- ISRCTN09433624. High-intensity interval exercise training prior to abdominal aortic aneurysm repair. isrctn.com/ISRCTN09433624 (first received 28 June 2013). [DOI: 10.1186/ISRCTN09433624] [DOI]
- Tew GA, Batterham AM, Colling K, Gray J, Kerr K, Kothmann E, et al. Randomized feasibility trial of high-intensity interval training before elective abdominal aortic aneurysm repair. British Journal of Surgery 2017;104:1791–801. [DOI] [PubMed] [Google Scholar]
- Tew GA, Weston M, Kothmann E, Batterham AM, Gray J, Kerr K, et al. High-intensity interval exercise training before abdominal aortic aneurysm repair (HIT-AAA): protocol for a randomised controlled feasibility trial. BMJ open 2014;4(1):e004094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston M, Batterham AM, Tew GA, Kothmann E, Kerr K, Nawaz S, et al. Patients awaiting surgical repair for large abdominal aortic aneurysms can exercise at moderate to hard intensities with a low risk of adverse events. Frontiers in Physiology 2017;9(7):684. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Bailey 2018 {published data only}
- Bailey TG, Perissiou M, Windsor MT, Schulze K, Nam M, Magee R, et al. Effects of acute exercise on endothelial function in patients with abdominal aortic aneurysm. American Journal of Physiology - Heart and Circulatory Physiology 2018;314(1):H19-30. [DOI] [PubMed] [Google Scholar]
Barakat 2014 {published data only}
- Barakat HM, Shahin Y, Barnes R, Gohil R. Supervised exercise program improves aerobic fitness in patients awaiting abdominal aortic aneurysm repair. Annals of Vascular Surgery 2014;28(1):74-9. [DOI] [PubMed] [Google Scholar]
Gunasekera 2014 {published data only}
- Gunasekera RC, Moss J, Crank H, Mitchell PA, Nawaz S, Tew GA. Patient recruitment and experiences in a randomised trial of supervised exercise training for individuals with abdominal aortic aneurysm. Journal of Vascular Nursing 2014;32(1):4-9. [DOI] [PubMed] [Google Scholar]
Hayashi 2016 {published data only}
- Hayashi K, Hirashiki A, Kodama A, Kobayashi K, Yasukawa Y, Shimizu M, et al. Impact of preoperative regular physical activity on postoperative course after open abdominal aortic aneurysm surgery. Heart and Vessels 2016;31(4):578-83. [DOI] [PubMed] [Google Scholar]
Lo Sapio 2014 {published data only}
- Lo Sapio P, Chechi T, Gensini GF, Troisi N, Pratesi C, Chiti E, et al. Impact of two different cardiac work-up strategies in patients undergoing abdominal aortic aneurysm repair. International Journal of Cardiology 2014;175(1):e1-e3. [DOI] [PubMed] [Google Scholar]
Myers 2010 {published data only}
- Myers JN, White JJ Narasimhan B, Dalman R. Effects of exercise training in patients with abdominal aortic aneurysm: preliminary results from a randomized trial. Journal of Cardiopulmonary Rehabilitation and Prevention 2010;30(6):374-83. [DOI] [PubMed] [Google Scholar]
Myers 2014 {published data only}
- Myers J, McElrath M, Jaffe A, Smith K, Fonda H, Vu A, et al. A randomized trial of exercise training in abdominal aortic aneurysm disease. Medicine and Science in Sports and Exercise 2014;46(1):2-9. [DOI] [PubMed] [Google Scholar]
NCT00349947 {published data only}
- NCT00349947. Exercise therapy to treat adults with abdominal aortic aneurysms (AAA:STOP). clinicaltrials.gov/ct2/show/NCT00349947 (first received 10 July 2006).
NCT01234610 {published data only}
- NCT01234610. Feasibility study of exercise training for abdominal aortic aneurysm disease. clinicaltrials.gov/ct2/show/NCT01234610 (first received 4 November 2010).
NCT02097186 {published data only}
- NCT02097186. Preconditioning shields against vascular events in surgery (SAVES-F). clinicaltrials.gov/ct2/show/results/NCT02097186?view=results (first received 26 March 2014).
NCT02292927 {published data only}
- NCT02292927. Trimodal prehabilitation for aneurysm surgery study (T-PASS). clinicaltrials.gov/ct2/show/NCT02292927 (first received 18 November 2014).
NCT02767518 {published data only}
- NCT02767518. Prehabilitation for aortic repair patients (PREPARE). clinicaltrials.gov/ct2/show/NCT02767518 (first received 10 May 2016).
NCT02997618 {published data only}
- NCT02997618. The AAA Get Fit Trial: a pilot randomised controlled trial of community based exercise in patients with abdominal aortic aneurysms. clinicaltrials.gov/ct2/show/NCT02997618 (first received 20 December 2016).
NCT03985202 {published data only}
- NCT03985202. Structured exercise programme and abdominal aortic aneurysm surgery. clinicaltrials.gov/ct2/show/NCT03985202 (first received 13 June 2019).
Takeuchi 2016 {published data only}
- Takeuchi M, Matsumoto Y, Kawamoto S, Kumagai K, Amamizu H, Ohyama K, et al. Importance of preoperative pulmonary rehabilitation in patients with thoracic aortic diseases. Circulation 2016;134:A17793. [Google Scholar]
Tew 2012 {published data only}
- Tew GA, Moss J, Crank H, Mitchell PA, Nawaz S. Endurance exercise training in patients with small abdominal aortic aneurysm: a randomized controlled pilot study. Archives of Physical Medicine and Rehabilitation 2012;93(12):2148-53. [DOI] [PubMed] [Google Scholar]
UMIN000028237 {published data only}
- UMIN000028237. The study of the profitability and protective effect of cardiac rehabilitation on abdominal aortic aneurysm. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000032321 (first received 1 August 2017).
References to ongoing studies
NCT04169217 {published data only}
- NCT04169217. POWER: Prehabilitation workshop and mentored exercise programme in patients having elective aortic aneurysm repair (POWER). clinicaltrials.gov/ct2/show/NCT04169217 (first received 19 November 2019).
Additional references
Aherne 2015
- Aherne T, McHugh S, Kheirelseid EA, Lee MJ, McCaffrey N, Moneley D, et al. Comparing supervised exercise therapy to invasive measures in the management of symptomatic peripheral arterial disease. Surgery Research and Practice 2015;960402:960402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anjum 2012
- Anjum A, Allmen R, Greenhalgh R, Powell JT. Explaining the decrease in mortality from abdominal aortic aneurysm rupture. British Journal of Surgery 2012 May;99(5):637-45. [DOI] [PubMed] [Google Scholar]
Ashton 2002
- Ashton HA, Buxton MJ, Day NE, Kim LG, Marteau TM, Scott RA. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet 2002;360(9345):1531–9. [DOI] [PubMed] [Google Scholar]
Ashton 2007
- Ashton HA, Gao L, Kim LG, Druce PS, Thompson SG, Scott RA. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. British Journal of Surgery 2007;94(6):696–701. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barakat 2015
- Barakat HM, Shahin Y, McCollum PT, Chetter IC. Prediction of organ-specific complications following abdominal aortic aneurysm repair using cardiopulmonary exercise testing. Anaesthesia 2015;70(6):679-85. [DOI] [PubMed] [Google Scholar]
Blanchard 2000
- Blanchard JF, Armenian HK, Friesen PP. Risk factors for abdominal aortic aneurysm: results of a case-control study. American Journal of Epidemiology 2000;151(6):575-83. [DOI] [PubMed] [Google Scholar]
Boden 2014
- Boden WE, Franklin B, Berra K, Haskell WL, Calfas KJ, Zimmerman FH, et al. Exercise as a therapeutic intervention in patients with stable ischemic heart disease: an underfilled prescription. American Journal of Medicine 2014;127(10):905-11. [DOI] [PubMed] [Google Scholar]
Bown 2013
- RESCAN Collaborators, Bown MJ, Sweeting MJ, Brown LC, Powell JT, Thompson SG. Surveillance intervals for small abdominal aortic aneurysms: a meta-analysis. Journal of the American Medical Association 2013;309(8):806-13. [DOI] [PubMed] [Google Scholar]
Brewster 2003
- Brewster DC, Cronenwett JL, Hallett JW, Johnston KW, Krupski WC, Matsumura JS. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. Journal of Vascular Surgery 2003;37(5):1106–17. [DOI] [PubMed] [Google Scholar]
Brown 2012
- Brown LC, Powell JT, Thompson SG, Epstein DM, Sculpher MJ, Greenhalgh RM. The UK EndoVascular Aneurysm Repair (EVAR) trials: randomised trials of EVAR versus standard therapy. Health Technology Assessment 2012;16(9):1-218. [DOI] [PubMed] [Google Scholar]
Cosford 2007
- Cosford PA, Leng GC, Thomas J. Screening for abdominal aortic aneurysm. Cochrane Database of Systematic Reviews 2007, Issue 2. Art. No: CD002945. [DOI: 10.1002/14651858.CD002945.pub2] [DOI] [PubMed] [Google Scholar]
Filardo 2015
- Filardo G, Powell JT, Martinez MA, Ballard DJ. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database of Systematic Reviews 2015, Issue 2. Art. No: CD001835. [DOI: 10.1002/14651858.CD001835.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Forsdahl 2009
- Forsdahl SH, Singh K, Solberg S, Jacobsen BK. Risk factors for abdominal aortic aneurysms: a 7-year prospective study: the Tromsø study, 1994–2001. Circulation 2009;119:2202–8. [DOI] [PubMed] [Google Scholar]
Giles 2010
- Giles KA, Wyers MC, Pomposelli FB, Hamdan AD, Avery Ching Y, Schermerhorn ML. The impact of body mass index on perioperative outcomes of open and endovascular abdominal aortic aneurysm repair from the National Surgical Quality Improvement Program, 2005–2007. Journal of Vascular surgery 2010;52:1471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gommans 2015
- Gommans LN, Fokkenrood HJ, Dalen HC, Scheltinga MR, Teijink JA, Peters RJ. Safety of supervised exercise therapy in patients with intermittent claudication. Journal of Vascular Surgery 2015;61(2):512-8. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed January 2021. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Grant 2015
- Grant SW, Hickey GL, Wisely NA. Cardiopulmonary exercise testing and survival after elective abdominal aortic aneurysm repair. British Journal of Anaesthesia 2015;114:430–6. [DOI] [PubMed] [Google Scholar]
Greenhalgh 2010
- Greenhalgh RM, Brown LC, Powell JT, Thompson SG, Epstein D, Sculpher MJ. Endovascular versus open repair of abdominal aortic aneurysm. New England Journal of Medicine 2010;362(20):1863-71. [DOI] [PubMed] [Google Scholar]
Gunnarsson 2016
- Gunnarsson K, Wanhainen A, Djavani Gidlund K, Björck M, Mani K. Endovascular versus open repair as primary strategy for ruptured abdominal aortic aneurysm: a national population-based study. European Journal of Vascular and Endovascular Surgery 2016;51(1):22-8. [DOI] [PubMed] [Google Scholar]
Hawthorne 1999
- Hawthorne G, Richardson J, Osborne R. The Assessment of Quality of Life (AQoL) instrument: a psychometric measure of health-related quality of life. Quality of Life Research 1999;8(3):209-24. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
Hirsch 2006
- Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management). Circulation 2006;113(11):e463-654. [DOI] [PubMed] [Google Scholar]
Hoogeboom 2014
- Hoogeboom TJ, Dronkers JJ, Hulzebos EH, Meeteren NL. Merits of exercise therapy before and after major surgery. Current Opinion in Anaesthesiology 2014;27(2):161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Howard 2015
- Howard DP, Banerjee A, Fairhead JF, Handa A, Silver LE, Rothwell PM. Age-specific incidence, risk factors and outcome of acute abdominal aortic aneurysms in a defined population. British Journal of Surgery 2015;102(8):907-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacomelli 2016
- Jacomelli J, Summers L, Stevenson A, Lees T, Earnshaw JJ. Impact of the first 5 years of a national abdominal aortic aneurysm screening programme. British Journal of Surgery 2016;103(9):1125-31. [DOI] [PubMed] [Google Scholar]
Jahangir 2015
- Jahangir E, Lipworth L, Edwards TL, Kabagambe EK, Mumma MT, Mensah GA, et al. Smoking, sex, risk factors and abdominal aortic aneurysms. Journal of Epidemiology and Community Health 2015;69(5):481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kato 2019
- Kato M, Kubo A, Green FN, Takagi H. Meta-analysis of randomized controlled trials on safety and efficacy of exercise training in patients with abdominal aortic aneurysm. Journal of Vascular Surgery 2019;63(3):933-43. [DOI] [PubMed] [Google Scholar]
Kent 2010
- Kent KC, Zwolak RM, Egorova NN, Riles TS, Manganaro A, Moskowitz AJ, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. Journal of Vascular Surgery 2010;2(3):539-48. [DOI] [PubMed] [Google Scholar]
Larsson 2009
- Larsson E, Granath F, Swedenborg J, Hultgren R. A population-based case-control study of the familial risk of abdominal aortic aneurysm. Journal of Vascular Surgery 2009;49(1):47-50; discussion 51. [DOI] [PubMed] [Google Scholar]
Lederle 1997
- Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D, et al. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Prevalence and associations of abdominal aortic aneurysm detected through screening. Annals of Internal Medicine 1997;126:441–9. [DOI] [PubMed] [Google Scholar]
Lederle 2002
- Lederle FA, Wilson SE, Johnson GR, Reinke DB, Littooy FN, Acher CW, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. New England Journal of Medicine 2002;346:1437–44. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
LeFevre 2014
- LeFevre ML. Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force recommendation statement. Annals of Internal Medicine 2014;161(4):281–90. [DOI] [PubMed] [Google Scholar]
Li 2013
- Li X, Zhao G, Zhang J, Duan Z, Xin S. Prevalence and trends of the abdominal aortic aneurysms epidemic in general population - a meta-analysis. PLoS ONE 2013;8(12):e81260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindholt 2005
- Lindholt JS, Juul S, Fasting H, Henneberg EW. Screening for abdominal aortic aneurysms: single centre randomised controlled trial. BMJ 2005;330:750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moll 2011
- Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M, et al. Management of Abdominal Aortic Aneurysms Clinical Practice Guidelines of the European Society for Vascular Surgery. European Journal of Vascular and Endovascular Surgery 2011;41:S1eS58. [DOI] [PubMed] [Google Scholar]
Moran 2016
- Moran J, Wilson F, Guinan E, McCormick P, Hussey J, Moriarty J. Role of cardiopulmonary exercise testing as a risk-assessment method in patients undergoing intra-abdominal surgery: a systematic review. British Journal of Anaesthesia 2016;116(2):177-91. [DOI] [PubMed] [Google Scholar]
Mousa 2016
- Mousa AY, Bozzay J, Yacoub M, Stone PA, Najundappa A, Bates MC, et al. Novel risk score model for prediction of survival following elective endovascular abdominal aortic aneurysm repair. Vascular and Endovascular Surgery 2016;116(2):261-9. [DOI] [PubMed] [Google Scholar]
Myers 2011
- Myers J, Powell A, Smith K, Fonda H, Dalman RL. Cardiopulmonary exercise testing in small abdominal aortic aneurysm: profile, safety, and mortality estimates. European Journal of Cardiovascular Prevention and Rehabilitation 2011;18(3):459–66. [DOI] [PubMed] [Google Scholar]
NICE 2020
- NICE. Abdominal aortic aneurysm: diagnosis and management. nice.org.uk/guidance/ng156 2020.
Nordon 2011
- Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nature Reviews Cardiology 2011;8:92-102. [DOI] [PubMed] [Google Scholar]
Norman 2004
- Norman PE, Jamrozik K, Lawrence-Brown MM, Le MT, Spencer CA, Tuohy RJ, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ 2004;329(7477):1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Older 2013
- Older P. Anaerobic threshold, is it a magic number to determine fitness for surgery? Perioperative Medicine 2013;21(2):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paravastu 2014
- Paravastu SC, Jayarajasingam R, Cottam R, Palfreyman SJ, Michaels JA, Thomas SM. Endovascular repair of abdominal aortic aneurysm. Cochrane Database of Systematic Reviews 2014, Issue 1. Art. No: CD004178. [DOI: 10.1002/14651858.CD004178.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2016
- Patel R, Sweeting MJ, Powell JT, Greenhalgh RM, EVAR trial investigators. Endovascular versus open repair of abdominal aortic aneurysm in 15-years' follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet 2016;388(10058):2366-74. [DOI] [PubMed] [Google Scholar]
Pavy 2006
- Pavy B, Iliou MC, Meurin P, Tabet JY, Corone S. Functional evaluation and cardiac rehabilitation Working Group of the French Society of Cardiology safety of exercise training for cardiac patients: results of the French registry of complications during cardiac rehabilitation. Archives of Internal Medicine 2006;166(21):2329-34. [DOI] [PubMed] [Google Scholar]
Petersen 2005
- Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. Journal of Applied Physiology (Bethesda, Md. : 1985) 2005;98(4):1154-62. [DOI] [PubMed] [Google Scholar]
Pouwels 2015
- Pouwels S, Willigendael EM, Sambeek MR, Nienhuijs SW, Cuypers PW, Teijink JA. Beneficial effects of pre-operative exercise therapy in patients with an abdominal aortic aneurysm: a systematic review. European Journal of Vascular and Endovascular Surgery 2015;49(1):66-76. [DOI] [PubMed] [Google Scholar]
Powell 2008
- Powell JT, Brown LC, Greenhalgh RM, Thompson SG. The rupture rate of large abdominal aortic aneurysms: is this modified by anatomical suitability for endovascular repair? Annals of Surgery 2008;247(1):173-9. [DOI] [PubMed] [Google Scholar]
Powell 2011
- Powell JT, Gotensparre SM, Sweeting MJ, Brown LC, Fowkes FG, Thompson SG. Rupture rates of small abdominal aortic aneurysms: a systematic review of the literature. European Journal of Vascular and Endovascular Surgery 2011 Jan;41(1):2-10. [DOI] [PubMed] [Google Scholar]
Prentis 2012
- Prentis JM, Trenell MI, Jones, DJ. Submaximal exercise testing predicts perioperative hospitalization after aortic aneurysm repair. Journal of Vascular Surgery 2012;56:1564–70. [DOI] [PubMed] [Google Scholar]
Prinssen 2004
- Prinssen M, Verhoeven EL, Buth J, Cuypers PW, Sambeek MR, Balm R, et al, on behalf of the Dutch Randomized Endovascular Aneurysm Management (DREAM) Trial Group. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. New England Journal of Medicine 2004;351(16):1607-18. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. The Cochrane Collaboration, 2020.
Rughani 2012
- Rughani G, Robertson L, Clarke M. Medical treatment for small abdominal aortic aneurysms. Cochrane Database of Systematic Reviews 2012, Issue 9. Art. No: CD009536. [DOI: 10.1002/14651858.CD009536.pub2] [DOI] [PubMed] [Google Scholar]
Salzler 2015
- Salzler GG, Meltzer AJ, Mao J, Isaacs A, Connolly PH, Schneider DB, et al. Characterizing the evolution of peri-operative outcomes and costs of endovascular abdominal aortic aneurysm repair. Journal of Vascular Surgery 2015;62(5):1134-9. [DOI] [PubMed] [Google Scholar]
Schermerhorn 2012
- Schermerhorn ML, Bensley RP, Giles KA, Hurks R, Oʼmalley AJ, Cotterill P, et al. Changes in abdominal aortic aneurysm rupture and short-term mortality, 1995-2008: a retrospective observational study. Annals of Surgery 2012;256(4):651-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scott 2002
- Scott RA, Bridgewater SG, Ashton HA. Randomized clinical trial of screening for abdominal aortic aneurysm in women. British Journal of Surgery 2002;89:283–5. [DOI] [PubMed] [Google Scholar]
Smith 2009
- Smith TB, Stonell C, Purkayastha S, Paraskevas P. Cardiopulmonary exercise testing as a risk assessment method in non cardio-pulmonary surgery: a systematic review. Anaesthesia 2009;64(8):883-93. [DOI] [PubMed] [Google Scholar]
Sonesson 2018
- Sonesson B, Dias N, Resch T. Is there an age limit for abdominal aortic aneurysm repair? Journal of Cardiovascular Surgery 2018;59(2):190-4. [DOI] [PubMed] [Google Scholar]
Spark 2001
- Spark JI, Baker JL, Vowden P, Wilkinson D. Epidemiology of abdominal aortic aneurysms in the Asian community. British Journal of Surgery 2001;88(3):382-4. [DOI] [PubMed] [Google Scholar]
Svensjö 2014
- Svensjö S, Mani K, Björck M, Lundkvist J, Wanhainen A. Screening for abdominal aortic aneurysm in 65-year-old men remains cost-effective with contemporary epidemiology and management. European Journal of Vascular and Endovascular Surgery 2014;47(4):357-65. [DOI] [PubMed] [Google Scholar]
Sweeting 2015
- Sweeting MJ, Balm R, Desgranges P, Ulug P, Powell JT. Ruptured Aneurysm Trialists. Individual-patient meta-analysis of three randomized trials comparing endovascular versus open repair for ruptured abdominal aortic aneurysm. British Journal of Surgery 2015;102(10):1229-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thompson 2011
- Thompson AR, Peters N, Lovegrove RE. Cardiopulmonary exercise testing provides a predictive tool for early and late outcomes in abdominal aortic aneurysm patients. Annals of the Royal Collage of Surgery England 2011;93:474–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ulug 2016
- Ulug P, Powell JT, Sweeting MJ, Bown MJ, Thompson SG, SWAN Collaborative Group. Meta-analysis of the current prevalence of screen-detected abdominal aortic aneurysm in women. British Journal of Surgery 2016;103(9):1097-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vardulaki 1999
- Vardulaki KA, Prevost TC, Walker NM, Day NE, Wilmink AB, Quick CR, et al. Incidence among men of asymptomatic aortic abdominal aneurysms: estimates from 500 screen detected cases. Journal of Medical Screening 1999;6(1):50–4. [DOI] [PubMed] [Google Scholar]
Wanhainen 2019
- Wanhainen A, Verzini F, Van Herzeele I, Allaire E, Bown M, Cohnert T, et al. Editor's choice–European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. European Journal of Vascular and Endovascular Surgery 2019;57(1):8-93. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware J, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Medical Care 1992;30(6):473-83. [PubMed] [Google Scholar]
Ware 1996
- Ware JE, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care 1996;34(3):220-33. [DOI] [PubMed] [Google Scholar]
Waton 2018
- Waton S, Johal A, Heikkila K, Cromwell D, Boyle J, Miller F. National Vascular Registry: 2018 annual report. London: The Royal College of Surgeons of England, 2018. [Google Scholar]
Wee 2019
- Wee IJ, Choong AM. A systematic review of the impact of preoperative exercise for patients with abdominal aortic aneurysms. Journal Vascular Surgery 2019;71(6):2123-2131.e1. [DOI: 10.1016/j.jvs.2018.09.039] [DOI] [PubMed] [Google Scholar]
Wild 2013
- Wild JB, Stather PW, Biancari F, Choke EC, Earnshaw JJ, Grant SW, et al. A multicentre observational study of the outcomes of screening detected sub-aneurysmal aortic dilatation. European Journal of Vascular and Endovascular Surgery 2013;45(2):128-34. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Fenton 2020
- Fenton C, Abaraogu UO, Tan AR, McCaslin JE. Prehabilitation exercise therapy before abdominal aortic aneurysm repair. Cochrane Database of Systematic Reviews 2020, Issue 6. Art. No: CD013662. [DOI: 10.1002/14651858.CD013662] [DOI] [PMC free article] [PubMed] [Google Scholar]


