Abstract
We used the nationwide claims database to calculate the incidence of thrombotic events and predict their overall 2-week incidence. From 2006 to 2020, the incidence of deep vein thrombosis (DVT), pulmonary embolism (PE), and disseminated intravascular coagulation (DIC) tended to increase. Unlike intracranial venous thrombosis (ICVT) and intracranial thrombophlebitis (ICTP), which showed no age difference, other venous embolism, and thrombosis (OVET), DIC, DVT, and PE were significantly more common in over 65 years. The overall 2-week incidence of ICVT was 0.21/1,000,000 (95% confidence interval [CI], 0.11–0.32). ICTP, OVET, DIC, DVT and PE were expected to occur in 0.08 (95% CI, 0.02–0.14), 7.66 (95% CI, 6.08–9.23), 5.95 (95% CI, 4.88–7.03), 13.28 (95% CI, 11.92–14.64), 14.09 (95% CI, 12.80–15.37) per 1,000,000, respectively. To date, of 8,548,231 patients vaccinated with ChAdOx1 nCoV-19 in Korea, two had confirmed thrombosis with thrombocytopenia syndrome within 2 weeks. The observed incidence of ICVT after vaccination was 0.23/1,000,000.
Keywords: COVID-19, Thrombosis with Thrombocytopenia Syndrome, Vaccination, Vaccine Adverse Events
Graphical Abstract
Several unusual thrombotic events have been reported after vaccination with ChAdOx1 nCoV-19 (AstraZeneca) and Ad26.COV2.S (Johnson & Johnson/Janssen), two adenoviral vector vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1,2,3 These events share distinct clinical features including thrombosis in unusual locations and thrombocytopenia, occurring more frequently in young adults and women.1,4 Furthermore, anti-platelet factor 4-heparin antibody was detected in most patients, suggesting a similar pathogenesis to that of autoimmune heparin-induced thrombocytopenia.1
After a thorough review of epidemiological and clinical data, the European Medicine Agency announced a possible link to the very rare cases of unusual blood clots with low blood platelets, later named thrombosis with thrombocytopenia syndrome (TTS).5,6 This assessment was followed by age-based restrictions on ChAdOx1 nCoV-19 in various European countries. Approximately a week later, the US Centers for Disease Control and Prevention and Food and Drug Administration recommended a pause in vaccination with Ad26.COV2.S after reports of a small number of rare thrombotic events similar to those associated with ChAdOx2 nCoV-19.
Multiple factors should be considered when deciding on the use of the two vaccines—whether to continue their use and in whom. The baseline incidence of similar conditions must be considered as the risk of TTS appears extremely low. Furthermore, rare thrombotic events and TTS incidence is unclear among Asians.
We examined the nationwide claims database to estimate the expected incidence of rare thrombotic events in Korea.
We extracted biweekly numbers of rare thrombotic events from the National Health Insurance Service medical claims database, which provides comprehensive information on healthcare use in Korea. Study conditions were identified from the primary diagnoses of all hospitalizations from 2006 through 2020 using the International Classification of Diseases and Related Health Problems, 10th edition.
Six categories of thrombotic events were selected: intracranial venous thrombosis (ICVT; I63.6, I67.6), intracranial thrombophlebitis (ICTP; G08), other venous embolism and thrombosis (OVET; I82.2, I82.3, I82.8, I82.9), disseminated intravascular coagulation (DIC; D65), pulmonary embolism (PE; I26, I26.0, I26.9), and deep vein thrombosis (DVT; I80.1–80.3) (Supplementary Table 1).
The biweekly incidence per 1,000,000 population was calculated from the number of each event and mid-year population of each year. We constructed prediction models using the autoregressive integrated moving average (ARIMA) from the observed incidence. Model parameters were determined using the “auto.arima” function in the “forecast” package of R software. The predicted annual incidence in the year 2021 was divided by 26 to yield 2-week incidence, as most cases of TTS were reported to occur within 14 days post-vaccination. Subgroup analysis was conducted for age groups in compliance with the US Post-licensure Rapid Immunization Safety Monitoring Program.7 The ARIMA model’s parameters are listed in Supplementary Table 2. Statistical analysis was performed using R (version 4.0.4; R Foundation for Statistical Computing, Vienna, Austria).
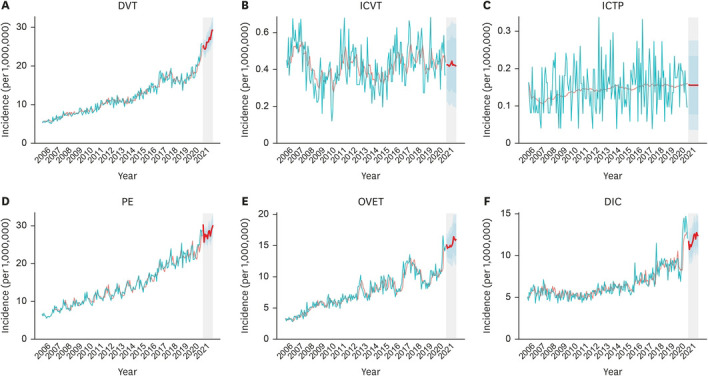
The predicted overall 2-week incidence of ICVT was 0.21/1,000,000 (95% confidence interval [CI], 0.11–0.32). ICTP and OVET were expected to occur in 0.08 (95% CI, 0.02–0.14) and 7.66 (95% CI, 6.08–9.23) per 1,000,000, respectively (Table 1, Fig. 1).
Table 1. Predicted incidence of thromboembolic events.
| Condition | Predicted incidence per 1,000,000 | ||
|---|---|---|---|
| 2-week | Annual | ||
| Overall | |||
| Intracranial venous thrombosis | 0.21 (0.11–0.32) | 5.10 (2.49–7.71) | |
| Intracranial thrombophlebitis | 0.08 (0.02–0.14) | 1.87 (0.43–3.31) | |
| Other venous embolism and thrombosis | 7.66 (6.08–9.23) | 183.72 (145.91–221.53) | |
| Disseminated intravascular coagulation | 5.95 (4.88–7.03) | 142.84 (116.95–168.74) | |
| Deep vein thrombosis | 13.28 (11.92–14.64) | 318.60 (285.97–351.23) | |
| Pulmonary embolism | 14.09 (12.80–15.37) | 337.99 (307.15–368.83) | |
| Age 20–49 yr | |||
| Intracranial venous thrombosis | 0.23 (0.12–0.35) | 5.54 (2.79–8.29) | |
| Intracranial thrombophlebitis | 0.07 (0.00–0.14) | 1.56 (0.00–3.31) | |
| Other venous embolism and thrombosis | 4.34 (3.27–5.41) | 104.15 (78.43–129.86) | |
| Disseminated intravascular coagulation | 4.30 (3.21–5.39) | 103.12 (77.04–129.19) | |
| Deep vein thrombosis | 5.72 (4.75–6.69) | 137.19 (113.96–160.41) | |
| Pulmonary embolism | 4.80 (3.98–5.63) | 115.22 (95.48–134.96) | |
| Age 50–64 yr | |||
| Intracranial venous thrombosis | 0.27 (0.01–0.54) | 6.33 (0.10–12.78) | |
| Intracranial thrombophlebitis | 0.10 (0.00–0.25) | 2.38 (0.00–6.02) | |
| Other venous embolism and thrombosis | 8.64 (6.23–11.06) | 207.41 (149.47–265.35) | |
| Disseminated intravascular coagulation | 7.62 (5.76–9.48) | 182.83 (138.18–227.48) | |
| Deep vein thrombosis | 13.02 (10.90–15.14) | 312.44 (261.51–363.36) | |
| Pulmonary embolism | 12.01 (10.34–13.68) | 288.08 (248.02–328.14) | |
| Age ≥ 65 yr | |||
| Intracranial venous thrombosis | 0.40 (0.00–1.07) | 9.45 (0.00–25.65) | |
| Intracranial thrombophlebitis | 0.16 (0.00–0.43) | 3.76 (0.00–10.28) | |
| Other venous embolism and thrombosis | 21.28 (15.59–26.96) | 510.57 (374.14–646.99) | |
| Disseminated intravascular coagulation | 12.90 (9.72–16.07) | 309.50 (233.30–385.71) | |
| Deep vein thrombosis | 43.63 (37.64–49.63) | 1,047.17 (903.25–1,191.10) | |
| Pulmonary embolism | 54.10 (48.23–59.97) | 1,298.28 (1,157.38–1,439.19) | |
Prediction models were constructed using autoregressive integrated moving average from observed incidence during 2006–2019.
Fig. 1. Observed and predicted incidences of the study conditions. (A) DVT; (B) ICVT; (C) ICTP; (D) PE; (E) OVET; (F) DIC.
DIC = disseminated intravascular coagulation, DVT = deep vein thrombosis, ICTP = intracranial thrombophlebitis, ICVT = intracranial venous thrombosis, PE = pulmonary embolism, OVET = other venous embolism, and thrombosis.
There were no significant differences between age groups in the incidence of ICVT and ICTP. However, OVET, DIC, DVT, and PE were significantly more common among those aged ≥ 65 years (Supplementary Figs. 1, 2, 3).
By June 16, 2021, there were two confirmed cases of TTS within 2 weeks after vaccination with ChAdOx1 nCoV-19 in Korea among 8,548,231 people who were vaccinated with the same vaccine. The observed incidence of ICVT after vaccination was 0.23/1,000,000. No occurrence of ICVT or other rare thrombotic events has been reported among those who received mRNA-based vaccines (BNT162b2, Pfizer-BioNTech).8
We measured and followed the trajectories of the incidence of a total of six conditions that could potentially be associated with rare and severe thrombotic events. Based on these analyses, we predicted the annual and 2-week incidence of the conditions for the year 2021. The incidence of most diseases tended to increase over time; in particular, there was a marked increase in DVT, PE, and DIC.
In the Netherlands, Coutinho et al investigated cases of adults with cerebral venous thrombosis (CVT) in a retrospective cross-sectional study.9 The incidence rate was calculated using population figures from 2008 to 2010 in two Dutch provinces. The overall incidence was 1.32/100,000 person-years (95% CI, 1.06–1.61). In particular, in women aged between 31 and 50 years, the incidence was 2.78 (95% CI, 1.98–3.82). In Australia, the incidence of CVT was investigated in adults admitted to all public hospitals in Adelaide from 2005 to 2011. A total of 105 patients were diagnosed with CVT using brain imaging among 955,390 hospitalized patients, which translates to an incidence of 15.7 cases per million per year (95% CI, 12.9–19.0).10 Fifty-five cases occurred in women, and the relative risk of developing CVT in women of reproductive age was higher than that in men (1.18; 95% CI, 0.94–1.48).
A retrospective study11 was conducted in the United States to determine the incidence of cerebral venous sinus thrombosis (CVST) using medical data from Olmsted County adult residents from 1990 to 2015. The incidence rates were adjusted according to the age and sex distribution of the US white population in 2010. A total of 46 patients (mean age, 39.9; women, 65%) with CVST were identified, and the overall age- and sex-adjusted incidence of CVST was 1.47/100,000 per year (95% CI, 1.03–1.91). The incidence was 1.16/100,000 per year for men (95% CI, 0.57–1.75), and 1.78/100,000 per year for women (95% CI, 1.13–2.43). Among women with CVST, 53.3% were receiving hormonal therapy. In Germany, a cohort study12 using claims data estimated the incidence of CVST per 100,000 per year from 2015 to 2019. The average incidence rate during the observation period was calculated by estimating the average annual number of cases divided by the average annual number of people at risk. Approximately 3.2 million adults per year were included in the study. The mean annual incidence of CVST was 1.9/100,000 (95% CI, 1.4–2.3) between 2015 and 2019. In the people aged 18–59 years, the incidence of CVST was higher in women than in men (women: 2.4/100,000 [95% CI, 1.5–3.4], men: 1.0/100.000 [95% CI, 0.4–1.7]). However, in patients > 60 years old, the incidence of CVST was similar between women and men.
In this study, the baseline incidence of each condition over two weeks under general conditions was calculated for the entire Korean population. In the past 15 years, ICVT and ICTP had very low incidence in Koreans and did not have a specific pattern; however, it was confirmed that the incidence of most thromboembolic events increased over time in this study. The baseline incidence of thromboembolic events predicted in this study can be used to evaluate the possibility of an increased risk of rare and serious adverse events after COVID-19 vaccination.
This study had similar limitation applied to previous study13 that measured the baseline occurrence of 11 conditions listed in the US Vaccine Adverse Event Reporting System. Because this study does not provide detailed subgroups, it is insufficient to assess risk factors in a specific population. In addition, there are inherent limitations in epidemiological studies using health insurance claim data. Also, it should be noted that TTS associated with the COVID-19 vaccine can be reliably diagnosed with a specific antibody test. Thus, confirmed cases of vaccine-associated TTS are excess incidences, to which our results cannot be compared directly.
In conclusion, the baseline incidence of thromboembolic events is increasing annually, and the incidence of ICVT, which can appear as one feature of the TTS, is very low. These results can be used for causality assessment and monitoring of potential adverse events after vaccination.
Ethics statement
The study protocol was approved by the Institutional Review Board of the Gil Medical Center, Gachon University College of Medicine (GFIRB2020-284). and the requirement to obtain written consent was waived due to the retrospective nature of this study; patients and the public were not involved in the study design, data collection, analysis, or interpretation of data. All study methods were carried out based on the Declaration of Helsinki.
Footnotes
Funding: This study was supported by grants from the Gachon University Gil Medical Center (grant number 2019–11). Also this work was supported by a grant of the National IT Industry Promotion Agency (NIPA) and Institute of Information & Communications Technology Planning & Evaluation (IITP) (grant number 2020-0-01907), funded by the Ministry of Science and ICT. The sponsors of the study were not involved in the study design, analysis, interpretation of data, writing of the report, or decision to submit the findings of the study for publication.
Disclosure: The authors have no conflicts of interest to declare.
- Conceptualization: Peck KR, Jung J.
- Data curation: Huh K, Na Y, Kim YE.
- Formal analysis: Kim YE.
- Methodology: Na Y, Kim YE.
- Software: Radnaabaatar M.
- Writing - original draft: Huh K, Na Y, Kim YE.
- Writing - review & editing: Radnaabaatar M, Peck KR, Jung J.
SUPPLEMENTARY MATERIALS
ICD-10 codes used to capture the thrombotic conditions of interest
Model parameters and mean absolute percentage error of the autoregressive integrated moving average model constructed from observed incidence during 2006–2019
Observed and predicted incidences of the study conditions in population aged 20–49 years.
Observed and predicted incidences of the study conditions in population aged 50–64 years.
Observed and predicted incidences of the study conditions in population aged over 65 years.
References
- 1.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dotan A, Shoenfeld Y. Perspectives on vaccine induced thrombotic thrombocytopenia. J Autoimmun. 2021;121:102663. doi: 10.1016/j.jaut.2021.102663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muir KL, Kallam A, Koepsell SA, Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med. 2021;384(20):1964–1965. doi: 10.1056/NEJMc2105869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yocum A, Simon EL. Thrombotic thrombocytopenic purpura after Ad26.COV2-S vaccination. Am J Emerg Med. doi: 10.1016/j.ajem.2021.05.001. Forthcoming 2021. DOI: 10.1016/j.ajem.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Medicines Agency. AstraZeneca's COVID-19 vaccine: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets. [Updated 2021]. [Accessed May 31, 2021]. https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood.
- 6.European Medicines Agency. COVID-19 Vaccine Janssen: assessment of very rare cases of unusual blood clots with low platelets continues. [Updated 2021]. [Accessed May 31, 2021]. https://www.ema.europa.eu/en/news/covid-19-vaccine-janssen-assessment-very-rare-cases-unusual-blood-clots-low-platelets-continues.
- 7.Baker MA, Nguyen M, Cole DV, Lee GM, Lieu TA. Post-licensure rapid immunization safety monitoring program (PRISM) data characterization. Vaccine. 2013;31(Suppl 10):K98–112. doi: 10.1016/j.vaccine.2013.04.088. [DOI] [PubMed] [Google Scholar]
- 8.Korea Disease Control and Prevention Agency. Press release (6.16) [Updated 2021]. [Accessed June 16, 2021]. http://www.kdca.go.kr/board/board.es?mid=a20501000000&bid=0015&list_no=713681&act=view.
- 9.Coutinho JM, Zuurbier SM, Aramideh M, Stam J. The incidence of cerebral venous thrombosis: a cross-sectional study. Stroke. 2012;43(12):3375–3377. doi: 10.1161/STROKEAHA.112.671453. [DOI] [PubMed] [Google Scholar]
- 10.Devasagayam S, Wyatt B, Leyden J, Kleinig T. cerebral venous sinus thrombosis incidence is higher than previously thought: a retrospective population-based study. Stroke. 2016;47(9):2180–2182. doi: 10.1161/STROKEAHA.116.013617. [DOI] [PubMed] [Google Scholar]
- 11.Fairbanks A, Chodnicki K, Lesser E, Hodge D, Leavitt J, Garrity J, et al. ARVO Annual Meeting Abstract. Rockville, MD: Association for Research in Vision and Ophthalmology; 2018. Population-based incidence and visual outcomes of cerebral venous sinus thrombosis. [Google Scholar]
- 12.Jacob J, Klamroth R, Ploner T, Harder T, Koch J, Wichmann O, et al. Incidence of cerebral venous sinus thrombosis in adults in Germany – a retrospective study using health claims data. Res Sq. Forthcoming 2021. DOI: 10.21203/rs.3.rs-428469/v2. [Google Scholar]
- 13.Huh K, Kim YE, Radnaabaatar M, Lee DH, Kim DW, Shin SA, et al. Estimating baseline incidence of conditions potentially associated with vaccine adverse events: a call for surveillance system using the Korean National Health Insurance claims data. J Korean Med Sci. 2021;36(9):e67. doi: 10.3346/jkms.2021.36.e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ICD-10 codes used to capture the thrombotic conditions of interest
Model parameters and mean absolute percentage error of the autoregressive integrated moving average model constructed from observed incidence during 2006–2019
Observed and predicted incidences of the study conditions in population aged 20–49 years.
Observed and predicted incidences of the study conditions in population aged 50–64 years.
Observed and predicted incidences of the study conditions in population aged over 65 years.