Abstract
Introduction
Rapid antigen tests are convenient for diagnosing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); however, they have lower sensitivities than nucleic acid amplification tests. In this study, we evaluated the diagnostic performance of Quick Chaser® Auto SARS-CoV-2, a novel digital immunochromatographic assay that is expected to have higher sensitivity than conventional antigen tests.
Methods
A prospective observational study was conducted between February 8 and March 24, 2021. We simultaneously obtained two nasopharyngeal samples, one for evaluation with the QuickChaser® Auto SARS-CoV-2 antigen test and the other for assessment with reverse transcription PCR (RT-PCR), considered the gold-standard reference test. The limit of detection (LOD) of the new antigen test was compared with those of four other commercially available rapid antigen tests.
Results
A total of 1401 samples were analyzed. SARS-CoV-2 was detected by reference RT-PCR in 83 (5.9%) samples, of which 36 (43.4%) were collected from symptomatic patients. The sensitivity, specificity, positive predictive value, and negative predictive value were 74.7% (95% confidence interval (CI): 64.0–83.6%), 99.8% (95% CI: 99.5–100%), 96.9% (95% CI: 89.2–99.6%), and 98.4% (95% CI: 97.6–99.0%), respectively. When limited to samples with a cycle threshold (Ct) < 30 or those from symptomatic patients, the sensitivity increased to 98.3% and 88.9%, respectively. The QuickChaser® Auto SARS-CoV-2 detected 34–120 copies/test, which indicated greater sensitivity than the other rapid antigen tests.
Conclusions
QuickChaser® Auto SARS-CoV-2 showed sufficient sensitivity and specificity in clinical samples of symptomatic patients. The sensitivity was comparable to RT-PCR in samples with Ct < 30.
Keywords: Digital immuno-chromatographic antigen test, QuickChaser Auto, FUJI DRI-CHEM, Silver amplification, SARS-CoV-2, COVID-19
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a global pandemic and continues to place an immense burden on healthcare systems [1] despite the introduction of effective vaccines [2]. Since rapid and accurate testing is a critical element in containing viral transmission [3], the development of reliable point-of-care testing is necessary.
Nucleic acid amplification tests (NAATs) are the gold standard for diagnosing coronavirus disease 2019 (COVID-19) because of their high diagnostic performance [4]. However, several limitations have reduced their test capacity and clinical utility, including long processing times and the need for expensive equipment and skilled staff [3]. By contrast, antigen tests are convenient and have moderate sensitivities and high specificities [4]. These tests have made it possible to diagnose COVID-19 in low-resource settings [5], despite the possibility of missing a certain proportion of infected patients [6]. Therefore, increasing sensitivity should enhance the clinical utility of antigen tests.
Quick Chaser® Auto SARS-CoV-2 (Mizuho Medy, Saga, Japan) is a new antigen test based on the silver amplification method. This test uses the same reagent as FUJI DRY-CHEM IMMUNO AG Cartridge COVID-19 Ag (Fujifilm, Tokyo, Japan), and is tailored for digital immuno-chromatographic assays. The test provides results in 15 min when used with the QuickChaser Immuno Reader II dedicated reader (Mizuho Medy). Both the silver amplification method and digital immuno-chromatographic assays were reported to increase the sensitivity of antigen tests for the influenza virus [7]. Although Quick Chaser® Auto SARS-CoV-2 is expected to have higher sensitivity than conventional antigen tests, its diagnostic performance for detecting SARS-CoV-2 has not been evaluated in clinical samples.
In this study, we evaluated the diagnostic performance of Quick Chaser® Auto SARS-CoV-2 and QuickChaser Immuno Reader II with nasopharyngeal specimens, and performed comparisons with the reverse transcription PCR (RT-PCR) method.
2. Methods
This study was carried out as an extension of our previous research [8] and followed a similar protocol. The investigation was performed between February 8 and March 24, 2021, at Tsukuba Medical Center Hospital (TMCH), a tertiary hospital in Ibaraki Prefecture, Japan. Nasopharyngeal samples and clinical information were gathered from individuals who had possibly contracted SARS-CoV-2. The enrolled patients were referred from 67 nearby clinics and a local public health center, and by healthcare workers at TMCH. All patients provided informed consent to participate in the study, which was approved by the ethics committee of TMCH (approval number: 2020-071).
2.1. Procedures for sample collection and antigen test
Two nasopharyngeal samples were obtained from each patient for further testing: one with a sponge swab™ (NIPRO, Osaka, Japan) for antigen testing, and the other with FLOQSwab™ (Copan Italia S.p.A., Brescia, Italy) for the RT-PCR assay. After sample collection, antigen testing was performed immediately using the QuickChaser® Auto SARS-CoV-2 and QuickChaser Immuno Reader II. FLOQSwab samples were diluted in 3 mL of Universal Transport Medium™ (UTM™) (Copan Italia) for in-house RT-PCR and reference RT-PCR.
2.2. RT-PCR assay for SARS-CoV-2
magLEAD 6gC (Precision System Science, Chiba, Japan) was used for extraction and purification of RNA from UTM™ samples. GENECUBE and GENECUBE® HQ SARS-CoV-2 were used for in-house RT-PCR [9]. The purified samples were then transferred to Mizuho Medy for reference real-time RT-PCR. The N2 primer/probe set (Nihon Gene Research Laboratories, Miyagi, Japan) was employed for reference RT-PCR as suggested by the “Manual for the Detection of Pathogen 2019-nCoV Ver. 2.9.1” issued by the National Institute of Infectious Diseases of Japan [10]. The RT-PCR assays were performed on a Thermal Cycler Dice III (Takara Bio, Kusatsu, Japan) using One Step PrimeScript™ III RT-qPCR Mix (Takara Bio) with the following cycling conditions: reverse transcription at 52 °C for 5 min and at 95 °C for 10 s, and 45 cycles at 95 °C for 5 s and at 60 °C for 30s. The absolute viral copy number was determined by serially diluted RNA control targeting the N2 gene of SARS-CoV-2 (Nihon Gene Research Laboratories). If the in-house and reference RT-PCR showed conflicting results, GeneXpert® for SARS-CoV-2 (Cepheid, Sunnyvale, CA, USA) was used to re-examine the sample for the final decision.
Limits of detection of QuickChaser® Auto SARS-CoV-2 and four commercially available rapid antigen tests.
We compared the limit of detection (LOD) of QuickChaser® Auto SARS-CoV-2 with those of four commercially available rapid antigen tests (Espline® SARS-CoV-2, Fujirebio, Tokyo, Japan; QuickNavi™-COVID19 Ag, Denka, Tokyo Japan; Panbio™ COVID-19 Ag Rapid Test Device, Abbott Diagnostics, Illinois, USA; SARS-CoV-2 Rapid Antigen Test, Roche Diagnostics, Rotkreuz, Switzerland). Two SARS-CoV-2–positive, cryopreserved, nasopharyngeal swab specimens were serially diluted two-fold with UTM™. The diluted solution was collected with the swabs included in each antigen test kit and was added to the extraction reagent solution of each antigen test. After that, these extracted samples were dropped into test cartridges. The LOD of each antigen test was evaluated according to the measurement method described in the package insert, and the results were jointly evaluated by three researchers.
The numbers of viral copies contained in the UTM™ samples were determined by RT-PCR with viral RNA extraction performed using a QIAamp Viral RNA Mini Kit (QIAGEN N.V., Hilden, Germany).
2.3. Statistical analyses
The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of QuickChaser® Auto SARS-CoV-2 were calculated using the Clopper and Pearson method, with 95% confidence intervals (CIs). All calculations were conducted using the R 4.0.3 software program (www.r-project.org).
3. Results
During the study period, 1416 nasopharyngeal samples were initially included. Samples with missing clinical data (n = 14) or measurement errors (n = 1) were excluded. A total of 1401 samples were eventually analyzed.
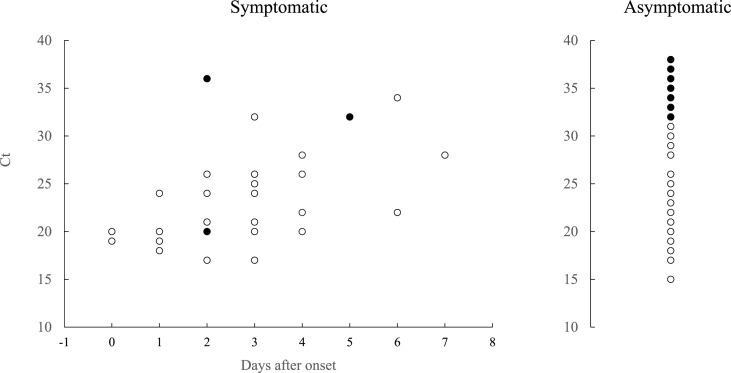
Reference real-time RT-PCR detected SARS-CoV-2 in 83 (5.9%) of the 1401 samples. The results of reference and in-house RT-PCR were consistent in all but one sample, which was negative by in-house RT-PCR and positive by reference RT-PCR. This sample was re-evaluated by in-house RT-PCR and GeneXpert® using preserved UTM. Both tests showed positive results, and the sample was finally considered to be positive for SARS-CoV-2. Of the 83 samples, 36 (43.4%) were collected from symptomatic patients, and 47 (56.6%) were obtained from asymptomatic participants. The relationship between the interval from symptom onset and the sensitivity of QuickChaser® Auto SARS-CoV-2 is shown in Fig. 1 .
Fig. 1.
Difference in sensitivity of QuickChaser® Auto SARS-CoV-2 stratified by the day after symptoms onset. Two patients with unknown onset dates were excluded. White circles indicate positive samples, and black circles indicate negative samples for the antigen test.
3.1. Comparison of LODs
The results of LOD tests using clinical specimens are summarized in Table 1 . Among the five antigen tests, the QuickChaser® Auto SARS-CoV-2 had the lowest LOD: QuickChaser® Auto SARS-CoV-2, 34–120 copies/test; Espline SARS®-CoV-2, 481–549 copies/test; QuickNavi™-COVID19 Ag, 4394 copies/test; Panbio™ COVID-19 Ag Rapid Test Device, 1098–1924 copies/test; and SARS-CoV-2 Rapid Antigen Test, 549–1924 copies/test.
Table 1.
Limits of detection tests using nasopharyngeal swab samples.
| Dilution factor | Copies/test | Quick Chaser |
Espline |
QuickNavi |
Panbio |
Rapid Antigen Test |
||
|---|---|---|---|---|---|---|---|---|
| 15 min | 30 min | 15 min | 15 min | 15 min | 30 min | |||
| Sample 1 | 20 | 8787 | + | + | + | + | + | + |
| 40 | 4394 | + | + | + | + | + | + | |
| 80 | 2197 | + | + | – | + | + | + | |
| 160 | 1098 | + | + | – | + | + | + | |
| 320 | 549 | + | + | – | – | + | – | |
| 640 | 275 | + | – | – | – | – | – | |
| 1280 | 137 | + | – | – | – | – | – | |
| 2560 | 69 | + | n.t. | n.t. | n.t. | n.t. | n.t. | |
| 5120 | 34 | + | n.t. | n.t. | n.t. | n.t. | n.t. | |
| 10,240 | 17 | – | n.t. | n.t. | n.t. | n.t. | n.t. | |
| Sample 2 | 20 | 3849 | + | + | – | + | + | + |
| 40 | 1924 | + | + | – | + | + | + | |
| 80 | 962 | + | + | – | – | – | – | |
| 160 | 481 | + | + | – | – | – | – | |
| 320 | 241 | + | – | – | – | – | – | |
| 640 | 120 | + | n.t. | n.t. | n.t. | n.t. | n.t. | |
| 1280 | 60 | – | n.t. | n.t. | n.t. | n.t. | n.t. | |
| 2560 | 30 | – | n.t. | n.t. | n.t. | n.t. | n.t. | |
| 5120 | 15 | – | n.t. | n.t. | n.t. | n.t. | n.t. | |
+, Positive; −, negative; n.t., not tested.
QuickChaser™, QuickChaser® Auto SARS-CoV-2; Espline™, Espline® SARS-CoV-2 (Fujirebio, Tokyo, Japan); QuickNavi™, QuickNavi™-COVID19 Ag (Denka, Tokyo, Japan); Panbio™, Panbio™ COVID-19 Ag Rapid Test Device (Abbott Diagnostics Medical, Illinois, USA); Rapid Antigen Test, SARS-CoV-2 Rapid Antigen Test (Roche Diagnostics, Rotkreuz, Switzerland).
3.2. Sensitivity, specificity, PPV, and NPV of QuickChaser® Auto SARS-CoV-2
The clinical performance of QuickChaser® Auto SARS-CoV-2 is summarized in Table 2 and 3. Sixty-two of the 83 samples that were positive by reference RT-PCR were also positive by the antigen test. The sensitivity, specificity, PPV, and NPV were 74.7% (95% CI: 64.0–83.6%), 99.8% (95% CI: 99.5–100%), 96.9% (95% CI: 89.2–99.6%), and 98.4% (95% CI: 97.6–99.0%), respectively (Table 2).
Table 2.
Sensitivity and specificity of QuickChaser® Auto SARS-CoV-2 among overall subjects.
| Overall subjects |
Real-time RT-PCR |
||
|---|---|---|---|
| Positive |
Negative |
||
| QuickChaser® Auto SARS-CoV-2 | Positive |
62 |
2 |
| Negative | 21 | 1316 | |
| Sensitivity (%) | 74.7 (64.0–83.6) | ||
| Specificity (%) | 99.8 (99.5–100) | ||
| Positive predictive value (%) | 96.9 (89.2–99.6) | ||
| Negative predictive value (%) | 98.4 (97.6–99.0) | ||
RT-PCR, reverse transcription polymerase chain reaction.
Data in parentheses are 95% confidence intervals.
In samples from symptomatic patients, 32 of 36 reference RT-PCR–positive samples were also positive by antigen testing (Table 3 a). The sensitivity, specificity, PPV, and NPV were 88.9% (95% CI: 73.9–96.9%), 100% (95% CI: 99.3–100%), 100% (95% CI: 84.2–100%), and 99.5% (95% CI: 98.8–99.9%), respectively.
Table 3a.
Sensitivity and specificity of QuickChaser® Auto SARS-CoV-2 among symptomatic patients.
| Symptomatic subjects |
Real-time RT-PCR |
||
|---|---|---|---|
| Positive |
Negative |
||
| QuickChaser® Auto SARS-CoV-2 | Positive |
32 |
0 |
| Negative | 4 | 825 | |
| Sensitivity (%) | 88.9 (73.9–96.9) | ||
| Specificity (%) | 100 (99.3–100) | ||
| Positive predictive value (%) | 100 (84.2–100) | ||
| Negative predictive value (%) | 99.5 (98.8–99.9) | ||
RT-PCR, reverse transcription polymerase chain reaction.
Data in parentheses are 95% confidence intervals.
In samples from asymptomatic individuals, 30 of 47 reference RT-PCR–positive samples were positive by antigen testing (Table 3 b). The sensitivity, specificity, PPV, and NPV were 63.8% (95% CI: 48.5–77.3%), 99.6% (95% CI: 98.5–100%), 93.8% (95% CI: 79.2–99.2%), and 96.7% (95% CI: 94.7–98.0%), respectively.
Table 3b.
Sensitivity and specificity of QuickChaser® Auto SARS-CoV-2 among asymptomatic patients.
| Asymptomatic subjects |
Real-time RT-PCR |
||
|---|---|---|---|
| Positive |
Negative |
||
| QuickChaser® Auto SARS-CoV-2 | Positive |
30 |
2 |
| Negative | 17 | 491 | |
| Sensitivity (%) | 63.8 (48.5–77.3) | ||
| Specificity (%) | 99.6 (98.5–100) | ||
| Positive predictive value (%) | 93.8 (79.2–99.2) | ||
| Negative predictive value (%) | 96.7 (94.7–98.0) | ||
RT-PCR, reverse transcription polymerase chain reaction.
Data in parentheses are 95% confidence intervals.
The sensitivities of the antigen test stratified by Ct value are shown in Table 4 .
Table 4.
QuickChaser® Auto SARS-CoV-2 sensitivities stratified by the Ct values.
| Ct value (N2 gene) | Antigen test |
Sensitivity | ||
|---|---|---|---|---|
| Positive (n = 62) | Negative (n = 21) | |||
| <20 | 16 | 0 | 100.0% | (71.3–100%) |
| 20–24 | 28 | 1 | 96.6% | (82.2–99.9%) |
| 25–29 | 13 | 0 | 100.0% | (66.1–100%) |
| ≥30 | 5 | 20 | 20.0% | (6.8–20.7%) |
Data in parentheses are 95% confidence intervals.
Ct, cycle threshold.
Detailed data of samples with discrepant results between QuickChaser® Auto SARS-CoV-2 and reference RT-PCR assay.
Of the 23 discrepant samples, two were positive by the antigen test and negative by reference RT-PCR (false positive). Both samples were collected from patients who were referred to the PCR center from a local public health center due to a contact history with COVID-19 patients. They were asymptomatic and were not referred to our PCR center again during the study period. Of the 21 samples that were negative by the antigen test and positive by reference RT-PCR (false negative), 20 had Ct values > 30, and one had a Ct value of 20. For a patient with a false-negative result despite a Ct value of 20, we retested the preserved UTM™ sample with QuickChaser® Auto SARS-CoV-2. The antigen test provided a positive result for UTM™ samples that were diluted approximately 40 fold.
4. Discussion
In this prospective study, Quick Chaser® Auto SARS-CoV-2 using nasopharyngeal specimens demonstrated a sensitivity of 74.7% and a specificity of 99.8%. In patients with Ct values < 30, the sensitivity was almost identical to RT-PCR. Furthermore, Quick Chaser® Auto SARS-CoV-2 had a lower LOD than other antigen tests currently approved in Japan.
Several antigen tests have been developed, with generally high specificity and variable sensitivity [5]. While Quick Chaser® Auto SARS-CoV-2 had the lowest LOD in this study, QuickNavi [8] and Panbio [11] had comparable sensitivities. The diagnostic performance of antigen tests may differ between experimental (UTM™) and clinical samples [12]. Also, the current LOD evaluation was performed by three researchers without a blinded manner, and therefore observer bias was a significant concern. Additionally, antigen concentrate dilution, which is the method recommended in package inserts for testing UTM samples using the Espline SARS-CoV-2 Assay, was not used in this study. Therefore, direct comparison using clinical samples should be conducted to evaluate the real-life performance of each test.
Viral load influences overall sensitivity, as shown by the fact that the sensitivity of antigen tests generally plummets in samples with Ct > 30 [8,13]. Samples with Ct > 30 comprised 30.1% (25/83) of our study population, which may have decreased the overall sensitivity of Quick Chaser® Auto SARS-CoV-2. Another factor that may influence sensitivity is the swab type used. Quick Chaser® Auto SARS-CoV-2 includes sponge swabs, although flocked-type swabs can collect samples more efficiently [14]. Despite the aforementioned challenges, this antigen test successfully detected SARS-CoV-2 in all but one of the samples with Ct < 30. The remaining case was a false negative, and considering that re-examination with the UTM™ sample showed a positive result, the original finding may have been caused by a low viral concentration due to a flawed sample collection technique. The good performance of this test indicates that it can accurately identify contagious patients, given that those with Ct values < 30 are considered to be highly infectious [15].
We observed false positives in only two samples, and the specificity of Quick Chaser® Auto SARS-CoV-2 was over 99%. False positives should be avoided as they lead to unnecessary further testing or quarantine measures [16]; thus, the specificity is recommended to be over 97% [5]. Positive results should be cautiously interpreted, especially when the prevalence of SARS-CoV-2 or the possibility of infection is low, especially if samples were obtained from asymptomatic individuals or patients with atypical symptoms for COVID-19, such as diarrhea without respiratory symptoms.
To maximize its sensitivity, Quick Chaser® Auto SARS-CoV-2 uses two methods: silver amplification and digital interpretation of the results. Similar to many antigen tests [5], Quick Chaser® Auto SARS-CoV-2 implements sandwich methods using labeled antibodies and capture antibodies. Antibodies labeled with gold colloid attach to specific antigens in a sample. The labeled antigens are then sandwiched by capture antibodies, which indicate the positive bands. The silver amplification method generates large silver particles using the gold colloid as a catalyst, and thus enhances the visibility of the labeled antibody complex [7]. A previous study showed that among antigen tests for the influenza virus, those that used this method had higher sensitivity than those that did not (type A, 91.2% vs. 86.8%, respectively; type B, 94.4% vs. 81.2%, respectively), without compromising specificity [17]. Similarly, digital scans of the test reagents can improve the accuracy of antigen detection. Digital scans also increase the objectivity of test result interpretation by removing the necessity for visual inspection. A systematic review suggested that digital immunoassays increased sensitivities by 25.5% and 23.5% for influenza A and B, respectively [18]. Although digital immunoassays require special equipment, the additional cost is much cheaper than that of NAATs, and is compensated for by the increase in sensitivity [18].
There are several limitations regarding this study. First, reference real-time RT-PCR used frozen samples. While samples were stored at −80 °C, their viral load may have decreased during storage process. Second, we did not investigate whether mutations in SARS-COV-2 affected the diagnostic performance. Third, we did not evaluate saliva or anterior nasal cavity samples. Saliva collection and anterior nasal swabs cause less pain and coughing than nasopharyngeal swabs [19]. Future studies should compare the diagnostic performance of samples obtained using each of these methods.
In conclusion, Quick Chaser® Auto SARS-CoV-2 showed satisfactory diagnostic performance of symptomatic patients. The sensitivity was especially high in samples of Ct < 30, indicating that the test can accurately detect highly infectious patients.
Author's contribution
All authors meet the ICMJE authorship criteria. Contributor Yoko Kurihara drafted the manuscript and performed the statistical analyses. Shigeyuki Notake, Atsuo Ueda, and Koji Nakamura collected samples and operated the equipment. Yoshihiko Kiyasu, Yuto Takeuchi, Yusaku Akashi, Kenji Narahara, Sunao Mori, and Tomonori Takeshige analyzed the data and revised the manuscript. Hiromichi Suzuki supervised the project. All authors contributed to the writing of the final manuscript.
Declaration of competing interest
Mizuho Medy provided fees for research expenses and provided the Quick Chaser® Auto SARS-CoV-2 without charge. Kenji Narahara, Sunao Mori, and Tomonori Takeshige are employed by Mizuho Medy, the developer of the Quick Chaser® Auto SARS-CoV-2. Hiromichi Suzuki received consultation fee from Mizuho Medy, and Fujifilm.
Acknowledgments
We thank the staff in the Department of Clinical Laboratory of Tsukuba Medical Center Hospital for their intensive support of this study. We thank all of the medical institutions for providing their patients' clinical information.
References
- 1.World Health Organization Coronavirus disease (COVID-19) weekly epidemiological update and weekly operational update. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports accessed March 30, 2021.
- 2.Creech C.B., Walker S.C., Samuels R.J. SARS-CoV-2 vaccines. J Am Med Assoc. 2021;325:1318–1320. doi: 10.1001/jama.2021.3199. [DOI] [PubMed] [Google Scholar]
- 3.Younes N., Al-Sadeq D.W., Al-Jighefee H., Younes S., Al-Jamal O., Daas H.I., et al. Challenges in laboratory diagnosis of the novel coronavirus SARS-CoV-2. Viruses. 2020;12:582. doi: 10.3390/v12060582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Diagnostic testing for SARS-CoV-2: interim guidance, 11 September 2020. https://www.who.int/publications/i/item/diagnostic-testing-for-sars-cov-2 accessed March 20, 2021.
- 5.SARS-CoV-2 antigen-detecting rapid diagnostic tests: an implementation guide. World Health Organization; Geneva: 2020. https://apps.who.int/iris/handle/10665/337948 Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 6.Dinnes J., Deeks J.J., Berhane S., Talor M., Adriano A., Davenport C., et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2021;3:CD013705. doi: 10.1002/14651858.CD013705.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wada A., Sakoda Y., Oyamada T., Kida H. Development of a highly sensitive immunochromatographic detection kit for H5 influenza virus hemagglutinin using silver amplification. J Virol Methods. 2011;178:82–86. doi: 10.1016/j.jviromet.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi Y., Akashi Y., Kato D., Kuwahara M., Muramatsu S., Ueda A., et al. The evaluation of a newly developed antigen test (QuickNaviTM-COVID19 Ag) for SARS-CoV-2: a prospective observational study in Japan. J Infect Chemother. 2021;27:890–894. doi: 10.1016/j.jiac.2021.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiyasu Y., Akashi Y., Sugiyama A., Takeuchi Y., Notake S., Naito A., et al. A prospective evaluation of the analytical performance of GENECUBE® HQ SARS-CoV-2 and GENECUBE® FLU A/B. Mol Diagn Ther. 2021:1–10. doi: 10.1007/s40291-021-00535-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shirato K., Nao N., Katano H., Takayama I., Saito S., Kato F., et al. Development of genetic diagnostic methods for detection for novel coronavirus 2019(nCoV-2019) in Japan. Jpn J Infect Dis. 2020;73:304–307. doi: 10.7883/yoken.JJID.2020.061. [DOI] [PubMed] [Google Scholar]
- 11.Torres I., Poujois S., Albert E., Colomina J., Navarro D. Evaluation of a rapid antigen test (PanbioTM COVID-19 Ag rapid test device) for SARS-CoV-2 detection in asymptomatic close contacts of COVID-19 patients. Clin Microbiol Infect. 2021;27:636.e1–636.e4. doi: 10.1016/j.cmi.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gremmels H., Winkel B.M.F., Schuurman R., Rosingh A., Rigter N.A.M., Rodriguez O., et al. Real-life validation of the PanbioTM COVID-19 antigen rapid test (Abbott) in community-dwelling subjects with symptoms of potential SARS-CoV-2 infection. EClinicalMedicine. 2021;31:100677. doi: 10.1016/j.eclinm.2020.100677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fenollar F., Bouam A., Ballouche M., Fuster L., Prudent E., Colson P., et al. Evaluation of the panbio COVID-19 rapid antigen detection test device for the screening of patients with COVID-19. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.02589-20. e02589-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daley P., Castriciano S., Chernesky M., Smieja M. Comparison of flocked and rayon swabs for collection of respiratory epithelial cells from uninfected volunteers and symptomatic patients. J Clin Microbiol. 2006;44:2265–2267. doi: 10.1128/JCM.02055-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bullard J., Dust K., Funk D., Strong J.E., Alexander D., Garnett L., et al. Predicting infectious severe acute respiratory syndrome coronavirus 2 from diagnostic samples. Clin Infect Dis. 2020;71:2663–2666. doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogawa T., Fukumori T., Nishihara Y., Sekine T., Okuda N., Nishimura T., et al. Another false-positive problem for a SARS-CoV-2 antigen test in Japan. J Clin Virol. 2020;131:104612. doi: 10.1016/j.jcv.2020.104612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitamura K., Shimizu H., Yamazaki M., Ichikawa M., Nagai K., Katada J., et al. Clinical evaluation of highly sensitive silver amplification immunochromatography systems for rapid diagnosis of influenza. J Virol Methods. 2013;194:123–128. doi: 10.1016/j.jviromet.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Merckx J., Wali R., Schiller I., Caya C., Gore G.C., Chartrand C., et al. Diagnostic accuracy of novel and traditional rapid tests for influenza infection compared with reverse transcriptase polymerase chain reaction: a systematic review and meta-analysis. Ann Intern Med. 2017;167:394–409. doi: 10.7326/M17-0848. [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi Y., Akashi Y., Kato D., Kuwahara M., Muramatsu S., Ueda A., et al. Diagnostic performance and characteristics of anterior nasal collection for the SARS-CoV-2 antigen test: a prospective study. Sci Rep. 2021;11:10519. doi: 10.1038/s41598-021-90026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]