Abstract
Vaccine manufacturers from developing countries have a proven track record of developing, producing, and supplying high-quality vaccines globally. However, due to the complexity of vaccine manufacturing, numerous stakeholder organizations support manufacturers across a variety of functions. To optimize the support from stakeholders it is instrumental to first understand which manufacturing processes these manufacturers require support for and what support functions are most beneficial. To this end, the Developing Countries Vaccine Manufacturers Network designed a comprehensive survey to assess the specific needs of the Network’s member organizations.
We found that almost all sampled manufacturers are interested in obtaining funding or technology transfers for COVID-19 vaccines. Furthermore, results indicated that manufacturers have a strong appetite for modern technology platforms, particularly RNA technologies. Scale-up, phase III clinical trials, and formulation were also key processes for which manufacturers require support.
Keywords: COVID-19, Vaccine supply, Technology transfer, Clinical trials
1. Introduction
The Developing Countries Vaccine Manufacturers Network (DCVMN) is an international alliance representing public and private organizations engaged in research, development, manufacturing, and supply of high-quality affordable vaccines. Comprising of 41 member manufacturers based in developing countries, the Network operates under the mandate to produce and supply vaccines against known and emerging infectious diseases globally.
According to an internal survey, DCVMN members had an estimated capacity of around 3.5 billion doses in 2019 and supplied over 50 distinct vaccine types globally [1]. These manufacturers have a proven track record of producing high-quality vaccines. Notably 13 member manufacturers have vaccine products prequalified by WHO making them eligible for procurement by UN agencies [2]. Beyond producing high-quality, affordable vaccines in large quantities, DCVMN members are increasingly developing innovative vaccine products, both in respect to antigen attributes and presentations, subsequently increasing coverage and programmatic ease of use [3]. Members’ engagements in COVID-19 vaccine development highlights these manufacturers' commitment to innovation. As of April 20, 2021, twelve members have vaccine candidates in clinical trials or approved for emergency use [4]. Furthermore, member manufacturers have the capabilities to upscale COVID-19 vaccine candidates, a key success story being the partnership between the Serum Institute of India, Oxford University and AstraZeneca to produce COVID-19 vaccines for developing countries1 .
Despite the contribution of developing country vaccine manufacturers many challenges persist. Vaccine manufacturing is a highly complex process, requiring scientific and technical expertise, high levels of investment, stringent regulatory standards, quality control and quality assurance. Vaccine products can take 10–20 years to develop, with manufacturers facing risks during development but also once the product goes to market given variable and unpredictable demand [5]. Due to this complexity, there is no ‘one glove fits all’ business model for vaccine manufacturers. Nevertheless, to remain viable manufacturers must achieve consistent production of multiple vaccines and do so at scale to offset the high fixed costs associated with vaccine development and production. External to an individual organization, systems of local and international bodies must be in place to support technology adoption and research and development and enable healthy environments for investment and collaboration [6].
To reduce the global impact of infectious diseases numerous organizations support vaccine manufacturers to meet the high standards required to develop, produce, and supply vaccines globally. The Gates Foundation, CEPI, PATH, WHO, and Gavi, among others critically provide support through funding, training programs and other initiatives to foster healthy markets and advance public health. While these agencies support some DCVMN member manufacturers, to reduce the global burden of infectious disease an increased level of support must be accessible to more manufacturers globally.
Importantly, to best support manufacturers, stakeholder organizations must understand what types of support are required and for which specific manufacturing processes. To build a clear case for vaccine manufacturers from developing countries, we designed a comprehensive survey to assess the needs of DCVMN member manufacturers. A broad assessment of these needs will provide a foundation to develop evidence-based strategies on how to best support manufacturers. The remainder of this report will discuss the results of the described survey and their implications while drawing on the literature.
2. Method
To understand the needs of member manufacturers, the DCVMN secretariat designed a 17-question survey accessing key topics related to vaccine manufacturing (Appendix A)
For each topic respondents selected specific elements where their organization required support (respondents were able to select any number of elements). Then, respondents were asked what type of support is needed for each topic (respondents could select any number of the following: tech transfer, training, funding, or other)2 . Furthermore, the survey asked manufacturers to share which technology platforms they were in a position (and prepared) to provide in the form of a technology transfer.
The survey was circulated, by email, to all 41 DCVMN members on March 18th 2021. The final response was recorded on April 12th 2021.
3. Results
Of the Network’s 41 members, 27 completed the survey (~66%). Responding manufacturers represent a mixture of public and private organizations from 13 countries and territories spanning Africa, Asia, Europe and South America. A more detailed overview of the survey respondents is available as supplementary material (Appendix B).
3.1. Vaccine types and technology platforms
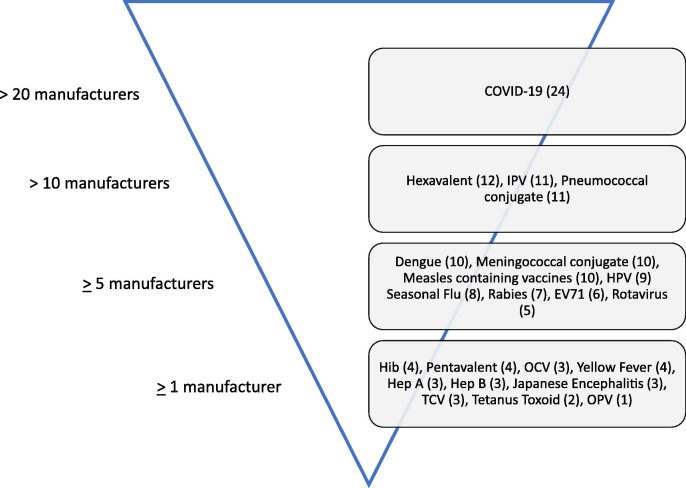
Sampled manufacturers were asked for which vaccine types their organizations were interested in acquiring funding and/or technological support. Almost 90% of the sampled (24 manufacturers) indicated interest in obtaining support for COVID-19 vaccines.
Following COVID-19 vaccines, twelve members indicated interest in obtaining support for Hexavalent (DTP-HepB-Hib-IPV) vaccines. DCVMN members also indicated strong interest for two conjugate vaccines: pneumococcal conjugate vaccines (11 manufacturers) and meningococcal conjugate vaccines (10 manufacturers). A complete breakdown of the vaccine types sampled manufacturers require support for is illustrated in Fig. 1 .
Fig. 1.
Number of manufacturers interested in obtaining funding/technology support for the listed vaccine types. Respondents were able to select any number of vaccine types. Specific number of manufacturers for each vaccine type is indicated in parentheses. Four manufacturers selected ‘Other’ (Chikungunya, GBS, AMR, Cancer).
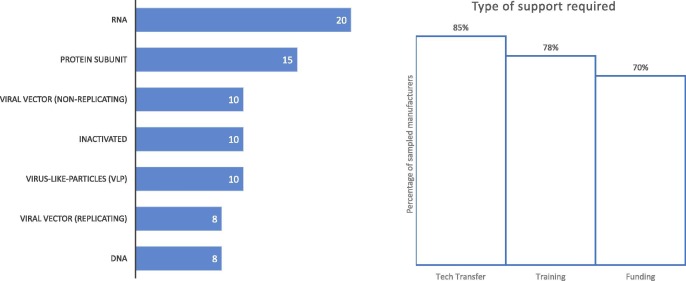
In concurrence with the high level of interest in COVID-19 vaccines, the most sought-after vaccine technology vaccine was RNA platforms (20 manufacturers). Support for protein subunit vaccines was also frequently requested (15 manufacturers), while for the remaining five technology platforms under consideration, ten manufacturers required support for inactivated, virus-like particles and non-replicating viral vector technology platforms respectively and eight manufacturers require support for each DNA and replicating viral vector technology platforms (Fig. 2 ).
Fig. 2.
Number of sampled manufacturers who require support with the listed technology platforms (left) and the percentage of manufacturers who require each type of specific support (right). Respondents were able to select any number of technology platforms and support types. For technology platforms one manufacturer selected ‘Other’ (Conjugation Technology) and for type of support required two manufacturers selected ‘Other’ (Consultancy Support and Co-Development).
When assessing what type of support is required technology transfers was prominent (23 manufacturers). Sampled manufacturers also indicated that training (21 manufacturers) and funding (19 manufacturers) are fundamental support mechanisms (Fig. 2).
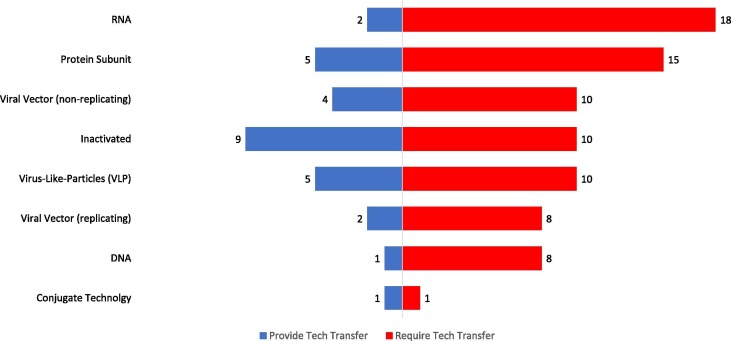
Additionally, we assessed manufacturers’ ability and preparedness to provide technology transfers. Among the sampled manufacturers preparedness to transfer inactivated vaccine technologies was most frequent (9 manufacturers) followed by protein subunit and virus-like particle vaccine platforms (5 manufacturers each). A breakdown, comparing the technology platforms sampled manufacturers can provide and those which the manufacturers require support for is illustrated in Fig. 3 .
Fig. 3.
Matching Analysis - Number of manufacturers who can provide tech transfer and number who require tech transfer for the listed technology platforms. Respondents were able to select any number of technology platforms. For providing technology transfers six manufacturers selected ‘Other’ (Live Attenuated (4), Polysaccharide Subunit (1), Protein Subunit – For COVID-19 (1)).
3.2. Vaccine development, production, supply and regulatory processes
In assessing drug substance processes, support in up-scaling was most frequently requested (by approximately 74% of sampled manufacturers) followed by upstream and downstream processes (70% each) (Table 1 ). In terms of specific support required, funding was prominent followed by technology transfer and training (Table 2 ). For drug product processes, two thirds of manufacturers require formulation support. Other drug product processes were selected at lower frequencies suggesting that manufacturers have sufficient capabilities in these areas. Overall, the support required for drug product processes was equal for three factors: technology transfer, training, and funding (Table 2).
Table 1.
Manufacturing processes for which sampled manufacturers require support.
| Assessed manufacturing processes | Number of manufacturers requiring support | Percentage |
|---|---|---|
| Drug Substance | ||
| Scale-up | 20 | 74.1% |
| Upstream processes | 19 | 70.4% |
| Downstream processes | 19 | 70.4% |
| Facility modification | 11 | 40.7% |
| Drug Product | ||
| Formulation | 18 | 66.7% |
| Filling | 10 | 37.0% |
| Packaging | 3 | 11.1% |
| Storage | 2 | 7.4% |
| Supply | ||
| Shipping Validation | 12 | 44.4% |
| Cold chain equipment | 8 | 29.6% |
| Logistics | 4 | 14.8% |
| Clinical Trials | ||
| Phase III | 19 | 70.4% |
| Protocols Development | 13 | 48.1% |
| Phase I | 11 | 40.7% |
| Phase II | 11 | 40.7% |
| Post-marketing Surveillance | 9 | 33.3% |
| Contract Research Organizations | 5 | 18.5% |
| Regulatory | ||
| Guidance on EUA | 16 | 59.3% |
| PQ | 16 | 59.3% |
| Collaborative Registration Procedure | 14 | 51.9% |
| Post Approval Changes | 7 | 25.9% |
The number and percentage of sampled manufacturers who require support for the listed processes.
Respondents were able to select any number of the listed processes.
Table 2.
Types of support sampled manufacturers require for the listed processes.
| Type of support required (%) |
|||
|---|---|---|---|
| Assessed manufacturing processes | Tech Transfer | Training | Funding |
| Drug Substance | 70% | 70% | 74% |
| Drug Product | 63% | 63% | 63% |
| Supply | 48% | 52% | |
| Clinical Trials | 44% | 81% | |
| Regulatory | 85% | 44% | |
Percentage of the sampled manufacturers requiring the given type of support for each manufacturing process assessed.
Respondents were able to select any number of the listed support mechanisms.
Processes related to vaccine supply were less frequently indicated by the sampled manufacturers as areas where support was needed. Almost half of the manufacturers required support for shipping validation, 30% require support for equipment used in the cold chain, and just under 15% need logistical support (Table 1). In supporting these manufacturers in supply processes funding (52%) and training (48%) were selected by manufacturers as most important (Table 2).
For clinical trial processes, over 70% of manufacturers indicated they require support in conducting Phase III trials. Protocol development (48%), and Phase I and Phase II trials 40% each) were also notable processes requiring support (Table 1). Specifically, over 80% of manufacturers require funding support, and to a lesser extent approximately 44% require training (Table 2).
Guidance on Emergency Use Authorization (EUA) and support for WHO prequalification were the two regulatory processes manufacturers require support for (almost 60% of the sampled manufacturers for each) while just over 50% of manufacturers indicated they require support for the Collaborative Registration Procedure (CRP) (Table 1). Regulatory training was selected as needed by 85% manufacturers (Table 2).
Lastly, in Appendix C we analysed the results by separating the sampled manufacturers into two groups: manufacturers with a vaccine product prequalified by WHO (WHO PQ) and those who do not. By segregating the sampled manufacturers into two groups we identified how their needs differed across different manufacturing processes. Results highlight those manufacturers without WHO PQ require more support for almost all of the manufacturing processes we assessed in this study with notably exceptions being nucleic acid vaccine technologies, hexavalent vaccines, upstream processes and guidance of EUA. A complete breakdown and summary of this additional analysis can be found in Appendix C.
4. Discussion
Several DCVMN members are producing COVID-19 vaccines: Sinovac Biotech (China) reported supplying 260 million doses of their inactivated COVID-19 vaccine globally3 , Sinopharm (China), intends to reach an annual production capacity of 1 billion doses in 2021 for their inactivated COVID-19 vaccine4 , Bharat Biotech (India), through support of the Government of India, has scaled-up manufacturing capacity of their COVID-19 vaccine to 700 million doses per annum5 and the Serum Institute of India, producing the AstraZenaca-Oxford non-replicating viral vector vaccine, achieved the distinction of being first and largest supplier to the COVAX facility which has already shipped vaccines to over 114 Countries, ensuring low-income economies have access to COVID-19 vaccines [7]. Furthermore, DCVMN members have been actively engaged in the development COVID-19 vaccines with 12 manufacturers having candidates in clinical trials [4].
Despite these advancements made by developing countries manufacturers, our findings indicate that manufacturers require funding and/or technology transfers for COVID-19 vaccines, particularly in respect to RNA vaccine technology (Fig. 2). Messenger RNA (mRNA) technologies provide advantages of accelerated immunogen discovery and response times [8]. The first two approved COVID-19 vaccines were based on this modern technology [9] and 43% of COVID-19 vaccine doses produced globally were mRNA vaccines6 . The importance of novel mRNA vaccine technologies and the need for global manufacturing scale-up highlight the need for technology transfers. Recognizing this need, the World Health Organisation (WHO) has launched an expression of interest to establish a COVID-19 mRNA vaccine technology transfer hub to “expand the capacity of Low- and middle-income countries to produce COVID-19 vaccines” [10]. The initiative has rapidly yielded results with the WHO announcing on June 21st 2021 that a South African consortium, including DCVMN member manufacturer Biovac, will establish the first technology transfer hub to scale up production and access to COVID vaccines [11]
The COVID-19 pandemic has severely impacted immunization programmes globally [12]. Before the pandemic, coverage for DTP3 and measles vaccines was stagnant at 85% and it is now estimated that less than 20% of children will receive their recommended vaccines by the age of 5 [13]. Reports state that 41 countries suspended their measles campaigns for 2020 and 2021, greatly increasing the risk of future outbreaks7 . Post-pandemic, coverage gaps must be filled to reduce vaccine preventable deaths globally. Our results show that many DCVMN members have expressed their interest in obtaining funding and/or technology transfers for hexavalent and measles containing vaccines (Fig. 1). Increasing production of these combination vaccines, for local and international use (through UNICEF supply channels) will go a long way in restoring immunization programs globally.
In addition to increasing coverage, by obtaining funding or technology transfers, developing country manufacturers can impact market dynamics with the ability to make vaccines more affordable. A key example is the WHO pre-qualified pneumococcal conjugate vaccine from the Serum Institute of India which greatly lowered prices to use in low-and middle-income countries [14]. DCVMN member manufacturers expressed high interest in pneumococcal conjugate vaccines and HPV vaccines, both historical high-priced vaccines and products whose markets are expected to grow substantially [15]. With more manufacturers producing these vaccines at scale, increased supply may drive prices down, increasing affordability.
Furthermore, our findings emphasize that developing countries vaccine manufacturers require support for Phase III clinical trials and to scale up their vaccine production, particularly funding support (Table 1, Table 2). Phase III trials involve enrolling thousands of participants to assess the efficacy and safety of a vaccine product [16]. Required for registration and approval to market a vaccine, phase III trials are a final step in vaccine development however are highly expensive to conduct [17]. Some DCVMN members have received funding from their local governments and/or international agencies to advance clinical trials and scale up facilities for COVID-19 vaccine candidates but in small proportions relative to MNCs [18]. However, DCVMN members have the ability to develop and produce vaccines at large scale rapidly to reach populous countries and achieve better vaccine equity globally.
Given the high-cost and complexity of in-house development, often manufacturers engage in licensing agreements and joint ventures to access new technologies and expertise. Recent research has revealed that partnerships for novel and improved vaccines between DCVMN members is particularly low, with manufacturers electing to engage with biotechnology companies and academic institutions [19]. Our matching analysis, illustrated in Fig. 3, suggests that there are ample opportunities for ‘South-South’ collaborations. For all technology platforms that were assessed at least one DCVMN member is positioned (and prepared) to provide technology transfer to other organizations. Opportunities for South-South collaborations are an area DCVMN and other stakeholders must explore. More generally, in assessing technology platforms, tech transfers were the support mechanism most frequently request by the sampled manufacturers (compared to funding and training) (Fig. 2). DCVMN members have frequently been technology recipients and subsequently these manufacturers increased supply and access to vaccines globally [20]. Nevertheless, as the technology transfer process is highly complex, requiring time and expertise alignment from stakeholders to support and catalyse technology transfer initiatives will be imperative to increase access to COVID-19 vaccines and novel vaccine platforms.
In selecting which support mechanisms they would value, DCVMN members did not select more tech transfers, funding, or training exhaustively for each and every manufacturing process. The finding is indicative of the significant advancements in DCVMN member manufacturers capabilities for developing and producing vaccines, and also the fact that several DCVMN members are specialized in key areas e.g., R&D or bulk manufacturing and are less likely to require support for other manufacturing processes. Subsequently, the responses from sampled manufacturers highlight the key priority areas which, without the support of external stakeholders, are true ‘pain points’ for DCVMN members.
In conclusion, this study served to draw attention to the requirements of developing country vaccine manufacturers and to the type of support that international stakeholders can provide. Identifying gaps across a range of manufacturing processes will provide stakeholders with an evidence-based foundation to develop strategies and initiatives to further support vaccine manufacturers and immunization globally.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We would like to thank all DCVMN members who completed the survey this paper is based on. We thank the DCVMN Board and all members of the DCVMN Secretariat for their ongoing support, guidance, and collaboration.
Disclaimer
The authors alone are responsible for the views expressed in this article, which do not necessarily represent the views, decisions or policies of any mentioned institutions with which the authors are affiliated.
Footnotes
https://www.gavi.org/news/media-room/100-million-covid-19-vaccine-doses-available-low-and-middle-income-countries-2021 (Accessed 20 April 2021)
Tech Transfer was only an option when applicable. If a respondent selected ‘Other’ they were required to share what was their interpretation of ‘Other’
https://www.reuters.com/business/healthcare-pharmaceuticals/sinovac-supplied-260-mln-covid-19-vaccine-doses-globally-2021–04-20/ (Accessed 22 April 2021)
https://www.reuters.com/article/us-health-coronavirus-vaccine-sinopharm-idUSKBN2BI27Z (Accessed 22 April 2021)
https://www.bharatbiotech.com/images/press/bharat-biotech-covaxin-capacity-expansionto-worldwide.pdf (Accessed 22 April 2021)
https://www.ifpma.org/wp-content/uploads/2021/03/Airfinity_global_summit_master_final.pdf (Accessed 22 April 2021)
https://www.cdc.gov/globalhealth/measles/data/global-measles-outbreaks.html (Accessed 22 April 2021)
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.07.007.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Pagliusi, et al. Emerging manufacturers engagements in the COVID −19 vaccine research, development and supply. Vaccine. 2020;38(34):5418–5423. doi: 10.1016/j.vaccine.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization: Prequalification. Available at https://extranet.who.int/pqweb/vaccines/prequalified-vaccines [accessed 20 April 2021].
- 3.Hayman B., Pagliusi S. Emerging vaccine manufacturers are innovating for the next decade. Vaccine X. 2020 doi: 10.1016/j.jvacx.2020.100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization: Draft landscape and tracker of COVID-19 candidate vaccines. Available at https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines [accessed 20 April 2021].
- 5.Plotkin, et al. The complexity and cost of vaccine manufacturing – an overview. Vaccine. 2017 doi: 10.1016/j.vaccine.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luter, et al. An updated methodology to review developing-country vaccine manufacturer viability. Vaccine. 2017;35(31):3897–3903. doi: 10.1016/j.vaccine.2017.04.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization: COVAX updates participants on delivery delays for vaccines from Serum Institute of India (SII) and AstraZeneca. Article. 25 March 2021. Available at https://www.who.int/news/item/25-03-2021-covax-updates-participants-on-delivery-delays-for-vaccines-from-serum-institute-of-india-(sii)-and-astrazeneca [accessed 22 April 2021].
- 8.Jackson, et al. The promise of mRNA vaccines: a biotech and industrial perspective. Nature Partner J. – Vaccines. 2020 doi: 10.1038/s41541-020-0159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee, et al. Current status of COVID-19 vaccine development: focusing on antigen design and clinical trials on later stages. Immune Network. 2021 doi: 10.4110/in.2021.21.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization: Establishment of a COVID-19 mRNA Vaccine Technology Transfer Hub to Scale Up Global Manufacturing. Article. 16 April 2021. Available at https://www.who.int/news-room/articles-detail/establishment-of-a-covid-19-mrna-vaccine-technology-transfer-hub-to-scale-up-global-manufacturing [accessed 22 April 2021].
- 11.World Health Organization: WHO supporting South African consortium to establish first COVID mRNA vaccine technology transfer hub. Article. 21 June 2021. Available at https://www.who.int/news/item/21-06-2021-who-supporting-south-african-consortium-to-establish-first-covid-mrna-vaccine-technology-transfer-hub [accessed 28 June 2021].
- 12.Lassi, et al. The impact of the COVID-19 pandemic on immunization campaigns and programs: a systematic review. Int J Environ Res Publ Health. 2021 doi: 10.3390/ijerph18030988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization: WHO and UNICEF warn of a decline in vaccinations during COVID-19. Press release. 15 July 2020. Available at https://www.who.int/news/item/15-07-2020-who-and-unicef-warn-of-a-decline-in-vaccinations-during-covid-19 [accessed 22 April 2021].
- 14.Alderson, M., Dhere, R., 2019. A new pneumococcal vaccine is here! Why this matters. PATH. Available at https://www.path.org/articles/new-pneumococcal-vaccine/ [accessed 22 April 2021].
- 15.World Health Organization: Global Vaccine Market Report. Working Draft – December 2020. Available at https://www.who.int/immunization/programmes_systems/procurement/mi4a/platform/module2/2020_Global_Vaccine_Market_Report.pdf?ua=1 [accessed 29 April 2021].
- 16.Singh K., Mehta S. The clinical development process for a novel preventive vaccine: an overview. J Postgrad Med. 2016 doi: 10.4103/0022-3859.173187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Black S. The costs and effectiveness of large Phase III pre-licensure vaccine clinical trials. Expert Rev Vaccines. 2015 doi: 10.1586/14760584.2015.1091733. [DOI] [PubMed] [Google Scholar]
- 18.Wouters O.J., et al. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet. 2021 doi: 10.1016/S0140-6736(21)00306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayman, et al. Advancing innovation for vaccine manufacturers from developing countries: prioritization, barriers, opportunities. Vaccine. 2021;39(8):1190–1194. doi: 10.1016/j.vaccine.2020.12.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization: Increasing Access to Vaccines Through Technology Transfer and Local Production, 2011. Available at https://www.who.int/phi/publications/Increasing_Access_to_Vaccines_Through_Technology_Transfer.pdf [accessed 28 June 2021].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.