Abstract
Understanding the mechanism for heat tolerance is important for the hot pepper breeding program to develop heat-tolerant cultivars in changing climate. This study was conducted to investigate physiological and biochemical parameters related to heat tolerance and to determine leaf heat damage levels critical for selecting heat-tolerant genotypes. Seedlings of two commercial cultivars, heat-tolerant ‘NW Bigarim’ (NB) and susceptible ‘Chyung Yang’ (CY), were grown in 42 °C for ten days. Photosynthesis, electrolyte conductivity, proline content were measured among seedlings during heat treatment. Photosynthetic rate was significantly reduced in ‘CY’ but not in ‘NB’ seedlings in 42 °C. Stomatal conductivity and transpiration rate was significantly higher in ‘NB’ than ‘CY’. Proline content was also significantly higher in ‘NB’. After heat treatment, leaf heat damages were determined as 0, 25, 50 and 75% and plants with different leaf heat damages were moved to a glasshouse (30–32/22–24 °C in day/night). The growth and developmental parameters were investigated until 70 days. ‘NB’ was significantly affected by leaf heat damages only in fruit yield while ‘CY’ was in fruit set, number and yield. ‘NB’ showed fast recovery after heat stress compared to ‘CY’. These results suggest that constant photosynthetic rate via increased transpiration rate as well as high proline content in heat stress condition confer faster recovery from heat damage of heat-tolerant cultivars in seedlings stages.
Subject terms: Biochemistry, Physiology, Plant sciences, Environmental sciences
Introduction
The heat stress caused by high temperatures induces morphological, anatomical, physiological, biochemical and genetic modifications in plants1–4. Photosynthetic and biochemical parameters can be good indicators for detecting effects of heat stress on plants because photosynthesis, growth and yield are closely related5,6. Therefore, it is vital to elucidate mechanisms of plants in response to heat stress, which may result in reducing reproductive success and destroying physiological activity, and consequently cause yield loss in agriculture2–4. Many plants have tolerance to heat stress but underlying mechanisms are not well understood yet4,7,8.
Hot pepper (Capsicum annuum L.) is one of the important vegetable crops of global significance. In changing climate, developing heat-tolerant cultivars has become a key research topic in the field of hot pepper breeding. In hot pepper, the temperature more than 30 °C not only significantly reduces the pollen viability, and fruit set9,10 and yield11,12, but induces the abscission of floral buds, flowers and immature fruits11,13. Various hot pepper accessions with diverse genetic backgrounds significantly differ in response to heat stress14.
A lot of screening methods for identifying heat-tolerant genotypes in different developmental stages and day and/or night temperatures have been developed for various crops in Solanaceae family4,6,9,12,15. Heat tolerance is a developmentally regulated, stage-specific phenomenon and its mechanism in hot pepper is still unknown. Heat tolerance at one developmental stage can be related16 or not to tolerance at the other developmental stages17. Therefore, breeding programs should focus on determining developmental stages susceptible to heat stress -to develop hot pepper cultivars with heat tolerance. Plants are usually susceptible to high temperature in early growth stages and, therefore, seedlings in the appropriate stage for transplanting can be proper starting materials to reveal heat tolerance mechanism in hot pepper.
The present study was conducted to understand the mechanisms to confer upon hot pepper plants the heat tolerance in seedling stages. Seedlings of two commercial pepper cultivars, one tolerant and the other susceptible to heat stress, were treated with severe temperature regime of 42 °C in day and night for ten days and their physiological changes were estimated. In addition, the growth and fruit yield parameters of seedlings with different leaf heat damages were investigated 70 days after heat treatment.
Results
Difference in physiological responses to heat treatment between heat-tolerant and susceptible seedlings
Plant height and shoot and root fresh weight were significantly reduced in seedlings grown in 42 °C compared to normal temperature regardless of heat-tolerant or susceptible cultivars (Supplementary Table S1). However, the heat-tolerant cultivar, ‘NB’ showed more reduction in shoot fresh weight than ‘CY’, the susceptible cultivar, but ‘CY’ showed more reduction in root fresh weight than ‘NB’ (Supplementary Table S1). Chlorophyll content significantly decreased as days of heat treatment (HT) increased in both cultivars (Supplementary Fig. S1) and the decrease was more prominent in a heat-susceptible cultivar, ‘CY’, than ‘NB’, after 2nd day of HT although the difference was not significant (see Supplementary Fig. S1).
Photosynthetic rate was significantly reduced in a heat-susceptible cultivar compared to a tolerant one after seven days of HT (Fig. 1a). Although photosynthetic rate of ‘CY’ was significantly higher than that of ‘NB’ before HT, it decreased more in ‘CY’ than ‘NB’, after short increase in 2nd day, showing similar photosynthetic rate in both cultivars in 7th day of HT (Fig. 1a). Photosynthetic rate was not significantly different in ‘NB’, but significantly reduced in ‘CY’, between 0 and 7th days of HT (Fig. 1a).
Figure 1.
Effect of heat treatment of 42 °C on photosynthetic rate (a), stomatal conductivity (b), intercellular CO2 concentration (c) and transpiration rate (d) of hot pepper seedlings. Vertical bars represent standard deviation (n = 3). Different letters indicate significant differences by Duncan’s multiple range test at p < 0.05.
The steady and significant increase in stomatal conductivity (Fig. 1b), the intercellular CO2 concentration (Fig. 1c) and transpiration rate (Fig. 1d) were observed in both cultivars when HT was prolonged. However, there were significant difference among them between two cultivars. Before HT, stomatal conductivity and transpiration rate was not different between two cultivars but significantly higher thereafter in ‘NB’ than ‘CY’, heat-tolerant and susceptible cultivars, respectively (Fig. 1b,d). In intercellular CO2 concentration, although there was initial significant difference between two cultivars before HT, the difference was not significant 7th day of HT (Fig. 1c).
The cell membrane thermo-stability, calculated by electrolyte conductivity (EC), differ between two cultivars (Fig. 2a). EC was significantly higher in ‘NB’ before and 1st day of HT but reversed in 2nd day and no significant difference was observed between two cultivars after 5th day (Fig. 2a). However, there were different pattern in proline content (Fig. 2b). Proline content of ‘CY’ was significantly higher than that of ‘NB’ before and 1st day of HT but was significantly lower after 2nd day of HT than that of ‘NB’ (Fig. 2b). The proline content of ‘NB’ continuously increased from 1st to 7th days of HT but no distinct trend was observed in ‘CY’ (Fig. 2b).
Figure 2.

Electrolyte conductivity (a) and proline content (b) as affected by different heat treatment days in seedlings of two hot pepper cultivars grown in 42 °C. Vertical bars represent standard deviation (n = 3). NS, *, ** and *** indicate not significant and significant at the P < 0.05, P < 0.01 and P < 0.001 levels in t-test, respectively.
Effects of leaf heat damage levels on the growth and yield of heat-tolerant and susceptible cultivars
A leaf heat damage (LHD) increased significantly in both cultivars as days of HT increased (Supplementary Fig. S2). There was significantly difference in LHD in 10th day of HT, showing significantly higher in ‘NB’ (67.4%), a tolerant, than ‘CY’ (47.6%), a susceptible cultivar. It implies that ‘CY’ seems more tolerant to heat stress than ‘NB’ in seedling stages.
The development, not the growth, of heat-tolerant and susceptible cultivars were significantly affected by LHD levels in the seedling stage (Fig. 3). Plant height in 70 days after transplanting (DAT) in a greenhouse (30–32/22–24 °C in day/night) was not affected by LHD levels although there was innate difference between two cultivars (Fig. 3a). In a heat susceptible cultivar, ‘CY’, LHD 75% significantly affected the fruit set while all LHD levels did not affect the fruit set of a heat-tolerant cultivar, ‘NB’ (Fig. 3b). The number of fruits in ‘CY’ was significantly affected by LHD levels, showing steady and significant decrease as LHD level increased (Fig. 3c). However, the number of fruits in a heat-tolerant cultivar, ‘NB” was not significantly affected by LHD levels (Fig. 3c). Total yield was significantly affected by LHD 25% in both cultivars but significantly higher in ‘NB’ than ‘CY’ in all LHD levels (Fig. 3d).
Figure 3.
Effect of leaf heat damage levels on plant height (a), fruit set (b), the number of fruits (c) and total yield (d) in hot pepper cultivars. Vertical bars represent standard deviation (n = 3). Different letters indicate significant differences by Duncan’s multiple range test at p < 0.05. All parameters were measured on 70 days after transplanting.
EC was significantly higher in ‘CY’ than ‘NB’ at LHD 0 and 25% but significantly lower at LHD 75% (Fig. 4a). There was no abrupt changes in EC in both cultivars, showing relative small changes within the range between 17 to 26% (Fig. 4a). However, the proline content increased as LHD levels increased from 0 to 75% (Fig. 4b). However, the differences between the two cultivars were not significant, except for LHD 25%, which was significantly higher in ‘NB’, a heat-tolerant cultivar.
Figure 4.

Electrolyte conductivity (a) and proline content (b) as affected by different leaf heat damages in seedlings of two hot pepper cultivars grown in 42 °C for 9 to 10 days. Vertical bars represent standard deviations (n = 3). NS, * and *** indicate not significant and significant at the P < 0.05 and P < 0.001 levels in t-test, respectively.
The plant growth was recovered faster in a heat tolerant than susceptible cultivar (Figs. 5, 6). The plant height was significantly lower in plants with LHD than those without LHD (plants did not go through HT) in ‘CY’ in early growth stages (Figs. 5a, 6a) and it was not until 70 DAT that all plants with different LHD levels showed similar plant height (Fig. 5a). ‘CY’ plants with LHD 25% were recovered earlier than other LHD levels (Fig. 5a). In contrast to ‘CY’, the plant height of ‘NB’ was not significantly different in 56 DAT among plants with LHD 0, 25, 50 and 70% (Figs. 5b, 6b), showing 14 days faster recovery than ‘CY’, except for LHD 25% (Fig. 5b).
Figure 5.
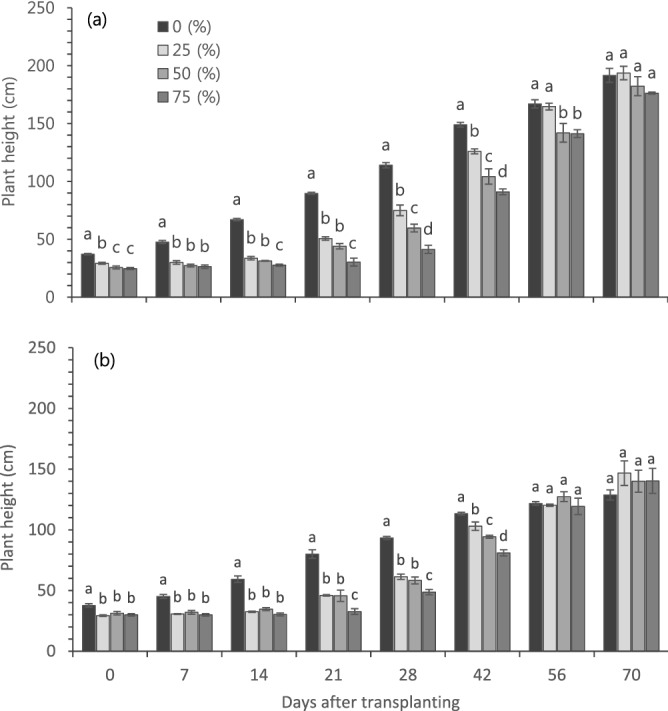
Effect of different leaf heat damage levels on plants growth rate in hot pepper cultivars susceptible (a, ‘Chyung Yang’) and tolerant (b, ‘NW Bigarim’) to heat stress. Vertical bars represent standard deviation (n = 3). Different letters indicate significant differences by Duncan’s multiple range test at p < 0.05.
Figure 6.
Effect of different leaf heat damages on the recovery of hot pepper plants. Cultivars ‘Chyung Yang’ in 30 days (a) and 55 days (b), and ‘NW Bigarim’ in 30 days (c) and 55 days (d) after heat treatment in 42 °C.
Discussion
Heat-tolerant cultivars are required to mitigate the adverse impact of changing climate without the yield reduction that was generally observed in heat susceptible cultivars18. The difference in heat tolerance can be related to different responses to high temperatures among genotypes and development stages8,16,19. The present study investigated physiological and biochemical changes due to high temperature using heat-tolerant and susceptible cultivars to understand the heat tolerance mechanisms in hot pepper seedlings.
The range and duration of high temperature influence the physiological parameters of plants11,20,21. Chlorophyll content in leaves decreased as HT days increased, and the decrease was faster in a heat-susceptible cultivar than a tolerant cultivar (Supplementary Fig. S1), as was observed in hot pepper9,11 and tomato17,22. The higher chlorophyll content in heat-tolerant cultivar gives better photosynthetic stability than heat-susceptible cultivars16,17,22.
In our study, HT significantly decrease photosynthetic rate in a heat-susceptible cultivar but not in a heat-tolerant cultivar (Fig. 1a). The stable photosynthetic rate of heat-tolerant cultivar in HT might be due to the increased stomatal conductivity (Fig. 1b) and transpiration rate (Fig. 1d). In tomato, high stomatal conductivity and transpiration rate under heat stress improves leaf cooling in heat-tolerant genotypes, providing better protection for chlorophyll and maintaining relatively high photosynthetic rate16. Also, a heat-tolerant cultivar had greener leaves than a heat-susceptible cultivar (see Supplementary Fig. S3) and this may contribute to better and stable photosynthesis17. The stay-green trait is also an important agronomical characteristic that contributes to higher yield under heat stress in tomato23.
Heat-tolerant cultivars may show more cell membrane thermostability than susceptible ones2 and cell membrane thermostability has been often linked to photosynthetic and transpiration rates24. However, membrane thermosstability seems not to contribute the photosynthesis and transpiration in hot pepper. Significant decrease in photosynthetic rate in a heat-susceptible cultivar (Fig. 1a) and significantly higher transpiration in a heat-tolerant cultivar (Fig. 1d) were observed while there was no difference in EC between both cultivars in 7th day (Fig. 2b).
In response to environmental stresses, plants accumulate osmoprotectants including proline17,25 and they are generally found in large amounts under any stress conditions15,19,23. Our results are in line with previous studies. Proline content increased as LHD increased regardless of cultivars (Fig. 4b). Interestingly, proline content that was significantly lower in early days of HT became higher in a heat-tolerant cultivar than heat-susceptible one from 5th day of HT (Fig. 2b). This indicate that heat tolerant cultivar responses more to the duration of HT than the magnitude of leaf heat damage in terms of proline accumulation.
HT significantly affect the plant height in early growth stages and it was recovered in later growth stages; however, the recovery was faster in a heat-tolerant cultivars than susceptible one (Fig. 5). This is possibly due to the stable photosynthetic rate in a heat-tolerant cultivar throughout the entire days of HT (Fig. 1a). The fruit set is the main indicator for screening the response of genotype on abiotic stress condition1,23,24, and heat stress also significantly affect fruit set in other Solanaceae crops including tomato4,17,19,26,27. In hot pepper, fruit set of the heat-tolerant cultivar was not affected by HT (Fig. 3b). In addition, the number of fruits was also not affected by HT in a heat-tolerant cultivar (Fig. 3c).
The heat-tolerant cultivar was not affected by LHD levels in terms of plant height (Fig. 3a), fruit set (Fig. 3b) and the number of fruits (Fig. 3c), total yield was significantly reduced in LHD 25% (Fig. 3d). Therefore, more than LHD 25% can be critical level for selecting heat-tolerant genotypes in seedling stages of hot pepper.
In conclusion, the present study suggests mechanism for heat tolerance in hot pepper via constant photosynthetic rate possibly due to increased stomatal conductivity and transpiration rate in high temperature, which can improve leaf cooling. The steady photosynthetic rate and high proline content in high temperature can provide faster recovery from heat damage in seedlings of heat-tolerant hot pepper plants, which resulted in stable fruit set and the number of fruits, followed by high yield. In addition, LHD levels over 25% in seedling stage was critical for selecting heat-tolerant genotypes in hot pepper breeding program.
Materials and methods
Plant materials and heat treatment conditions
The seeds of two commercial pepper cultivars ‘CY’ and ‘NB’, which are susceptible and tolerant to high temperature, respectively, were sown in 31st March, 2020 in plastic trays (52 × 26 cm in size, 6 × 6 cm cells with pot volume 5 L) containing 1:1 ratio of sand and commercial bed soil (Bio Sangto, Seoul, Korea) containing coco peat (47.2%), peat moss (35%), zeolite (7%), vermiculite (10.0%), dolomite (0.6%), humectant (0.006%) and fertilizers (0.194%). A liter of water was provided to each tray daily, and the trays were placed in a glasshouse (26/18 °C in day/night with relative humidity within 65–70%) in National institute of Horticultural and Herbal Science, Wanju, Korea.
Hot pepper seedlings with 8–10 true leaves were transferred on 11 May 2020 to growth chamber, maintaining day and night temperatures of 42 °C, light intensity 800 µmol m−2 s−1 for 16 h and 60–70% relative humidity. For each cultivar, a total of 32 seedlings were grown in the growth chamber for 10 days and watered twice a day (total two liters) to avoid drought stress. Plant height, shoot fresh weight and, root length and fresh weight were measured 10th day in HT with three replications.
Photosynthesis in heat treated seedlings
The photosynthetic rate (μmol CO2 m−2 s−1), stomatal conductance (mol H2O m−2 s−1), intercellular CO2 concentration (μmol CO2 mol−1) and transpiration rate (mmol H2O m−2 s−1) were measured from 5 to 6th leaves of 0, 2 and 7 days after HT between 10:00–12:00 am. Data were recorded in three plants per cultivar using a portable photosynthesis measurement system (LI-6400, LI-COR Bioscience, Lincoln, NE, USA). Light response curves was set to 800 μmol m −2 s−1, the leaf chamber temperature was set to 25 °C, and the intercellular CO2 concentration was maintained at 400 μmol mol−1. The photosynthetic rate was measured automatically at each irradiation level after 3–4 min light exposure28.
Chlorophyll content, electrolyte conductivity, proline content in leaves of heat treated seedlings
All measurements for chlorophyll content, EC and proline content were conducted in 0, 1, 2, 5 and 7 days in HT with biological (three plants) and technical replications. Chlorophyll content were estimated using SPAD meter (Konica Minolta, Japan) in hot pepper leaves between 5–6 internodes with three technical replications.
EC in hot pepper leaves was measured according to Camejo et al.2 with minor modifications. Five leaves per seedling were perforated into discs with a radius of 5.5 mm, and each disc was placed in a 15-mL tube containing 10 mL of deionized water and then incubated on a shaking table at 25 °C for 30 min. At this time, the conductivity (EC1) of water was measured using a STARA-HB conductivity meter (Thermo Orion, Waltham, MA, USA). The tube was heated in a boiling water bath for 30 min and cooled at room temperature for 20 min, and then the conductivity (EC2) was measured again. Final EC content was expressed as the percentage of EC1/EC2.
Proline content in hot pepper leaves was measured using colorimetric assay25. Leaf samples from heat treated and non-treated seedlings were lyophilized (− 72 °C) in a freezer dryer (IlShin BioBase, Korea) for three days. Each leaf sample (100 mg in dry weight) was homogenized with 2 ml of 3% (w/v) aqueous sulfosalicylic acid solution. The homogenate was centrifuge at 14,000 rpm for 7 min and 1 ml of supernatant was transferred to 5 ml micro tubes containing 1 ml of acid ninhydrin. Acid ninhydrin was prepared by adding ninhydrin (2.5 g/100 ml) to solution containing glacial acetic acid, distilled water and 6 M ortho-phosphoric acid 85% at ratio of 6:3:1, receptively. The reaction mixtures were kept immediately in boiling water bath (95 °C) for one hour. The reaction was stopped (boiled micro tubes were kept) at 4 °C for 20 min and reading were taken at wavelength of 546 nm by spectrophotometer (EON, BioTek Instruments, USA).
Evaluation of leaf heat damage levels among seedlings
LHD levels of hot pepper plants after 10 days of HT were identified according to the visual injuries. Leaf damage was calculated by measuring the percentage of leaf area that was dried or light yellow-white colored and classified into four levels of LHD 0% (no heat treatment), LHD 25% with leaf damages from 11 to25%, LHD 50% from 25 to 50% and LHD 75% from 50 to 75%. Seedlings with LHD 25% were collected in 9th day and those with LHD 50 and 75% were collected in 10th day of HT. After 9–10 days of HT, seedlings were transferred to glasshouse condition described above and maintained for three days to recover.
Electrolyte conductivity, proline content, growth and yield of seedlings with different leaf heat damage levels
After three days of recovery, seedlings were transplanted to plastic pots with the same substrate described above and all plants were maintained in a glasshouse condition (30–32/22–24 °C in day/night). All plants were watered once a day and fertilized weekly with 1 L of water containing 1 mL of N-6, P-10 and K-5 (HYPONeXm, Japan). EC and proline content of seedlings with LHD 0, 25, 50 and 75% were measured eight days after HT with the same methods described above.
The measurement of plant height were started 70 days after transplanting (DAT) with seven days interval during the first four weeks and then every 14 days. Fruit set (FS, %) was calculated as follows:
The number of fruits and flowers was determined by counting those from first to fourth internodes and total yield was determined by the sum of fresh weight of all fruits. All measurements were conducted with three biological replications.
Statistical analysis
The arrangement of hot pepper plants was completely randomized. Analysis of variance were performed using the SAS Enterprise Guide 7.1 (SAS Institute Inc., NC, USA) for the data of the physiological and agronomical parameters. The mean values were compared with a significance level of 5% using Duncan’s multiple range test or Student’s t-test at the P < 0.05, P < 0.01 and P < 0.001 levels.
Informed consent
This article does not contain any studies involving animals or human participants as objects of research.
Supplementary Information
Abbreviations
- HT
Heat treatment
- LHD
Leaf heat damage
- EC
Electrolyte conductivity
- DAT
Days after transplanting
Author contributions
E.Y.Y., M.C.C., and S.N.R., designed experiments; S.N.R., S.Y.C., and H.J.B., performed experiments and analyzed the data; W.B.C. and S.N.R. contributed to interpretation of results and writing of the manuscript. All authors reviewed and agreed to the published version of the manuscript.
Funding
This study was supported by a grant (Project No: PJ01267102 "Study on the physiological mechanism of temperature adaptable pepper lines") from National Institute of Horticultural and Herbal Science, Rural Development Administration.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on responsible request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Sherzod Nigmatullayevich Rajametov and Eun Young Yang.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-93697-5.
References
- 1.Sato S, Peet MM, Thomas JF. Physiological factors limit fruit set of tomato (Lycopersicon esculentum Mill.) under chronic, mild heat stress. Plant. Cell Environ. 2000;23:719–726. doi: 10.1046/j.1365-3040.2000.00589.x. [DOI] [Google Scholar]
- 2.Camejo D, Rodriguez P, Morales MA, Dell’Amico JM, Torrecillas A, Alarcon JJ. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. Plant Physiol. 2005;162:281–289. doi: 10.1016/j.jplph.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Munir S, Liu H, Xing Y, Hussain S, Ouyang B, Zhang Y, Li H, Ye Z. Overexpression of calmodulinlike (ShCML44) stress-responsive gene from Solanum habrochaites enhances tolerance to multiple abiotic stresses. Sci. Rep. 2016;6:31772. doi: 10.1038/srep31772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu J, Wolters-Arts M, Mariani C, Huber H, Rieu I. Heat stress affects vegetative and reproductive performance and trait correlations in tomato (Solanum lycopersicum) Euphytica. 2017;213:156. doi: 10.1007/s10681-017-1949-9. [DOI] [Google Scholar]
- 5.Rivero RM, Mestre TC, Mittler R, Rubio F, Garcia-Sanchez F, Martinez V. The combined effect of salinity and heat reveals a specific physiological, biochemical and molecular response in tomato plants. Plant Cell Environ. 2014;37:1059–1073. doi: 10.1111/pce.12199. [DOI] [PubMed] [Google Scholar]
- 6.Zhou R, Wu Z, Wang X, Rosenqvist E, Wang Y, Zhao T, Ottosen CO. Evaluation of temperature stress tolerance in cultivated and wild tomatoes using photosynthesis and chlorophyll fluorescence. Hortic. Environ. Biotechnol. 2018;59:499. doi: 10.1007/s13580-018-0050-y. [DOI] [Google Scholar]
- 7.Zinn KE, Tunc-Ozdemir M, Harper JF. Temperature stress and plant sexual reproduction: Uncovering the weakest links. J. Exp. Bot. 2010;61:1959–1968. doi: 10.1093/jxb/erq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rieu I, Twell D, Firon N. Pollen development at high temperature: From acclimation to collapse. Plant Physiol. 2017;173:1967–1976. doi: 10.1104/pp.16.01644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnaoudova Y, Topalova E, Todorova V. High temperature effect on the male gametophyte and the photosynthetic activity of two Capsicum annuum L. cultivars. Bulgar. J. Agric. Sci. 2020;26(2):409–415. [Google Scholar]
- 10.Erickson AN, Markhart AH. Flower developmental stage and organ sensitivity of bell pepper (Capsicum annuum L.) to elevated temperature. Plant Cell Environ. 2002;25:123–130. doi: 10.1046/j.0016-8025.2001.00807.x. [DOI] [Google Scholar]
- 11.Utami D, Aryanti E. Impact of heat stress on germination and seedling growth of chili pepper (Capsicum annuum L) Earth Environ. Sci. 2021;637:012032. doi: 10.1088/1755-1315/637/1/012032. [DOI] [Google Scholar]
- 12.Pagamas P, Nawata E. Sensitive stages of fruit and seed development of chili pepper (Capsicum annuum L. var. Shishito) exposed to high-temperature stress. Sci. Horticult. 2008;117:21–25. doi: 10.1016/j.scienta.2008.03.017. [DOI] [Google Scholar]
- 13.AVRDC Development of high yielding, diseaseresistant chilli peppers. In: AVRDC Progress report - 2003. Asian Vegetable Research and Development Centre, Shanhua, Taiwan. pp. 41–46 2003.
- 14.Ghai N, Kaur J, Jindal SK, Dhaliwal MS, Pahwa K. Physiological and biochemical response to higher temperature stress in hot pepper (Capsicum annuum L.) J. Appl. Nat. Sci. 2016;8(3):1133–1137. doi: 10.31018/jans.v8i3.930. [DOI] [Google Scholar]
- 15.Bhandari SR, Kim YH, Lee JG. Detection of temperature stress using chlorophyll fluorescence parameters and stress-related chlorophyll and proline content in paprika (Capsicum annuum L.) seedlings. Korean J. Hortic. Sci. Technol. 2018;36(5):619. doi: 10.12972/kjhst.20180062. [DOI] [Google Scholar]
- 16.Zhou R, Kjaer KH, Rosenqvist E, Yu X, Wu Z, Ottosen CO. Physiological response to heat stress during seedling and anthesis stage in tomato genotypes differing in heat tolerance. Agron. Crop. Sci. 2017;203:68–80. doi: 10.1111/jac.12166. [DOI] [Google Scholar]
- 17.Rajametov SN, Yang EY, Bong JH, Cho MC, Chae SY, Paudel N. Heat treatment in two tomato cultivars: a study if the effect on physiological and growth recovery. Horticulturae. 2021;7:119. doi: 10.3390/horticulturae7050119. [DOI] [Google Scholar]
- 18.Solankey SS, Singh RK, Baranwal DK, Singh DK. Genetic expression of tomato for heat and drought stress tolerance: An overview. Intl J. Veg. Sci. 2015;21(5):496–515. doi: 10.1080/19315260.2014.902414. [DOI] [Google Scholar]
- 19.Din JU, Khan SU, Khan A, Qayyum A, Abbasi KS, Jenks MA. Evaluation of potential morpho-physiological and biochemical indicators in selecting heat-tolerant tomato (Solanum lycopersicum Mill.) genotypes. Hortic. Environ. Biotechnol. 2015;56(6):769–776. doi: 10.1007/s13580-015-0098-x. [DOI] [Google Scholar]
- 20.Garruna-Hernandez R, Orellana R, Larque-Saaverda A, Canto A. Understanding the physiological responses of a tropical crop (Capsicum Chinense Jacq) at high temperature. PLoS ONE. 2014;3:9. doi: 10.1371/journal.phone.0111402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hussain T, Ayyub CM, Amjad M, Hussain M. Analysis of morpho-physiological changes occurring in Chilli genotypes (Capsicum spp.) under high temperature conditions. Pak. J. Agric. Sci. 2021;58(1):43–50. doi: 10.21162/PAKJAS/21.7185. [DOI] [Google Scholar]
- 22.Poudyal D, Rosenqvist E, Ottosen CO. Phenotyping from lab to field—tomato lines screened for heat stress using Fv/Fm maintain high fruit yield during thermal stress in the field. Funct. Plant Biol. 2018 doi: 10.1071/FP17317. [DOI] [PubMed] [Google Scholar]
- 23.Zhou R, Yu X, Kjær KH, Rosenqvist E, Ottosen C-O, Wu Z. Screening and validation of tomato genotypes under heat stress using Phenotyping tomato for heat tolerance Functional Plant Biology K Fv/Fm to reveal the physiological mechanism of heat tolerance. Envir. Exp. Bot. 2015;118:1–11. doi: 10.1016/j.envexpbot.2015.05.006. [DOI] [Google Scholar]
- 24.Wahid A, Gelani S, Ashraf M, Foolad MR. Heat tolerance in plants: An overview. Environ. Expt. Bot. 2007;61:199–223. doi: 10.1016/j.envexpbot.2007.05.011. [DOI] [Google Scholar]
- 25.Claussen W. Proline as a measure of stress in tomato plants. Plant Sci. 2005;168:241–248. doi: 10.1016/j.plantsci.2004.07.039. [DOI] [Google Scholar]
- 26.Kaur N, Dhaliwal MS, Jindal S, Singh S. Evaluation of hot pepper (Capsicum annuum L.) genotypes for heat tolerance during reproductive phase. Intl. J. Bio-Resour. Stress Manag. 2016;7(1):126. doi: 10.5958/0976-4038.2016.00020.8. [DOI] [Google Scholar]
- 27.Rajametov S, Yang E, Cho M, Chae S, Chae W. Physiological traits associated with high temperature tolerance differ by fruit types and sizes in tomato (Solanum lycopersicum L.) Hortic. Environ. Biotechnol. 2020;61:837–847. doi: 10.1007/s13580-020-00280-4. [DOI] [Google Scholar]
- 28.Shah Jahan M, Wang Y, Sheng S, Zhong M, Chen Z, Wu J, Sun J, Guo Sh. Exogenous salicylic acid increases the heat tolerance in Tomato (Solanum lycopersicum L.) by enhancing photosynthesis efficiency and improving antioxidant defense system through scavenging of reactive oxygen species. Sci. Horticult. 2019;247:421–429. doi: 10.1016/j.scienta.2018.12.047. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on responsible request.





