Abstract
Pancreatic duct stenting is a well-established method for reducing post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis. However, there is no consensus on the optimal type of plastic stent. This study aimed to evaluate the feasibility and safety of a new 4-Fr plastic stent for pancreatic duct stenting. Forty-nine consecutive patients who placed the 4-Fr stent into the pancreatic duct (4Fr group) were compared with 187 consecutive patients who placed a conventional 5-Fr stent (control group). The primary outcome was technical success. Complications rate, including post-ERCP pancreatitis (PEP) were the secondary outcomes. Propensity score matching was introduced to reduce selection bias. The technical success rate was 100% in the 4Fr group and 97.9% in the control group (p = 0.315). Post-ERCP amylase level was significantly lower in the 4-Fr group than the control group before propensity score matching (p = 0.006), though without statistical significance after propensity score matching (p = 0.298). The rate of PEP in the 4Fr group (6.1%) was lower than the control group (15.5%), though without statistical significance before (p = 0.088) and after (p = 1.00) propensity score matching. Pancreatic duct stenting using a novel 4-Fr plastic stent would be at least similar or more feasible and safe compared to the conventional plastic stent.
Subject terms: Pancreatitis, Acute pancreatitis
Introduction
Post endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis is the most common complication of ERCP and can occasionally become severe or fatal. While the reported frequency of post-ERCP pancreatitis (PEP) varies between 3 and 5%, a recent systematic review reported an incidence of 14.7% in high-risk patients1–3. The known mechanisms of PEP comprise impaired drainage from the pancreatic duct caused by papillary edema and/or spasm of the sphincter of Oddi after the procedure4–7. Pancreatic duct stenting guarantees unhindered drainage of the pancreatic secretions. Moreover, it is a well-established method of reducing PEP, particularly in high-risk patients8–15. Endoscopic pancreatic duct stenting has also been proven effective in patients with obstructive pancreatitis16,17.
The size and length of the stents vary. Furthermore, there are no guidelines or consensus on an optimal plastic stent18–20. The use of smaller-diameter stents may result in less ductal irritation and changes, particularly in non-dilated pancreatic duct cases18. Despite reports on the efficacy of 3-Fr plastic stents, smaller-diameter stents have not been accepted in clinical practice21. This can be partially attributed to the requirement of a smaller-caliber 0.018- or 0.021-in. guidewire, which can be difficult to maneuver around the tortuous pancreatic duct compared to the standard 0.025-in. wire. This, in turn, likely results in a higher rate of PEP6,19,21.
Novel 4-Fr plastic stents with the ability to place over a standard 0.025-in. guidewire have been recently developed in our country. The present study aimed to examine the feasibility and safety of a new 4-Fr single-pigtail pancreatic plastic stent by comparing it with the conventional 5-Fr plastic stent.
Results
A total of 236 patients met the eligibility criteria for study inclusion; of these, The 4-Fr plastic stent was placed 49 in patients (4-Fr group), and the conventional 5-Fr plastic stent was placed in 186 patients (conventional stent group). Table 1 summarizes the baseline characteristics, including sex, age, history of ERCP related procedures, indication for stent placement, and indication for ERCP. Indication for stent showed significant differences between groups regarding placement before propensity score matching; however, significant differences were not observed in any of these characteristics after propensity score matching.
Table 1.
Baseline characteristics of the study patients.
| Characteristics | Before propensity score matching | After propentisy score matching | ||||
|---|---|---|---|---|---|---|
| 4 Fr group (n = 49) |
Conventional stent group (n = 187) |
P-value | 4 Fr group (n = 47) |
Conventional stent group (n = 47) |
P-value | |
| Sex (M/F), n | 35/14 | 111/76 | 0.122 | 33/14 | 35/12 | 0.818 |
| Age, y, median (quantile) | 69.0 (57.5–75.0) | 67.0 (55.0–74.0) | 0.521 | 69.0 (58.0–75.0) | 68.0 (52.0–75.0) | 0.555 |
| History of ERCP related procedures, n (%) | 5 (10.2) | 7 (3.7) | 0.135 | 3 (6.4) | 2 (4.3) | 0.646 |
| Indication for stent placement, n (%) | 0.028 | 0.928 | ||||
| Papillectomy | 26 (53.1) | 138 (73.8) | 26 (55.3) | 28 (59.6) | ||
| Unintentional pancreatic guidewire passage | 18 (36.7) | 38 (20.3) | 18 (38.3) | 15 (31.9) | ||
| Argon plasma coagulation | 3 (6.1) | 2 (1.1) | 2 (4.3) | 1 (2.1) | ||
| Post EST bleeding | 0 (0) | 3 (1.6) | 0 (0) | 1 (2.1) | ||
| Divisum | 1 (2.0) | 4 (2.1) | 1 (2.1) | 1 (2.1) | ||
| Obstructive pancreatitis | 1 (2.0) | 2 (1.1) | 0 (0) | 1 (2.1) | ||
| Indication for ERCP, n (%) | 0.26 | 0.889 | ||||
| Ampulla of Vater adenoma | 29 (59.2) | 140 (74.9) | 28 (59.6) | 29 (61.7) | ||
| Malignant biliary obstruction | 5 (10.2) | 7 (2.0) | 5 (10.6) | 4 (8.5) | ||
| Biliary stone | 12 (24.5) | 28 (15.0) | 12 (25.5) | 10 (21.3) | ||
| Benign biliary stricture | 1 (2.0) | 3 (1.6) | 1 (2.0) | 1 (2.1) | ||
| Divisum | 1 (2.0) | 4 (2.1) | 1 (2.1) | 1 (2.1) | ||
| Obstructive pancreatitis | 1 (2.0) | 2 (1.1) | 0 (0) | 1 (2.1) | ||
| Post EST bleeding | 0 (0) | 3 (1.6) | 0 (0) | 1 (2.1) | ||
ERCP, endoscopic retrograde cholangiopancreatography; EST, endoscopic sphincterotomy.
Table 2 shows the study populations’ evaluation details, divided into endoscopic papillectomy (EP) related procedures and ERCP-related procedures other than EP. The number of patients that underwent EP and endoscopic sphincterotomy (EST) was significantly lower in 4-Fr group than conventional stent group, before (19.2% [5/26] vs 83.3% [115/138], respectively; P < 0.01) and after (19.2% [5/26] vs 82.1% [23/28], respectively; P < 0.01) propensity score matching. While, the number of patients that underwent EP and endoscopic biliary drainage (EBD) was significantly higher in 4-Fr group than conventional stent group, before (57.6% [15/26] vs 5.1% [7/138], respectively; P < 0.01) and after (57.6% [15/26] vs 14.2% [4/28], respectively; P = 0.001) propensity score matching. No significant differences were evident between the two groups for the other evaluation variables.
Table 2.
Examination details of study populations.
| Examinations | Before propensity score matching | After propentisy score matching | |||||
|---|---|---|---|---|---|---|---|
| 4 Fr group (n = 49) |
Conventional stent group (n = 187) |
P-value | 4 Fr group (n = 47) |
Conventional stent group (n = 47) |
P-value | ||
| EP related procedure, n | 26 | 138 | 26 | 28 | |||
| EP alone, n (%) | 5 (19.2%) | 11 (8.0%) | 0.139 | 5 (19.2%) | 1 (3.6%) | 0.095 | |
| EP + EST, n (%) | 5 (19.2%) | 115 (83.3%) | < 0.01 | 5 (19.2%) | 23 (82.1%) | < 0.01 | |
| EP + EBD, n (%) | 15 (57.6%) | 7 (5.1%) | < 0.01 | 15 (57.6%) | 4 (14.2%) | 0.001 | |
| EP + EST + EBD, n (%) | 1 (3.8%) | 5 (3.6%) | 0.956 | 1 (3.8%) | 0 (0%) | 0.481 | |
| ERCP related procedure other than EP, n | 23 | 49 | 21 | 19 | |||
| EST, n (%) | 7 (30.4%) | 15 (30.6%) | 0.988 | 7 (33.3%) | 5 (26.3%) | 0.629 | |
| EST + EBD, n (%) | 1 (4.3%) | 5 (10.2%) | 0.402 | 1 (4.8%) | 4 (21.1%) | 0.172 | |
| Non EST + EBD, n (%) | 4 (17.3%) | 8 (16.3%) | 0.91 | 4 (19.0%) | 1(5.3%) | 0.345 | |
| Argon plasma coagulation, n (%) | 2 (8.7%) | 3 (6.1%) | 0.319 | 2 (9.5%) | 1(5.3%) | 0.609 | |
| IDUS (bile duct), n (%) | 4 (17.3%) | 6 (12.2%) | 0.716 | 4 (19.0%) | 3 (15.8%) | 0.787 | |
| POCS, n (%) | 2 (8.7%) | 2 (4.1%) | 0.588 | 2 (9.5%) | 0 (0%) | 0.488 | |
EP, endoscopic papillectomy; EST, endoscopic sphincterotomy; EBD, endoscopic biliary drainage; IDUS, intraductal ultrasonography; POCS, peroral cholangioscopy.
Technical success and adverse events
Table 3 presents the procedure outcomes of both groups. The technical success rate was 100% (49/49) in the 4-Fr group and 98.4% (184/187) in the conventional stent group; there was no significant difference (p = 0.372). These results remained unchanged after propensity score matching (100% [47/47] vs 97.9% [46/47], respectively; P = 0.315). A significant difference between the 4-Fr group and conventional stent group was observed in stents lengths selection before (p < 0.01) and after propensity score matching (p < 0.01). The median stenting duration was 7 days in both group, and there was no difference in stenting duration between the two groups after propensity score matching (p = 0.288). However, the duration of the 4-Fr group was significantly shorter than the conventional stent group before propensity score matching(p = 0.027).
Table 3.
Outcomes of pancreatic stent placement in each group.
| Before propensity score matching | After propentisy score matching | |||||
|---|---|---|---|---|---|---|
| 4 Fr group (n = 49) |
Conventional stent group (n = 187) |
P-value | 4 Fr group (n = 47) |
Conventional stent group (n = 47) |
P-value | |
| Technical success, no. (%) | 49 (100) | 184 (98.4) | 0.372 | 47 (100%) | 46 (97.9%) | 0.315 |
| Length of PS | < 0.01 | < 0.01 | ||||
| 3 cm, no. (%) | 8 (16.3) | 0 (0) | 8 (17.0) | 0 (0) | ||
| 5 cm, no. (%) | 21 (42.9) | 31 (16.8) | 20 (42.6) | 11 (23.9) | ||
| 7 cm, no. (%) | 16 (32.7) | 141 (76.2) | 15 (31.9) | 32 (69.6) | ||
| 9 cm, no. (%) | 4 (8.2) | 13 (7.0) | 4 (8.5) | 3 (6.5) | ||
| Stenting duration, days, median (quantile) | 7.0 (6.0–7.0) | 7.0 (7.0–10.0) | 7.0 (6.0–7.0) | 7.0 (7.0–7.0) | ||
| 1–4 days, no. (%) | 11 (22.9) | 11 (6.0) | 0.027 | 10 (21.7) | 4 (8.7) | 0.288 |
| 5–8 days, no. (%) | 29 (60.4) | 127 (69.0) | 29 (63.0) | 33 (71.7) | ||
| 9–14 days, no. (%) | 3 (6.3) | 20 (10.9) | 3 (6.5) | 4 (8.7) | ||
| 15- days, no. (%) | 5 (10.4) | 26 (14.1) | 4 (8.7) | 5 (10.9) | ||
| Complications during pancreatic stent placement | ||||||
| Post-ERCP pancreatitis, no. (%) | 3 (6.1) | 29 (15.5) | 0.088 | 3 (6.4) | 3 (6.4) | 1 |
| Hemorrhage, no. (%) | 3 (6.1) | 23 (12.3) | 0.219 | 3 (6.4) | 3 (6.4) | 1 |
| Spontaneous stent dislodgment, no. (%) | 2 (4.1) | 2 (1.1) | 0.191 | 1 (2.1) | 0 (0) | 0.315 |
| Delayed onset retroperitoneal perforation, no. (%) | 0 (0) | 5 (2.7) | 0.587 | 0 (0) | 0 (0) | – |
| Stent migration, no. (%) | 0 (0) | 1 (0.5) | 0.608 | 0 (0) | 1 (2.1) | 0.315 |
| Complications after removal of pancreatic stent | ||||||
| Pancreatitis due to obstruction of pancreatic duct orifice, no. (%) | 0 (0) | 6 (3.2) | 0.349 | 0 (0) | 0 (0) | – |
PS, plastic stent.
The PEP rate in the 4-Fr group (6.1% [3/49]) was lower than the conventional stent group (15.5% [29/187]); however, no statistical significance (p = 0.088) before propensity score matching. After propensity score matching, the PEP rate in the 4-Fr group (6.4% [3/47]) was the same as the conventional stent group (6.4% [3/47]). No significant differences were evident between the two groups regarding other complications during pancreatic stent placement. Six cases showed pancreatitis due to obstruction of pancreatic duct orifice after removing the pancreatic stent in the conventional stent group; conversely, no cases were observed in the 4-Fr group (p = 0.349) before propensity score matching.
Table 4 showed both groups’ PEP details. Post-ERCP amylase level was significantly lower in the 4-Fr group than conventional stent group before propensity score matching (165.0 vs 295.0, respectively; p = 0.006), though no statistical significance after propensity score matching (150.0 vs 240.0, respectively; p = 0.298). Asymptomatic hyperamylasemia rate was lower in the 4-Fr group (46.9% [23/49]) than the conventional group (57.2% [107/187]), though without statistical significance before (p = 0.198) and after (p = 0.536) propensity score matching. All patients with PEP recovered conservatively, including the patients with severe status in both groups.
Table 4.
Post-ERCP pancreatitis in each group.
| Before propensity score matching | After propentisy score matching | |||||
|---|---|---|---|---|---|---|
| 4 Fr group (n = 49) |
Conventional stent group (n = 187) |
P-value | 4 Fr group (n = 47) |
Conventional stent group (n = 47) |
P-value | |
| Pre-ERCP amylase, median (quantile) | 76.5 (49.8–128.8) | 77.0 (62.0–107.0) | 0.589 | 75.0 (45.3–125.8) | 79.0 (56.5–100.0) | 0.789 |
| Post-ERCP amylase, median (quantile) | 165.0 (110.5–369.0) | 295.0 (142.5–539.0) | 0.006 | 150.0 (106.0–383.0) | 240.0 (111.0–432.0) | 0.298 |
| Asymptomatic hyperamylasemia, no. (%) | 23 (46.9) | 107 (57.2) | 0.198 | 21 (44.7) | 24 (51.1) | 0.536 |
| Post-ERCP pancreatitis, no. (%) | 3 (6.1) | 29 (15.5) | 0.088 | 3 (6.4) | 3 (6.4) | 1 |
| Mild, no. (%) | 2 (4.1) | 22 (11.7) | 2 (4.3) | 3 (6.4) | ||
| Moderate, no. (%) | 0 (0) | 5 (2.7) | 0 (0) | 0 (0) | ||
| Severe, no. (%) | 1 (2.0) | 2 (1.1) | 1 (2.1) | 0 (0) | ||
The supplementary table shows PEP's rate in each characteristic comparing the 4-Fr group with the conventional stent group. However, no significant differences were evident between the two groups regarding each characteristic.
Discussion
This study demonstrated that a newly designed 4-Fr plastic pancreatic duct stent shows similar feasibility and safety compared to the conventional 5-Fr plastic stent. The new stent offered the following advantages: (1) less injury to the pancreatic duct because of small-diameter stents; (2) the ability to pass over a standard 0.025-in. guidewire, despite the small diameter; (3) easy advancement of the tapered and straight distal tip via the pancreatic duct, and (4) the role of the three flanges and a single pigtail in anchoring the stent and preventing outward and inward migration. This is the first report on the evaluation of a new 4-Fr plastic pancreatic duct stent.
Multiple clinical trials and a meta-analysis have demonstrated that pancreatic duct stenting in high-risk patients effectively reduces the incidence of PEP8–14. Therefore, the consensus guidelines recommend pancreatic duct stenting in high-risk patients22,23. However, there are controversies on the best type of plastic stent. According to previous reports, 3-or 4-Fr stents were more effective than the traditionally used 5-Fr stents. This can be attributed to their smaller diameter that causes less ductal or parenchymal pancreatic changes18,21. Despite the advantages above, smaller diameter stents are not widely used because of their need for a smaller caliber 0.018- or 0.021-in. guidewire. This, in turn, is more challenging to work with, thus adding to the difficulty of the procedure. Consequently, there can be a higher rate of failure of stent placement, thereby increasing the incidence of pancreatitis24. Despite a smaller diameter, the novel 4-Fr stents were placed into the pancreatic duct over a user-friendly standard 0.025-in. guidewire in all cases, without difficulty. The tapered and straight distal tip also enabled the easy placement of the stent in the pancreatic duct. The technical ease of stent placement is considered an important factor in selecting the prophylactic pancreatic duct stent25. In addition, post-ERCP amylase level and the rate of PEP in the 4-Fr stent group were lower than the conventional stent group, though without statistical significance. This 4-Fr plastic stent is expected to be at least similar or more effective for PEP prophylaxis compared to the conventional plastic stent.
Stent migration into the pancreatic duct is another adverse event associated with the procedure mentioned above. It results in stent-induced pancreatic duct changes, the need for several endoscopic attempts to retrieve the stent and an occasional surgical intervention during the failure of ERCP retrieval26. Inward migration did not occur in any of the cases mentioned above. The flanges and single pigtail on the proximal side of the stent might have contributed to preventing an inward migration.
The 4-Fr plastic stents have a flange at the distal end that prevents stent dislodgment. Several reports recommended the prophylactic placement of pancreatic stents without flanges. Spontaneous dislodgment occurred within 7 days in most of these cases, thus reducing the need for the re-insertion of an endoscope for stent removal11,12,21,27. However, early stent dislodgment may result in delayed-onset pancreatitis14. Delayed-onset pancreatitis because of the secondary obstruction of flow after resection or a direct burn effect in the pancreatic parenchyma is a serious complication, particularly in cases of EP28,29. Therefore, the inner flange is important for preventing early dislodgment, though the optimal stenting time is still uncertain30. In addition, stents with an inner flange were effective in cases with obstructive pancreatitis or divisum, requiring pancreatic duct drainage. Thus, the 4-Fr plastic stent with inner flange would effectively secure the pancreatic duct drainage route for the patients with a high PEP risk, including EP.
Our study had several limitations. First, it was a single-center retrospective study. Therefore, the sample size was relatively small. Second, the retrospective design might have introduced some selection bias. The study population included more patients who underwent EP than in previous studies. However, the risk of pancreatitis is high after EP28,29,31. The rates of PEP in our heterogeneous study population were comparable to that reported in high-risk patients with pancreatic duct stents11,13,21. Third, pancreatic duct stent placement was uncommon in all cases. The length of the stents and the timing of the stent removal had been selected by an operator. This might have influenced our results.
In conclusion, pancreatic duct stenting using a novel 4-Fr plastic stent is feasible and safe. Large-scale, multicenter trials are warranted to validate our results.
Methods
Study design
This single-center, retrospective case–control study was conducted at the Tokyo Medical University Hospital. Written informed consent was obtained from each patient before endoscopic treatment. This study was approved by the institutional review board of the Tokyo Medical University (T2020-0206), and was conducted in accordance with the ethical standards described in the latest revision of the Declaration of Helsinki. Informed consent for patient participation was received in the form of an opt-out in-hospital notice.
Study participants
A total of 895 consecutive ERCP procedures were performed between November 2019 and March 2021. We selected 49 consecutive patients who had a 4-Fr plastic stent placed in their pancreatic duct. We also included 187 consecutive patients who had a conventional 5-Fr pancreatic duct stent with internal flanges (Geenen, Pancreatic Stent Sets, Cook Medical, Bloomington, IN, USA) in their pancreatic duct between November 2013 and October 2019 as the control group. The study primarily included the following patients who were considered at a high risk of developing PEP: (1) those receiving unintentional pancreatic guidewire passages, (2) requiring EP, (3) requiring argon plasma coagulation (APC) for residual lesions of the papilla tumor after EP, (4) those showing post-EST bleeding. The remaining patients were placed on a stent for pancreatic duct drainage in divisum or obstructive pancreatitis. The exclusion criteria were as follows: (1) age < 18 years and (2) refusal to participate in the study. A history of biliary or pancreatic drainage during patient selection was not considered. We compared the 4-Fr group and control group with respect to outcomes. Propensity score matching was introduced to reduce selection bias.
Endoscopic procedures
All patients underwent ERCP using a duodenoscope (TJF-260V; Olympus Medical Systems, Tokyo, Japan) under conscious sedation. Cannulation was attempted using a standard injection catheter (ERCP catheter, MTW Co., Dusseldorf, Germany) or sphincterotome (CleverCut; Olympus Medical Systems, Tokyo, Japan) with a 0.025-in. guidewire (VisiGlide 2, Olympus Medical Systems, Tokyo, Japan). We used a newly designed 4-Fr pancreatic duct stent with varying lengths of 3, 5, 7, and 9 cm. The stent had a tapered tip, three internal flanges (one at the distal end and two at the proximal end), and a single external pigtail (Fit Stent 025: Gadelius Medical Co., Ltd., Tokyo, Japan) (Fig. 1). The stent could pass over a 0.025-in. guidewire under fluoroscopic guidance.
Figure 1:
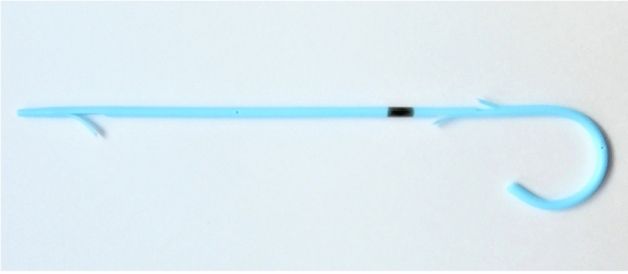
4-Fr pancreatic stent. The stent has a tapered tip, three internal flanges (one at the distal end and two at the proximal end), and a single external pigtail with a black marker on the proximal side.
The patients underwent subsequent pancreatic duct cannulation, contrast injection, and guidewire insertion for stent placement. We selected the length of the stent based on the degree of flexion and the length of the pancreatic duct in the head of the pancreas. The stent was passed over a guidewire under fluoroscopic guidance (Fig. 2). Following ERCP, the patients were requested to fast until their blood tests confirmed no pancreatitis or other complications, the following day. All patients were hospitalized for ERCP and observation. The stent was planned to be removed duodenoscopically between the third to seventh day in cases of prophylactic pancreatic stent placement, if it had not been dislodged. However, it was planned to be removed based on the symptoms in case of pancreatic duct drainage. The conventional 5-Fr pancreatic duct stent placement was the same as those for 4-Fr pancreatic duct stent. All ERCP procedures were performed by experts (> 5 years of ERCP experience) or by trainees (< 5 years of ERCP experience) under the direction of an expert.
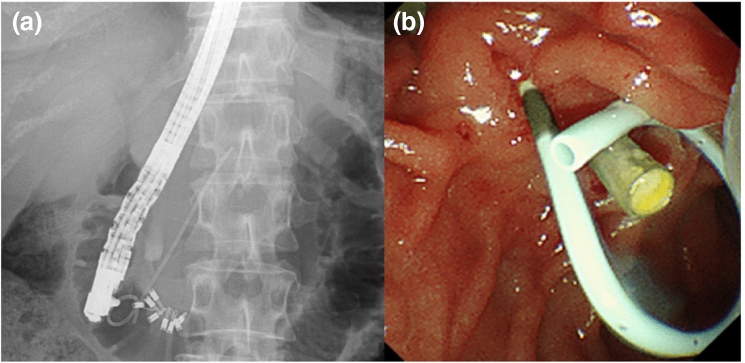
Figure 2.
A case of pancreatic duct stenting using the novel 4-Fr plastic stent. (a) Fluoroscopy, The 4-Fr stent has been placed in the pancreatic duct; (b) Endoscopy, The single-pigtail and the black marker of the proximal side are exposed to the duodenum.
Measured outcomes
The primary outcome was technical success, defined as the successful placement of the stent. The secondary outcomes comprised the frequency and severity of PEP, hyperamylasemia, rate and duration of spontaneous stent dislodgment, stent migration, and other complications. We also evaluated the PEP rate and analyzed various risk factor;. 4-Fr group and control group were compared regarding these outcomes. All ERCP-related complications were graded according to the severity grading system of the American Society for Gastrointestinal Endoscopy Lexicon32. PEP was defined as pancreatic pain and hyperamylasemia within 24 h of the procedure. Hyperamylasemia was defined as an increase in the serum amylase level to more than thrice the upper normal limit. We defined “stent migration” as the inward migration of the stent into the pancreatic duct. We defined “stent dislodgment” as the outward migration of the stent to the duodenal side.
Statistical analyses
Categorical variables were expressed as numbers and percentages, and they were compared using the χ2 test (with Yates’ correction) or the Fisher’s exact test. Continuous variables were expressed as medians and interquartile ranges, and they were compared using the Mann–Whitney U-test. We used propensity score matching to adjust baseline differences between the two groups. Sex, age, history of ERCP related procedures, indication for pancreatic stent placement, and indication for ERCP were selected as the observed covariates. Based on this set of covariates, propensity scores were estimated using a logistic regression model. Groups were matched using 1:1 nearest neighbor-matching, within a caliper width of 0.2 of the standard deviation of the propensity score logit. After propensity matching, differences in clinical outcomes were compared between the two groups. All analyses were performed using IBM SPSS software (version 27; IBM Corp., Armonk, NY, USA). For all analyses, P < 0.05 was considered statistically significant.
Ethical statement
This study was approved by the institutional review board of the Tokyo Medical University (T2020-0206), and was conducted in accordance with the ethical standards described in the latest revision of the Declaration of Helsinki.
Patient consent for patient participation and publication
Informed consent for patient participation was received in the form of an opt-out in-hospital notice.
Supplementary Information
Author contributions
K.N. wrote the paper; A.S., T.T., K.I., R.T., R.T., S.M., K.Y., Y.M., Y.A., T.K., H.K., H.M., and T.H. performed patient care. All authors reviewed the manuscript. A.K. and T.I. supervised the manuscript preparation, made important revisions, and approved the final manuscript.
Competing interests
Takao Itoi and Akio Katanuma are consultants of Gadelius Medical Co., Ltd. The other authors declare that they have no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-92811-x.
References
- 1.Freeman ML, et al. Complications of endoscopic biliary sphincterotomy. N. Engl. J. Med. 1996;335:909–918. doi: 10.1056/NEJM199609263351301. [DOI] [PubMed] [Google Scholar]
- 2.Glomsaker T, et al. Patterns and predictive factors of complications after endoscopic retrograde cholangiopancreatography. Br. J. Surg. 2013;100:373–380. doi: 10.1002/bjs.8992. [DOI] [PubMed] [Google Scholar]
- 3.Kochar B, et al. Incidence, severity, and mortality of post-ERCP pancreatitis: A systematic review by using randomized, controlled trials. Gastrointest. Endosc. 2015;81:143–149. doi: 10.1016/j.gie.2014.06.045. [DOI] [PubMed] [Google Scholar]
- 4.Fazel A, Quadri A, Catalano MF, Meyerson SM, Geenen JE. Does a pancreatic duct stent prevent post-ERCP pancreatitis? A prospective randomized study. Gastrointest. Endosc. 2003;57:291–294. doi: 10.1067/mge.2003.124. [DOI] [PubMed] [Google Scholar]
- 5.Tarnasky PR, Palesch YY, Cunningham JT, Mauldin PD, Cotton PB, Hawes RH. Pancreatic stenting prevents pancreatitis after biliary sphincterotomy in patients with sphincter of Oddi dysfunction. Gastroenterology. 1998;115:1518–1524. doi: 10.1016/S0016-5085(98)70031-9. [DOI] [PubMed] [Google Scholar]
- 6.Smithline A, et al. Effect of prophylactic main pancreatic duct stenting on the incidence of biliary endoscopic sphincterotomy-induced pancreatitis in high-risk patients. Gastrointest. Endosc. 1993;39:652–657. doi: 10.1016/S0016-5107(93)70217-5. [DOI] [PubMed] [Google Scholar]
- 7.Singh P, et al. Does prophylactic pancreatic stent placement reduce the risk of post-ERCP acute pancreatitis? A meta-analysis of controlled trials. Gastrointest. Endosc. 2004;60:544–550. doi: 10.1016/S0016-5107(04)02013-9. [DOI] [PubMed] [Google Scholar]
- 8.Choudhary A, et al. Pancreatic stents for prophylaxis against post-ERCP pancreatitis: A meta-analysis and systematic review. Gastrointest. Endosc. 2011;73:275–282. doi: 10.1016/j.gie.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 9.Mazaki T, Masuda H, Takayama T. Prophylactic pancreatic stent placement and post-ERCP pancreatitis: A systematic review and meta-analysis. Endoscopy. 2010;42:842–853. doi: 10.1055/s-0030-1255781. [DOI] [PubMed] [Google Scholar]
- 10.Fan JH, Qian JB, Wang YM, Shi RH, Zhao CJ. Updated meta-analysis of pancreatic stent placement in preventing post-endoscopic retrograde cholangiopancreatography pancreatitis. World J. Gastroenterol. 2015;21:7577–7583. doi: 10.3748/wjg.v21.i24.7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sofuni A, et al. Endoscopic pancreatic duct stents reduce the incidence of post-endoscopic retrograde cholangiopancreatography pancreatitis in high-risk patients. Clin. Gastroenterol. Hepatol. 2011;9:851–858. doi: 10.1016/j.cgh.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 12.Tsuchiya T, et al. A temporary inner unflanged 5Fr pancreatic duct stent to prevent post-ERCP pancreatitis. A preliminary and single center randomized controlled trial study. J. Hepatobiliary Pancreat. Surg. 2007;14:302–307. doi: 10.1007/s00534-006-1147-8. [DOI] [PubMed] [Google Scholar]
- 13.Sofuni A, et al. Prophylaxis of post-endoscopic retrograde cholangiopancreatography pancreatitis by an endoscopic pancreatic spontaneous dislodgement stent. Clin. Gastroenterol. Hepatol. 2007;5:1339–1346. doi: 10.1016/j.cgh.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Njei B, et al. Comparative effectiveness of pharmacologic and endoscopic interventions for prevention of post-ERCP pancreatitis: A network meta-analysis. Endosc. Int. Open. 2020;8:E29–E40. doi: 10.1055/a-1005-6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawaguchi Y, Ogawa M, Omata F, Ito H, Shimosegawa T, Mine T. Randomized controlled trial of pancreatic stenting to prevent pancreatitis after endoscopic retrograde cholangiopancreatography. World J. Gastroenterol. 2012;18:1635–1641. doi: 10.3748/wjg.v18.i14.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito T, et al. Evidence-based clinical practice guidelines for chronic pancreatitis 2015. J. Gastroenterol. 2016;51:85–92. doi: 10.1007/s00535-015-1149-x. [DOI] [PubMed] [Google Scholar]
- 17.Löhr JM, et al. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU) United Eur. Gastroenterol. J. 2017;5:153–199. doi: 10.1177/2050640616684695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rashdan A, Fogel EL, Mchenry L, Jr, Sherman S, Temkit MH, Lehman GA. Improved stent characteristics for prophylaxis of post-ERCP pancreatitis. Clin. Gastroenterol. Hepatol. 2004;2:322–329. doi: 10.1016/S1542-3565(04)00062-X. [DOI] [PubMed] [Google Scholar]
- 19.Chahal P, et al. Short 5Fr vs long 3Fr pancreatic stents in patients at risk for post-endoscopic retrograde cholangiopancreatography pancreatitis. Clin. Gastroenterol. Hepatol. 2009;7:834–839. doi: 10.1016/j.cgh.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Zolotarevsky E, et al. Prophylactic 5-Fr pancreatic duct stents are superior to 3-Fr stents: A randomized controlled trial. Endoscopy. 2011;43:325–330. doi: 10.1055/s-0030-1256305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee TH, et al. Prophylactic temporary 3F pancreatic duct stent to prevent post-ERCP pancreatitis in patients with a difficult biliary cannulation: A multicenter, prospective, randomized study. Gastrointest. Endosc. 2012;76:578–585. doi: 10.1016/j.gie.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Chandrasekhara V, et al. ASGE Standards of Practice Committee. Adverse events associated with ERCP. Gastrointest. Endosc. 2017;85:32–47. doi: 10.1016/j.gie.2016.06.051. [DOI] [PubMed] [Google Scholar]
- 23.Testoni PA, et al. Papillary cannulation and sphincterotomy techniques at ERCP: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2016;48:657–683. doi: 10.1055/s-0042-108641. [DOI] [PubMed] [Google Scholar]
- 24.Sherman S, Hawes RH, Savides TJ, et al. Stent-induced pancreaticductal and parenchymal changes: Correlation of endoscopic ultrasound with ERCP. Gastrointest. Endosc. 1996;44:276–282. doi: 10.1016/S0016-5107(96)70164-5. [DOI] [PubMed] [Google Scholar]
- 25.Freeman ML, Overby C, Qi D. Pancreatic stent insertion: Consequences of failure and results of a modified technique to maximize success. Gastrointest. Endosc. 2004;59:8–14. doi: 10.1016/S0016-5107(03)02530-6. [DOI] [PubMed] [Google Scholar]
- 26.Sotoudehmanesh R, et al. Pharmacological prophylaxis versus pancreatic duct stenting plus pharmacological prophylaxis for prevention of post-ERCP pancreatitis in high risk patients: A randomized trial. Endoscopy. 2019;51:915–921. doi: 10.1055/a-0977-3119. [DOI] [PubMed] [Google Scholar]
- 27.Lawrence C, Cotton PB, Romagnuolo J, Payne KM, Rawls E, Hawes RH. Small prophylactic pancreatic duct stents: An assessment of spontaneous passage and stent-induced ductal abnormalities. Endoscopy. 2007;39:1082–1085. doi: 10.1055/s-2007-966815. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto K, Iwasaki E, Itoi T. Insights and updates on endoscopic papillectomy. Expert Rev. Gastroenterol. Hepatol. 2020;14:435–444. doi: 10.1080/17474124.2020.1766965. [DOI] [PubMed] [Google Scholar]
- 29.Minami K, et al. A long (7 cm) prophylactic pancreatic stent decreases incidence of post-endoscopic papillectomy pancreatitis: A retrospective study. Endosc. Int. Open. 2019;7:E1663–E1670. doi: 10.1055/a-1010-5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pahk A, Rigauxm J, Poreddym V, Smithm J, Al-Kawas F. Prophylactic pancreatic stents: Does size matter? A comparison of 4-Fr and 5-Fr stents in reference to post-ERCP pancreatitis and migration rate. Dig. Dis. Sci. 2011;56:3058–3064. doi: 10.1007/s10620-011-1695-x. [DOI] [PubMed] [Google Scholar]
- 31.Harewood GC, Pochron NL, Gostout CJ. Prospective, randomized, controlled trial of prophylactic pancreatic stent placement for endoscopic snare excision of the duodenal ampulla. Gastrointest. Endosc. 2005;62:367–370. doi: 10.1016/j.gie.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 32.Cotton PB, et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest. Endosc. 2010;71:446–454. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.