Abstract
Background & Aims:
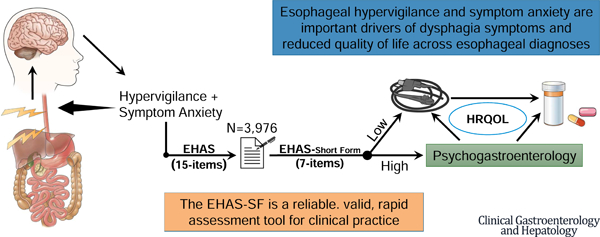
Esophageal hypervigilance and anxiety are emerging as important drivers of dysphagia symptoms and reduced quality of life across esophageal diagnoses. The esophageal hypervigilance and anxiety scale (EHAS) is a validated measure of these cognitive-affective processes. However, its length may preclude it from use in clinical practice. We aimed to create a short form version of the EHAS using established psychometric practices.
Methods:
A retrospective review of a registry of patients who visited a university-based esophageal motility clinic for diagnostic testing was conducted. Patients were included if they completed the 15-item EHAS and questionnaires assessing dysphagia severity and health-related quality of life (HRQOL) at the time of motility testing. Principle components factor analysis identified items for possible removal. Tests for reliability and concurrent validity were performed on the full EHAS and short-form version (EHAS-SF).
Results:
3,976 adult patients with confirmed esophageal disease were included: 30% with achalasia or EGJOO, 13% with EoE, 13% with GERD, 39% normal motility. Eight items were removed from the scale based on a factor loading of > 0.70, resulting in a single scale 7-item EHAS-SF scored from 0 to 28. The EHAS-SF demonstrated excellent internal consistency (α= 0.91) and split-half reliability (0.88) as was found in the full EHAS in the current study and prior validation. Concurrent validity existed between the EHAS-SF and measures of dysphagia (r= 0.33) and HRQOL (r = −0.73, both p<.001).
Conclusions:
The EHAS-SF is a 7-item scale to assess esophageal hypervigilance and symptom-specific anxiety that performs as well as the original 15-item version. Shorter questionnaires allow for implementation in clinical practice. The EHAS-SF is a useful tool for clinicians to quickly assess how hypervigilance and anxiety may be contributing to their patients’ clinical presentations.
Keywords: Hypervigilance, Symptom-Specific Anxiety, Esophageal Disease
Graphical Abstract
Introduction
Chronic esophageal diseases, including gastroesophageal reflux disease (GERD), achalasia, eosinophilic esophagitis (EoE), and esophageal disorders of gut-brain interaction (DGBI) represent a significant proportion of visits and cost in outpatient gastroenterology practices in the United States1–4. Many patients report ongoing symptoms in spite of medical or surgical management, resulting in significant morbidity and mortality5–7. Patients with any esophageal disease, not simply those with DGBIs, may present for follow-up when diagnostic findings conflict with symptoms. Up to 45% of patients with GERD report persistent symptoms in spite of endoscopic and acid-monitoring normalization7. Approximately half of patients with esophageal DGBIs do not respond to neuromodulator therapies8 while 25% to 50% of patients with EoE may experience symptoms with histological remission5, 9. Many patients with esophageal conditions also experience degradations of HRQOL10–12 which further compounds the costs of these conditions.
Psychological and behavioral processes are important considerations in chronic digestive disease13. A growing body of evidence suggests gut-brain axis disruptions are important drivers of symptoms, especially in DGBIs, but also organic conditions14–16. Cognitive-affective processes compound neuronal hypersensitivity in the digestive tract and centrally mediated pain processing in the brain17, 18. Subsequently, the patient’s symptoms may be amplified19, their thoughts may predict catastrophic outcomes, and once-useful avoidance behaviors become chronic and counterproductive. Two of these cognitive-affective processes, hypervigilance and symptom-specific anxiety20, are common. Hypervigilance is the tendency to overly focus on bodily sensations to the point normal signals from the gut may be interpreted as uncomfortable or even painful. Anxiety, including chronic worry and fear response, not only impairs psychological functioning but amplifies autonomic nervous system activity which is an integral part of the gut-brain axis. Both hypervigilance and anxiety are of particular interest due to being highly amendable to psychogastroenterology interventions such as cognitive-behavioral therapy (CBT) and gut-directed hypnotherapy (GDH)15.
Our group developed the esophageal hypervigilance and anxiety scale (EHAS) in 201821. The scale was validated across several esophageal conditions and has since been used to identify the importance of hypervigilance and anxiety in esophageal symptoms. In a sample of 236 patients, symptom-specific anxiety and hypervigilance explained a greater percentage of variance in dysphagia symptoms than having a major motor disorder19. Standard physiological assessment including high-resolution manometry and endoscopic measures of EJG pressure were not significant predictors of dysphagia. These findings underscore the importance of assessing hypervigilance and symptom-specific anxiety in esophageal patients as a main driver of symptom reporting.
For a measure to be clinically useful, it should use the minimum number of items to measure a construct to facilitate fast administration and scoring. The current version of the EHAS includes 15 questions, which may preclude it being widely used in clinical practice. Therefore, we aim to develop a short form of the EHAS while maintaining its reliability and construct validity.
Methods
Patients retrospectively identified using a query of the Esophageal Center at Northwestern Motility Laboratory Registry, which includes English-speaking patients 18–85 years old evaluated at Northwestern with high-resolution manometry (HRM) and/or functional luminal imaging probe (FLIP) during esophagogastroduodenoscopy (EGD) and/or ambulatory reflux monitoring between 2015 and 2020. Participants completed paper-based questionnaires prior to their procedure. Basic demographic information (age, gender), clinical details, including indication for HRM and current use of proton-pump inhibitors (PPI), were obtained. Esophageal motility diagnoses were designated from ten supine swallows in accordance with the Chicago Classification v3.0 (CCv3)22. Eosinophilic esophagitis diagnosis was based on histological findings of >15 eosinophils per high power field from esophageal biopsies23, and the endoscopic reference score (EREFS)24. A waiver of informed consent was obtained. The study protocol was approved by the Northwestern University Institutional Review Board.
Study Measures
The Esophageal Hypervigilance and Anxiety Scale (EHAS)21:
The EHAS is a 15-item measure with two factors evaluating esophageal hypervigilance and symptom specific anxiety scored on a 0–4 Likert scale. Items are summed to yield a total score ranging from 0 – 60 (Greater hypervigilance/anxiety). Two sub-scores can also be generated: Esophageal Hypervigilance (HV; sum of six items) and symptom-specific anxiety (ANX; sum of nine items).
Brief Esophageal Dysphagia Questionnaire (BEDQ)25:
The BEDQ is a 10-item measure of esophageal dysphagia. The frequency and difficulty with swallowing solid foods, soft foods, and liquids are rated on a 5-point Likert scale for 8 items over the past 30 days. Scores range from 0–40 (Greater dysphagia).
Northwestern Esophageal Quality of Life Scale (NEQOL)26:
HRQOL was measured using the NEQOL, a 14-item measure assessing social function, emotional distress, eating impact, sleep, and financial burden in patients with esophageal symptoms over the past 2 weeks via a 5-point Likert scale ranging from 4=“Not True at all” to 0=“Very True”. Higher scores denote greater HRQOL.
Statistical Analyses
Data were entered into SPSS v. 26 for Macintosh (Chicago, IL). Evaluation for normal distribution was assessed via skewness and kurtosis (+/− 2.0). Continuous variables are presented as mean(SD) and categorical as percentage(N). Principal components factor analysis (PCFA) with varimax rotation evaluated the EHAS scale structure. Internal consistency was measured using Cronbach alpha and split-half reliability via Guttman statistic on the full version of the EHAS and again after item removal.
To identify items for exclusion, all EHAS items were first entered into a single factor PCFA with cutoff for item-to-scale correlation being > 0.69. Any items loading below this cutoff were considered for removal. The PCFA was repeated with retained items entered with an Eigenvalue >1 set to determine whether subscale scores (hypervigilance, anxiety) were retained for the EHAS short form (EHAS-SF). Four study authors (TT, LG, LK, JP) reviewed the removed items and came to a consensus on the final EHAS-SF.
Pearson’s correlations evaluated the full EHAS and EHAS-SF relationships with the BEDQ and NEQOL. These correlations were compared to previously published data to ensure no significant changes occurred. Independent sample t-Tests and One-Way Analysis of Variance (ANOVA) with Tukey post-hoc test evaluated between group differences for gender, PPI use, EoE diagnosis, indication for manometry, and CCv3 diagnosis (for applicable patients). Separate linear regression models estimated the predictive qualities of the EHAS and EHAS-SF for HRQOL and symptom severity. For HRQOL, either the EHAS or EHAS-SF was entered into the model with BEDQ score. For symptom severity, either the EHAS or EHAS-SF was entered into the model with CCv3, coded 1 (normal) to 4 (Disorders of EGJ Outflow). Patients without a CCv3 diagnosis were excluded from the regression samples. Adjusted R squared, converted to percentage, and standardized β weights are reported for each model. Forest plots represent regression model β weights with 95% confidence intervals. Statistical significance was set to P < .05 for all analyses.
Results
Study Sample
The study sample included 3,976 patients (Table 1). The majority was female and middle aged. Half of patients’ primary symptom was dysphagia, followed by heartburn/reflux. Most of the sample (85%) underwent HRM with 39.3% of patients having a CCv3 of normal motility, then EGJ outflow obstruction (EGJOO) and ineffective esophageal motility (IEM). 34.2% had other esophageal conditions (e.g. GERD, functional heartburn) or prior foregut surgery and not classified via CCv3. Approximately 13% had EoE. On average, patients scored a mean BEDQ score of 8.66(9.27), Range: 0 – 40 and a mean NEQOL score of 35.19(14.61), Range: 0 – 56. Mean scores for each questionnaire by CCv3 category are in Table 2.
Table 1.
Demographic and Clinical Characteristics of Sample
| N = 3976 | |
|---|---|
|
| |
| Age in years | 52.9 (16.2) |
|
| |
| Male Gender | 40.4% (1121) |
|
| |
| Chicago Classification 3.0 Diagnosis | |
| Disorders of EGJ Outflow Obstruction | 30.4% (797) |
| Achalasia I | 4.2% (110) |
| Achalasia II | 5.0% (131) |
| Achalasia III | 3.5% (92) |
| EGJOO | 17.7% (464) |
| Major Motor Disorders | 12.5% (328) |
| Absent Contractility | 9.1% (238) |
| Jackhammer Esophagus | 1.6% (41) |
| Distal Esophageal Spasm | 1.9% (49) |
| Minor Disorders of Peristalsis | 17.6% (462) |
| Fragmented Peristalsis | 1.2% (31) |
| Ineffective Esophageal Motility | 16.4% (431) |
| Normal Esophageal Motility | 39.4% (1031) |
|
| |
| Other Esophageal Disorders | 34.2% (1358) |
| GERD | 12.9% (511) |
| Prior Foregut Surgery | 18.8% (747) |
|
| |
| Eosinophilic Esophagitis | 12.9% (109) |
|
| |
| Diagnostic Tests | |
| High Resolution Manometry | 84.6% (3365) |
| FLIP | 22.6% (898) |
|
| |
| Indication for Evaluation | |
| Dysphagia | 49.1% (1528) |
| Heartburn/Reflux | 24.0% (745) |
| Chest pain | 6.5% (202) |
| Pre-Operative | 7.7% (240) |
| Follow-Up | 12.7% (395) |
|
| |
| On PPI Therapy | 64.4% (1227) |
Notes: 1906 patients had any PPI use in the registry.
Table 2.
Mean Scores for Study Measures by Chicago Classification 3.0 Category
| BEDQ 0–40 | NEQOL 0–56 | EHAS 0–60 | EHAS-SF 0–28 | P | |
|---|---|---|---|---|---|
| 1 Normal Motility | 7.36 (8.4) |
35.37 (13.9) |
29.31 (14.4) |
12.94 (7.7) |
|
| 2 Minor Peristalsis Disorder | 7.63 (8.8) |
34.76 (15.6) |
28.82 (15.4) |
12.72 (7.9) |
|
| 3 Major Motor Disorder | 7.87 (8.4) |
38.14 (13.5)c |
26.70 (14.1)b |
11.19 (7.4)a |
a3 < all, p< .05 b3 < 1, p=.03, 4 p< .001 c3 > all p< .05 |
| 4 Disorders of EGJOO | 12.3 (10.5)e |
32.86 (14.9)d |
30.80 (14.5) |
13.83 (7.8) |
d4 < 1, p=.008 e4 > all, p<.001 |
Factor Structure of EHAS-SF
Entering the 15 questions from the EHAS into PCFA with one factor identified 8 items falling below the correlation cutoff of 0.70 (Table 3). When PCFA was repeated, a single-factor structure emerged on the EHAS-SF with increases in factor loading coefficients for all items except question 11 which demonstrated a slight decline but was still above the 0.70 cutoff. Inter-item correlations for EHAS-SF ranged from 0.424 to 0.740 (all p< .001) suggesting each question measures a distinct but related construct. Questions 7 and 8 demonstrated multicollinearity (r = 0.740), however due to the difference in the question content (“There is nothing I can do to reduce symptoms”, “When I feel discomfort in my esophagus, it frightens me”) these items were retained.
Table 3.
Principle Components Factor Analyses for EHAS and EHAS-SF with Reliability Statistics
| EHAS Item | EHAS Subscale | PCFA 1 EHAS | PCFA 2 EHAS-SF |
|---|---|---|---|
| Retained for EHAS-SF | |||
| I keep track of my symptom levels | HV | 0.802 | 0.816 |
| As soon as I awake, I worry I will have discomfort in my throat/chest/esophagus during the day | ANX | 0.800 | 0.860 |
| There is nothing I can do to reduce the intensity of my symptoms | ANX | 0.797 | 0.857 |
| These symptoms are terrible and I think things are never going to get better | ANX | 0.778 | 0.806 |
| When I feel discomfort in my throat/chest/esophagus it frightens me | ANX | 0.773 | 0.826 |
| I am aware of sudden or temporary changes in my esophagus | HV | 0.749 | 0.726 |
| These symptoms are awful and they overwhelm me | ANX | 0.716 | 0.724 |
| Removed | |||
| I can’t seem to keep my symptoms out of my mind | ANX | 0.683 |

|
| I am very sensitive to esophageal sensations such as heartburn or chest pain | HV | 0.681 | |
| I notice my symptoms even if I am busy with another activity | HV | 0.670 | |
| I often worry about problems in my throat/chest/esophagus | ANX | 0.663 | |
| I am quick to notice changes in the location or extent of my symptoms | HV | 0.653 | |
| I anxiously want the symptoms to go away | ANX | 0.650 | |
| I have a difficult time enjoying myself because I cannot get my mind off the discomfort in my throat/chest/esophagus | ANX | 0.617 | |
| I focus on esophageal sensations | HV | 0.590 | |
| Reliability Statistics | |||
| Cronbach Alpha | 0.929 | 0.908 | |
| Guttman Split Half | 0.854 | 0.877 | |
Reliability and Construct Validity of EHAS-SF
Reliability statistics for the EHAS and EHAS-SF were very good (Cronbach α >0.80) to excellent (Cronbach α >0.90). There was a small decrease in internal consistency (0.929 to 0.908) and a small increase in split-half reliability (0.854 to 0.877) for EHAS-SF as compared to the full EHAS. These scores aligned closely with the original EHAS validation study (Cronbach α = 0.931, split-half reliability = 0.873). The total score for the EHAS-SF highly correlated with the EHAS (r = 0.96, p<.001) as expected.
Concurrent validity was tested by correlational analyses between the EHAS, EHAS-SF, BEDQ, and NEQOL. Both the EHAS and EHAS-SF were highly correlated with HRQOL (EHAS r = −0.72, EHAS-SF r = −0.73, both p< .001) and moderately with dysphagia severity (EHAS r = 0.36, EHAS-SF r = 0.33, both p< .001). Correlation coefficients for EHAS-SF were similar to the validation study (BEDQ r = 0.37, NEQOL r = −0.69). Separate hierarchical linear regression analyses assessed the EHAS and EHAS-SF as a predictor of HRQOL when controlling for dysphagia severity (Figure 1). Additional regression analyses were performed to assess the EHAS and EHAS-SF as a predictor of dysphagia when controlling for CCv3 (Figure 2). In both models, the EHAS and EHAS-SF were a much greater predictor of HRQOL than symptom severity (β = −0.21). The standardized beta weights for EHAS-SF (β = −0.657) were slightly larger than EHAS (β = −0.641) and the percentage of the variance in NEQOL score was comparable (EHAS: 51%, EHAS-SF: 53%). For dysphagia severity, EHAS-SF predicted 11% of reported symptoms versus 4% for motility classification; similar findings existed for the full EHAS. Standardized beta weights were larger for the EHAS/EHAS-SF than CCv3.
Figure 1.
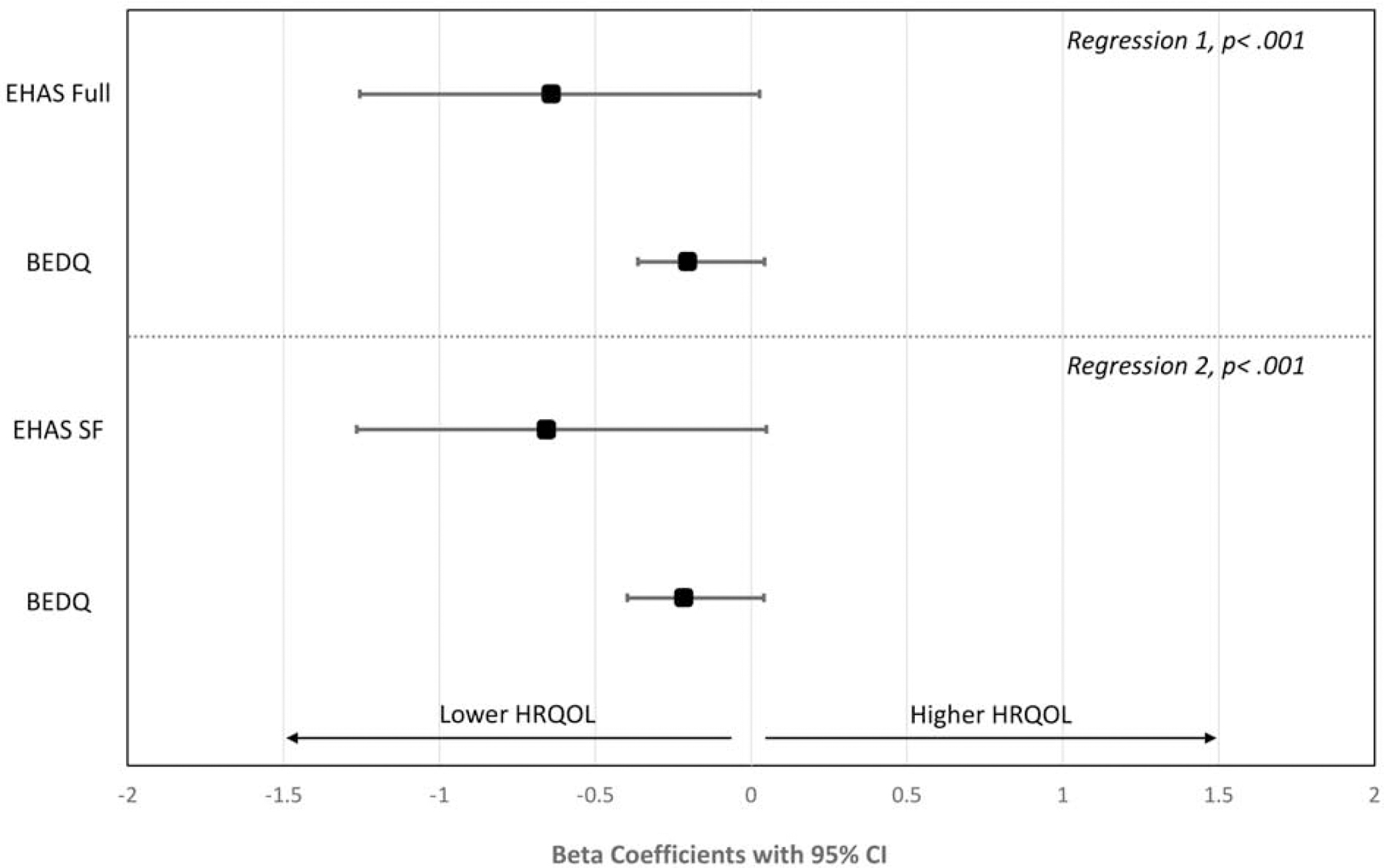
Standardized Beta Weights with 95% Confidence Intervals for EHAS or EHAS-SF and BEDQ as Predictors of HRQOL
Figure Note: Only patients with a CCv3 are included; N=2618.
Figure 2.
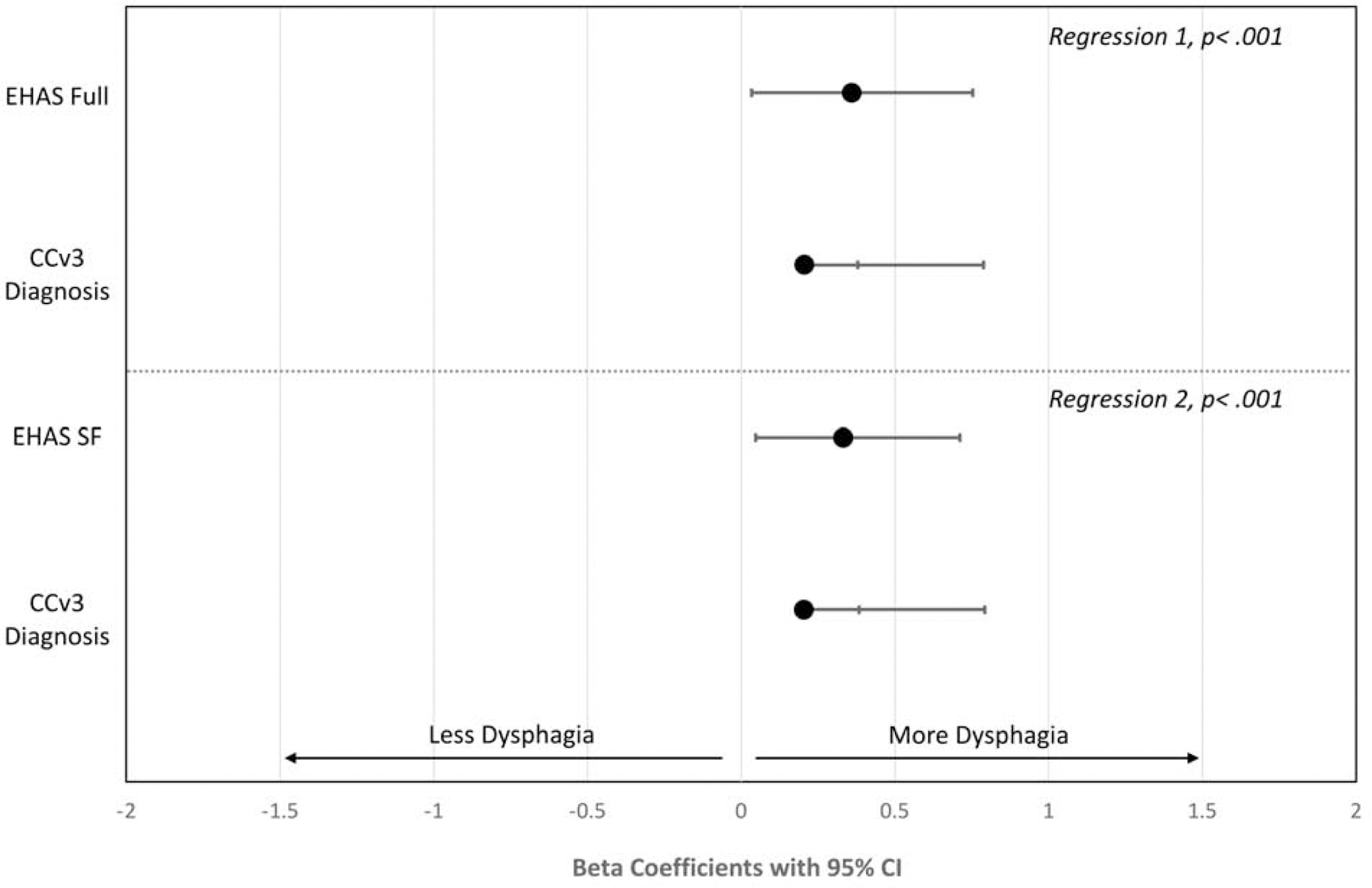
Standardized Beta Weights with 95% CI for EHAS or EHAS-SF and CCv3 as Predictors of Dysphagia Severity
Figure Note: Only patients with a CCv3 are included; N=2618.
Subgroup analyses were performed for EoE, Achalasia/EGJOO, and GERD using the same regression methods for HRQOL, with the exception of replacing BEDQ score with GERDQ score for the GERD group. For EoE and Achalasia, both EHAS-SF and BEDQ remained predictors of NEQOL score (EoE: EHAS-SF β = −0.70, BEDQ β = −0.23; Achalasia/EGJOO: EHAS-SF β = −0.65, BEDQ β = 0.26, both p< .001). However, for GERD, only EHAS-SF predicted NEQOL score (EHAS-SF β = −0.68, p< .001, GERDQ p = .770). Patients whose primary symptom was “heartburn/reflux” were evaluated separately for BEDQ analyses to ensure dysphagia was relevant in this group. No differences were found for standardized beta weights for EHAS or EHAS-SF.
Comparisons of EHAS and EHAS-SF on Demographic and Clinical Variables
Additional analyses evaluated how the total score for EHAS and EHAS-SF potentially differed by age, gender, indication for workup, PPI use, EoE diagnosis, and CCv3 (Figure 3). The only disparity in group differences was for gender, where EHAS-SF failed to identify significant differences between males and females found on the full EHAS. All other clinical and demographic variables demonstrated similar mean differences and statistical significance.
Figure 3.
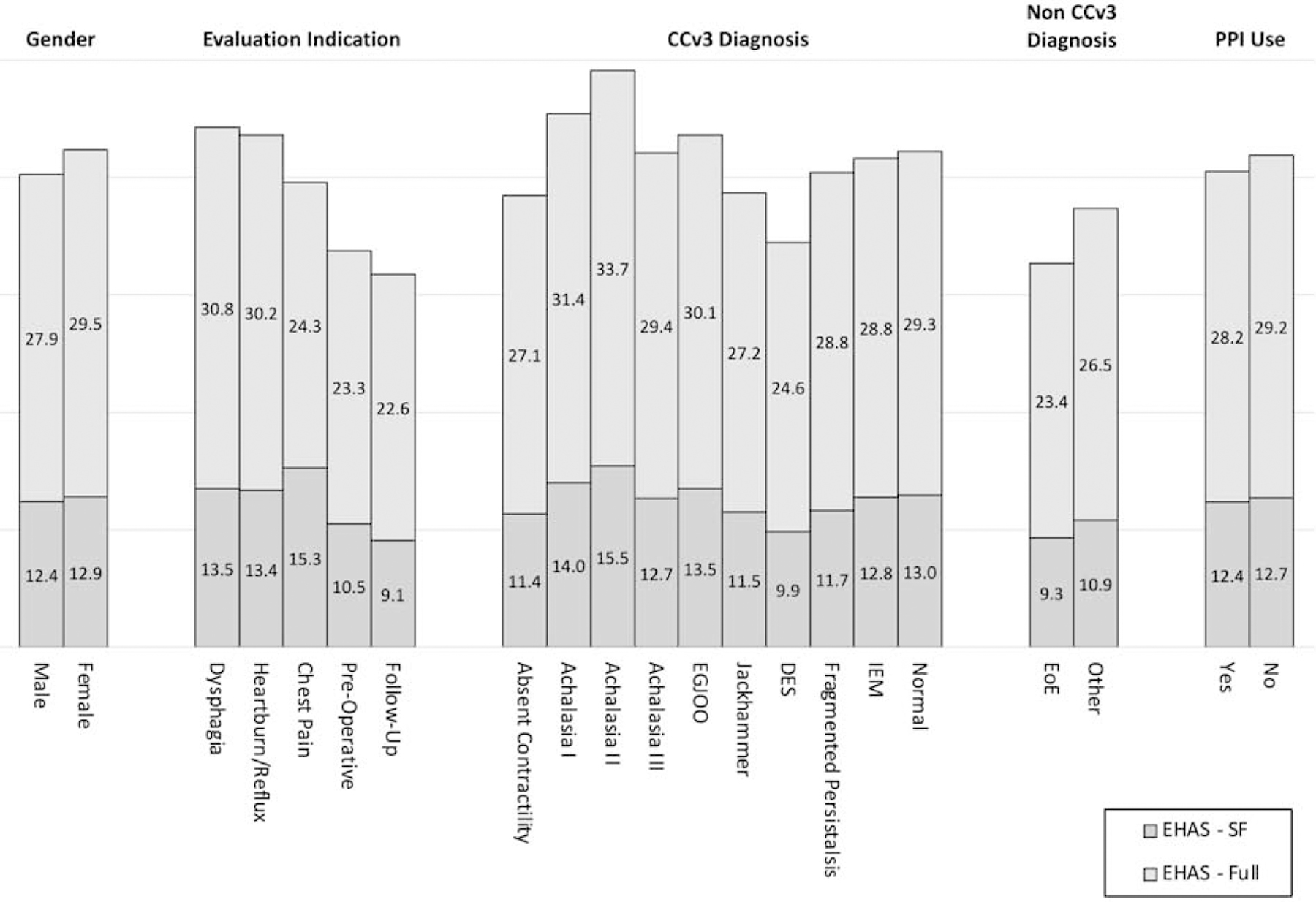
Mean Scores for EHAS-SF and EHAS by Gender, Indication for Evaluation, Diagnosis, and PPI Use
Figure Note: EGJOO= Esophagogastric Junction Outflow Obstruction; DES = Diffuse Esophageal Spasm; IEM = Ineffective Esophageal Motility; EoE = Eosinophilic Esophagitis; CCv3 = Chicago Classification 3.0.
Potential Cutoff Score to Recommend Referral for Mental Health Evaluation
Due to the lack of a definitive psychiatric diagnosis associated with esophageal hypervigilance and anxiety, it is challenging to produce sensitivity and specificity data via ROC curve analyses for a cutoff score of “high” hypervigilance and anxiety. In the EHAS validation study, median split categorized high/low symptom groups. When replicated on EHAS-SF, the median score is 12.88 (IQR = 10). As such, a cutoff score of 13 out of 28 may prompt consideration for mental health evaluation. When using 13, 51.1% of the study sample reported high levels of hypervigilance and anxiety via EHAS-SF.
Discussion
We aimed to develop a short form version of the EHAS to improve its clinical utility by reducing administration time while retaining original psychometric properties. Using validated techniques, the 15-item EHAS was reduced to the 7-item EHAS-SF which takes approximately 2 minutes to complete. The recommended cutoff score for referral for psychological evaluation for clinically elevated hypervigilance and anxiety is 13 based on median split. Future studies should evaluate this finding. It is unclear whether elevated scores on EHAS-SF should guide clinical practice, including physiologic testing and treatment planning. Prospective studies of the performance of EHAS-SF over time are needed to generate these recommendations.
Overall, EHAS-SF performed as well for measures of reliability and concurrent validity as the full version in the present sample and original validation study21. Specifically, EHAS-SF demonstrated a large relationship with HRQOL and moderate associations with dysphagia severity. This suggests a patient’s underlying vigilance about the presence of any symptoms, as well as anxiety about the symptoms’ significance or threat, may be more important than the underlying quantitative burden defined by symptom frequency and intensity19.
Screening patients for esophageal symptom hypervigilance and anxiety is proving to be an important part of the clinical workup of all esophageal conditions. Historically, research and clinical practice related to psychological and behavioral moderators of illness experiences has focused on functional digestive conditions, now disorders of gut-brain interaction (DGBI) per Rome IV criteria27, 28. While certainly an important consideration in DGBIs, more recent research in conditions with an organic etiology elucidates similar cognitive-affective processes contributing to patient symptom reporting15, 17. Regardless of whether symptoms are due to visceral hypersensitivity, as in functional heartburn, or repeated acid exposure, as in GERD, hypervigilance to those sensations combined with anxiety will likely amplify their intensity and complicate the clinical picture.
A secondary goal of this study was to test interactions between hypervigilance, symptom-specific anxiety, and HRQOL in this large patient population. Our results support a significant association, and these constructs were more important than patient-reported symptom frequency and intensity. Approximately 50% of the variance in HRQOL could be explained by the EHAS compared to a significant but small relationship between dysphagia severity and HRQOL. Thus, our data suggests it is not necessarily the frequency or severity of symptoms, but instead our perception and subsequent interpretation of the symptoms as harmful, threatening, or scary, which leads to negative impacts on HRQOL. This is similar to the fear-avoidance model of chronic pain, which postulates it is not simply the presence of pain that leads to disability, but instead motivation to escape or avoid pain drives unhelpful behavior and results in negative impacts on HRQOL29.
These findings hold relevant clinical implications. Providers may understandably assume patients reporting high symptom frequency and/or severity will have high disability. Conversely, and arguably more troublesome, patients who exhibit lower symptom reporting may be erroneously thought of as functioning well with minimal impacts on HRQOL. Such possibilities underscore the importance of using subjective measures, like EHAS-SF, to screen patients during clinical encounters, to offer insight into patient symptom experience that cannot be gathered through symptom reporting alone.
Our data also suggests the EHAS has a direct relationship with reported symptom severity that is greater than underlying physiologic perturbation reflected by HRM per CCv3; this is consistent with our previously reported results in a multicenter international study assessing the relationship between symptom severity and HRM findings19. These results do not suggest motility patterns are irrelevant as the disease states trigger referral for associated symptoms, rather hypervigilance and visceral anxiety may amplify symptoms or reduce overall HRQOL more than esophageal function. Significant differences in EHAS score existed across motility diseases and it was interesting non-spastic achalasia syndromes had the highest EHAS scores, suggesting some interaction between hypervigilance and anxiety and the severity of the disease state as achalasia is typically associated with the most overt presentation.
Brief measures are favorable for clinical practice to reduce patient burden and infringement upon encounter time30–32. The EHAS-SF can easily be administered during a typical outpatient visit and may serve as a discussion point to help gastroenterologists understand patients’ symptom experiences, especially when symptoms appear refractory to treatment or physiological improvement. Elevations on EHAS-SF do not necessarily mean the patient is in need of psychological services. Physicians and nurses can reduce anxiety through education and clarifying information the patient may not understand. A strong doctor-patient relationship may also help reduce anxiety as the patient trusts a more serious condition was not missed33–35. Teaching simple relaxation strategies, such as diaphragmatic breathing, can be done by medical providers and does not necessitate a referral for psychological services15. If preliminary strategies do not reduce EHAS-SF, then referral to a mental health practitioner is warranted.
There are some limitations to consider when evaluating these findings. While prospectively collected as part of a patient registry, data were retrospectively analyzed. Since the EHAS was only collected once, causality between the EHAS and other variables cannot be determined. We also cannot present how the EHAS may change over time especially after treatment. The EHAS-SF is unable to distinguish between the original two EHAS subscales, symptom-specific anxiety and hypervigilance. While similar, these are differing psychological constructs. In research settings, it is useful to examine the individual impact of the two subscales. However, EHAS-SF was developed for a clinical setting to provide a general indication of the degree to which psychological factors are impacting a patient’s experience. Thus, researchers may choose to utilize the EHAS in studies where defining between different psychological constructs is valuable. Lastly, our study does not contain detailed socioeconomic information, specifically race and ethnicity, which are important to evaluate.
Conclusion
In conclusion, EHAS-SF is a reliable and valid tool for quick assessment of hypervigilance and anxiety about esophageal symptoms. As these constructs consistently demonstrate an important role in both dysphagia severity and HRQOL, it behooves clinicians to assess their presence in every esophageal patient. This is even more important in treatment refractory patients, whether PPI for GERD, swallowed steroids for EoE, or tricyclic antidepressants for functional heartburn. Baseline scores from EHAS-SF may be relevant considerations for treatment planning, including if/when psychogastroenterology services are integrated into patient care. Early mitigation of significantly elevated hypervigilance or anxiety may improve response to medical or dietary management. Intermittent assessment is also warranted to gauge effects of interventions on patient outcomes. Established cognitive behavioral interventions are designed to target anxiety and perseverative thinking (i.e. hypervigilance) about symptoms. Some of these strategies can be taught by physicians, nurse practitioners, or nurses with referral for psychogastroenterology services for appropriate patients.
What you need to know:
Background:
The psychological processes, hypervigilance and symptom-specific anxiety, contribute to symptom report in patients with esophageal disease. The esophageal hypervigilance and anxiety scale (EHAS) is a validated, 15-item measure of these constructs but it’s length may limit utility in clinical practice.
Findings:
The EHAS-short form (EHAS-SF) is as reliable and valid as the original EHAS as reflected by relationships with symptom severity and quality of life. The EHAS-SF is an efficient tool for gastroenterologists to use in clinical practice to gauge patient anxiety and hypervigilance.
Implications for patient care:
Understanding patient hypervigilance and anxiety regarding esophageal symptoms is an important but often missed aspect of patients’ clinical presentations. Evaluating these constructs quickly can give critical information about patient outcomes, especially in refractory patients.
Grant Support:
This study is supported by the NIH-NIDDK 1P01DK117824-01
Appendix A. Final Version of the EHAS-SF
| 0 Strongly Disagree | 1 Somewhat Disagree | 2 Neither Agree nor Disagree | 3 Somewhat Agree | 4 Strongly Agree | |
|---|---|---|---|---|---|
| 1. I keep track of my symptom levels | |||||
| 2. As soon as I awake, I worry I will have discomfort in my throat/ chest/ esophagus during the day | |||||
| 3. There is nothing I can do to reduce the intensity of my symptoms | |||||
| 4. These symptoms are terrible, and I think things are never going to get better | |||||
| 5. When I feel discomfort in my throat/ chest/ esophagus it frightens me | |||||
| 6. I am aware of sudden or temporary changes in my esophagus | |||||
| 7. These symptoms are awful, and they overwhelm me |
Scoring: Sum the value for each row based on coding with Likert scale. Minimum score = 0, Maximum score = 28.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: JP: Speaking/Consulting for Sandhill, Medtronic, Torax, Ironwood, Impleo, Trimedyne; LK: Consulting for Pfizer, Abbvie. Co-Founder, Trellus Health; TT: Consulting for Abbvie; DC, LG, WK: Nothing to disclose.
References
- 1.Dellon ES. Cost-effective care in eosinophilic esophagitis. Ann Allergy Asthma Immunol 2019;123:166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jamshed S, Bhagavathula AS, Zeeshan Qadar SM, et al. Cost-effective Analysis of Proton Pump Inhibitors in Long-term Management of Gastroesophageal Reflux Disease: A Narrative Review. Hosp Pharm 2020;55:292–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nenshi R, Takata J, Stegienko S, et al. The cost of achalasia: quantifying the effect of symptomatic disease on patient cost burden, treatment time, and work productivity. Surg Innov 2010;17:291–4. [DOI] [PubMed] [Google Scholar]
- 4.Brook RA, Kleinman NL, Choung RS, et al. Excess comorbidity prevalence and cost associated with functional dyspepsia in an employed population. Dig Dis Sci 2012;57:109–18. [DOI] [PubMed] [Google Scholar]
- 5.Dellon ES, Liacouras CA. Advances in clinical management of eosinophilic esophagitis. Gastroenterology 2014;147:1238–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruley des Varannes S, Lofman HG, Karlsson M, et al. Cost and burden of gastroesophageal reflux disease among patients with persistent symptoms despite proton pump inhibitor therapy: an observational study in France. BMC Gastroenterol 2013;13:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Serag H, Becher A, Jones R. Systematic review: persistent reflux symptoms on proton pump inhibitor therapy in primary care and community studies. Aliment Pharmacol Ther 2010;32:720–37. [DOI] [PubMed] [Google Scholar]
- 8.Talley NJ, Locke GR, Saito YA, et al. Effect of Amitriptyline and Escitalopram on Functional Dyspepsia: A Multicenter, Randomized Controlled Study. Gastroenterology 2015;149:340–9 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirano I, Furuta GT. Approaches and Challenges to Management of Pediatric and Adult Patients With Eosinophilic Esophagitis. Gastroenterology 2020;158:840–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukkada V, Falk GW, Eichinger CS, et al. Health-Related Quality of Life and Costs Associated With Eosinophilic Esophagitis: A Systematic Review. Clin Gastroenterol Hepatol 2018;16:495–503 e8. [DOI] [PubMed] [Google Scholar]
- 11.Kaji M, Fujiwara Y, Shiba M, et al. Prevalence of overlaps between GERD, FD and IBS and impact on health-related quality of life. J Gastroenterol Hepatol 2010;25:1151–6. [DOI] [PubMed] [Google Scholar]
- 12.Ross D, Richter J, Velanovich V. Health-related quality of life and physiological measurements in achalasia. Dis Esophagus 2017;30:1–5. [DOI] [PubMed] [Google Scholar]
- 13.Keefer L, Mandal S. The potential role of behavioral therapies in the management of centrally mediated abdominal pain. Neurogastroenterol Motil 2015;27:313–23. [DOI] [PubMed] [Google Scholar]
- 14.Chey WD. Integrated care for disorders of gut-brain interaction. Lancet Gastroenterol Hepatol 2020;5:876–877. [DOI] [PubMed] [Google Scholar]
- 15.Keefer L, Palsson OS, Pandolfino JE. Best Practice Update: Incorporating Psychogastroenterology Into Management of Digestive Disorders. Gastroenterology 2018;154:1249–1257. [DOI] [PubMed] [Google Scholar]
- 16.Farrell KE, Callister RJ, Keely S. Understanding and targeting centrally mediated visceral pain in inflammatory bowel disease. Front Pharmacol 2014;5:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keefer L, Drossman DA, Guthrie E, et al. Centrally Mediated Disorders of Gastrointestinal Pain. Gastroenterology 2016. [DOI] [PubMed]
- 18.Chen CL. Visceral hypersensitivity in non-erosive reflux disease: neurogenic overwhelming in esophagus? Dig Dis Sci 2013;58:2131–2. [DOI] [PubMed] [Google Scholar]
- 19.Carlson DA, Gyawali CP, Roman S, et al. Esophageal Hypervigilance and Visceral Anxiety Are Contributors to Symptom Severity Among Patients Evaluated With High-Resolution Esophageal Manometry. Am J Gastroenterol 2020;115:367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKinnon AC, Van Oudenhove L, Tack J, et al. The association of personality, appraisal, catastrophising and vigilance with gastrointestinal symptom-specific anxiety. J Health Psychol 2015;20:456–65. [DOI] [PubMed] [Google Scholar]
- 21.Taft TH, Triggs JR, Carlson DA, et al. Validation of the oesophageal hypervigilance and anxiety scale for chronic oesophageal disease. Aliment Pharmacol Ther 2018;47:1270–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spergel JM, Dellon ES, Liacouras CA, et al. Summary of the updated international consensus diagnostic criteria for eosinophilic esophagitis: AGREE conference. Ann Allergy Asthma Immunol 2018;121:281–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dellon ES, Cotton CC, Gebhart JH, et al. Accuracy of the Eosinophilic Esophagitis Endoscopic Reference Score in Diagnosis and Determining Response to Treatment. Clin Gastroenterol Hepatol 2016;14:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taft TH, Riehl M, Sodikoff JB, et al. Development and validation of the brief esophageal dysphagia questionnaire. Neurogastroenterol Motil 2016;28:1854–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bedell A, Taft TH, Keefer L, et al. Development of the Northwestern Esophageal Quality of Life Scale: A Hybrid Measure for Use Across Esophageal Conditions. Am J Gastroenterol 2016;111:493–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drossman DA, Hasler WL. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology 2016;150:1257–61. [DOI] [PubMed] [Google Scholar]
- 28.Schmulson M How to use Rome IV criteria in the evaluation of esophageal disorders. Curr Opin Gastroenterol 2018;34:258–265. [DOI] [PubMed] [Google Scholar]
- 29.Vlaeyen JWS, Crombez G. Behavioral Conceptualization and Treatment of Chronic Pain. Annu Rev Clin Psychol 2020;16:187–212. [DOI] [PubMed] [Google Scholar]
- 30.Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn’s Relapse Prevention Trial. Am J Gastroenterol 1996;91:1571–8. [PubMed] [Google Scholar]
- 31.Melzack R The short-form McGill Pain Questionnaire. Pain 1987;30:191–7. [DOI] [PubMed] [Google Scholar]
- 32.Backonja MM, Krause SJ. Neuropathic pain questionnaire--short form. Clin J Pain 2003;19:315–6. [DOI] [PubMed] [Google Scholar]
- 33.Jayaraman T, Wong RK, Drossman DA, et al. Communication breakdown between physicians and IBS sufferers: what is the conundrum and how to overcome it? J R Coll Physicians Edinb 2017;47:138–141. [DOI] [PubMed] [Google Scholar]
- 34.Saypol B, Drossman DA, Schmulson MJ, et al. A review of three educational projects using interactive theater to improve physician-patient communication when treating patients with irritable bowel syndrome. Rev Esp Enferm Dig 2015;107:268–73. [PubMed] [Google Scholar]
- 35.Drossman DA. 2012 David Sun lecture: helping your patient by helping yourself--how to improve the patient-physician relationship by optimizing communication skills. Am J Gastroenterol 2013;108:521–8. [DOI] [PubMed] [Google Scholar]


