Abstract
Binge drinking is a widespread alcohol consumption pattern commonly engaged by youth. Here, we present the first systematic review of emotional processes in relation to binge drinking. Capitalizing on a theoretical model describing three emotional processing steps (emotional appraisal/identification, emotional response, emotional regulation) and following PRISMA guidelines, we considered all identified human studies exploring emotional abilities among binge drinkers. A literature search was conducted in PubMed, Scopus, and PsychINFO, and a standardized methodological quality assessment was performed for each study. The main findings offered by the 43 studies included are, 1) regarding emotional appraisal/identification, binge drinking is related to heightened negative emotional states, including greater severity of depressive and anxiety symptoms, and have difficulties in recognizing emotional cues expressed by others; 2) regarding emotional response, binge drinkers exhibit diminished emotional response compared with non-binge drinkers; 3) regarding emotional regulation, no experimental data currently support impaired emotion regulation in binge drinking. Variability in the identification and measurement of binge drinking habits across studies limits conclusions. Nevertheless, current findings establish the relevance of emotional processes in binge drinking and set the stage for new research perspectives to identify the nature and extent of emotional impairments in the onset and maintenance of excessive alcohol use.
Keywords: emotional identification, emotional response, alcohol
1. Introduction
Binge drinking consists of drinking large quantities (more than 60 gr of pure ethanol on one occasion, leading to a blood alcohol concentration level of at least 0.08%) in a short time interval – usually less than two hours (Courtney & Polich, 2009; NIAAA1, 2004; WHO2, 2018). This drinking pattern is common to youth from adolescence to young adulthood in most Western countries (ESPAD3, 2016; SAMHSA4, 2016). Recent statistics show that almost 90% of young people (18 years old or older) have already drunk alcohol, and 30% engaged in binge drinking (NIAAA, 2018). Moreover, nearly 12% of youth before the legal age (12–20 years old, United States) and 40% of college students (18–22 years old) report binge drinking habits (NIAAA, 2018). As bingers drink heavily but irregularly, this habit is also characterized by withdrawal episodes. The repeated alternation between high intake and withdrawal, known to be particularly detrimental for brain functioning (Alaux-Cantin et al., 2013; Pascual, Blanco, Cauli, Miñarro, & Guerri, 2007), has guided some authors to propose that binge drinking might lead to cerebral impairments similar to those reported in severe Alcohol Use Disorder (AUD) (e.g., Stephens & Duka, 2008). Comparable impairments between binge drinkers and patients with severe AUD have actually been identified by neuropsychological (see Carbia, López-Caneda, Corral, & Cadaveira, 2018a for a systematic review), neuroimaging, and electrophysiological (see Cservenka & Brumback, 2017; Maurage, Petit, & Campanella, 2013a for reviews) studies. Nevertheless, whereas emotion research constitutes a burgeoning field in severe AUD, explaining excessive drinking episodes and relapse risks (e.g., Bora & Zorlu, 2017; Le Berre, 2019), no paper has reviewed available data to determine the role of emotional processes in binge drinking. Emotional alterations play a role in excessive alcohol use but also in the development of comorbid affective disorders that influence control over drinking (Boden & Fergusson, 2011). Thus, identifying alterations in emotional control and related processes may enhance a fundamental understanding of youthful hazardous drinking. Moreover, the neurotoxic effects of alcohol on the developing brain together with emotional and stressful events occurring during adolescence may increase the propensity of emotional disturbances (Agoglia & Herman, 2018; Elsayed et al., 2018) and create a self-perpetuating disorder.
Dual-process Approach of Binge Drinking
Dominant neuroscientific models of addictive behaviors and models of binge drinking have focused on drug-driven emotions and inhibitory control (e.g., Goldstein & Volkow, 2011; Koob, 2015), without integrating emotional processes per se. Indeed, most studies capitalized on a dual-process view, proposing an interaction between two types of processes related to specific brain systems (e.g., Blanco-Ramos, Cadaveira, Folgueira-Ares, Corral, & Rodríguez Holguín, 2019; Carbia, Corral, Doallo, & Caamaño-Isorna, 2018b; Castellanos-Ryan, Rubia, & Conrod, 2011; Lannoy, Billieux, & Maurage, 2014; Oei & Morawska, 2004; Peeters et al., 2012). The first one, System A, is sustained by the (bottom-up) limbic brain network (Hampton, Adolphs, Tyszka, & O’Doherty, 2007) and involves processes such as automatic/motivational tendencies (e.g., positive bias towards alcohol; Carbia et al., 2018b; reward-seeking, expectancies towards alcohol; Castellanos-Ryan et al., 2011; Oei and Morawska, 2004). The second one, System B, is sustained by the (top-down) prefrontal brain network (Daw, Niv, & Dayan, 2005) and encompasses cognitive processes such as executive functions or more specifically the ability to control alcohol consumption (Lannoy, Maurage, D’Hondt, Billieux, & Dormal, 2018a; Oei & Morawska, 2004). In line with what has been found in severe AUD, results showed an imbalance between these systems in binge drinking: high alcohol bias/expectancies towards alcohol combined with poor executive control predicts binge drinking (Carbia et al., 2018b; Morawska & Oei, 2005; Peeters et al., 2012). Studies focusing on adolescence also speculated that the interaction between Systems A and B would be explained by a brain maturation imbalance: (a) limbic and paralimbic brain areas mature during early adolescence, following hormonal changes, and this modification results in increased reward sensitivity; (b) conversely, prefrontal and parietal cortices mature gradually during late adolescence, and this later maturity explains poor control abilities (Shulman, Harden, Chein, & Steinberg, 2015; Steinberg, 2007). The interaction between heightened reward sensitivity and poor control abilities might therefore lead to risk-taking behaviors in adolescence (Casey & Jones, 2010; Somerville, Hare, & Casey, 2011; Steinberg, 2007), including binge drinking (Noël, 2014; Peeters, Vollebergh, Wiers, & Field, 2014). These proposals, however, have been based primarily on studies exploring System A functioning through cue-reactivity, System B functioning through memory/executive function abilities, or both systems. To address the dearth of knowledge regarding the contribution of emotional processes, this review considers how emotional processing adds value to the current understanding of binge drinking.
1.1. The Role of Emotion
Emotion plays an essential role in the emergence and maintenance of psychopathological disorders. A dominant theoretical view for describing emotion depicts it as a multidimensional response comprising multiple components of emotional processing (Phillips, Drevets, Rauch, & Lane, 2003a). The constellation of components combines three successive steps, usually following a stimulus presentation: emotional appraisal and identification, emotional response, and emotional regulation (Phillips et al., 2003a). As this model identifies distinct emotional processes and has received strong support (e.g., Pessoa, 2017), we will use it as a theoretical framework for the present review. First, emotional appraisal and identification allow assessing an emotional stimulus or situation. Emotional stimuli may be internal (self-emotional states) or external (situation or other individuals’ emotional expressions). In human research, this process has been investigated by self-report questionnaires requiring one to identify internal states (for internal stimuli) or by paradigms requiring the identification of emotionally salient stimuli; e.g., facial emotional expressions, emotional scenes (for external stimuli). Specific brain regions are involved in this external identification, namely the amygdala, insula, ventral striatum, thalamus, and hypothalamus (Britton et al., 2006; Murphy, Nimmo-Smith, & Lawrence, 2003; Pessoa, 2017; Phillips et al., 2003a). Second, emotional response is a reaction to the emotional situation. The inference of the emotional experience leads to feelings and reactions. This response is often described by cognitive, physiological, and behavioral correlates (e.g., danger-related thoughts, accelerated heartbeats, increased sweat, behavioral approach/avoidance tendencies). In human research, this process has been investigated by paradigms inducing affective states (e.g., mood induction, fear conditioning). The brain regions associated with this process are the amygdala, ventral striatum, insula, and orbitofrontal cortex (Britton et al., 2006; Murphy et al., 2003; Phillips et al., 2003a). The final stage of emotional processing is the emotional regulation of affective states and action tendencies (e.g., voluntarily slowing breath, using relaxation or cognitive restructuring). This step is critical for personal and social adaptation, poor emotional regulation being considered a central transdiagnostic process explaining several psychopathological states (see Sloan et al., 2017 for a review). In human research, emotional regulation has been investigated by paradigms involving response to or control from responding to emotional stimuli. The specific neural correlates associated with this process are the anterior cingulate and dorsomedial prefrontal cortices (Esperidião-Antonio et al., 2017; Murphy et al., 2003; Phillips et al., 2003a; Stevens, 2011).
These emotional processing steps enable disentanglement of the complexity of emotions and allow the exploration of individual abilities to process and react to emotional stimuli. It is worth noting that these steps are frequently intertwined, but can also be reported independently (e.g., an emotional response may occur without a specific emotional stimulus presentation and evaluation). Critically, this model describes emotional processing as the successive steps occurring in a specific situation, but the processing of emotional signals may also lead to long-term emotional outcomes (e.g., depressive symptoms; Phillips, Drevets, Rauch, & Lane, 2003b). The relationship between alcohol and depression being crucial to better understand the onset and perpetuation of alcohol misuse (Boden & Fergusson, 2011), this paper will consider both short-term (e.g., self-reported emotional states at the time of the study) and long-term (e.g., persistent and significant depressive or anxious symptoms) perspectives.
In severe AUD, the role of emotional processing is critical and may explain the maintenance of substance abuse (Koob, 2015). Although patients can exhibit emotional deficits resulting from the alcohol’s effects on the brain (Bora & Zorlu, 2017), these emotional deficits can also be involved in relapse (Le Berre, 2019). Patients with severe AUD are impaired for the whole emotional processing stream, with deficits in emotional identification and regulation particularly salient (Le Berre, 2019). In binge drinking, the study of emotional processes only emerged recently. It has been proposed that the repeated alternations between high alcohol consumption and abstinence led to deleterious brain effects in binge drinking, especially in the prefrontal cortex and amygdala, which might produce similar emotional impairments than those observed in severe AUD (Stephens & Duka, 2008). The aim of this review is to highlight how emotion research may bring insight into understanding the antecedents and consequences of binge drinking. We aim to explore which emotional processing steps are altered in binge drinking and how emotional impairments may be related to alcohol-related outcomes in a non-clinical population. For this purpose, we adopted a comprehensive and systematic approach to review emotion studies in binge drinking for the first time. We expect that emotional difficulties will be reported in binge drinking, particularly for emotional identification and response, as these stages appear particularly related to the effects of alcohol (Bora & Zorlu, 2017; Stephens & Duka, 2008).
2. Methods
2.1. Inclusion Criteria and Articles Selection
The systematic research has been conducted according to the model of emotional processing previously described (Phillips et al., 2003a; 2003b). The reliability of this framework is supported in various studies (e.g., Caparelli et al., 2017; Rutter et al., 2019) and offers a theoretically driven research including distinct emotional processes. As this research field is nascent, lenient inclusion criteria were used to offer a broad representation of the field. To determine inclusion criteria, we used a modified PICOS (Population, Intervention, Comparator, Outcome, Study design/setting) procedure for observational studies (Liberati et al., 2009): (1) the Population referred to human participants (adolescents and adults, identified age range: 13–74 years old) with current binge drinking (as a pattern of alcohol consumption) or having presented at least one binge drinking episode. Studies referring to other patterns of alcohol use (e.g., severe AUD, maternal binge drinking) that may bias the current results were excluded. We also excluded animal studies. In order to offer a comprehensive view of the relationship between binge drinking and emotional processing, no exclusion criterion was related to personal, demographic, and socio-economic characteristics. Regarding psychopathological conditions, we included studies assessing depression and anxiety, as these psychopathological symptoms are closely related to impaired affective processing and thus of critical importance when considering the links between emotional variables and binge drinking. The presence of other psychopathological disorders not related to emotional processes (e.g., Attention Deficit Hyperactivity Disorder, Posttraumatic Stress Disorder) constituted an exclusion criterion as they may bias the interpretation of emotional difficulties (e.g., the difficulties observed may not be specifically related to binge drinking but rather, at least partly, to the comorbid pathological condition, which is not directly related to emotional processing and, therefore, out of the scope of the present review); (2) regarding Intervention, we focused on alcohol drinking and considered both the immediate (i.e., emotional processing after drinking alcohol; acute consumption) and long-term alcohol influence (i.e., stable emotional processing among binge drinkers, outside the intoxication episodes). As the definition of binge drinking varies widely across studies, we included all studies referring to the concept of binge drinking, recognizing that the specificity of alcohol use criteria would be evaluated in the quality assessment; (3) as Comparator, we considered two types of studies: those presenting experimental comparisons (between binge drinking and control groups or between emotional and control conditions) and those evaluating the relationship between binge drinking and emotional processing (without group comparison), controlling for personal, demographic, and psychopathological variables when statistically appropriate; (4) the Outcome focused on emotion. Accordingly, research using self-report measures, behavioral tasks, electrophysiological, or neuroimaging data were included if participants were required to process emotional information or if emotional measures were taken; (5) the Study design included correlational or experimental studies but excluded research focused on the evaluation of clinical interventions as well as single-case or case series studies or publications without experimental data (e.g., comments, reviews).
The guidelines of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) were followed (Moher, Liberati, Tetzlaff, & Altman, 2009), as reported in the PRISMA checklist (see supplementary material). Inclusion criteria included peerreviewed articles published in English between January 1st, 2000 and June 1st, 2020. The systematic research was conducted in three databases (Pubmed, Scopus, PsychINFO). Keywords were determined following the theoretical proposal of Phillips and colleagues (2003a) for emotion (emotional appraisal OR emotional identification OR emotional response OR emotional regulation OR emotion OR emotions) and related to binge drinking (binge drinking OR heavy drinking OR social drinking OR college drinking). The initial search produced 575 papers, 43 of which met inclusion criteria for this review (see Figure 1 for a flowchart of article selection). As an example, the search for emotional response AND binge drinking in PubMed led to 72 results, 18 were removed because they were already found in other research (i.e., emotional appraisal, emotional identification) and 54 abstracts were screened. Articles were excluded when they did not focus on human studies (n=11), emotional processing (n=16), or binge drinking (n=10). We read 17 full-texts and excluded six other articles (intervention studies, no binge drinking measure). Then, four articles were included, related to internal emotional identification (i.e., Bekman, Winward, Lau, Wagner, & Brown, 2013; Pape & Norström, 2016; Scaife & Duka, 2009, Strine et al., 2008). The final research led to the identification of seven articles, included for the evaluation of emotional response. This procedure was applied for all searches. The first author performed the search in the databases and the first and last authors (SL and PM) conducted the articles’ selection based on exported PDF files.
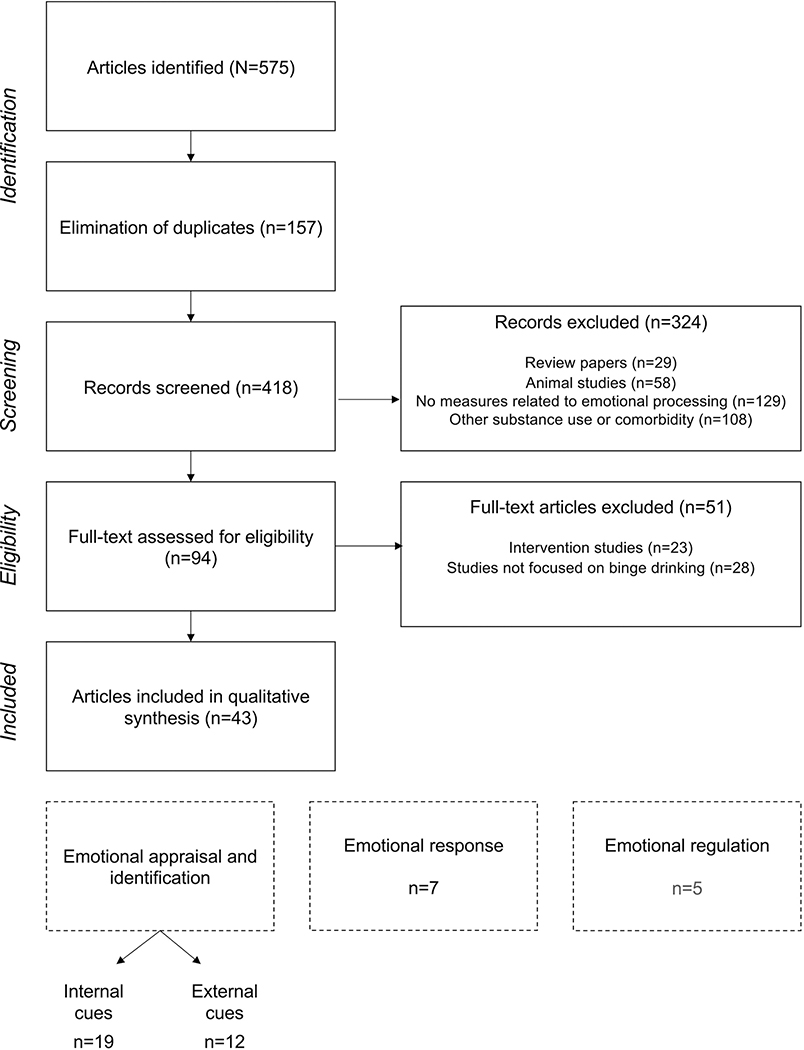
Figure 1.
PRISMA flowchart of articles selection
Figure 1 illustrates the different steps of article selection and inclusion through the PRISMA guidelines (identification, screening, eligibility, inclusion), and the number of articles kept and excluded at each selection step. The procedure leads to the inclusion of 43 articles, 19 related to internal emotional identification, 12 related to external emotional identification, 7 related to emotional response, among which one also informs about emotional regulation, and 5 more related to emotional regulation.
2.3. Methodological Quality Assessment
The existing literature describes many ways to assess the quality of research studies without a specified standard (Zeng et al., 2015). Indeed, such assessments may appear subjective and have to be adapted according to the specific aims of each review. Consequently, for the present review, identified articles were assessed based on an adapted version of the AXIS criteria (Downes, Brennan, Williams, & Dean, 2016). This tool was developed to be adapted across all scientific disciplines, and its reliability was ensured by a Delphi panel (validation of 18 experts) (Downes et al., 2016). Its non-specificity affords a simple and clear way for a critical appraisal of the literature, which matched our aim to include studies using a variety of approaches (e.g., neuroscience, psychology) to assess emotional processing.
The final criteria appear in Table 1, with the detailed evaluation of each study. In summary, six items were deleted or modified from the original scale because they were not directly relevant: items 8 and 9 were combined in a unique item (item 13 in the adapted scale, referring to manipulation of the dependent variable and its appropriateness), evaluation of sample size was added in item 3 (to determine what a sufficient sample size was, we referred to previous studies and defined a minimum of 25 participants for group studies; Carbia et al., 2018a, and 52 participants per predictors for correlational studies; Maxwell, 2000), and items 13, 15, and 19 were deleted as they referred to non-response bias, internal consistency (already assessed in other items), and conflicts of interest, respectively. Several adaptations were also conducted regarding selection criteria and representativeness of the population to meet the specific needs of the present paper (e.g., evaluation of timeframe, intensity, and frequency of binge drinking). To increase the procedure reliability, this quality assessment was performed by two independent judges (authors SL and PM). The total agreement between the assessors was 87.9% (= 605/688), which can be evaluated as very strong (McHugh, 2012). Assessment discrepancies were related to two items (6 and 10). Thus, the minimum quantity of alcohol in a binge drinking episode (i.e., the consumption of at least 60 gr of pure ethanol on one occasion, 56 gr was considered if authors used distinct categorization for girls; binge drinking score) and the reliable ways to determine statistical significance to reach a consensus (e.g., p-values, confidence intervals) were discussed. A score (i.e., percentage of “Yes” answers) was computed to provide an overview of the quality associated with each study (i.e., poor quality for scores below 50%, fair quality for scores between 50 and 69%, good quality for scores between 70% and 79%, strong quality for scores of 80% and beyond; Black et al., 2017; Maurage, Masson, Bollen, & D’Hondt, 2020).
Table 1.
Studies scoring using the adapted quality assessment AXIS (Downes et al., 2016).
| Authors | Date | AXIS Items |
% score |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3a/b | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |||
|
| ||||||||||||||||||
| Balodis et al. | 2011 | Y | Y | N/N | Y | N | N | N | Y | Y | Y | Y | N | Y | Y | Y | Y | 64.71 |
| Bekman et al. | 2013 | Y | Y | Y/N | Y | N | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | 82.35 |
| Carbia et al. | 2020 | Y | Y | Y/N | N | N | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 76.47 |
| Chen & Feeley | 2015 | Y | N | Y/N | N | N | N | N | N | N | Y | Y | Y | Y | N | Y | N | 41.18 |
| Cohen-Gilbert et al. | 2017 | Y | Y | N/N | Y | N | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | 76.47 |
| Connell et al. | 2015 | Y | Y | N/N | Y | N | N | N | Y | Y | Y | Y | Y | Y | Y | Y | N | 64.71 |
| Ehlers et al. | 2007 | Y | Y | Y/N | Y | N | N | N | N | Y | Y | Y | Y | Y | Y | Y | Y | 70.59 |
| Ehret et al. | 2013 | Y | Y | Y/N | Y | N | N | N | N | N | Y | Y | Y | Y | Y | Y | Y | 64.71 |
| Ewing et al. | 2010 | Y | Y | Y/N | Y | N | Y | N | Y | Y | Y | Y | Y | N | Y | Y | Y | 76.47 |
| Gowin et al. | 2020 | Y | Y | Y/N | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 88.24 |
| Hartley et al. | 2004 | Y | Y | N/Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 88.24 |
| Haynes et al. | 2005 | Y | Y | Y/N | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | 88.24 |
| Hefner et al. | 2013 | Y | Y | N/N | Y | N | N | N | N | N | Y | Y | Y | Y | Y | Y | Y | 58.82 |
| Herman et al. | 2018 | Y | Y | N/N | Y | N | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | 70.59 |
| Howland et al. | 2010 | Y | Y | Y/Y | Y | N | Y | N | Y | Y | Y | Y | Y | N | Y | Y | Y | 82.35 |
| Huang et al. | 2018 | Y | Y | Y/N | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N | 76.47 |
| Khan et al. | 2018 | Y | Y | N/N | Y | N | Y | N | Y | Y | Y | Y | Y | N | Y | Y | Y | 70.59 |
| Laghi et al. | 2018 | Y | Y | Y/N | Y | N | Y | N | N | N | Y | Y | Y | Y | Y | Y | Y | 70.59 |
| Lannoy et al. | 2017 | Y | Y | N/N | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 82.35 |
| Lannoy et al. | 2018 | Y | Y | N/N | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 82.35 |
| Lannoy et al. | 2018 | Y | Y | N/N | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 82.35 |
| Lannoy et al. | 2019 | Y | Y | Y/N | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 88.24 |
| Leganes-Fonteneau et al. | 2020 | Y | Y | N/N | Y | N | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | 70.59 |
| Lindgren et al. | 2018 | Y | Y | Y/N | Y | N | N | N | N | N | Y | Y | Y | Y | Y | Y | Y | 64.71 |
| Loeber & Duka | 2009 | Y | Y | N/N | Y | N | N | N | N | N | Y | Y | Y | Y | Y | Y | Y | 58.82 |
| Maurage et al. | 2009 | Y | Y | N/N | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 82.35 |
| Maurage et al. | 2013 | Y | Y | N/N | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 82.35 |
| Mngoma et al. | 2020 | Y | N | Y/N | N | N | N | N | Y | Y | N | Y | Y | N | N | Y | Y | 47.06 |
| Mushquash et al. | 2013 | Y | Y | Y/N | Y | Y | N | N | N | N | Y | Y | Y | N | Y | Y | Y | 64.71 |
| Nourse et al. | 2017 | Y | Y | Y/N | Y | N | N | N | Y | Y | Y | Y | Y | N | N | Y | Y | 64.71 |
| Pape & Norström | 2016 | Y | Y | Y/N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 94.12 |
| Pedersen | 2013 | Y | N | Y/N | Y | N | N | N | N | N | Y | Y | Y | N | Y | Y | N | 47.06 |
| Poncin et al. | 2017 | Y | Y | N/N | Y | N | Y | N | N | N | N | Y | Y | Y | Y | Y | Y | 58.82 |
| Rose & Grunsell | 2008 | Y | Y | N/N | Y | N | Y | Y | N | N | Y | Y | Y | Y | N | N | Y | 58.82 |
| Ruiz et al. | 2020 | Y | Y | Y/N | N | N | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | 76.47 |
| Scaife & Duka | 2009 | Y | Y | Y/N | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | 82.35 |
| Stephens et al. | 2005 | Y | Y | N/N | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | 76.47 |
| Stickley et al. | 2014 | Y | Y | Y/N | Y | Y | N | N | N | N | Y | Y | Y | N | Y | Y | Y | 64.71 |
| Strine et al. | 2008 | Y | Y | Y/N | N | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | N | 76.47 |
| Townshend & Duka | 2005 | Y | Y | Y/N | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 88.24 |
| Trojanowski et al. | 2019 | Y | Y | Y/N | Y | N | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | 82.24 |
| Venerable & Fairbairn | 2020 | Y | Y | Y/Y | Y | Y | N | N | N | N | Y | Y | Y | Y | Y | Y | Y | 76.47 |
| Wichaidit et al. | 2020 | Y | Y | Y/N | N | N | N | N | Y | Y | Y | Y | Y | N | Y | Y | Y | 64.71 |
Legend: N = No; Y = Yes
Note: Question related to each item:
Introduction
(1) Were the aims/objectives of the study clear?
Methods
(2) Was the study design appropriate for the stated aim(s)?
(3) Was the sample size (a) sufficient (i.e., at least 25 subjects per group or 52 per predictor) and (b) justified (i.e., power analysis, reference to samples or effect sizes reported in earlier studies)?
(4) Was the target/reference population clearly defined (Is it clear who the research was about?)?
(5) Were measures undertaken to address and categorize non-responders?
(6) Were the selection criteria clearly defined and selective enough (e.g., alcohol intensity) to focus on binge drinkers?
(7) Was the timeframe of binge drinking habits enough (i.e., 6 months), so that one could reasonably expect to see an association between binge drinking and emotional processes?
(8) Were key potential confounding variables considered (e.g., included in pre-selection criteria or adjusted statistically)?
(9) Were key potential confounding and outcome variables measured appropriately?
(10) Is it clear what was used to determine statistical significance and/or precision estimates (p-values, CIs)?
(11) Were the methods (including statistical methods) sufficiently described to enable them to be repeated/reproduced?
Results
(12) Were the basic measures (i.e. demographics) adequately described?
(13) Were the dependent variables (i.e. emotional processes) manipulated, and was this manipulation adequately described?
Discussion
(14) Were the authors’ discussions and conclusions justified by the results?
(15) Were the limitations of the study discussed?
Other
(16) Was ethical approval or consent of participants attained?
3. Results
3.1. Description of the Studies
For each study, data were extracted systematically using the PICOS framework. All details regarding the sample, inclusion/exclusion criteria, or emotional processes and measures are reported in Tables 2–4. The Results section describes the key conclusions related to each research study and is divided into three sub-sections, organized according to the theoretical model of emotional processing (Phillips et al., 2003a): emotional appraisal and identification (subdivided in self/internal emotional identification and other/external emotional identification), emotional response, and emotional regulation.
Table 2.
Description and main results of studies evaluating emotional appraisal and identification in binge drinking.
| Authors (year) | Participants | Intervention | Comparator | Experimental design | Outcomes | Scoring | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample (n) | Age | Gender ratio (% of males) | Inclusion criteria | Binge drinking criteria | Control group/variable | Processes measured | Task/scale | Stimuli | Main results | Limits | ||
| Emotional identification of internal cues | ||||||||||||
| Bekman et al. (2013) | 39 BD | Range 16–18 Mean 17.74 |
51% | No recent substance consumption No psychiatric or neurological disorders |
Drinking alcohol on more than 100 occasions at lifetime. At least 3 binge drinking episodes (≥ 70 alcohol gr for boys and ≥ 56 gr for girls on one occasion) during the past month At least 1 withdrawal symptom following a recent drinking episode |
26 non-BD (no history of binge drinking or alcohol use problems) | Anxiety and depression Mood |
6-week research following abstinence: two assessments (start and end) and daily follow-up (3–6 times/day) The Hamilton Rating Scales for Anxiety and Depression State-Trait Anxiety Inventory Self-reported negative affects (down, angry, and stressed) |
N/A | Negative affect at early stage of abstinence: BD > non-BD Depression, anxiety: BD > non-BD Correlations between negative affect and maximum number of drinks consumed on one occasion and total number of drinks |
No information about affective states prior the onset of alcohol use | 82.35 |
| Chen & Feeley (2015) | 179 college students | Range 18–29 Mean 19.76 |
46.9% | N/A | Number of binge drinking episodes in the past 2-week (≥ 70 alcohol gr for boys and ≥ 56 for girls in 2 hours) | Analyses were controlled for age, gender, ethnicity, and health status | Stress Loneliness |
2-week research Perceived Stress Scale UCLA Loneliness Scale |
N/A | Students with higher stress depicted increased binge drinking 2 weeks later No associations were found with loneliness |
Convenience sample | 41.18 |
| Ewing et al. (2010) | 45 college students | Range 21–33 Mean 22.8 |
44.44% | No MRI contraindication, personal or family history of psychopathological disorder, current medical condition, mental retardation, left handedness, or current substance use No alcohol, caffeine, and nicotine use prior scanning |
At least 42 alcohol gr (men) or 28 (women) per drinking occasion, 2–5 times per week, for 4 weeks Number of binge drinking episodes (at least 70 gr) in the past month |
N/A | Depressive symptoms Anxiety symptoms |
AUDIT Beck Depression Inventory Beck Anxiety Inventory fMRI: taste-cue paradigm (alcohol and non-alcohol appetitive cues) |
N/A | Depression was positively associated with brain activations (insula, cingulate, ventral tegmentum, striatum, and thalamus) while viewing alcohol cues Anxiety was positively associated with brain activations (striatum, thalamus, insula, and inferior frontal, mid-frontal, and cingulate gyri) while viewing alcohol cues |
No control group | 76.47 |
| Hartley et al. (2004) | 14 BD | Range 18–23 Mean 21.13 |
64.3% | N/A | ≥ 80 alcohol gr on one occasion Binge drinking score ≥ 24 |
13 teetotalers | Trait anxiety and depression Mood |
The Hospital Anxiety and Depression Scale Visual analogue rating scales (alertness, well-being, anxiety) |
N/A | Trait anxiety and depression: BD < teetotalers NS for mood rating |
Comparison with a group of non-drinkers (i.e., it assesses the effect of alcohol consumption but not a specific effect of binge drinking) | 88.24 |
| Haynes et al. (2005) | 8,580 adults at baseline | 18–74 | N/A | Absence of mental disorder at baseline | ≥ 48 alcohol gr on one occasion at least once a month | Analyses were adjusted for age, gender, socio-demographic and -economic variables, other substance use and mental health | Depression | 18-month study Clinical Interview Schedule at baseline (n=2,413 who completed the follow-up have no mental disorder) AUDIT Severity of Alcohol Dependence Questionnaire |
N/A | Hazardous drinking, binge drinking, and severe alcohol use disorders were not related to anxiety or depression at follow-up Sub-threshold of anxiety and depression at baseline was related to the onset of severe alcohol use disorders (weak evidence) |
No information about the evaluation of depression and anxiety during the 18 months (e.g., recovery) | 88.24 |
| Howland et al. (2010) | 193 college students | Range 21–24 Mean 21.47 |
No alcohol problems or other substance use, no medical condition No night shifts work, no regnancy, no travel across two or more time zones in the prior month |
At least one binge drinking episode (≥ 70 alcohol gr for boys or ≥ 56 for girls) in the past month Alcohol administration: 1.068 g/kg for boys and 0.915 g/kg for girls with cans of beer in one-hour interval. Non-alcoholic beer as placebo condition. |
Analyses were controlled for gender and session Alcohol administration versus placebo |
Mood | 2-week research (alcohol versus placebo) and assessment following beverage administration (morning and afternoon) Profile of Mood States |
N/A | Mood was affected the morning after alcohol consumption (BAC of 12%) | Not reported | 82.35 | |
| Mngoma et al. (2020) | 355 youth | Range 14–24 Mean 18.6 |
100% | N/A | No specific binge drinking criteria | N/A | Anxiety Depression |
Brief Symptom Inventory Rosenberg Self-Esteem Scale Social Provisions Scale Substance use |
N/A | Suicidal thoughts were associated with depression, anxiety, worthlessness, and binge drinking | No inclusion of women | 47.06 |
| Mushquash et al. (2013) | 191 women | Mean 19.9 | N/A | N/A | Dichotomic alcohol measure: 0: no more than 56 gr in 2 hours 1: more than 56 gr in 2 hours at least once in the past week |
N/A Use of structural equation modeling |
Depression Mood |
4-week research Depression Adjective Checklist Center for Epidemiological Studies Depression Scale Profile of Mood States |
N/A | Depressive symptoms predicted binge drinking over one week but binge drinking did not predict depression. | No inclusion of men | 64.71 |
| Nourse et al. (2017) | 201 college students | Mean 21.1 | 25.4% | N/A | AUDIT score ≥ 7 | N/A | Anxiety Depression |
Generalized Anxiety Questionnaire Patient Health Questionnaire AUDIT |
N/A | No association between hazardous drinking and depression or anxiety | No inclusion of men Small convenience sample |
64.71 |
| Pape & Norström (2016) | 2,171 youth people | Range 13–17 Time 1 Mean 14.9 |
43% | N/A | Frequency of alcohol use and intoxication feelings in the past 12 months | Separate analyses according to age (to consider developmental trajectories) and gender | Anxiety Depression Loneliness (as a control measure at the longitudinal level) |
13-year research, 4 assessment times The Hopkins Symptom Check List The Depressive Mood Inventory UCLA Loneliness Scale |
N/A | Emotional distress was not associated with binge drinking in early adolescence From adolescence to adulthood (mean age: 16.4 yo to 21.8 yo) and in late adulthood (mean age: from 21.8 yo to 28.3 yo), depression, but not anxiety, was positively associated with binge drinking |
Subjectivity related to the binge drinking measure | 94.12 |
| Pedersen (2013) | 248 college students | Range 18–29 Mean 20.83 |
37.09% | N/A | Number of binge drinking episodes (≥ 70 alcohol gr for boys or ≥ 56 for girls) in the past month | Separate analyses for men and women. Analyses controlled for class level and employment status | Depressive mood Stress |
Behavioral Risk Factor Surveillance System (BRFSS) School stress scale |
N/A | In men, binge drinking was positively related to depression In women, the relation between binge drinking and depression was explained by class level (first university year), employment status (higher number of work hours) and school-related stress |
Single-item measure of depression | 47.01 |
| Rose & Grunsell (2008) | 10 BD | Range 18–25 Mean 21.5 |
50% | No psychiatric or substance use disorder, no current medication No alcohol drinking, caffeine, or fat meal before the experiment |
≥ 80 alcohol gr per week Binge drinking score ≥ 24 Alcohol administration: 0.6 g/kg for boys and 0.5 g/kg for girls with lemonade in a 500 ml solution drank in a 30-minute period. In the control condition, 500 ml of lemonade with drops of ethanol (≤ 5 ml) |
10 non-BD (binge drinking score ≤ 16) Alcohol administration versus placebo |
Mood | Visual Analog Scale (alert, content, relaxed, stimulated, lightheaded, and irritable), completed (1) before alcohol drinking and (2) 30 minutes after drinking alcohol | N/A | Ratings of “stimulated” decreased between baseline and post-preload: (1) placebo condition, BD < non-BD (2) alcohol condition, BD > non-BD Ratings of “lightheaded” following alcohol preload, BD < non-BD |
Not reported | 58.82 |
| Ruiz et al. (2020) | 1505 participants | Range 18–30 Mean 23.25 |
25% | N/A | Number of binge drinking episodes (≥ 70 alcohol gr for boys or ≥ 56 for girls on one occasion) in the last 12 months Late drinkers (first alcohol use after 15 yo) and early drinkers (first alcohol use before 14 yo) |
Analyses were controlled for the total volume of alcohol consumed and sex | Emotional distress | AUDIT Alcohol consequences questionnaire Kessler Scale of psychological distress (anxiety, depression, and non-specific distress) Doherty Scale of Emotional Contagion |
N/A | Psychological distress: Early drinkers > Late drinkers Significant relationship between psychological distress and negative consequences of alcohol Psychological distress was not associated with binge drinking but with negative consequences of alcohol |
Not reported | 76.47 |
| Scaife & Duka (2009) | 30 BD | Range 18–29 Mean 20.6 |
60% | No use of illicit drug or medication 1 week before the experiment, no alcohol drinking 12 hours before the experiment | Binge drinking score > 31 (median split) | 30 non-BD (binge drinking score < 31) | Mood | Profile of Mood States | N/A | No significant difference between BD and controls, only a gender effect showed higher arousal in female | Not reported | 82.35 |
| Stickley et al. (2014) | 4,045 adolescents | Range 13–15 | 47.4% | N/A | Drinking more than 70 alcohol gr on one occasion at least once in the past month | Analyses controlled for age, parental education, family structure, and depressive symptoms. | Loneliness | Adapted Center for Epidemiological Studies Depression Scale | N/A | Loneliness was associated with binge drinking in the last month among adolescents in the US | Single-item measure of loneliness | 64.71 |
| Strine et al. (2008) | 217,379 adults | 18 or older | N/A | N/A | ≥ 70 alcohol gr for boys and ≥ 56 for girls on one occasion in the past month Heavy drinking: > 28 gr per day for boys and > 14 for girls |
Analyses were adjusted by sex, age, socio-demographic and -economic status | Anxiety and depression | Patient Health Questionnaire Evaluation of smoking habits, height, weight, physical activity, and alcohol consumption |
N/A | Adults who presented current depression or had a lifetime history of depression or anxiety exhibited increased smoking, obesity, physical inactivity, binge, and heavy drinking | No information on the causality link between anxiety/ depression and alcohol use | 76.47 |
| Townshend & Duka (2005) | 38 BD | Range 18–30 Mean 20.9 |
60.5% | No psychopathological disorder, neurological disorder, or substance use disorder No use of drug, sleeping tablet, hay fever and alcohol prior the experiment |
≥ 48 alcohol gr per week Binge drinking score ≥ 24 |
34 non-BD (binge drinking score ≤ 16) | Mood | Profile of Mood States | N/A | BD had less positive mood | Self-reported alcohol use | 88.24 |
| Venerable & Fairbairn (2020) | 60 BD | Range 21–28 Mean 22.5 |
50% | No medical contraindication for alcohol drinking No severe alcohol use disorders, extreme body mass index, and no pregnant women |
Drinking at least 2 times/ week, 56 alcohol gr per occasion Number of binge drinking episodes (≥ 70 alcohol gr for boys or ≥ 56 for girls on one occasion) in the past 30 days Alcohol administration 0.82 g/kg for boys and 0.74 g/kg for girls (mix of cranberry and vodka) served in three equal parts at 0, 12, and 24 min. In the control condition, isovolumic amount of cranberry juice |
Comparison between alcohol and placebo sessions Analyses exploring the effects of alcohol on mood were controlled for predrink mood and lagged mood |
Mood | 7-day study, 18-month follow-up Self-report mood, anxiety, and alcohol-related stimulation and sedation Transdermal sensors (7 days) Mood scale: positive (upbeat, content, happy, euphoric, energized) and negative (nervous, sad, irritated, lonely, bored) mood after alcohol use Short Inventory of Problems |
N/A | When controlling for baseline drinking, greater negative mood reduction after alcohol drinking predicted drinking problems at follow-up Greater positive mood after alcohol drinking also predicted drinking problems and binge drinking at follow-up |
Less BD compared to non-BD answered at follow-up | 76.47 |
| Wichaidit et al. (2020) | 38,186 students | Age range 12–17 Mean 15.2 |
45.5% | N/A | At least one binge drinking episode (≥ 60 alcohol gr for boys and 50 for girls on one occasion) in the past month | Analyses were controlled for socio-demographic and -economic variable, substance use and psychopathology | Mood | Substance (tobacco, alcohol, illicit drug) and behaviors (gambling, sexual behaviors, gaming, and social media use) Patient Health Questionnaire |
N/A | Depressed mood was significantly associated with alcohol drinking in the past year, the past month, and with past-month binge drinking | No information on the causality | 64.71 |
| Emotional identification of external cues | ||||||||||||
| Carbia et al. (2020) | 180 college students at follow-up | Range 18–20 Mean 18.01 |
46.67% | No personal or family history of severe alcohol use disorder, illicit drug use, neurological or psychiatric disorders | Number of binge drinking episodes (≥ 60 alcohol gr for boys and ≥ 40 gr for girls) in the last 3 months | Analyses were controlled for cannabis use, tobacco use, and psychopathology | Emotional memory | 2-year research AUDIT Alcohol Timeline Followback Emotional Verbal Learning Test (assessed at follow-up) |
Positive, negative, and neutral words | Boys: no significant effect Girls: BD had an emotional memory bias for negative words, lower recall for positive and neutral words, increased false alarms for negative emotional distractors. |
No neuropsychological assessment at baseline | 76.47 |
| Ehlers et al. (2007) | 30 BD 59 BD and drug users |
Range 18–25 Mean 19.91 |
50% | No psychiatric disorder | At least one binge drinking episode (> 70 alcohol gr) during adolescence; with or without drug consumption | 36 non-BD and non-drug users | Emotional identification | Facial discrimination task (answer to happy or sad faces and do not answer to neutral) EEG recording: event-related potentials (P3a and P3b) |
Happy, neutral, and sad faces | Binge drinking + drug use history: decreased P3a latency during the view of all faces Binge drinking and binge drinking + drug use: decreased P3b amplitude during the view of happy faces |
Cross-sectional alcohol use data | 70.59 |
| Gowin et al. (2020) | 177 BD | Range 22–35 Mean 27.9 |
72.31% | No lifetime history of alcohol abuse or dependence | At least one binge drinking episode (≥ 70 alcohol g for boys or ≥ 56 for girls on one occasion) per week in the last year | 309 non-BD | Emotional identification | Penn Emotion Recognition Test (happy, sad, angry, scared or neutral emotional faces) fMRI measures: Emotional task (matching of faces with angry, fearful, or neutral emotional expressions) |
Emotional facial expressions of happiness, sadness, anger, and fear compared to neutral ones | Machine learning: Emotion processing did not perform better than chance The best model to identify BD compared to non-BD included social and language processing |
Heterogeneity in the BD sample | 88.24 |
| Huang et al. (2018) | 32 BD | Range 18–30 Mean 23.3 |
50% | No history of neurological or neuropsychiatrie disorder, visual or auditive problem, learning difficulty, left-handed participant No drug or medication use before the study |
At least 5 binge drinking episodes (≥ 84 alcohol gr for boys or ≥ 70 for girls within 2-hour) in the last 6 months | 32 non-BD (no more than one binge drinking episode in the last 6 months) | Emotional appraisal | Appraisal of emotional images (9-point Likert scale) EEG recording: event-related theta power at frontal, central, and parietal sides |
Emotional scenes: negative, positive, erotic, and neutral stimuli | No behavioral difference in the ratings of emotional images. Modulation of event-related theta power (early and later processing): BD < non-BD |
Not reported | 76.47 |
| Khan et al. (2018) | 147 college students | Range 18–23 Mean 19.92 |
/ | No psychiatric disorder (inclusion of moderate depression), illicit substance use, or significant cognitive deficit | At least one binge drinking episode (≥ 50 alcohol gr for boys or ≥ 40 for girls in two hours) in the past month | Analyses were controlled for gender and drinking quantity | Distress tolerance | The Structured Clinical Interview for DSM-IV The Distress Tolerance Scale Timeline Follow back Brief Young Adult Alcohol Consequences Questionnaire Beck Depression Inventory |
Appraisal of distress tolerance predicted alcohol-related problems in BD, when controlling for drinking quantity and sex differences. The relationship between alcohol-related problems and distress tolerance, absorption, and regulation was mediated by drinking to cope. |
No information on the causality | 70.50 | |
| Lannoy et al. (2017) | 20 BD | Range 18–23 Mean 19.73 |
45% | No personal or family history of severe alcohol use disorder, psychological, neurological or medical disorders, medication or drug use, normal visual and auditory abilities | Binge drinking score ≥ 16 | 20 non-BD (binge drinking score ≤ 12) | Emotional crossmodal identification | Emotional crossmodal task (identification of emotional stimuli of anger and happiness based on facial and vocal processing) | Emotional facial expression of anger and happiness Emotional bursts of anger and happiness |
No significant group difference Non-BD were slower than BD for the recognition of emotional faces |
Subjective evaluation of alcohol use (drunkenness) | 82.35 |
| Lannoy et al. (2018c) | 17 BD | Range 18–29 Mean 20.52 |
58.8% | No personal or family history of severe alcohol use disorder, psychological, neurological or medical disorders, medication or drug use, normal visual and auditory abilities | Binge drinking score ≥ 16, ≥ 60 alcohol gr per occasion, ≥ 20 gr per hour, 2–4 times per week | 17 non-BD (binge drinking score between 1 and 12, ≤ 30 alcohol gr per occasion, ≤ 3 times per week) 19 teetotalers |
Emotional crossmodal identification | Emotional crossmodal task (identification of emotional stimuli of anger and happiness based on facial and vocal processing) EEG recording |
Emotional facial expression of anger and happiness Emotional bursts of anger and happiness |
No significant behavioral difference N100 latency, anger: BD > non-BD, teetotalers P3b amplitude, congruent happiness: BD > non-BD, teetotalers in Crossmodal integration for anger in incongruent trials Latency: BD > non-BD, teetotalers Amplitude: BD>non-BD, teetotalers |
Small sample size | 82.35 |
| Lannoy et al. (2018b) | 23 BD | Range 18–27 Mean 20.02 |
47.8% | No personal or family history of severe alcohol use disorder, psychological, neurological or medical disorder, medication or drug use, normal visual abilities | Binge drinking score ≥ 16 | 23 non-BD (binge drinking score ≤ 12) | Emotional recognition | Facial emotional recognition test (morphed stimuli) | Facial emotional expressions of anger, contempt, disgust, fear, happiness, and sadness | Overall emotion recognition: BD < non-BD No specific effects of emotion |
Small sample size | 82.35 |
| Lannoy et al. (2019) | 52 BD | Range 18–27 Mean 21.09 |
65.4% | No personal or family history of severe alcohol use disorder, or psychiatric disorder | At least one binge drinking episode (> 60 alcohol gr) per month Binge drinking score ≥ 16 |
42 non-BD (no binge drinking episode in the last year, binge drinking score ≤ 12) | Emotional recognition | Facial emotional recognition test (morphed stimuli) | Facial emotional expressions of anger, contempt, disgust, fear, happiness, and sadness | Recognition of fear and sadness: BD < non-BD These deficits concerned 21.15 and 15.38% of the sample, respectively |
Possible influence of impaired participants on the group results | 88.24 |
| Leganes-Fonteneau et al. (2020) | 48 participants | Students Mean 21.2 Youth Mean 15.4 |
50% | No psychological or neurological disorder, normal visual abilities | High and low BD: median split on the binge drinking score Students, median score = 15.8 Youth, median score = 8.5 |
Comparison between high and low BD | Emotional identification | Emotional identification (matching of emotional word with emotional face; congruent or incongruent) Emotional perception threshold |
Facial emotional expressions of fear, anger, happiness, surprise, sadness, and disgust | Emotion identification, fear: low BD > high BD Emotional perception Sadness: low BD < high BD Happiness: low BD < high BD |
Recruitment of two BD groups that did not have the same consumption patterns | 70.59 |
| Maurage et al. (2009) | 18 BD at time 2 | Mean 18.16 | 38.9% | No positive family history of severe alcohol use disorder, tobacco or drug use, psychiatric, medical or neurological problem, auditory impairment | Baseline: low alcohol use, no binge drinking episode Time 2: distinction between BD (> 200 alcohol gr per week) and controls |
18 non-BD (< 30 alcohol gr per week) | Emotional identification | 9-month, two assessments: Emotional valence detection task (auditory stimuli, positive or negative valence) EEG recording: event-related potentials (P1, N2, P3) |
The word “paper” pronounced with prosody of anger and happiness | After 9 months, P1, N2, P3 latencies: BD > controls | No comparison between emotional cognitive event-related potentials | 82.35 |
| Maurage et al. (2013) | 12 BD | Range 19–32 Mean 23.8 |
58.3% | No positive personal or family history of severe alcohol use disorder, medical, psychiatric, or neurological problem, drug or tobacco use, auditory impairment, left-handedness participant | Consumption of more than 50 alcohol gr per occasion, at least 3 times a week; with consumption speed 20 gr per hour | 12 non-BD (< 20 alcohol gr per occasion, < 1 per week, < 10 gr per hour) | Emotional identification | Two-alternative forced choice task (morphed stimuli: fear – anger continuum) fMRI recording, whole brain |
Auditory stimuli expressing negative affective bursts related to fear and anger | Behavioral categorization: BD < non-BD Bilateral superior temporal gyrus: BD < non-BD Right middle frontal gyrus: BD > non-BD |
Small sample size | 82.35 |
Note. All alcohol units have been converted in grams of pure ethanol, according to the number of grams per unit in each country. BD = binge drinkers; AUDIT = Alcohol Use Disorders Identification Test; DSM = Diagnostic and Statistical Manuel of mental disorders; fMRI = functional Magnetic Resonance Imaging; EEG = electroencephalogram; yo = years old.
Table 4.
Description and main results of studies evaluating emotional regulation in binge drinking.
| Authors (year) | Participants | Intervention | Comparator | Experimental design | Outcomes | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample (n) | Age | Gender ratio (% of males) | Inclusion criteria | Binge drinking criteria | Control group/variable | Processes measured | Task/scale | Stimuli | Main results | Limits | ||
| Cohen-Gilbert et al. (2017) | 23 college students | Range 18–20 Mean 18.80 |
Not reported | No MRI contraindication, neurological disorder, and use of illicit drugs. Low use of marijuana and tobacco No alcohol use before the experiment |
The number of binge drinking episodes (≥ 70 alcohol gr for boys or ≥ 56 for girls in one occasion) in the three-past month | Continuous view of binge drinking (0 – 19 BD episodes in the past three months) Contrast between emotional images (positive and negative) and neutral ones |
Impact of emotional scenes on the ability to inhibit an automatic response | Structured Clinical Interview Counseling Center Assessment of Psychological Symptoms Go/No-Go task (letters as target stimuli; emotional images as preliminary background) fMRI recording |
Positive, negative, and neutral images from the IAPS | Negative emotional background: higher binge drinking episodes related to decreased activation of the dorsolateral prefrontal cortex, dorsomedial prefrontal cortex, and anterior cingulate cortex. Positive emotional background: non-significant results |
Small sample size | 76.47 |
| Ehret et al. (2013) | 1,084 college students | Mean age of 20.1 | 37% | N/A | At least one binge drinking episode (≥ 70 alcohol gr for boys or ≥ 56 for girls) in the last month | Analyses were adjusted for gender, membership affiliation in a fraternity or sorority, and typical weekly drinking | Emotional regulation | Daily Drinking Questionnaire The Rutgers Alcohol Problems Protective Behavioral Strategies Drinking Refusal Self-Efficacy (social pressure, emotional relief, opportunistic) Drinking Motives (enhancement, social, coping, conformity) |
N/A | Greater binge drinking in participants with lower protective behavioral strategies, poor drinking refusal self-efficacy for social pressure or emotional regulation Participants with high drinking refusal self-efficacy in social and emotional contexts: protective behavioral strategies were not related to alcohol consequences |
No information on the causality | 64.71 |
| Herman et al. (2018) | 30 college students | Range 18–37 Mean 23.40 |
30% | No MRI contradiction, mental or neurological disorder, no significant impairment of vision | Binge drinking score as a continuous variable | Continuous view of binge drinking Control by comparing fearful expressions to neutral ones |
Inhibition of fear (facial expressions) Impact of fearful emotional expressions on decision-making abilities (delay discounting) |
Barratt Impulsiveness Scale Alcohol Use Questionnaire Affective Stop Signal Task (fearful and neutral facial expressions as target stimuli) Affective Delay Discounting Task (fearful and neutral facial before target trials) fMRI recording |
Emotional facial expressions of fear and neutral facial expressions | Successful inhibition of fear: higher binge drinking scores related to decreased activation in frontal and parietal brain areas Delayed reward after the fearful presentation: higher binge drinking scores related to decreased frontopolar activation |
No evaluation of socio-emotional functioning | 70.59 |
| Laghi et al. (2018) | 1,004 high-school students | Range 16–21 Mean 17.90 |
39.34% | N/A | At least one binge drinking episode (≥ 50 alcohol gr for boys or ≥ 40 for girls on one occasion) in the past two weeks | Comparison of three groups: 227 BD, 89 binge eaters, 37 participants presenting both binge behaviors | Emotion regulation | The binge eating scale Drinking quantity and frequency The Emotion Regulation Questionnaire (expression suppression and cognitive reappraisal) |
N/A | Cognitive reappraisal: no group difference Expression suppression: BD < binge eaters and participants with both binge behaviors |
No consideration of confounding variables (e.g., negative emotions) | 70.59 |
| Trojanowski et al. (2019) | 776 college students | Range 17–22 Mean 18.79 |
20.10% | N/A | At least one binge drinking episode (≥ 70 alcohol gr for boys or ≥ 56 for girls on one occasion) in the past month | Mixture modeling was used to create four groups: BD, binge eaters, both bingers, and low bingers | Emotion regulation | Eating Disorder Examination Drinking Timeline Follow-back Drinking Motives and Eating Motives Questionnaire Thinness and Restricting Expectancies Inventory UPPS-P (negative and positive urgency, lack of premeditation, lack of perseverance, sensation seeking) Difficulties in Emotion Regulation Scale Beck Depression Inventory AUDIT Quality of Life Inventory |
N/A | Depression, eating disorders, impulsivity, emotion regulation, quality of life: Low binge < binge eaters and both bingers Social and enhancement motives: BD > low binge |
No information on the causality | 82.24 |
Note. All alcohol units have been converted in grams of pure ethanol, according to the number of grams per unit in each country. BD = binge drinkers; AUDIT = Alcohol Use Disorders Identification Test; fMRI = functional Magnetic Resonance Imaging.
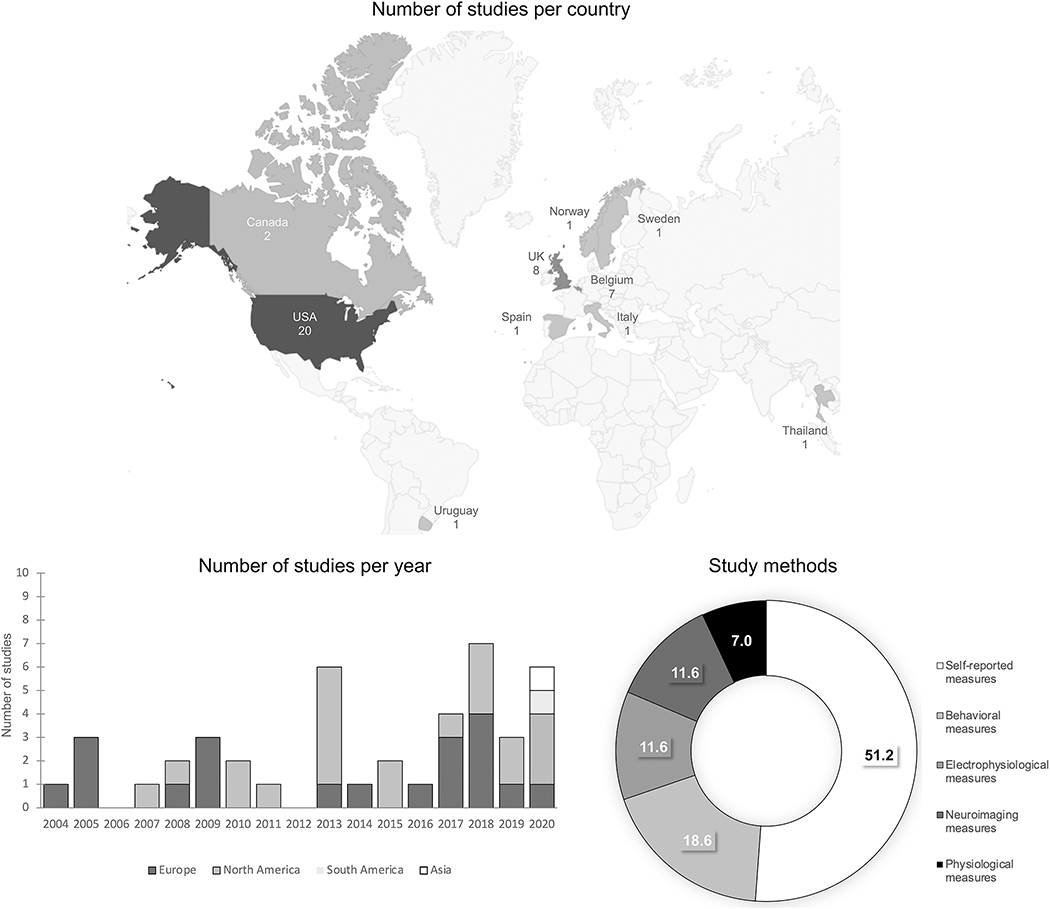
Study characteristics are illustrated in Figure 2. Regarding the geographical distribution (i.e., affiliation of the first author), the papers selected were mainly from North America (the United States of America and Canada) and Europe (the United Kingdom, Belgium, Sweden, Spain, and Italy), one from Uruguay, and one from Thailand. The yearly number of publications increased after 2012 and most studies were cross-sectional (81.4%). Among longitudinal studies (18.6%), seven were related to the evaluation of internal emotional states and its association with binge drinking and one with external emotional identification. Self-reported measures were mainly used to assess internal emotional identification and emotional regulation, whereas studies assessing external emotional appraisal/identification and emotional response used behavioral tasks or a combination of behavioral and neuroimaging/(electro)physiological measures. Only six studies (13.9%) focused on the effects of acute alcohol intake at binge-drinking level or among binge drinkers for the evaluation of emotional experience and emotional response. Finally, in 16 of the studies included (37.2%), participants had to process emotional stimuli (emotional scenes, emotional facial expressions, emotional words, or emotional voices).
Figure 2.
Description of the studies
Figure 2 illustrates the distribution of the studies included in this systematic review: the number of studies per country, the number of studies per year (studies conducted in Europe in dark grey, studies conducted in North America in light grey, studies conducted in South America in middle grey, and studies conducted in Asia in white), and the study methods (from white to black: 51.2% of studies used self-reported measures, 18.6% behavioral measures, 11.6% electrophysiological measures, 11.6% neuroimaging measures, and 7% physiological measures).
3.1. Quality Assessment
The methodological quality of the 43 studies (Table 1) was globally estimated as good according to the applied criteria (i.e., only three studies had a score below 50%). All studies had clear research objectives and the majority took the influence of confounding factors into account (e.g., depression, anxiety, drug use) (65.1%). The selected studies covered various designs and methodologies, but the vast majority proposed clear study aims and a justified experimental protocol. Nevertheless, most studies did not justify their sample size based on a priori power computation or previous experiments. Studies comparing groups with experimental measures also had small sample sizes (i.e., 60% had fewer than 25 participants per groups) with often unbalanced gender ratio (e.g., some studies focusing only on women). Moreover, we found a poor evaluation of binge drinking habits in 58.1% of the papers, few studies combining sufficient quantity (i.e., at least 56/60 gr) and timeframe (i.e., at least six months) measures to evaluate habit validly.
3.2. Emotional Processing in Binge Drinking
3.2.1. Emotional Appraisal and Identification (Table 2)
Emotional identification of internal cues.
This section comprises 19 studies that evaluated the identification of internal emotions. This subsection has been divided into two categories: 1) the current emotional states (i.e., those felt during the evaluation like stress or short-term negative/positive affect); 2) the longer-term and prolonged emotional states or mood disorders, like depression and anxiety.
First, the evaluation of current emotional states showed that binge drinking is differentially associated with loneliness, stress, and short-term affect according to age. In adolescents (13–15 years old), loneliness was related to past 30-day binge drinking (Stickley, Koyanagi, Koposov, Schwab-Stone, & Ruchkin, 2014), but in college students (18–29 years old) perceived stress predicted binge drinking two weeks later (Chen & Feeley, 2015). Regarding mood, an initial study indicated that binge drinkers (18–30 years old) reported less positive mood than non-binge drinkers (Townshend & Duka, 2005), but results were not supported in other studies among similar populations (Hartley et al., 2004; Scaife & Duka, 2009). Another study evaluating mood considered the effects of school-related stress and sex in college students (mean age: 20.83 years old). In men, school-related stress was indirectly related to binge drinking through its positive relation with depressive mood, which was directly related to binge drinking. In women, depressive mood was indirectly related to binge drinking through school stress, which was directly and negatively related to binge drinking (Pedersen, 2013). The relationship between binge drinking and mood is thus influenced by stress and differs according to sex. Interestingly, a study evaluated whether alcohol abstinence was related to negative mood in young binge drinkers (16–18 years old). The prevalence of negative mood was related to the specific number of drinks consumed on one occasion and to the total number of drinks consumed before the abstinence period (Bekman et al., 2013). Further, depressive and anxiety symptoms were more pronounced in binge drinkers than in non-binge drinkers during the early stages of abstinence (before 4 weeks) but disappeared by the end of abstinence. In general, these studies suggest that current emotional states (loneliness, stress, mood) are associated with binge drinking and with early stage of alcohol abstinence in binge drinkers, but relevant parameters including age and the type of emotional states need to be determined.
Additional studies have explored whether mood impairments were observed after drinking alcohol at binge levels (acute alcohol consumption). Rose and Grunsell (2008) showed that mood evaluation differed between binge drinkers and non-binge drinkers (18–25 years old) following both alcohol and placebo intakes. Binge drinkers showed diminished feelings of stimulation in the placebo condition, and more lightheadedness in the alcohol condition (Rose & Grunsell, 2008). The mood alterations after drinking alcohol were supported and extended by another study indicating impaired mood the morning after drinking alcohol (average of 0.12 g% breath alcohol concentration) in young people (21–24 years old) who presented at least one binge-drinking episode in the past month (Howland et al., 2010). Longitudinally, alcohol consumption affected both positive and negative mood in young adults (21–28 years old), higher alcohol-induced positive mood was associated with more frequent binge drinking and more alcohol-related problems after 18-month, and higher negative mood reduction from alcohol predicted more alcohol-related problems (Venerable & Fairbairn, 2020). Together, these findings suggest that drinking alcohol (acute intoxication) impairs mood in binge drinkers and has implications for understanding differential effects of current and long-term emotional states in binge drinking (long-term effects of alcohol).
Second, cross-sectional studies showed that binge drinking is related to depression and anxiety in different age ranges and countries. In all studies, the diagnosis of depression and anxiety was based on cutoff scores from validated questionnaires but was not formally performed by a psychiatrist. A large-scale study conducted in the United States evaluated the prevalence of binge drinking in adults (18 years old or older) with current or previous depression diagnosis. Participants with current depression or having a lifetime history of depression or anxiety exhibited more binge drinking than controls (Strine et al., 2008). In Thailand, another large-scale study conducted among adolescents (12–17 years old) supported the association between binge drinking and depression, particularly among girls (Wichaidit, Pruphetkaew, & Assanangkornchai, 2020). In South Africa, suicidal thoughts were associated with depression, anxiety, worthlessness, and binge drinking in young men (Mngoma, Ayonrinde, Fergus, Jeeves, & Jolly, 2020). In Uruguayan college students (18–30 years old), however, psychological distress (i.e., anxiety, depression, distress) was not associated with binge drinking but rather with negative alcohol consequences (Ruiz, Pilatti, & Pautassi, 2020). Longitudinal studies enable specifying antecedents of these relations. In college students (mean age of 19.9 years), depressive symptoms predicted binge drinking over one week in women (Mushquash et al., 2013). Longer-term longitudinal studies indicated that sub-clinical anxious and depressive symptoms in the general population (18–74 years old) were related to the onset of AUD but not of binge drinking at 12-month follow-up (Haynes, Farrell, Singleton, Meltzer, & Aya, 2005). However, in the transition from adolescence to adulthood (13–17 years old at baseline), a 13-year longitudinal study specified that depressive symptoms were positively associated with binge drinking (Pape & Norström, 2016). Interestingly, depressive and anxiety symptoms in binge drinkers were related to greater activations in limbic and fronto-striatal regions when viewing alcohol cues compared to other appetitive cues, supporting the relationship between depression, anxiety, and the potential reinforcement of alcohol drinking (Ewing, Filbey, Chandler, & Hutchison, 2010).
Finally, the relationships identified between internal emotional states and binge drinking did not address the specificity of these emotional states in binge drinkers relative to comparison groups (either non-binge drinkers5 or teetotalers6). For example, it has been shown that young adult binge drinkers (18–23 years old) exhibited lower trait-anxiety and depression than teetotalers (Hartley, Elsabagh, & File, 2004). Yet, the comparison between binge drinkers and teetotalers may lead to unexpected results, as teetotalers may exhibit higher levels of somatic anxiety and aggressive mood (Gil-Hernandez & Garcia-Moreno, 2016). Further, in comparison with non-binge drinkers, adolescent binge drinkers (16–18 years old) presented greater anxiety and depressive symptoms (Bekman et al., 2013), but this result was not replicated among young adults (mean age of 21.1; Nourse, Adamshick, & Stoltzfus, 2017).
Interim summary:
The investigation of internal emotional identification shows that both current emotional states as well as depression and anxiety symptoms are related to binge drinking. Overall, findings seem to indicate that loneliness is more prevalent in early adolescence, whereas high stress levels are more frequent in late adolescence. The evaluation of mood leads to consistent results in studies evaluating alcohol drinking and abstinence, showing an augmentation of positive mood immediately after alcohol drinking and the emergence of negative mood the day after drinking or when adolescents quit drinking. Finally, the relation between binge drinking and depression or anxiety is supported in several studies but also seems influenced by other alcohol outcomes, such as severe AUD or alcohol-related problems. These findings are particularly important for healthcare providers, as emotional identification of internal cues is related to binge drinking in prospective studies.
Emotional identification of external cues.
External emotional identification has been investigated in binge drinking by 12 studies using various measures. This subsection has been divided into two categories. First, external emotional appraisal, namely the ability of binge drinkers to appraise emotional situations (e.g., emotional situations or scenes), was measured in two studies. Secondly, emotional identification, the ability of binge drinkers to identify emotional states expressed by other individuals (e.g., emotional faces), was targeted in ten studies. For the sake of clarity, we first describe in each subsection the results obtained with self-reported and behavioral measures, before shifting to the studies based on brain-functioning measures.
Regarding emotional appraisal, a first study underlined that poor self-reported distress appraisal predicted alcohol-related problems in binge drinkers (18–23 years old). This relationship was explained by a propensity to drink to deal with negative emotions (Khan et al., 2018). Then, emotional appraisal was assessed through brain electrophysiological activity (electroencephalographic recordings) while participants rated positive, negative, erotic, and neutral images (Huang, Holcomb, Cruz, & Marinkovic, 2018). Results comparing binge drinkers and non-binge drinkers (18–30 years old) showed no group difference in the self-reported appraisal of emotional images. However, reduced sensitivity in event-related theta power was found in binge drinkers during emotional appraisal, both at early and later processing stages. During emotional appraisal, binge drinkers also presented attenuated differences between neutral and emotional conditions in comparison with non-binge drinkers. This indicates that attentional resources during emotional appraisal are reduced in binge drinkers, potentially limiting their ability to evaluate and react to emotional stimuli accurately (Huang et al., 2018).
Regarding the identification of others’ emotional states, recent studies present behavioral insights about the binge drinkers’ abilities to identify and recognize emotional expressions. Performing a crossmodal identification task (facial and vocal stimuli depicting anger and happiness), binge drinkers did not differ from non-binge drinkers (18–23 years old; Lannoy, Dormal, Brion, Billieux, & Maurage, 2017). However, in a more complex task, binge drinkers (18–27 years old) exhibited poorer performance than non-binge drinkers in recognizing emotional categories presented at different intensities (Lannoy et al., 2018b). This first study produced a group effect nonspecific to emotional category, whereas a second exploration in a larger sample specified that binge drinkers had impairments in recognizing fear and sadness (Lannoy et al., 2019). Individual single-case analyses have also been conducted in this larger sample to explore the percentage of binge drinkers who actually presented a clinically significant emotional recognition deficit compared to a group of matched control participants: 21.15% of binge drinkers were identified as having a deficit for fear recognition and 15.38% for sadness recognition. These studies thus illustrate that binge drinkers have overall difficulties to recognize emotions, while clinical deficits were identified in a subsample of binge drinkers. Then, comparing binge drinkers of different intensities (high and low binge-drinking score) among college students (mean age of 21.2) and youth (mean age of 15.4), another study indicated that low binge drinkers had difficulties to detect sadness whereas high binge drinkers correctly detected sadness but poorly recognized fear (Leganes-Fonteneau, Pi-Ruano & Tejero, 2019). Finally, evaluating the impact of emotional processing on memory, it has been shown that college students with binge drinking (18–20 years old) had a negative emotional recall bias. Results showed no significant effects in males but an emotional memory bias for negative words in females; i.e., higher recall of negative words, increased false alarms for negative emotional distractors, and lower recall of positive and neutral words (Carbia, Corral, Caamaño-Isorna, & Cadaveira, 2020). These findings thus support the proposal of impaired processing of external emotional contents in binge drinkers and show possible specific difficulties with negative emotions.
In addition to these results, studies have evaluated emotional identification through electrophysiological brain measures. Indeed, behavioral studies were not always able to detect subtle differences between binge drinkers and healthy controls (e.g., Lannoy et al., 2017). Electrophysiological measures thus offer a complementary observation of the brain correlates during external emotional identification. Ehlers and colleagues (2007) focused on young adults who had experienced at least one binge drinking episode or had both binge drinking and drug use histories and compared them to non-bingeing and non-drug-consuming controls (18–25 years old). Electrophysiological activity was explored during the identification of happy, sad, and neutral faces, and results suggested a reduced recruitment of attentional resources during emotional identification in young adults with a binge drinking history (i.e., decreased amplitude of the late P3 component). Young adults with binge drinking and drug history also depicted a faster latency of the early P3 component for all stimuli compared with controls, this being interpreted as a possible effect of alcohol and drug use on developmental changes in the P3. Another study (Lannoy et al., 2018c) explored emotional crossmodal integration as described above and showed differences in electrophysiological activity in binge drinkers compared to both teetotalers and non-binge drinkers (18–29 years old). In particular, greater brain activity was observed for anger processing than for happiness in binge drinkers, they also presented increased brain activity during incongruency (e.g., a happy face presented together with an angry voice). Differences in electrophysiological activity were finally observed when binge drinkers had to process vocal stimuli consisting of the emotional enunciation of a semantically neutral word. Results indicated that after nine months of binge drinking, participants (mean age of 18.17 years old at baseline) who did not present a binge drinking pattern at baseline depicted delayed latency of the ERP components related to early perceptive-attentional (P1, N2) and late decision-related (P3) processing (Maurage, Pesenti, Philippot, Joassin, & Campanella, 2009), supporting that short-term binge drinking lead to modification of brain activity during emotional identification of anger and fear.
Eventually, the occurrence of brain modifications during emotional identification has been supported by a neuroimaging study (Maurage, Bestelmeyer, Rouger, Charest, & Belin, 2013b) that used morphed auditory stimuli (fear-anger continuum). Relative to non-binge drinkers, binge drinkers (19–32 years old) presented poorer ability to categorize emotional contents at the behavioral level and disrupted brain activation during emotional identification, namely lower activation of the bilateral superior temporal gyrus and increased activation of the right middle frontal gyrus. Authors proposed that the lower temporal activation observed in bingers indicated an impaired processing of affective sounds, whereas the greater frontal activation reflected a compensatory activity. Brain responses during emotional identification (i.e., emotional faces of anger, fear, or neutral expressions) were also used to classify binge drinkers and non-binge drinkers (22–35 years old) using machine learning algorithms. Results, however, showed that emotional processes were not among the variables offering a reliable distinction between groups. Neural correlates of social cognition were conversely related to a trustworthy group classification (Gowin, Manza, Ramchandani, & Volkow, 2020).
Interim summary:
The investigation of external emotional identification highlights emotional difficulties related to binge drinking. Emotional appraisal was only documented in two studies. Results showed lower attentional resources to appraise emotional images in binge drinkers, while poor appraisal of distress was related to alcohol-related problems. Lower attentional resources to identify emotional images were also linked to binge drinking. Regarding facial expressions processing, findings highlight consistent difficulties to recognize fear, while impairments in the recognition of sadness need to be further supported. Brain activity during emotional facial expressions processing appear to be disturbed in binge drinking, but this impairment is not sufficient to distinguish binge drinkers from non-binge drinkers. In order to delineate clinical perspectives, longitudinal findings are still needed to determine whether disrupted external emotional identification is a consequence of binge drinking (alcohol’s effect on the brain) and/or a risk factor for excessive drinking.
3.2.2. Emotional Response (Table 3)
Table 3.
Description and main results of studies evaluating emotional response in binge drinking.
| Authors (year) | Participants | Intervention | Comparator | Experimental design | Outcomes | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample (n) | Age | Gender ratio (% of males) | Inclusion criteria | Binge drinking criteria | Control group/variable | Processes measured | Task/scale | Stimuli | Main results | Limits | ||
| Balodis et al. (2011) | 87 college students | Range 19–27 Mean 20.00 |
33.33% | No allergic reaction to alcohol or contraindication to drink alcohol, cardiovascular disease, or neurological disorder. No food before the experiment |
Drinking at least once per month (mean occasions/month = 6.08, SD=4.3; mean alcohol gr/occasion = 74.2) Alcohol administration: BAC level of 0.08%, fresca soda and Vodka (alcohol), Fresca soda and flattened tonic water (placebo), Fresca soda (soft); 3 glasses, 10–15 min intervals |
Contrast between stress and non-stress conditions Alcohol administration versus placebo versus soft drink |
Physiological stress level Mood |
Stress (public task) and No-stress (crossword puzzles) Cortisol and alpha-amylase Risk-taking task Profile of Mood States Mood evaluation |
N/A | Stress condition, tension, anxiety, increase in cortisol: alcohol, placebo < soft groups Risk-taking: alcohol, placebo > soft groups Risk-taking was not related to stress |
Small sample size | 64.71 |
| Connell et al. (2015) | 10 BD 9 BD with depressive symptoms |
Range 18–22 | 40% | N/A | At least one binge drinking episode (≥ 70 alcohol gr for boys or ≥ 56 for girls) in the past year | 11 non-binge drinkers (no binge drinking episode in the past year) 12 controls with depressive symptoms |
Electrophysiological emotional response | Passive viewing of neutral, positive, and negative emotional images Electrophysiological recording: event-related potentials (EPN, P3, LPP) |
Neutral, positive, and negative images from the IAPS | LPP amplitudes, negative images: BD < non-BD EPN amplitude, negative and neutral images: BD > non-BD Reduced later processing P3 and LPP, all emotional: depressed BD < non-depressed BD |
Small sample size | 64.71 |
| Hefner et al. (2013) | 72 college students | Range 21–35 Mean 21.60 |
50% | No history of alcohol-related problem, medical or psychopathological disorder No alcohol/food use before the experiment |
Alcohol use: ≥ 42 alcohol gr for boys and 28 for girls on one occasion in the last year Alcohol administration: BAC level of 0.08%, fruit juice and vodka (alcohol), fruit juice and water (placebo), fruit juice (soft); 2 glasses, 15min interval |
Alcohol administration versus placebo versus soft drink | Startle response to anxiety and fear | Shock tolerance threshold assessment Experimental task (color square cues; predicted (fear elicitation) and unpredicted (anxiety elicitation) shocks Electromyographic activity |
Electric shocks of intensity (maximum tolerance for each participant) | Startle response in predicted condition: BD < placebo, soft groups Alcohol stress-response dampening, specific to anxiety and persistent in time |
No inclusion of attentional measures | 58.82 |
| Lindgren et al. (2018) | 149 college students | Range 21–25 Mean 21.55 |
53.29% | No major medical problem or alcohol use disorder. No alcohol, drug, or food use before the experiment |
At least one binge drinking episode (≥ 70 alcohol gr for boys or ≥ 56 for girls) in the last month | Control by contrasting emotional videos (positive, negative, and neutral) | Emotional and alcohol-related responses | Implicit alcohol-related association test (alcohol excite, alcohol approach, and drinking identity) Alcohol Self-Concept Scale Mood induction (video clips) Mood evaluation after the video Alcohol taste test |
Emotional videos inducing sadness, happiness, or neutral state. | Alcohol approach or drinking identity associations: non-significant Sad mood moderated the negative relation between implicit alcohol excite associations and drinking Happy and neutral mood moderated the positive relation between implicit alcohol excite associations and drinking |
No assessment of baseline mood | 64.71 |
| Loeber & Duka (2009) | 36 moderate social drinkers | Mean 21.6 | 52.78% | Body mass index between 18 and 28, no pregnant or breastfeeding women, heavy smoker (≥ 20 cigarettes/day), dyslexia, mental or neurological disorder, drug use No illicit drugs, medication, and alcohol use before the experiment |
At least 80 alcohol gr per week (≤ 320) Alcohol administration: Alcohol dose of 0.8 g/kg, 90% v/v alcohol, tonic water, and Angostura Bitter (alcohol), tonic water and Angostura Bitter (placebo); 10×50 ml, 3 min intervals |
Control by contrasting emotional positive and negative words Alcohol administration versus placebo |
Emotional response to aversive noise Inhibition of emotional information after the auditory aversive procedure |
Abstract stimuli with eye tracking measures: occurrence of aversive (102 db, S+) or no noise (S−) after specific stimuli Instrumental training: same procedure with possibility to avoid the noise Stop Signal task Affective Go/No-Go task |
Bursts of 102 db Presentation of positive or negative words during the affective Go/No-Go task |
Avoidance in S+ trials: alcohol < placebo Stop-signal performance: alcohol < placebo Go/No-Go, positive versus negative words: alcohol < placebo Reaction time, negative words: alcohol > placebo |
The alcohol group guessed they received alcohol (compared to the placebo group) | 58.82 |
| Poncin et al. (2017) 1 | 32 BD | Range 18–30 Mean 20.88 |
59.4% | No personal or family history of substance use disorder | Consumption > 60 alcohol gr per occasion, at least 2 times a week, with consumption speed > 20 gr per hour | 23 non-BD (consumption < 20 alcohol gr per week, < 0.5 occasion per week, consumption speed < 10 gr per hour) | Emotional response to distress | Anagram solution task (soluble and insoluble anagrams) Visual analogue scale (distress) Emotion regulation (self-blame, blaming others, rumination, catastrophizing, putting into perspective, positive refocusing, positive, reappraisal, acceptance, and refocusing on planning) Self-consciousness scale |
No emotional stimuli | No difference in distress rating Anagram induced distress predicted blaming others in the whole sample Acceptance: BD < non-BD Anagram induced distress was related to rumination and self-blame in BD |
No assessment of emotional states before distress induction | 58.82 |
| Stephens et al. (2005) 2 | 9 BD | Range 19–30 Mean 21.65 |
33.3% | N/A | Binge drinking score ≥ 27 | 9 non-BD (binge drinking score ≤ 13.2) Groups were matched on age, gender, depression, anxiety, and severity of severe alcohol use disorder |
Fear conditioning | Aversive auditory procedure (63-dB intensity with low, medium, and high frequencies; low and high tones as CS+ before an aversive burst of 97-dB, 40msec) Electromyographic recording and skin conductance |
Bursts of different intensities and frequencies | Electromyographic and skin conductance: impaired fear conditioning in BD BD had reduced abilities to discriminate aversive conditioned stimuli (also in later blocks) |
Not reported | 76.47 |
Note. All alcohol units have been converted in grams of pure ethanol, according to the number of grams per unit in each country. BD = binge drinkers.
This study also evaluates emotional regulation processing
This study describes animal and human experiments, but we focused on the human research in the current review.
Emotional response has been assessed in seven studies focused on binge drinking, using various measures and emotional induction procedures. We first describe results obtained with behavioral evaluations, then synthetize the insights offered by physiological and brain data, and finally we illustrate emotional responses after drinking alcohol.
A behavioral study demonstrated the effect of mood induction by short video clips (sad, happy, and neutral mood) on specific drinking responses (i.e., implicit and explicit alcohol-related associations and effective drinking in a taste test) in binge drinkers (21–25 years old; Lindgren et al., 2018). A positive relation was found between alcohol depressed associations (i.e., low excitement) and alcohol drinking and was stronger after the induction of sadness, whereas the link between alcohol excitement and alcohol drinking was stronger after the induction of happy or neutral mood (Lindgren et al., 2018). Beyond the potential effects of alcohol consumption in mood described earlier (i.e., drinking alcohol leads to negative mood), this study suggested that a specific emotional situation may influence drinking-related responses in binge drinkers (e.g., sad mood leads to drinking alcohol). However, these results only modestly predicted effective drinking in the taste test. Whereas this study showed the effects of both positive and negative emotional induction, another study explored distress induction using the anagram solution task (requiring participants to resolve soluble and insoluble anagrams); however, binge drinkers (18–30 years old) did not present higher self-reported distress than non-binge drinkers after the task (Poncin, Vermeulen, & de Timary, 2017).
Physiological measures (e.g., body changes related to a stimulation) presents an additional perspective on induced emotional responses. In one study, binge drinkers and non-binge drinkers (19–30 years old) were conditioned to an aversive auditory procedure (i.e., fear conditioning; a conditioned stimulus notifying the appearance of an aversive stimuli) while electromyographic data and skin conductance were recorded (Stephens et al., 2005). Binge drinkers exhibited impaired fear conditioning as they did not present 1) the classical increased amplitude of the startle response when the conditioned stimulus (aversive stimulus) was presented before the unpleasant noise related to fear conditioning; or 2) an increased galvanic skin response for the conditioned stimulus (Stephens et al., 2005). Second, electrophysiological brain responses to the passive viewing of neutral, positive, and negative emotional images were aimed at assessing the differential and combined effects of binge drinking and depressive symptoms (Connell, Patton, & McKillop, 2015). Binge drinkers (18–22 years old) had attenuated attentional and higher-order cognitive resources to negative emotional stimuli (i.e., lower Late Positive Potential (LPP) amplitude). The combination of binge drinking and depressive symptoms also showed an increased early attentional engagement (Early Posterior Negativity) for negative and neutral images, but a reduced later processing (P3 and LPP) for all emotional categories (Connell et al., 2015). Thus, physiological data support an overall blunted emotional response in binge drinkers.
Support of these results come from investigations of emotional response after drinking alcohol at binge levels. A study using an auditory aversive conditioned procedure revealed that after drinking alcohol at binge levels, social drinkers (mean age of 21.6 years old) presented less avoidance of aversive noise than participants who were not subjected to binge drinking levels acutely (Loeber & Duka, 2009). This study supported the presence of an impaired emotional response when binge alcohol drinking, leading to a decreased avoidance of negative stimuli. Beyond the discrimination noise procedure, acute drinkers had poorer behavioral control. Indeed, during prepotent response inhibition (i.e., control of automatic behavioral responses) of affective words, acute drinkers exhibited more commission errors in response to positive words and slower reaction times in response to negative words (Loeber & Duka, 2009), suggesting low control towards positive stimuli and slow (reduced) response towards negative stimuli.
Finally, studies have evaluated how the expectancies related to alcohol may alter emotional responses following stress in college students. In an initial study (Balodis, Wynne-Edwards, & Olmstead, 2011), three groups (19–27 years old) were compared: drinkers with alcohol intoxication at binge levels, placebo drinkers (who believed they were drinking alcohol) and sobers (knowing they were not drinking alcohol). Physiological and self-reported emotional responses were evaluated following two conditions: stress (speech task in public) and non-stress. Exposure to stress in the alcohol and placebo groups induced less tension and anxiety and a smaller increase in cortisol compared to the placebo, demonstrating that alcohol expectancies altered subjective and physiological responses to stress (Balodis et al., 2011). This reduced physiological response to stress after drinking alcohol at binge levels was also supported by a study evaluating the specific alcohol stress-response dampening (i.e., reduction of the stress reaction after drinking alcohol; Hefner, Moberg, Hachiya, & Curtin, 2013). In this study, participants (21–35 years old) performed a task while receiving predictable and unpredictable electric shocks (induction of fear and anxiety). The startle response was reduced in acute binge drinking in the unpredictable condition (anxiety elicitation); however, this result was observed in participants who actually drank alcohol but not in the placebo group, demonstrating a genuine alcohol-related dampening effect to uncertain threat.
Interim summary:
The investigation of emotional response highlights consistent results supporting that binge drinkers exhibit a attenuated emotional responses, shown by 1) reduced startle in response to fear; 2) low electrophysiological attentional engagement in response to negative images, and 3) less avoidance of aversive stimuli. Most results are observed with physiological data, behavioral evidence of impaired emotional response, and its consequences (e.g., inappropriate reactions such as initiation of fight or drinking alcohol excessively) is thus lacking. Longitudinal data are needed to understand the causal relationship between impaired emotional response and binge drinking.
3.2.3. Emotional Regulation (Table 4)
Emotional regulation was explored in five studies: three measured global emotion regulation strategies through self-reported measures, and two evaluated how the presentation of emotional stimuli affects cognitive abilities (conceptualized as a specific regulatory strategy) in binge drinking. A further study (Poncin et al. 2017) described earlier is also relevant to explicating emotion regulation strategies.
A study targeting emotion regulation strategies demonstrated that the distress induced by an insoluble anagram task was associated with self-reported maladaptive emotion regulation strategies (rumination and self-blame) among binge drinkers but not non-binge drinkers (Poncin et al., 2017). However, with no previous distress induction, a study evaluating cognitive reappraisal and expressive suppression as adaptive and maladaptive emotion regulation strategies respectively, showed that only adolescent binge drinkers (16–21 years old) who also presented binge eating had poor emotion regulation (Laghi, Liga, & Pompili, 2018). This observation was supported among young adults (17–22 years old), results showing that emotion regulation strategies did not differ between binge drinkers and non-binge drinkers (Trojanowski, 2019). Finally, when considering emotional regulation in the specific context of alcohol drinking, college students who had lower protective behavioral strategies (i.e., alcohol-related regulation strategies such as slowing the consumption speed) and poor drinking refusal self-efficacy reported higher binge drinking. The relationship between alcohol-related problems and emotional distress was mediated by coping-related drinking motivations (Ehret, Ghaidarov, & LaBrie, 2013), also reported previously (Khan et al., 2018).
Combining behavioral and neuroimaging measures, two studies evaluated whether the presentation of emotional stimuli can affect control ability (inhibition of automatic responses) and its relation to binge drinking (Cohen-Gilbert et al., 2017; Herman, Critchley, & Duka, 2018). Presenting positive and negative emotional scenes before inhibition targets, Cohen-Gilbert and colleagues (2017) showed that, among college students (19–20 years old), a higher number of binge-drinking episodes in the past three months was related to lower brain activations in the dorsolateral prefrontal cortex, dorsomedial prefrontal cortex, and anterior cingulate cortex when viewing negative emotional backgrounds. No association was found with positive versus neutral scenes. Using neutral and fear facial expressions, Herman and colleagues (2018) showed that higher binge drinking scores were related to lower activations in frontal and parietal brain areas during successful fear inhibition among a sample of adults (18–37 years old), suggesting that binge drinkers would be less distracted by negative emotional backgrounds, which actually leads to an increase of their cognitive abilities in a fearful context. In that sense, these results also support the inappropriate (reduced) emotional response in binge drinkers (see the above section). This study also evaluated the relevance of emotions on the ability to differ a reward (i.e., delay discounting task) and showed that higher binge drinking scores were related to lower frontopolar activations during delayed reward decision after the presentation of fear. Although these studies go beyond the regulation of emotional response per se, they suggest that the indirect processing of emotional stimuli before or during cognitive processing may affect binge drinkers’ abilities to control behavioral reactions (Cohen-Gilbert et al., 2017; Herman et al., 2018).
Interim summary:
The evaluation of emotional regulation has led to inconsistent results across studies, making it difficult to specify associations between emotional regulation disturbances and binge drinking. Studies using self-reported measures highlighted mixed results, with possible emotion regulation difficulties in specific contexts (e.g., after the insoluble anagram task or when emotional coping is needed). Studies using neuroimaging measures support the possibility of binge-related brain modifications related to regulatory processes affected by the processing of emotional stimuli.
4. Discussion
This systematic review describes the current state of emotion research in binge drinking. Emotional deficits have been largely documented in severe AUD, where they are considered a key factor explaining alcohol-related problems and relapse (e.g., Bora & Zorlu, 2017; Le Berre, 2019). Bingeing is a harmful drinking pattern, widespread in adolescence, with increased prevalence in young adults, possibly evolving toward AUD. The contribution of emotion research is, however, less straightforward in binge drinking than in severe AUD, only suggesting difficulties in specific emotional processes, such as impaired emotional response and reactivity associated with alcohol’s effects on the amygdala (Stephens & Duka, 2008). To offer a systematic exploration of emotional processes in binge drinking, we capitalized on the theoretical proposal of Phillips et al. (2003a), referring to three steps of emotional processing: emotional appraisal and identification (that has been split into two subsections related to internal and external emotional states), emotional response, and emotional regulation.
This review supports the proposal of impaired emotional processes in binge drinking by centrally showing that 1) internal emotional states as well as symptoms of depression and anxiety are associated with or predict alcohol use and binge drinking, and that binge drinkers have difficulties to identify emotions expressed by others; 2) binge drinkers also present a reduced behavioral and cerebral response to emotional stimulations or situations, but 3) binge drinkers do not appear to have impairments to regulate emotional responses. We have identified the need for longitudinal studies controlling for alcohol-related outcomes to advance our knowledge, but our systematic evaluation already has implications for fundamental research and clinical applications. Notably, these findings underline the role of emotional processes, which should be integrated in the current models of binge drinking. In the introduction, we noted that most proposals consider that addiction is related to heightened appetitive cues reactivity, reward seeking and poor executive functioning contributing to a poor control of alcohol-related behaviors (e.g., Lannoy et al., 2014; Voon et al., 2020). Recent systematic reviews focusing on binge drinking support this proposal (Carbia et al., 2018a; Courtney, Li, & Tapert, 2019; Lees et al., 2019). Here, we note the role of emotion with the model of Phillips et al. (2003a), which purports several conceptual considerations. Referring to dual-process models, emotion could be considered as part of System A and drives automatic behaviors (Steinberg, 2007; Volman, Roelofs, Koch, Verhagen, & Toni, 2011). However, this review clearly underlines that emotional processing does not present a total overlap with automatic processing, as it also involves regulatory processes. An alternative addiction model considering emotional processing is the triadic neurocognitive model, which postulates the existence of a third system modulating the interaction between Systems A and B, i.e., the insular cortex. According to this proposal (Noël, Brevers, & Bechara, 2013), the insula converts interoceptive information into subjective experiences (e.g., physiological sensations), which activates System A and reduces the cognitive resources of System B. The role of the insula is widely described in emotional processing and related to the identification of and response to emotional stimuli (Phillips et al., 2003a; Wilcox, Pommy, & Adinoff, 2016). Nevertheless, with regards to the model of Phillips et al. (2003a), emotional processing also requires cognitive abilities that are, in this model, encompassed in System B. These conceptual issues may be answered by considering a recent position that goes beyond dual or triadic models and refers to unitary models, in which all cognitive and emotional processes are conceptualized into a functionally integrated system. Various evidence has supported this unitary approach (Hommel & Wiers, 2017; Melnikoff & Bargh, 2018; Murphy et al., 2003; Pessoa, 2017), showing that the nature of psychological processes is composed of several features. This integrated proposal also comports with the emotion model (Phillips et al., 2003a), showing that emotional processing encompasses both automatic and cognitive processes. Indeed, the theoretical background approached in this review does not only consider emotion as a situation driving alcohol-related behaviors, but also as a multidimensional process bringing strong insights to the study of binge drinking and AUD.
In particular, the internal emotional state has a role in binge drinking (e.g., Bekman et al., 2013; Mushquash et al., 2013; Pape & Norström, 2016). Although the current literature does not inform about the ability to identify one’s own emotional states (i.e., detecting and labeling the emotions felt), it does show a consistent relationship between excessive drinking and the presence of symptoms of depression and anxiety. Although this association is well-known to researchers and clinicians working with patients presenting severe AUD (Boden & Fergusson, 2011), it remained uncertain in non-AUD binge drinkers. Whereas binge drinking is often considered as driven by positive emotions (Kuntsche, Knibbe, Gmel, & Engels, 2005), this literature review extends this consideration to negative emotions, the “dark side” of excessive drinking (Koob, 2015). Binge drinking was related to loneliness in adolescence and to depressive symptoms in the transition from adolescence to adulthood and during adulthood. It is thus possible that emotional dysregulation contributes to the escalation of binge drinking in late adolescence, when alcohol is readily available (Rowland et al., 2016) and positive expectancies abound (e.g., drinking to deal with negative emotions). Considering mood evaluation, available studies showed promising results, but no convincing conclusion can be drawn due to the limited number of studies and the inconsistent mood evaluation across them (see Table 2). Future studies should further the understanding of acute binge drinking effects on mood in order to determine how binge drinking may affect emotional states and how acute effects may predispose to potential chronic effects (e.g., Howland et al., 2010; Venerable& Fairbairn, 2020). One possibility is that the relationship between negative emotional states and future alcohol abuse may be induced by the repetition of excessive drinking episodes, which could support the late appearance of this association. Importantly, this association could also be explained by an impaired emotional processing, possibly leading to negative affect and social difficulties. Indeed, the current systematic review focused on a model of emotional processing (Phillips et al., 2003a), which enabled an understanding of the presence of disruptions related to external emotional identification and emotional responses in binge drinking. In contrast to this clear result, impairments in emotional regulation in both general and drinking contexts remain to be fully investigated.
Regarding the appraisal and identification of emotional states, several difficulties have been reported in young adult binge drinkers. Lower percentage of correct emotional identification were observed among binge drinkers in complex experimental paradigms, whereas increased brain activity was found in less demanding tasks (Lannoy et al., 2018c; Maurage et al., 2013b), suggesting that binge drinkers could show neural compensate for their emotional processing difficulties. With measures of electrophysiological activity, consistent results emphasized that binge drinkers have attentional difficulties in focusing on and efficiently appraising emotional content (e.g., Ehlers et al., 2007; Huang et al., 2017), which consequently hampers emotional identification (Lannoy et al., 2019). These findings constitute initial insight into the scope and limit of emotional difficulties in binge drinkers, which warrants considered at the clinical level, as a poor processing of emotional content has been associated with psychiatric symptoms (Phillips et al., 2003b).
Variability in emotional valences and intensities themselves and in the context of binge drinking need explication. The current literature indicates difficulties related to negative emotional processing (Huang et al., 2018; Lannoy et al., 2018c; Leganes-Fonteneau et al., 2019) but stops short of providing systematic evaluations and comparisons across emotional conditions. Indeed, although a tendency emerges for deficits in fear recognition (Lannoy et al., 2019; Leganes-Fonteneau et al., 2019; Stephens & Duka, 2008), it remains unclear whether the recognition of specific emotions is affected in binge drinking. Further, the production of emotional responses is impaired in binge drinkers, in particular in negative emotional context (Connell et al., 2015; Hefner et al., 2013; Herman et al., 2018; Loeber & Duka, 2009; Stephens et al., 2005). Such impairment is described by attenuated emotional response, both after acute alcohol consumption and among people without current intoxication but presenting a binge drinking pattern. Nevertheless, considering the model of Phillips et al. (2003a), it is unclear whether this altered response is the result of poor emotional appraisal and identification (e.g., reduced emotional response due to a misperception of the emotional significance) or a genuine emotional response impairment (e.g., inability to generate an appropriate emotional response). The consideration of this theoretical model emphasizes the emotional processes that are disturbed in binge drinking but also calls for an in-depth investigation of emotional processing to address the nature of these difficulties. Although some studies implicate the need to consider emotional regulation in binge drinking, despite of the relevance of this process in severe AUD (Petit et al., 2015), experimental results are lacking. Indeed, neuroimaging findings showed decreased brain activations during negative emotion-related inhibition (Cohen-Gilbert et al., 2017; Herman et al., 2018), but no finding supports that this deficiency is directly related to poor regulation of the emotional response, as proposed in emotional processing (Phillips et al., 2003a). Additionally, results based on self-reported measures do not demonstrate a specific impairment to regulate one’s own emotions in binge drinking (Laghi et al., 2018; Trojanowski, 2019), difficulties of binge drinkers being only related to specific contexts (e.g., Ehret et al., 2013; Poncin et al., 2017). Upcoming studies should thus further explore emotion regulation by considering the role of additional factors such as population heterogeneity and personality traits (Gierski et al., 2017; Maurage, Timary, & D’Hondt, 2017).
Taken together, these findings indicate that binge drinkers exhibit poor identification of emotional and social cues as well as poor emotional responses. These processes constitute risk factors for the perpetuation of excessive alcohol use (e.g., Rupp, Derntl, Osthaus, Kemmler, & Fleischhacker, 2017) and may lead to negative emotional states, reinforcing the vicious circle of AUD (Boden & Fergusson, 2011). Currently lacking are prospective evaluations that would enable investigation of whether disrupted emotional identification and emotional response in binge drinkers result from the effects of alcohol on brain structure and function and whether identified difficulties constitute risk factors for excessive drinking, as suggested in severe AUD (Bora & Zorlu, 2017; Le Berre, 2019; Rupp et al., 2017). In binge drinking, some proposals support the position that emotional difficulties are the result of drinking alcohol (e.g., Stephens & Duka, 2008), whereas others note modified frontostriatal and frontolimbic activations during emotional processing as a vulnerability (e.g., Heitzeg, Nigg, Yau, Zubieta, & Zucker, 2008).
Although emotional processes mature early (Steinberg, 2007), most studies exploring emotional abilities focused on late adolescence, 18 years old or older, and were cross-sectional, limiting observation of a potential interaction among age, development, and alcohol. Furthermore, in patients with severe AUD, low awareness of emotional deficits and high level of alexithymia have been recognized and contribute to explain family, social, and professional troubles as well as difficulties in initiating and maintaining abstinence (Le Berre, Fama, & Sullivan, 2017; Le Berre & Sullivan, 2016; Le Berre, 2019). Beyond the importance to explore emotional processing with controlled experimental tools, these results thus extend the interest to explore awareness of emotional difficulties and ability to feel and identify ones’ own emotions in binge drinkers. Investigations could also target overall processes of social cognition such as theory of mind (Gowin et al., 2020), which intersects with emotional recognition and hazardous drinking (Le Berre).
This narrative review highlights the relevance of emotion research in binge drinking and recognizes the limitations to determining causal relationships between behavioral outcomes and drinking. First, it is unfortunate that a poor definition/evaluation of binge-drinking habits was found in half of the papers reviewed. This might lead to biases in the current results and calls for future studies with a well-defined binge drinking conceptualization (e.g., Maurage et al., 2020b). Second, whereas we did not limit inclusion criteria according to age, all but one study identified in our searches were conducted in adolescents or young adults, leaving a gap in the relationship between emotional processing and binge drinking in older adulthood as emotional processing changes with age (Wirth et al., 2017). Indeed, recent studies highlight the increase of binge drinking in adulthood (Hingson, Zha, & White, 2017), especially among older populations (age 60+) (Breslow, Castle, Chen, & Graubard, 2017; Han, Moore, Ferris, & Palamar, 2019). Exploring the role of emotional processing in transition towards late life binging thus appears crucial. This review emphasizes the need to delineate and differentiate emotional processing associated with different ages, sex (Carbia et al., 2020; Erol & Karpyak, 2015), and drinking (Mushquash et al., 2013).
Supplementary Material
Acknowledgments
Role of funding sources
Séverine Lannoy is funded by the Belgian American Educational Foundation (BAEF), Séverine Lannoy and Edith V. Sullivan are supported by the National Institute on Alcohol Abuse and Alcoholism (grants NIAAA AA010723, AA017923, AA017347, AA021697), Carina Carbia has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie (No.754535), Eduardo López-Caneda is supported by the Portuguese Foundation for Science and Technology (PTDC/PSI-ESP/28672/2017), and Pierre Maurage was funded by the National Fund for Scientific Research (FRS-FNRS). However, these funds had no role in the study conceptualization, inclusion and interpretation of the literature review, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Conflict of Interest
None.
National Institute on Alcohol Abuse and Alcoholism
World Health Organization
European School Survey Project on Alcohol and Other Drugs
The Substance Abuse and Mental Health Services Administration
Individuals with regular drinking habits but without binge drinking pattern
Non-drinking participants
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agoglia AE, & Herman MA (2018). The center of the emotional universe: Alcohol, stress, and CRF1 amygdala circuitry. Alcohol, 72, 61–73. 10.1016/j.alcohol.2018.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, & Naassila M (2013). Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology, 67, 521–531. 10.1016/j.neuropharm.2012.12.007 [DOI] [PubMed] [Google Scholar]
- Balodis IM, Wynne-Edwards KE, & Olmstead MC (2011). The stress–response-dampening effects of placebo. Hormones and Behavior, 59(4), 465–472. 10.1016/j.yhbeh.2011.01.004 [DOI] [PubMed] [Google Scholar]
- Bekman NM, Winward JL, Lau LL, Wagner CC, & Brown SA (2013). The Impact of Adolescent Binge Drinking and Sustained Abstinence on Affective State. Alcoholism: Clinical and Experimental Research, 37(8), 1432–1439. 10.1111/acer.12096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black MH, Chen NTM, Iyer KK, Lipp OV, Bölte S, Falkmer M, … Girdler S (2017). Mechanisms of facial emotion recognition in autism spectrum disorders: Insights from eye tracking and electroencephalography. Neuroscience & Biobehavioral Reviews, 80, 488–515. 10.1016/j.neubiorev.2017.06.016 [DOI] [PubMed] [Google Scholar]
- Blanco-Ramos J, Cadaveira F, Folgueira-Ares R, Corral M, & Rodríguez Holguín S (2019). Electrophysiological correlates of an alcohol-cued Go/NoGo task: a dual-process approach to binge drinking in university students. International journal of environmental research and public health, 16(22), 4550. 10.3390/ijerph16224550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden JM, & Fergusson DM (2011). Alcohol and depression: Alcohol and depression. Addiction, 106(5), 906–914. 10.1111/j.1360-0443.2010.03351.x [DOI] [PubMed] [Google Scholar]
- Bora E, & Zorlu N (2017). Social cognition in alcohol use disorder: A meta-analysis: Social cognition in AUD. Addiction, 112(1), 40–48. 10.1111/add.13486 [DOI] [PubMed] [Google Scholar]
- Breslow RA, Castle IP, Chen CM, & Graubard BI (2017). Trends in Alcohol Consumption Among Older Americans: National Health Interview Surveys, 1997 to 2014. Alcoholism: Clinical and Experimental Research, 41(5), 976–986. 10.1111/acer.13365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Welsh RC, Berridge KC, & Liberzon I (2006). Neural correlates of social and nonsocial emotions: An fMRI study. NeuroImage, 31(1), 397–409. 10.1016/j.neuroimage.2005.11.027 [DOI] [PubMed] [Google Scholar]
- Caparelli EC, Ross TJ, Gu H, Liang X, Stein EA, & Yang Y (2017). Graph theory reveals amygdala modules consistent with its anatomical subdivisions. Scientific Reports, 7(1), 14392. 10.1038/s41598-017-14613-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbia C, Corral M, Caamaño-Isorna F, & Cadaveira F (2020). Emotional memory bias in binge drinking women. Drug and Alcohol Dependence, 209, 107888. 10.1016/j.drugalcdep.2020.107888 [DOI] [PubMed] [Google Scholar]
- Carbia C, Corral M, Doallo S, & Caamaño-Isorna F (2018b). The dual-process model in young adults with a consistent binge drinking trajectory into adulthood. Drug and Alcohol Dependence, 186, 113–119. 10.1016/j.drugalcdep.2018.01.023 [DOI] [PubMed] [Google Scholar]
- Carbia C, López-Caneda E, Corral M, & Cadaveira F (2018a). A systematic review of neuropsychological studies involving young binge drinkers. Neuroscience & Biobehavioral Reviews, 90, 332–349. 10.1016/j.neubiorev.2018.04.013 [DOI] [PubMed] [Google Scholar]
- Casey BJ, & Jones RM (2010). Neurobiology of the adolescent brain and behavior: Implications for substance use disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 49(12), 1189–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos-Ryan N, Rubia K, & Conrod PJ (2011). Response Inhibition and Reward Response Bias Mediate the Predictive Relationships Between Impulsivity and Sensation Seeking and Common and Unique Variance in Conduct Disorder and Substance Misuse: Disinhibition and externalizing problems in adolescence. Alcoholism: Clinical and Experimental Research, 35(1), 140–155. 10.1111/j.1530-0277.2010.01331.x [DOI] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality (2016). Key substance use and mental health indicators in the United States: Results from the 2015 National Survey on Drug Use and Health (HHS Publication No. SMA 16–4984, NSDUH Series H-51). Retrieved from http://www.samhsa.gov/data/
- Chen Y, & Feeley TH (2015). Predicting Binge Drinking in College Students: Rational Beliefs, Stress, or Loneliness? Journal of Drug Education, 45, 133–155. 10.1177/0047237916639812 [DOI] [PubMed] [Google Scholar]
- Cohen-Gilbert JE, Nickerson LD, Sneider JT, Oot EN, Seraikas AM, Rohan ML, & Silveri MM (2017). College Binge Drinking Associated with Decreased Frontal Activation to Negative Emotional Distractors during Inhibitory Control. Frontiers in Psychology, 8. 10.3389/fpsyg.2017.01650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell AM, Patton E, & McKillop H (2015). Binge drinking, depression, and electrocortical responses to emotional images. Psychology of Addictive Behaviors, 29(3), 673–682. 10.1037/adb0000071 [DOI] [PubMed] [Google Scholar]
- Courtney KE, Li I, & Tapert SF (2019). The effect of alcohol use on neuroimaging correlates of cognitive and emotional processing in human adolescence. Neuropsychology, 33(6), 781–794. 10.1037/neu0000555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, & Polich J (2009). Binge drinking in young adults: Data, definitions, and determinants. Psychological Bulletin, 135(1), 142–156. 10.1037/a0014414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, & Brumback T (2017). The Burden of Binge and Heavy Drinking on the Brain: Effects on Adolescent and Young Adult Neural Structure and Function. Frontiers in Psychology, 8. 10.3389/fpsyg.2017.01111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, Niv Y, Dayan P, 2005. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nat. Neurosci 8, 1704–1711. 10.1038/nn1560 [DOI] [PubMed] [Google Scholar]
- Downes MJ, Brennan ML, Williams HC, & Dean RS (2016). Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open, 6(12), e011458. 10.1136/bmjopen-2016-011458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E, Finnerman G, Gilder D, Lau P, & Criado J (2007). P3 components and adolescent binge drinking in Southwest California Indians. Neurotoxicology and Teratology, 29(1), 153–163. 10.1016/j.ntt.2006.11.013 [DOI] [PubMed] [Google Scholar]
- Ehret PJ, Ghaidarov TM, & LaBrie JW (2013). Can you say no? Examining the relationship between drinking refusal self-efficacy and protective behavioral strategy use on alcohol outcomes. Addictive Behaviors, 38(4), 1898–1904. 10.1016/j.addbeh.2012.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsayed NM, Kim MJ, Fields KM, Olvera RL, Hariri AR, & Williamson DE (2018). Trajectories of Alcohol Initiation and Use During Adolescence: The Role of Stress and Amygdala Reactivity. Journal of the American Academy of Child & Adolescent Psychiatry, 57(8), 550–560. 10.1016/j.jaac.2018.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erol A, & Karpyak VM (2015). Sex and gender-related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug and Alcohol Dependence, 156, 1–13. 10.1016/j.drugalcdep.2015.08.023 [DOI] [PubMed] [Google Scholar]
- Esperidião-Antonio V, Majeski-Colombo M, Toledo-Monteverde D, Moraes-Martins G, Fernandes JJ, Bauchiglioni de Assis M, … Siqueira-Batista R (2017). Neurobiology of emotions: An update. International Review of Psychiatry, 29(3), 293–307. 10.1080/09540261.2017.1285983 [DOI] [PubMed] [Google Scholar]
- Ewing SW, Filbey FM, Chandler LD, & Hutchison KE (2010). Exploring the Relationship Between Depressive and Anxiety Symptoms and Neuronal Response to Alcohol Cues. Alcoholism: Clinical and Experimental Research, 34(3), 396–403. 10.1111/j.1530-0277.2009.01104.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierski F, Benzerouk F, De Wever E, Duka T, Kaladjian A, Quaglino V, & Naassila M (2017). Cloninger’s Temperament and Character Dimensions of Personality and Binge Drinking Among College Students. Alcoholism: Clinical and Experimental Research, 41, 1970–1979. 10.1111/acer.13497 [DOI] [PubMed] [Google Scholar]
- Gil-Hernandez S, & Garcia-Moreno LM (2016). Executive performance and dysexecutive symptoms in binge drinking adolescents. Alcohol, 51, 79–87. 10.1016/j.alcohol.2016.01.003 [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, & Volkow ND (2011). Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nature Reviews Neuroscience, 12(11), 652–669. 10.1038/nrn3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowin JL, Manza P, Ramchandani VA, & Volkow ND (2020). Neuropsychosocial markers of binge drinking in young adults. Molecular Psychiatry. 10.1038/s41380-020-0771-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton AN, Adolphs R, Tyszka JM, & O’Doherty JP (2007). Contributions of the Amygdala to Reward Expectancy and Choice Signals in Human Prefrontal Cortex. Neuron, 55(4), 545–555. 10.1016/j.neuron.2007.07.022 [DOI] [PubMed] [Google Scholar]
- Han BH, Moore AA, Ferris R, & Palamar JJ (2019). Binge Drinking Among Older Adults in the United States, 2015 to 2017. Journal of the American Geriatrics Society 67(10), 2139–2144. 10.1111/jgs.16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley DE, Elsabagh S, & File SE (2004). Binge drinking and sex: Effects on mood and cognitive function in healthy young volunteers. Pharmacology Biochemistry and Behavior, 78(3), 611–619. 10.1016/j.pbb.2004.04.027 [DOI] [PubMed] [Google Scholar]
- Haynes JC, Farrell M, Singleton N, Meltzer H, & Aya RA (2005). Alcohol consumption as a risk factor for anxiety and depression: results from the longitudinal follow-up of the National Psychiatric Morbidity Survey. British Journal of Psychiatry,187, 544–551. 10.1192/bjp.187.6.544 [DOI] [PubMed] [Google Scholar]
- Hefner KR, Moberg CA, Hachiya LY, & Curtin JJ (2013). Alcohol stress response dampening during imminent versus distal, uncertain threat. Journal of Abnormal Psychology, 122(3), 756–769. 10.1037/a0033407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg MM, Nigg JT, Yau W-YW, Zubieta J-K, & Zucker RA (2008). Affective Circuitry and Risk for Alcoholism in Late Adolescence: Differences in Frontostriatal Responses Between Vulnerable and Resilient Children of Alcoholic Parents. Alcoholism: Clinical and Experimental Research, 32(3), 414–426. 10.1111/j.1530-0277.2007.00605.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman AM, Critchley HD, & Duka T (2018). Binge drinking is associated with attenuated frontal and parietal activation during successful response inhibition in fearful context. European Journal of Neuroscience, 50, 2297–2310. 10.1111/ejn.14108 [DOI] [PubMed] [Google Scholar]
- Hingson RW, Zha W, & White AM (2017). Drinking Beyond the Binge Threshold: Predictors, Consequences, and Changes in the U.S. American Journal of Preventive Medicine, 52(6), 717–727. https://doi.org?10.1016/j.amepre.2017.02.014. [DOI] [PubMed] [Google Scholar]
- Hommel B, & Wiers RW (2017). Towards a Unitary Approach to Human Action Control. Trends in Cognitive Sciences, 21(12), 940–949. 10.1016/j.tics.2017.09.009 [DOI] [PubMed] [Google Scholar]
- Howland J, Rohsenow DJ, Greece JA, Littlefield CA, Almeida A, Heeren T, … Hermos J (2010). The effects of binge drinking on college students’ next-day academic test-taking performance and mood state: Binge drinking and academic performance. Addiction, 105(4), 655–665. 10.1111/j.1360-0443.2009.02880.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Holcomb LA, Cruz SM, & Marinkovic K (2018). Altered oscillatory brain dynamics of emotional processing in young binge drinkers. Cognitive, Affective, & Behavioral Neuroscience, 18, 43–57. 10.3758/s13415-017-0551-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AJ, Pedrelli P, Shapero BG, Fisher L, Nyer M, Farabaugh AI, & MacPherson L (2018). The Association between Distress Tolerance and Alcohol Related Problems: The Pathway of Drinking to Cope. Substance Use & Misuse, 53(13), 2199–2209. 10.1080/10826084.2018.1464027 [DOI] [PubMed] [Google Scholar]
- Koob GF (2015). The dark side of emotion: The addiction perspective. European Journal of Pharmacology, 753, 73–87. 10.1016/j.ejphar.2014.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsche E, Knibbe R, Gmel G, & Engels R (2005). Why do young people drink? A review of drinking motives. Clinical Psychology Review, 25(7), 841–861. 10.1016/j.cpr.2005.06.002 [DOI] [PubMed] [Google Scholar]
- Kraus L, Guttormsson U, Leifman H, Arpa S, Molinaro S, Monshouwer K, et al. (2016). ESPAD Report 2015. Results from the European School Survey Project on Alcohol and Other Drugs. Lisbon: European Monitoring Centre for Drugs and Drug Addiction and the European School Survey Project on Alcohol and Other Drugs. Available online at: http://www.espad.org/sites/espad.org/files/ESPAD_report_2015.pdf [Google Scholar]
- Laghi F, Liga F, & Pompili S (2018). Adolescents who binge eat and drink: The role of emotion regulation. Journal of Addictive Diseases, 37(1–2), 77–86. 10.1080/10550887.2018.1553458 [DOI] [PubMed] [Google Scholar]
- Lannoy S, Billieux J, & Maurage P (2014). Beyond Inhibition: A Dual-Process Perspective to Renew the Exploration of Binge Drinking. Frontiers in Human Neuroscience, 8. 10.3389/fnhum.2014.00405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannoy S, Benzerouk F, Maurage P, Barrière S, Billieux J, Naassila M, … Gierski F (2019). Disrupted Fear and Sadness Recognition in Binge Drinking: A Combined Group and Individual Analysis. Alcoholism: Clinical and Experimental Research, 43, 1978–1985. 10.1111/acer.14151 [DOI] [PubMed] [Google Scholar]
- Lannoy S, D’Hondt F, Dormal V, Blanco M, Brion M, Billieux J, … Maurage P (2018c). Electrophysiological correlates of emotional crossmodal processing in binge drinking. Cognitive, Affective, & Behavioral Neuroscience, 18(6), 1076–1088. 10.3758/s13415-018-0623-3 [DOI] [PubMed] [Google Scholar]
- Lannoy S, Dormal V, Brion M, Billieux J, & Maurage P (2017). Preserved Crossmodal Integration of Emotional Signals in Binge Drinking. Frontiers in Psychology, 8. 10.3389/fpsyg.2017.00984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannoy S, Dormal V, Brion M, Gaudelus B, Billieux J, & Maurage P (2018b). Affective impairments in binge drinking: Investigation through emotional facial expression decoding. Comprehensive Psychiatry, 83, 59–63. 10.1016/j.comppsych.2018.03.004 [DOI] [PubMed] [Google Scholar]
- Lannoy S, Maurage P, D’Hondt F, Billieux J, & Dormal V (2018a). Executive Impairments in Binge Drinking: Evidence for a Specific Performance-Monitoring Difficulty during Alcohol-Related Processing. European Addiction Research, 24(3), 118–127. 10.1159/000490492 [DOI] [PubMed] [Google Scholar]
- Le Berre A-P (2019). Emotional processing and social cognition in alcohol use disorder. Neuropsychology, 33(6), 808–821. 10.1037/neu0000572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre A-P, Fama R, & Sullivan EV (2017). Executive Functions, Memory, and Social Cognitive Deficits and Recovery in Chronic Alcoholism: A Critical Review to Inform Future Research. Alcoholism: Clinical and Experimental Research, 41(8), 1432–1443. 10.1111/acer.13431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre A-P, & Sullivan EV (2016). Anosognosia for Memory Impairment in Addiction: Insights from Neuroimaging and Neuropsychological Assessment of Metamemory. Neuropsychology Review, 26(4), 420–431. 10.1007/s11065-016-9323-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees B, Mewton L, Stapinski LA, Squeglia LM, Rae CD, & Teesson M (2019). Neurobiological and Cognitive Profile of Young Binge Drinkers: A Systematic Review and Meta-Analysis. Neuropsychology Review, 29(3), 357–385. 10.1007/s11065-019-09411-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leganes-Fonteneau M, Pi-Ruano M, & Tejero P (2020). Early Signs of Emotional Recognition Deficits in Adolescent High-Binge Drinkers. Substance Use & Misuse, 55(2), 218–229. 10.1080/10826084.2019.1662810 [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, … Moher D (2009). The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Medicine, 6(7), e1000100. 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren KP, Ramirez JJ, Wiers RW, Teachman BA, Norris J, Olin CC, … Neighbors C (2018). Mood selectively moderates the implicit alcohol association–drinking relation in college student heavy episodic drinkers. Psychology of Addictive Behaviors, 32(3), 338–349. 10.1037/adb0000360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber S, & Duka T (2009). Acute alcohol decreases performance of an instrumental response to avoid aversive consequences in social drinkers. Psychopharmacology, 205(4), 577–587. 10.1007/s00213-009-1565-9 [DOI] [PubMed] [Google Scholar]
- Maurage P, Bestelmeyer PEG, Rouger J, Charest I, & Belin P (2013b). Binge drinking influences the cerebral processing of vocal affective bursts in young adults. NeuroImage: Clinical, 3, 218–225. 10.1016/j.nicl.2013.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurage P, Lannoy S, Mange J, Grynberg D, Beaunieux H, Banovic I, Gierski F, & Naassila M (2020). What We Talk About When We Talk About Binge Drinking: Towards an Integrated Conceptualization and Evaluation. Alcohol and Alcoholism, in press. 10.1093/alcalc/agaa041 [DOI] [PubMed] [Google Scholar]
- Maurage P, Masson N, Bollen Z, & D’Hondt F (2020). Eye tracking correlates of acute alcohol consumption: A systematic and critical review. Neuroscience & Biobehavioral Reviews, 108, 400–422. 10.1016/j.neubiorev.2019.10.001 [DOI] [PubMed] [Google Scholar]
- Maurage P, Pesenti M, Philippot P, Joassin F, & Campanella S (2009). Latent deleterious effects of binge drinking over a short period of time revealed only by electrophysiological measures. Journal of Psychiatry & Neuroscience, 34, 111–118. [PMC free article] [PubMed] [Google Scholar]
- Maurage P, Petit G, & Campanella S (2013a). Pathways to alcohol-induced brain impairment in young people: A review by Hermens et al., 2013. Cortex, 49(4), 1155–1159. 10.1016/j.cortex.2012.12.015 [DOI] [PubMed] [Google Scholar]
- Maurage P, Timary P. de, & D’Hondt F (2017). Heterogeneity of emotional and interpersonal difficulties in alcohol-dependence: A cluster analytic approach. Journal of Affective Disorders, 217, 163–173. 10.1016/j.jad.2017.04.005 [DOI] [PubMed] [Google Scholar]
- Maxwell SE (2000). Sample Size and Multiple Regression Analysis. Psychological Methods, 5(4), 432–458. 10.1037/1082-989X.5.4.434 [DOI] [PubMed] [Google Scholar]
- Melnikoff DE, & Bargh JA (2018). The Mythical Number Two. Trends in Cognitive Sciences, 22(4), 280–293. 10.1016/j.tics.2018.02.001 [DOI] [PubMed] [Google Scholar]
- McHugh ML (2012). Interrater reliability: The kappa statistic. Biochemia Medica, 22, 276–282. 10.11613/BM.2012.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mngoma NF, Ayonrinde OA, Fergus S, Jeeves AH, & Jolly RJ (2020). Distress, desperation and despair: Anxiety, depression and suicidality among rural South African youth. International Review of Psychiatry, 1–11. 10.1080/09540261.2020.1741846 [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, & Altman DG (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Medicine, 6(7), e1000097. 10.1371/journal.pmed.1000097.g001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska A, & Oei TPS (2005). Binge drinking in university students: A test of the cognitive model. Addictive Behaviors, 30(2), 203–218. 10.1016/j.addbeh.2004.05.011 [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, & Lawrence AD (2003). Functional neuroanatomy of emotions: A meta-analysis. Cognitive, Affective, & Behavioral Neuroscience, 3(3), 207–233. 10.3758/CABN.3.3.207 [DOI] [PubMed] [Google Scholar]
- Mushquash AR, Stewart SH, Sherry SB, Sherry DL, Mushquash CJ, & MacKinnon AL (2013). Depressive symptoms are a vulnerability factor for heavy episodic drinking: A short-term, four-wave longitudinal study of undergraduate women. Addictive Behaviors, 38(5), 2180–2186. 10.1016/j.addbeh.2012.11.008 [DOI] [PubMed] [Google Scholar]
- National Institute of Alcohol Abuse and Alcoholism [NIAAA] (2004). NIAAA Council Approves Definition of Binge Drinking. NIAAA Newsletter No. 3:3. Bethesda, MD: NIAAA. [Google Scholar]
- National Institute of Alcohol Abuse and Alcoholism [NIAAA] (2018). Alcohol Facts and Statistics. Report retrieved from: https://www.niaaa.nih.gov/sites/default/files/AlcoholFactsAndStats.pdf
- Noël X (2014). Why Adolescents Are at Risk of Misusing Alcohol and Gambling. Alcohol and Alcoholism, 49(2), 165–172. 10.1093/alcalc/agt161 [DOI] [PubMed] [Google Scholar]
- Noël X, Brevers D, & Bechara A (2013). A Triadic Neurocognitive Approach to Addiction for Clinical Interventions. Frontiers in Psychiatry, 4. 10.3389/fpsyt.2013.00179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourse R, Adamshick P, & Stoltzfus J (2017). College Binge Drinking and Its Association with Depression and Anxiety: A Prospective Observational Study. Eastern Asian Archive in Psychiatry, 27, 18–25. [PubMed] [Google Scholar]
- Oei TPS, & Morawska A (2004). A cognitive model of binge drinking: The influence of alcohol expectancies and drinking refusal self-efficacy. Addictive Behaviors, 29(1), 159–179. 10.1016/S0306-4603(03)00076-5 [DOI] [PubMed] [Google Scholar]
- Pape H, & Norström T (2016). Associations between emotional distress and heavy drinking among young people: A longitudinal study: Emotional distress and heavy drinking. Drug and Alcohol Review, 35(2), 170–176. 10.1111/dar.12290 [DOI] [PubMed] [Google Scholar]
- Pascual M, Blanco AM, Cauli O, Miñarro J, & Guerri C (2007). Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. European Journal of Neuroscience, 25(2), 541–550. 10.1111/j.1460-9568.2006.05298.x [DOI] [PubMed] [Google Scholar]
- Peeters M, Vollebergh WAM, Wiers RW, & Field M (2014). Psychological Changes and Cognitive Impairments in Adolescent Heavy Drinkers. Alcohol and Alcoholism, 49(2), 182–186. 10.1093/alcalc/agt162 [DOI] [PubMed] [Google Scholar]
- Peeters Margot, Wiers RW, Monshouwer K, van de Schoot R, Janssen T, & Vollebergh WAM (2012). Automatic processes in at-risk adolescents: The role of alcohol-approach tendencies and response inhibition in drinking behavior: Alcohol use in at-risk adolescents. Addiction, 107(11), 1939–1946. 10.1111/j.1360-0443.2012.03948.x [DOI] [PubMed] [Google Scholar]
- Pedersen DE (2013). Gender differences in college binge drinking: Examining the role of depression and school stress. The Social Science Journal, 50(4), 521–529. 10.1016/j.soscij.2013.03.003 [DOI] [Google Scholar]
- Pessoa L (2017). A Network Model of the Emotional Brain. Trends in Cognitive Sciences, 21(5), 357–371. 10.1016/j.tics.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit G, Luminet O, Maurage F, Tecco J, Lechantre S, Ferauge M, … de Timary P (2015). Emotion Regulation in Alcohol Dependence. Alcoholism: Clinical and Experimental Research, 39(12), 2471–2479. 10.1111/acer.12914 [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, & Lane R (2003a). Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biological Psychiatry, 54(5), 504–514. 10.1016/S0006-3223(03)00168-9 [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, & Lane R (2003b). Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biological Psychiatry, 54(5), 515–528. 10.1016/S0006-3223(03)00171-9 [DOI] [PubMed] [Google Scholar]
- Poncin M, Vermeulen N, & de Timary P (2017). Distress Response to the Failure to an Insoluble Anagrams Task: Maladaptive Emotion Regulation Strategies in Binge Drinking Students. Frontiers in Psychology, 8, 1795. 10.3389/fpsyg.2017.01795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AK, & Grunsell L (2008). The Subjective, Rather Than the Disinhibiting, Effects of Alcohol Are Related to Binge Drinking. Alcoholism: Clinical and Experimental Research, 32(6), 1096–1104. 10.1111/j.1530-0277.2008.00672.x [DOI] [PubMed] [Google Scholar]
- Rowland B, Evans-Whipp T, Hemphill S, Leung R, Livingston M, & Toumbourou JW (2016). The density of alcohol outlets and adolescent alcohol consumption: An Australian longitudinal analysis. Health & Place, 37, 43–49. 10.1016/j.healthplace.2015.11.004 [DOI] [PubMed] [Google Scholar]
- Ruiz P, Pilatti A, & Pautassi RM (2020). Consequences of alcohol use, and its association with psychological distress, sensitivity to emotional contagion and age of onset of alcohol use, in Uruguayan youth with or without college degree. Alcohol, 82, 91–101. 10.1016/j.alcohol.2019.09.001 [DOI] [PubMed] [Google Scholar]
- Rupp CI, Derntl B, Osthaus F, Kemmler G, & Fleischhacker WW (2017). Impact of Social Cognition on Alcohol Dependence Treatment Outcome: Poorer Facial Emotion Recognition Predicts Relapse/Dropout. Alcoholism: Clinical and Experimental Research, 41(12), 2197–2206. 10.1111/acer.13522 [DOI] [PubMed] [Google Scholar]
- Rutter LA, Scheuer L, Vahia IV, Forester BP, Smoller JW, & Germine L (2019). Emotion sensitivity and self‐reported symptoms of generalized anxiety disorder across the lifespan: A population‐based sample approach. Brain and Behavior, e01282. 10.1002/brb3.1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaife JC, & Duka T (2009). Behavioural measures of frontal lobe function in a population of young social drinkers with binge drinking pattern. Pharmacology Biochemistry and Behavior, 93(3), 354–362. 10.1016/j.pbb.2009.05.015 [DOI] [PubMed] [Google Scholar]
- Shulman EP, Harden KP, Chein JM, & Steinberg L (2015). Sex Differences in the Developmental Trajectories of Impulse Control and Sensation-Seeking from Early Adolescence to Early Adulthood. Journal of Youth and Adolescence, 44(1), 1–17. 10.1007/s10964-014-0116-9 [DOI] [PubMed] [Google Scholar]
- Sloan E, Hall K, Moulding R, Bryce S, Mildred H, & Staiger PK (2017). Emotion regulation as a transdiagnostic treatment construct across anxiety, depression, substance, eating and borderline personality disorders: A systematic review. Clinical Psychology Review, 57, 141–163. 10.1016/j.cpr.2017.09.002 [DOI] [PubMed] [Google Scholar]
- Somerville LH, Hare T, & Casey BJ (2011). Frontostriatal Maturation Predicts Cognitive Control Failure to Appetitive Cues in Adolescents. Journal of Cognitive Neuroscience, 23(9), 2123–2134. 10.1162/jocn.2010.21572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L (2007). Risk taking in adolescence: New perspectives from brain and behavioral science. Current Directions in Psychological Science, 16(2), 55–59. 10.1111/j.1467-8721.2007.00475.x [DOI] [Google Scholar]
- Stephens DN, & Duka T (2008). Cognitive and emotional consequences of binge drinking: Role of amygdala and prefrontal cortex. Philosophical Transactions of the Royal Society B: Biological Sciences, 363(1507), 3169–3179. 10.1098/rstb.2008.0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens David N., Ripley TL, Borlikova G, Schubert M, Albrecht D, Hogarth L, & Duka T (2005). Repeated Ethanol Exposure and Withdrawal Impairs Human Fear Conditioning and Depresses Long-Term Potentiation in Rat Amygdala and Hippocampus. Biological Psychiatry, 58(5), 392–400. 10.1016/j.biopsych.2005.04.025 [DOI] [PubMed] [Google Scholar]
- Stevens FL Hurley RA, Taber KH (2011). Anterior Cingulate Cortex: Unique Role in Cognition and Emotion. The Journal of Neuropsychiatry and Clinical Neurosciences, 23(2), 120–125. 10.1176/appi.neuropsych.23.2.121. [DOI] [PubMed] [Google Scholar]
- Stickley A, Koyanagi A, Koposov R, Schwab-Stone M, & Ruchkin V (2014). Loneliness and health risk behaviours among Russian and U.S. adolescents: A cross-sectional study. BMC Public Health, 14(1), 366. 10.1186/1471-2458-14-366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strine TW, Mokdad AH, Dube SR, Balluz LS, Gonzalez O, Berry JT, … Kroenke K (2008). The association of depression and anxiety with obesity and unhealthy behaviors among community-dwelling US adults. General Hospital Psychiatry, 30(2), 127–137. 10.1016/j.genhosppsych.2007.12.008 [DOI] [PubMed] [Google Scholar]
- Townshend JM, & Duka T (2005). Binge Drinking, Cognitive Performance and Mood in a Population of Young Social Drinkers: Alcoholism: Clinical & Experimental Research, 29(3), 317–325. 10.1097/01.ALC.0000156453.05028.F5 [DOI] [PubMed] [Google Scholar]
- Trojanowski PJ (2019). Understanding profiles of student binge drinking and eating_ The importance of motives. Addictive Behaviors, 96, 148–155. 10.1016/j.addbeh.2019.04.025. [DOI] [PubMed] [Google Scholar]
- Venerable WJ, & Fairbairn CE (2020). A multimodal, longitudinal investigation of alcohol’s emotional rewards and drinking over time in young adults. Psychology of Addictive Behaviors. 10.1037/adb0000567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volman I, Roelofs K, Koch S, Verhagen L, & Toni I (2011). Anterior Prefrontal Cortex Inhibition Impairs Control over Social Emotional Actions. Current Biology, 21(20), 1766–1770. 10.1016/j.cub.2011.08.050 [DOI] [PubMed] [Google Scholar]
- Voon V, Grodin E, Mandali A, Morris L, Weidacker K, Kwako L, … & Momenan R (2020). Addictions NeuroImaging Assessment (ANIA): towards an integrative framework for alcohol use disorder. Neuroscience & Biobehavioral Reviews, 113, 492–506. 10.1016/j.neubiorev.2020.04.004 [DOI] [PubMed] [Google Scholar]
- Wichaidit W, Pruphetkaew N, & Assanangkornchai S (2019). Variations by sex and age in the association between alcohol use and depressed mood among Thai adolescents. PLOS ONE, 14(12), e0225609. 10.1371/journal.pone.0225609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Pommy JM, & Adinoff B (2016). Neural Circuitry of Impaired Emotion Regulation in Substance Use Disorders. American Journal of Psychiatry, 173(4), 344–361. 10.1176/appi.ajp.2015.15060710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth M, Isaacowitz DM, & Kunzmann U (2017). Visual attention and emotional reactions to negative stimuli: The role of age and cognitive reappraisal. Psychology and Aging, 32(6), 543–556. 10.1037/pag0000188 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2018). Global status report on alcohol and health 2018. (ISBN 978–92-4–156563-9). Geneva: World Health Organization [Google Scholar]
- Zeng X, Zhang Y, Kwong JSW, Zhang C, Li S, Sun F, … Du L (2015). The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review: Methodological quality assessment tools. Journal of Evidence-Based Medicine, 8(1), 2–10. 10.1111/jebm.12141 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.