Abstract
Background/Objective:
Traumatic brain injury (TBI) is a leading cause of morbidity, mortality, and disability in the United States. While cardiopulmonary dysfunction can result in poor outcomes following severe TBI, the impact of acute kidney injury (AKI) is poorly understood. We examined the association between severe AKI with hospital mortality and healthcare utilization following isolate severe TBI.
Methods:
We conducted a retrospective cohort study using the National Trauma Data Bank (NTDB) from 2007-2014. We identified a cohort of adult patients with isolated severe TBI, and described the incidence of severe AKI, corresponding to Acute Kidney Injury Network stage 3 disease or greater. We examined the association of severe AKI with the primary outcome of hospital mortality using multivariable logistic regression models. In secondary analyses, we examined the association of severe AKI with dialysis catheter placement, tracheostomy and gastrostomy utilization, and hospital length of stay.
Results:
There were 37,851 patients who experienced isolated severe TBI during the study period. Among these patients, 787 (2.1%) experienced severe (Stage 3 or greater) AKI. In multivariable models, the development of severe AKI in the hospital was associated with in-hospital mortality (OR 2.03, 95% CI 1.64 – 2.52), need for tracheostomy (OR 2.10, 95% CI 1.52 – 2.89), PEG tube placement (OR 1.88, 95% CI 1.45 – 2.45), and increased hospital length of stay (p<0.001).
Conclusions:
The overall incidence of severe AKI is relatively low (2.1%), but is associated with increased mortality and multiple markers of increased healthcare utilization following severe TBI.
Keywords: traumatic brain injury, acute kidney injury, healthcare utilization
Introduction
Traumatic brain injury (TBI) is a leading cause of morbidity and mortality in the United States, and can lead to severe disability in survivors1. While TBI severity exists on a spectrum from mild to severe based on the presentation Glasgow Coma Scale (GCS) score, severe TBI has been associated with prolonged critical illness, significant healthcare resource utilization, and poor functional outcomes2. Recent advances in the understanding of TBI pathophysiology have revealed that severe TBI can lead to multi-organ system dysfunction in the setting of critical illness3,4.
Extracranial organ dysfunction following severe TBI may include cardiovascular, respiratory, hematologic, or renal organ systems5-8. While the direct trauma leads to primary brain injury, extracranial organ injury is postulated to occur via autonomic and inflammatory pathways as a response to the initial insult to the brain9. While pulmonary and circulatory dysfunction are the most common extracranial organ dysfunctions observed following severe TBI5,10, the impact on severe acute kidney injury (AKI) is poorly understood. Severe AKI can contribute to secondary brain injuries through worsening acidosis, electrolyte and volume disturbances that can further exacerbate brain tissue injury. Additionally, severe AKI complicates the pharmacological management of cerebral edema. To better understand the potential clinical implications of severe AKI following severe TBI, we assessed the association between severe AKI with hospital mortality and healthcare utilization following injury.
Methods
Study Design and Population
We conducted a retrospective cohort study using the National Trauma Data Bank (NTDB) from 2007-2014. The NTDB research data sets include information on over 7.5 million trauma patients from over 700 trauma facilities. The NTDB dataset files were used to identify adult patients (18 years of age or older) who suffered isolated severe TBI and did not have kidney disease at baseline, defined as having a co-morbidity of chronic kidney disease or end-stage renal disease. To examine the effect of the brain injury itself on severe AKI (rather than the impact of concurrent polytrauma), we examined patients with isolated severe TBI and excluded patients with mild or moderate TBI [Glasgow Coma Scale (GCS) score > 8 and head Abbreviated Injury Score (AIS) < 4] and severe extracranial injuries (non-head body region AIS > 2). We further excluded patients who were not admitted to the hospital, pediatric patients (age < 18 years), and patients who died within 48 hours of admission (as this likely indicates death from head injury alone, without time for severe AKI to develop or contribute meaningfully to the outcome). Lastly, we excluded patients from facilities that did not report hospital complications, as ascertained by a missing value in the first complications field. The NTDB is fully de-identified and, therefore, exempt from Institutional Review Board (IRB) review by the Duke University Health System IRB.
Exposures, Outcomes, and Covariates
To determine the incidence of severe AKI following severe TBI, our first research aim examined severe AKI as an outcome. The development of severe AKI was ascertained from the complications file of the NTDB dataset. Trained coders applied the following criteria to define severe AKI, which corresponds to the Acute Kidney Injury Network (AKIN) criteria to define clinical AKI of stage 3 or greater11: an increase of serum creatinine of at least 300% of baseline, an increase of serum creatinine levels to more than 4 mg/dL, a decrease in glomerular filtration rate of less than 35 mL/min per 1.73 m2 body surface area, reduction of urine output to less than 0.3 mL/kg/h for more than 24 hours, anuria for more than 12 hours, or the need for renal replacement therapy. Our second research aim focused on severe AKI as the exposure and in-hospital mortality (defined as death in the hospital or hospital discharge disposition indicating “hospice”) as the primary outcome. Secondary outcomes examined markers of healthcare utilization and included: 1) Dialysis catheter placement, indicating the need for renal replacement therapy during the hospitalization; 2) Tracheostomy and gastrostomy tube placement among survivors, as markers for a poor clinical course and additional healthcare utilization; and 3) Hospital length of stay among survivors. Covariates included demographic (age, gender, race), clinical (injury severity score, GCS, co-morbidities, mechanical ventilation, admission heart rate, admission blood pressure), and facility (region, hospital size, hospital teaching status, hospital Level 1 trauma center status) characteristics.
Statistical Analysis
Descriptive statistics were used to examine the demographic, clinical, and facility characteristics of the study group, stratified by the development of severe AKI. We calculated the cumulative incidence of severe AKI in the cohort. To examine the association of severe AKI with in-hospital mortality, we conducted multivariable analyses (with clustered standard error estimates, relaxing the assumption that observations from the same hospitals are independent) using logistic regression to examine adjusted odds ratios for mortality, tracheostomy utilization, and gastrostomy utilization and linear regression to examine adjusted mean differences in hospital and ICU length of stay. For the primary analysis, parsimonious models examining outcomes were adjusted for a core list of variables, considered the optimal risk adjustment model for examining mortality in studies using NTDB data12: injury severity, age, GCS, admission hypotension (defined as systolic blood pressure < 90mmHg), admission pulse, and the need for mechanical ventilation. To confirm the robustness of associations observed in the primary analysis, we conducted sensitivity analyses for each outcome using a model with the following additional covariates: gender, treatment year, presence of vascular co-morbidities, hospital region, hospital size, hospital teaching status, and hospital Level 1 trauma designation. Given that we pre-specified a single primary outcome (with the remainder of secondary outcomes considered as exploratory/hypothesis generating), no additional adjustments were made for multiple testing. All analyses were conducted using STATA 15.0 (College Station, Texas).
Results
Demographic, Clinical, and Facility Characteristics
After applying all inclusion/exclusion criteria, the study cohort included 37,851 patients who experienced isolated severe TBI during the study period. Among these patients, 787 (2.1%) experienced severe (Stage 3 or greater) AKI. Baseline characteristics of the study population are described in Table 1. The mean (SD) age of the cohort was 49.5(20.5) years, with patients that experienced severe AKI being slightly older compared to patients who did not develop severe AKI [55.8(19.6) versus 49.4(20.5) years, respectively]. Overall, 68% of patients were White, and falls (44%) were the most common mechanism of injury. The mean(SD) admission GCS score in the cohort was 4.2(1.8), with minimal differences in patients who did and did not develop severe AKI [4.2(1.8) versus 4.1(1.7), respectively]. The mean(SD) head AIS score in the cohort was 4.1(0.4), with a minimal difference in patients who did and did not develop severe AKI [4.2(0.4) versus 4.1(0.4), respectively]. Admission hemodynamics were similar between patients that did and did not develop severe AKI [mean(SD) systolic blood pressure: 147(39) versus 146(33) mmHg, respectively; mean(SD) heart rate: 93(27) versus 92(25) beats per minute, respectively]. Patients who developed severe AKI, compared to patients who did not develop severe AKI, had an increased burden of co-morbidities including: congestive heart failure (8.3% versus 2.9%, respectively), prior cerebrovascular accidents (7.0% versus 3.3%, respectively), diabetes (25.0% versus 10.8 %, respectively), and hypertension (38.9% versus 25.6%, respectively).
Table 1.
Demograhic and Clinical CharacteristicsB
| Patient Factors | No AKI | AKI | Total |
|---|---|---|---|
| Total Number of Patients 1 | 37064 (97.9) | 787 (2.1) | 37851 |
| Age, years 2 | 49.4 (20.5) | 55.8 (19.6) | 49.5 (20.5) |
| Gender 1 | |||
| Male | 27172 (73.3) | 628 (79.9) | 27800 (73.5) |
| Female | 9875 (26.7) | 158 (20.1) | 10033 (26.5) |
| Race/ethnicity 1 | |||
| White | 25235 (68.1) | 519 (66.0) | 27754 (68) |
| American Indian | 537 (1.5) | 7 (0.9) | 544 (1.5) |
| Asian | 707 (1.9) | 23 (2.9) | 730 (1.9) |
| African Am | 3913 (10.6) | 115 (14.6) | 4028 (10.6) |
| NHPI | 65 (0.2) | 0 | 65 (0.2) |
| Hispanic | 4218 (11.4) | 71 (9.0) | 4289 (11.3) |
| Other | 899 (2.4) | 27 (3.4) | 926 (2.5) |
| 2+ Races | 16 (0.04) | 0 | 16 (0.04) |
| Not reported | 341 (0.9) | 3 (0.4) | 344 (0.9) |
| Missing | 1133 (3.1) | 22 (2.8) | 1155 (3.06) |
| Injury Mechanism 1 | |||
| MVT (motorcyclist, cyclist, pedestrian, other) | 10197 (27.5) | 183 (23.3) | 10380 (27.4) |
| Fall | 16446 (44.4) | 413 (52.5) | 16859 (44.5) |
| Others | 10421 (28.2) | 191 (24.3) | 10602 (30.8) |
| Admission GCS 2 | 4.2 (1.8) | 4.1 (1.7) | 4.2 (1.8) |
| Head AIS score 2 | 4.1 (0.4) | 4.2 (0.4) | 4.1 (0.4) |
| Injury Severity score 2 | 20.3 (5.1) | 21.3 (6.0) | 20.4 (5.1) |
| SBP, mmHg 2 | 145.7 (32.6) | 146.7 (39.0) | 145.7 (32.7) |
| Admission HR, beats per minute 2 | 91.9 (24.8) | 92.8 (27.1) | 91.9 (24.8) |
| Patient Comorbidity 1 | |||
| Respiratory disease C | 1824 (4.9) | 58 (7.4) | 1882 (5.0) |
| Congestive heart failure | 1070 (2.9) | 65 (8.3) | 1135 (3.0) |
| Prior stroke | 1218 (3.3) | 55 (7.0) | 1273 (3.4) |
| Diabetes | 3987 (10.8) | 197 (25.0) | 4184 (11.1) |
| Hypertension | 9473 (25.6) | 306 (38.9) | 9779 (25.8) |
Continuous variables are expressed as means and standard deviations while categorial variables are expressed as count and percentage
Contains missing patient data from NTDB database, which is included in classification of ‘not reported,’ but not ‘other’; therefore, numbers may not add up to column total
Respiratory Disease defined as severe chronic lung disease, chronic asthma, cystic fibrosis, or chronic obstructive pulmonary disease (COPD)
Number (percentage)
Mean (standard deviation)
Facility characteristics of the cohort are described in Table 2. In this cohort, 43% of patients were treated in hospitals with greater than 600 beds. In addition, the majority of patients were treated in university/teaching hospitals (55%). Most patients in the cohort were treated in non-level 1 trauma centers, compared to level 1 trauma centers (57% versus 43%, respectively).
Table 2.
Geographical and Facility CharacteristicsB
| Patient Factors |
No AKI | AKI | Total |
|---|---|---|---|
| Total Number of Patients | 37064 (97.9) | 787 (2.1) | 37851 |
| Region A | |||
| Northeast | 5826 (16.1) | 121 (15.6) | 5947 (16.1) |
| Midwest | 8642 (23.9) | 170 (21.9) | 8812 (23.8) |
| West | 7911 (21.8) | 118 (15.2) | 8029 (21.7) |
| South | 13845 (38.2) | 366 (47.2) | 14211 (38.4) |
| Bedsize | |||
| ≤ 200 | 1077 (2.9) | 19 (2.4) | 1096 (2.9) |
| 201- 400 | 8303 (22.4) | 112 (14.2) | 8415 (22.2) |
| 401- 600 | 11706 (31.6) | 267 (33.9) | 11973 (31.6) |
| > 600 | 15978 (43.1) | 389 (49.4) | 16367 (43.2) |
| Teaching | |||
| Status | |||
| Non-teaching | 16719 (45.1) | 282 (35.8) | 17001 (44.9) |
| University | 20345 (54.9) | 505 (64.2) | 20850 (55.1) |
| Level 1 Trauma Status | |||
| Non-level 1 | 21028 (56.7) | 443 (56.3) | 21471 (56.7) |
| Level 1 | 16036 (43.3) | 344 (43.7) | 16380 (43.3) |
Contains missing patient data from NTDB database, therefore numbers may not add up to column total
All columns represented as counts and percentage
Association of Severe Acute Kidney Injury with Hospital Mortality and Healthcare Utilization
Clinical outcomes are described in Table 3 and Figure 1 (full model presented in Supplementary Table1). Among patients who developed severe AKI following severe TBI, 13% required dialysis catheter placement, 42% required tracheostomy placement, 40% required PEG tube placement, and 49% experienced hospital mortality. In parsimonious multivariable models, the development of severe AKI in the hospital was associated with in-hospital mortality (OR 2.03, 95% CI 1.64 – 2.52, p<0.001), need for tracheostomy (OR 2.10, 95% CI 1.52 – 2.89, p<0.001), and PEG tube placement (OR 1.88, 95% CI 1.45 – 2.45, p<0.001). These patients also had longer hospital length of stay (p<0.001). Sensitivity analyses (employing adjustment for additional covariates and co-morbidity) for all models were robust, with respect to direction/magnitude of risk estimates and statistical significance (Supplementary Table 2).
Table 3.
Association between Severe AKI and Clincal OutcomesB
| Outcome | Odds Ratio | 95% CI | P-Value | |
|---|---|---|---|---|
| Mortality | 2.03 | 1.64 | 2.52 | <0.001 |
| TracheostomyA | 2.10 | 1.52 | 2.89 | <0.001 |
| GastrostomyA | 1.88 | 1.45 | 2.45 | <0.001 |
| Mean Difference | 95% CI | P-Value | ||
| Hospital length of stay (days)A | 12.4 | 9.87 | 14.96 | <0.001 |
Among survivors
Results of multivariable logistic and linear regression models to examine the association of severe AKI with clinical outcomes, adjusted for the following covariates: age, injury severity score, admission Glasgow Coma Scale score, admission blood pressure, admission heart rate, and need for mechanical ventilation
Figure 1.
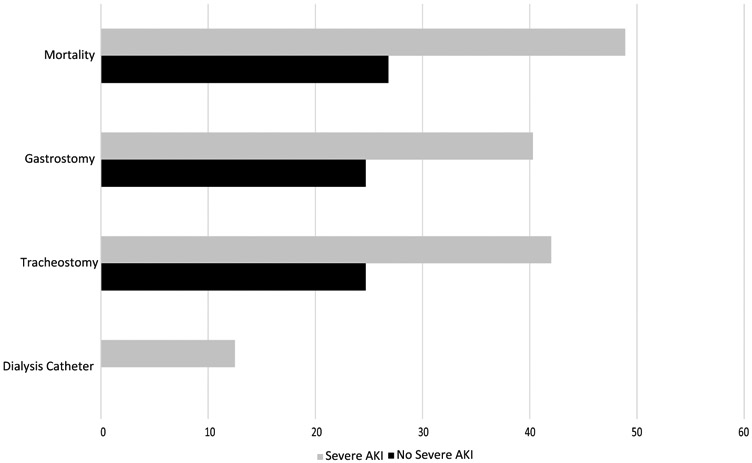
Outcomes following development of severe AKI
Discussion:
In this retrospective study, we examined a large multicenter population from the National Trauma Databank who suffered an isolated TBI. Overall, we found a relatively low incidence of severe AKI following isolated TBI (2.1%); despite this, patients who experienced severe AKI had increased mortality and multiple measures of increased healthcare utilization. Therefore, the development of severe AKI is potentially a modifiable risk factor for poor clinical outcomes following severe TBI.
Severe TBI is associated with the development of extracranial organ dysfunction. While the exact mechanisms underlying this are unknown, autonomic dysfunction, systemic inflammation, and poor renal perfusion from systemic hypotension are likely drivers of the process13-16. In this study, we examined patients without a known history of kidney disease, although patients who developed severe AKI had higher rates of other co-morbidities. Based on observations in this study, severe kidney injury may contribute to poor clinical outcomes following severe TBI, above and beyond baseline risk factors. While the incidence of severe AKI following isolated severe TBI patient was relatively low in our cohort, 12.5% of these patients required placement of a dialysis catheter during hospitalization, indicating the need for renal replacement therapy in a sizeable proportion of this population. Furthermore, these patients had experienced significantly increased odds of in-hospital mortality and multiple markers of increased healthcare utilization.
Previous studies have aimed to identify the risk of AKI following severe TBI, as well as its impact on mortality. Similar to our observations, two previous retrospective studies demonstrated an association of AKI following TBI with increased hospital mortality7,9. While these studies focused on the impact of the development of any AKI stage (and demonstrated a higher incidence of AKI following TBI, compared to our study), those studies did not focus on patients who developed severe AKI, who are at highest risk for poor clinical outcomes following critical illness17. Furthermore, prior studies did not examine healthcare utilization or focus on isolated TBI; and these studies included patients with polytrauma, which may have contributed to the development of AKI beyond the brain injury itself. Lastly prior studies have not examined the need for renal replacement therapy in the hospital in the setting of severe AKI development following TBI. In this study, we confirmed the association of severe AKI with increased hospital mortality in isolated severe TBI; in addition, we found that 13% of these patients required dialysis catheter placement, indicating a likely need for renal replacement therapy. Furthermore, patients who developed severe AKI experienced multiple markers of increased health care utilization (increased utilization of tracheostomy and gastrostomy tubes and longer ICU and hospital length of stays).
The mechanism by which TBI results in AKI has not been fully elucidated, although it is likely that the autonomic dysfunction and inflammatory response which is thought to contribute to other elements of extracranial organ dysfunction similarly drives AKI18, and that unmeasured or unknown pre-existing renal parameters predispose a subpopulation to develop severe AKI. The development of severe AKI may also be in part iatrogenic, as hypertonic saline and mannitol may predispose to kidney injury19. Sodium and chloride level increase with the amount of hypertonic solutions given, which can theoretically impact overall kidney function via vasoconstriction of the renal vasculature20,21. While preventing AKI development may represent a potentially modifiable risk factor to improve TBI outcomes, current therapeutic options for the prevention and treatment of severe AKI following TBI are limited. For example, an ICP-monitor directed hyperosmolar therapy reduced the incidence of AKI in a single-center study22. In addition, erythropoietin, which stimulates red blood cell production in patients with chronic kidney disease, was thought to potentially prevent the development of AKI following severe TBI; unfortunately, no renal protective effects were found23. Early identification of high-risk patients through improved predictive risk models and incorporation of sensitive biomarkers, coupled with aggressive maintenance of renal perfusion and avoidance of nephrotoxins, may represent a potential strategy to be examined in future studies.
There are several limitations to our study. First, detection of severe AKI in this study depended upon the accurate recognition and coding of this diagnosis; given that we examined administrative data, it was not possible to confirm this diagnosis via chart review. Second, our study did not evaluate the therapies used to manage patients with severe TBI. While the group that developed severe AKI and the group that did not develop severe AKI were similar in TBI severity, a limitation of this study is that the burden of elevated ICP and thus the need for interventions to treat elevated ICP was not captured. Third, the NTDB lacks data on functional outcomes, such as the Glasgow Outcome Scale. Fourth, the clinical course and pharmacologic treatment strategies of AKI were not captured in this administrative database; therefore, variables such as creatinine and urine output trajectory were unable to be ascertained. Fifth, given that AKI timing was not available in the database, it is possible that severe AKI was not the result of the TBI itself, but other critical care complications (such as pneumonia or sepsis). Sixth, despite ascertainment of patient co-morbidities, the severity of individual co-morbidities was unable to be ascertained. Seventh, the strategy used by the American College of Surgeons Committee on Trauma to define AKI for the NTDB includes only the development of severe AKI, and therefore we were unable to evaluate milder forms of AKI development. Eighth, despite robust data on injury severity, the NTDB lacks detailed physiologic severity scores (i.e. APACHE, SAPS); nevertheless, we attempted to included surrogate physiologic data in our statistical models, including the need for mechanical ventilation, the presence of shock, and heart rate. Lastly, despite adjustment for measured covariates, the observational nature of our study puts the analysis at risk for residual confounding from unmeasured covariates.
In conclusion, we found that the overall incidence of severe AKI is relatively low (2.1%), but is associated with increased mortality and multiple markers of increased healthcare utilization following severe TBI. Having established the incidence and clinical relevance of severe AKI development following isolated TBI, further research is necessary to understand the underlying mechanisms, as well as optimal prevention and treatment strategies.
Supplementary Material
Acknowledgments
Funding: National Institutes of Health - K23 NS109274 (Krishnamoorthy)
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of Interest: None
References:
- 1.Rutland-Brown W, Langlois JA, Thomas KE, Xi YL. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil 2006;21:544–8. [DOI] [PubMed] [Google Scholar]
- 2.Saatman KE, Duhaime AC, Bullock R, et al. Classification of traumatic brain injury for targeted therapies. J Neurotrauma 2008;25:719–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mascia L, Sakr Y, Pasero D, Payen D, Reinhart K, Vincent JL. Extracranial complications in patients with acute brain injury: a post-hoc analysis of the SOAP study. Intensive Care Med 2008;34:720–7. [DOI] [PubMed] [Google Scholar]
- 4.Jeremitsky E, Omert L, Dunham CM, Protetch J, Rodriguez A. Harbingers of poor outcome the day after severe brain injury: hypothermia, hypoxia, and hypoperfusion. J Trauma 2003;54:312–9. [DOI] [PubMed] [Google Scholar]
- 5.Manley G, Knudson MM, Morabito D, Damron S, Erickson V, Pitts L. Hypotension, hypoxia, and head injury: frequency, duration, and consequences. Arch Surg 2001;136:1118–23. [DOI] [PubMed] [Google Scholar]
- 6.Krishnamoorthy V, Rowhani-Rahbar A, Gibbons EF, et al. Early Systolic Dysfunction Following Traumatic Brain Injury: A Cohort Study. Crit Care Med 2017;45:1028–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore EM, Bellomo R, Nichol A, Harley N, Macisaac C, Cooper DJ. The incidence of acute kidney injury in patients with traumatic brain injury. Ren Fail 2010;32:1060–5. [DOI] [PubMed] [Google Scholar]
- 8.Maegele M Coagulopathy after traumatic brain injury: incidence, pathogenesis, and treatment options. Transfusion 2013;53 Suppl 1:28S–37S. [DOI] [PubMed] [Google Scholar]
- 9.Corral L, Javierre CF, Ventura JL, Marcos P, Herrero JI, Manez R. Impact of non-neurological complications in severe traumatic brain injury outcome. Crit Care 2012;16:R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S, Hwang H, Yamal JM, et al. IMPACT probability of poor outcome and plasma cytokine concentrations are associated with multiple organ dysfunction syndrome following traumatic brain injury. J Neurosurg 2019;131:1931–7. [DOI] [PubMed] [Google Scholar]
- 11.Lin CY, Chen YC. Acute kidney injury classification: AKIN and RIFLE criteria in critical patients. World J Crit Care Med 2012;1:40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haider AH, Hashmi ZG, Zafar SN, et al. Developing best practices to study trauma outcomes in large databases: an evidence-based approach to determine the best mortality risk adjustment model. J Trauma Acute Care Surg 2014;76:1061–9. [DOI] [PubMed] [Google Scholar]
- 13.Rosner MJ, Newsome HH, Becker DP. Mechanical brain injury: the sympathoadrenal response. J Neurosurg 1984;61:76–86. [DOI] [PubMed] [Google Scholar]
- 14.Koiv L, Merisalu E, Zilmer K, Tomberg T, Kaasik AE. Changes of sympatho-adrenal and hypothalamo-pituitary-adrenocortical system in patients with head injury. Acta Neurol Scand 1997;96:52–8. [DOI] [PubMed] [Google Scholar]
- 15.Rizoli SB, Jaja BN, Di Battista AP, et al. Catecholamines as outcome markers in isolated traumatic brain injury: the COMA-TBI study. Crit Care 2017;21:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonald SJ, Sharkey JM, Sun M, et al. Beyond the Brain: Peripheral Interactions after Traumatic Brain Injury. J Neurotrauma 2020;37:770–81. [DOI] [PubMed] [Google Scholar]
- 17.Wiersema R, Eck RJ, Haapio M, et al. Burden of acute kidney injury and 90-day mortality in critically ill patients. BMC Nephrol 2019;21:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramtinfar S, Chabok SY, Chari AJ, Reihanian Z, Leili EK, Alizadeh A. Early detection of nonneurologic organ failure in patients with severe traumatic brain injury: Multiple organ dysfunction score or sequential organ failure assessment? Indian J Crit Care Med 2016;20:575–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erdman MJ, Riha H, Bode L, Chang JJ, Jones GM. Predictors of Acute Kidney Injury in Neurocritical Care Patients Receiving Continuous Hypertonic Saline. Neurohospitalist 2017;7:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest 1983;71:726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maguigan KL, Dennis BM, Hamblin SE, Guillamondegui OD. Method of Hypertonic Saline Administration: Effects on Osmolality in Traumatic Brain Injury Patients. J Clin Neurosci 2017;39:147–50. [DOI] [PubMed] [Google Scholar]
- 22.Zeng J, Tong W, Zheng P. Decreased risk of acute kidney injury with intracranial pressure monitoring in patients with moderate or severe brain injury. J Neurosurg 2013;119:1228–32. [DOI] [PubMed] [Google Scholar]
- 23.Skrifvars MB, Moore E, Martensson J, et al. Erythropoietin in traumatic brain injury associated acute kidney injury: A randomized controlled trial. Acta Anaesthesiol Scand 2019;63:200–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


