Abstract
Parkinson’s disease (PD) is the second most common neurodegenerative disease, which manifests with both motor and non-motor symptoms. Circadian rhythm dysregulation, as one of the most challenging non-motor features of PD, usually appears long before obvious motor symptoms. Moreover, the dysregulated circadian rhythm has recently been reported to play pivotal roles in PD pathogenesis, and it has emerged as a hot topic in PD research. In this review, we briefly introduce the circadian rhythm and circadian rhythm-related genes, and then summarize recent research progress on the altered circadian rhythm in PD, ranging from clinical features to the possible causes of PD-related circadian disorders. We believe that future comprehensive studies on the topic may not only help us to explore the mechanisms of PD, but also shed light on the better management of PD.
Keywords: Circadian rhythm dysregulation, Circadian rhythm gene, Parkinson’s disease, REM sleep behavior disorder, Dopamine
Introduction
Parkinson's disease (PD) is the second most common neurodegenerative disease, affecting 1% of people aged over 60, and 3% of those older than 80 years [1, 2]. Many genetic and environmental factors have been identified to be involved in the extremely complicated pathogenesis of PD [3]. The degeneration of dopamine (DA) neurons in the substantia nigra and the deposition of Lewy bodies containing α-synuclein are its main pathological features [4, 5]. Clinically, PD is characterized by a wide range of motor symptoms (such as resting tremor, rigidity, bradykinesia, and postural and gait disturbance) and non-motor symptoms (such as mental and cognitive disorders, autonomic dysfunction, sensory impairment, and especially sleep disorders) [4, 6]. So far the diagnosis of PD mainly relies on the presence of motor symptoms as the disease progresses, but it is difficult to make an early diagnosis of this disease, since there are still no specific blood or other laboratory tests [7, 8]. Non-motor symptoms of PD usually occur earlier than motor symptoms and have been accepted as a promising research topic due to their close correlations with the progress of PD pathology [8], which may help the early diagnosis and treatment of PD [8, 9].
Non-motor symptoms of PD include sleep disorders, emotional disorders, autonomic dysfunction, neuroendocrine dysregulation and gastrointestinal (GI) dysfunctions [8]. Sleep disorders in PD patients usually manifest with disturbances of sleep structure and disorders of the sleep-wake cycle, mainly presenting with rapid eye movement (REM) sleep behavior disorder (RBD), restless legs syndrome (RLS), and excessive daytime sleepiness (EDS) [10–12]. Emotional disorders in PD include depression, anxiety, increased agitation, aggression, restlessness, and delirium [13]. Autonomic dysfunctions in PD include disturbances of the rhythm of blood pressure (BP), heart rate variability (HRV), and core-body temperature (CBT) [14–17]. Neuroendocrine function in PD is also disturbed and manifested by dysregulated hormones secretion [18]. The entire GI tract function is disturbed in PD patients featuring drooling, swallowing problems, delays in gastric emptying, and constipation [19, 20].
Interestingly, almost all non-motor symptoms in PD are associated to a certain extent with an impaired circadian rhythm [20]. Circadian rhythm is controlled by the neuronal circadian oscillators located in the central suprachiasmatic nucleus (SCN) of the hypothalamus and is executed by the interaction network of various circadian rhythm genes in almost all organs and tissues [21–23]. In this review, we update recent progress on the non-motor symptoms, pathologies, diagnosis, auxiliary examinations and treatments of PD, with a focus on circadian rhythm dysregulation. The implications of imaging and the electroencephalogram (EEG) for PD diagnosis are highlighted, and the impacts of pharmacological and non-pharmacological therapy for PD on the circadian rhythm system are also discussed. In addition, we present the recent findings on the alteration of circadian rhythm genes in PD, which may help us understand the mechanism of circadian rhythm dysregulation in this devastating disease. Our review provides an insight into the important roles of circadian rhythm dysregulation in PD pathogenesis and may help to establish a new diagnostic strategy for this disease.
Circadian Rhythm and Circadian Rhythm Genes
Circadian rhythms are 24-h cycles that are part of the body’s internal clock, running in the background to carry out essential functions and processes [24]. The circadian rhythm modulates many physiological processes including CBT, BP, pulse rate, oxygen consumption, hormone levels, metabolism, sleep-wake cycles, and GI function [20, 23]. The circadian rhythm is mostly governed by the pacemaker neurons in the SCN, and is regulated by various circadian rhythm genes distributed in almost all organs and tissues [21, 25, 26]. So far, many circadian rhythm genes have been identified, including circadian locomotor output cycle kaput (CLOCK), brain and muscle Arnt-like protein 1 (BMAL1, also called aryl hydrocarbon receptor nuclear translocator-like), period (PER, including 3 homologs PER1, PER2, and PER3), cryptochrome (CRY, including CRY1 and CRY2), neuronal PAS domain protein 2 (NPAS2), retinoic acid related orphan receptor (ROR, including RORα, RORβ, and RORγ), nuclear receptor subfamily 1 group D member 1 (NR1D1, also called Rev-erbα), basic helix-loop-helix family members e40 and e41, timeless (TIM), D-box-binding protein (DBP), and casein kinases 1 and 2 [21].
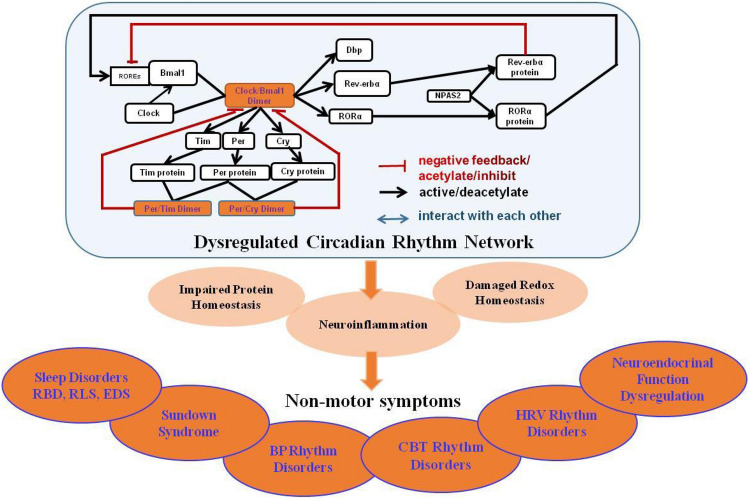
These circadian rhythm genes interact with each other to form complex networks, mainly via negative feedback loops, to maintain the homeostasis of the circadian rhythm at the levels of transcription, translation, and post-translation [21, 22]. It has been reported that at the beginning of the night, the expression of Per and Tim is activated by Clock/Bmal1 heterodimer, and at the end of the night, Per and Tim proteins are synthesized to form heterodimers [27, 28]. Per, Tim, or the Per/Tim heterodimer somehow restrain the activity of Clock and Bmal1 and their own transcription by entering the nucleus [27, 28]. CLOCK and BMAL1, two well-known control genes of the family of basic helix PAS transcription factors, guide the transcriptions of related genes and activate the expressions of PER and CRY genes to form heterodimers [29–31]. Once Per and Cry proteins accumulate to a certain level, they form a complex with Clock/Bmal1 heterodimer, thus inhibiting the transcription of their own genes [29–31]. At night, the Per/Cry complex is degraded to start another cycle [29–31]. DA can induce the expression of Per2 by promoting transcription of the Clock/Bmal1 complex [32]. NPAS2 promotes the expression of RORα and nuclear receptor Rev-erbα, main regulators of Bmal1 expression [33], to regulate the activity of the Clock/Baml1 heterodimer [34]. The underlying mechanism is that these receptors bind to the ROR response elements (RREs) located in the Bmal1 promoter, activate Bmal1 transcription through RORs, and inhibit Bmal1 transcription through Rev-erbs, leading to a Bmal1 rhythm [13, 34, 35] (Fig. 1). Changes in the expression spectrum or functions of circadian rhythm genes may result in a circadian rhythm dysregulation [36].
Fig. 1.
Dysregulated circadian rhythm network in Parkinson’s disease. Circadian rhythm genes interact with each other to form a complex network. The Clock/Bmal1 heterodimer activates Per and Tim gene expression, and the Per and Tim proteins form heterodimers. Per, Tim, or Per/Tim heterodimer restrains Clock and Bmal1 activity and their transcription. The Clock/Bmal1 heterodimer activates Per and Cry gene expression to form heterodimers. Once Per and Cry proteins accumulate to a certain extent, they form complexes with Bmal1/Clock heterodimers, and inhibit their own transcription. Then, the Per/Cry inhibitory complex degrades into the next cycle. NPAS2 mediates Bmal1 expression via the acid-related orphan receptor RORα and Rev-erbα, and further regulates the activity of Clock/Baml1 heterodimers. These receptors bind with retinoic acid-related orphan nuclear receptor response elements, which are located in the Bmal1 promoter, activate Bmal1 transcription through RORs and inhibit Bmal1 transcription through Rev-erbs. Circadian rhythm dysregulation may impact PD through the induction of neuroinflammation, impaired protein homeostasis, and redox homeostasis, manifested by various non-motor symptoms including sleep disorders such as RBD, RLS, EDS, sundown syndrome, BP rhythm disorders, CBT rhythm disorders, HRV rhythm disorders, neuroendocrine function dysregulation, and GI dysfunction. RBD rapid eye movement sleep behavior disorder, BP blood pressure, CBT core-body temperature, HRV Heart rate variability, RLS restless leg syndrome, EDS excessive daytime sleepiness, GI gastrointestinal.
Circadian Rhythm Dysregulation in Parkinson’s Disease
Almost all non-motor symptoms in PD are associated with impaired circadian rhythm [20]. Recently, increasing lines of evidence suggested that circadian rhythm dysregulation acts as the chief culprit leading to the non-motor symptoms of PD [20] (Fig. 1), especially sleep-wake disorders. The sleep-wake cycle is one of the most important and well-known circadian rhythms, regulated by the hypothalamus and reticular formation [8, 37]. About 60%–70% of PD patients suffer from sleep disorders [10–12]. Among the various sleep disorders, RBD is a potential early sign of PD and a parasomnia characterized by abnormal behaviors and loss of muscle atonia, manifesting in the main forms related to the REM-related dream-world of vocalizations, jerks, and motor behaviors during REM sleep [12, 38, 39]. RBD has a low incidence in the normal population but a high incidence in PD patients [40], causing a greater burden for care-givers and a higher mortality rate [40, 41]. The physiological nocturnal increase in REM sleep duration is lost in patients with PD and the increase of REM frequency across the night in PD patients with RBD is lost, supporting an alteration in the circadian system in RBD of PD patients [42]. Polysomnography reveals abnormal skeletal muscle atonia during RBD and consequently dream-enactment behavior marked by various redundant motor activity ranging from simple limb twitches to violent, complicated movements that may cause injury to the patient and/or the sleeping partner [10]. These behaviors can arise as early as 90 min after the first REM sleep episode, and occur more likely during a sleep session when REM sleep is more frequent, especially in the morning [12, 43]. RBD, as a potent predictor of PD and contributor to s poor prognosis, is more specific than any of the other prodromal markers of PD [38, 40].
RLS is also a frequent sleep disorder in PD [44]. Patients with RLS are characterized by the symptoms of moving the legs with or without sensory alterations which worsen at rest or with diminished activity and improve with movement, usually worse at night and in the evening [45, 46]. The diagnosis of RLS requires the patient’s symptoms to show circadian variation [47]. It has been reported that the rhythm of melatonin may be involved in RLS circadian variability [47, 48]. RLS patients who take melatonin in the evening often experience worse RLS symptoms, and bright light exposure may decrease melatonin secretion and reduce RLS motor symptoms [48]. EDS is also a common manifestation of sleep disorders in PD. Patients with EDS exhibit the most prominent impairment in circadian melatonin secretion, indicating an important role of circadian regulation in the manifestation of the EDS associated with PD [49].
PD patients who are often disturbed by disrupted sleep–wake circadian cycles and poor sleep quality may manifest emotional disorders including depression and anxiety [50]. Sleep, especially REM, is a process of emotional and brain homeostasis, optimally preparing the whole-body organs for the restoration of social and emotional function for the next day [51, 52]. Emotional disorders also affects subsequent sleep quality, especially the REM latency and REM duration, probably as a consequence of emotionally stressful events [53]. Approximately 45% of patients with PD suffer from depression, and 50% experience comorbidity of anxiety [54, 55]. Increasing lines of evidence have shown that depression and anxiety are associated with circadian rhythm dysregulation in PD patients [56, 57]. ‘Sundown syndrome’, also referred to as ‘nocturnal delirium’, which is used to describe mood disorders peaking in the late afternoon or evening in a daily pattern, can be found in PD [13], implying a strong relationship between circadian rhythm dysfunction and mood regulation impairment in patients with PD [58, 59]. Anxiety has been considered to be related to the pathology of the basal ganglia with DA and noradrenaline as the responsible neurotransmitters [8]. DAergic neurons in the midbrain ventral area innervate the prefrontal cortex, which is implicated in PD patients with mood disorders [59]. It has been reported that circadian rhythm dysregulation exacerbates DAergic neuronal loss in animal models of PD [60]. RRE overlaps with nerve growth factor inducible-B response element (NBRE), which is recognized by the nuclear receptor-related 1 protein (NURR1) in the tyrosine hydroxylase (TH) promoter [59, 61]. REV-ERBα antagonizes NURR1-induced activation of the TH promoter via binding to RRE/NBRE, driving circadian TH expression to regulate the circadian rhythmicity of the DAergic system [59, 61]. Rev-erbα is a key upstream regulator circadian rhythm gene to regulate mood via controlling the circadian rhythm of the DAergic system, suggesting that it is a potential therapeutic target for those PD patients with comorbid mood disorders [59, 61]. Polymorphisms of circadian rhythm genes have been shown to be associated with depression in PD patients [57]. Animal models of PD with emotional disorders need to be developed to explore the interaction between circadian rhythm dysregulation and emotion [56].
PD patients often manifest characteristic changes in the circadian rhythm of BP [14, 62, 63]. Nocturnal hypertension and postprandial hypotension are distinctive features in PD patients [14]. The difference between daytime and night-time BP is significantly smaller in PD than healthy control subjects [63]. In addition, the difference of CBT between the daytime and night is also significantly reduced in PD patients [17]. Moreover, the CBT change is significantly and negatively related to the severity of RBD [17]. Recently, it has been reported that changes in the thermo-regulatory circadian rhythm are associated with RBD rather than α-synucleinopathy [64], which may provide a prognostic means of evaluating the risk of developing PD in patients with idiopathic RBD (iRBD) [17, 65].
HRV is an index for measuring the autonomic, especially parasympathetic, functions [66]. A significantly decreased HRV has been found in PD patients all day but more sever at night [15, 16]. Moreover, this phenomenon becomes more profound with the motor symptoms, implying impaired autonomic, especially parasympathetic, cardiovascular regulation in PD [15, 16, 67, 68]. Moreover, HRV is significantly attenuated in patients with iRBD in the waking state, also indicating abnormalities of autonomic function in RBD [68]. Considering the important roles of iRBD in PD [65], the clinical value of impaired HRV as an early sign of cardiovascular autonomic circadian rhythm in PD should be emphasized [15, 68].
Neuroendocrine function has also been correlated with circadian rhythm. Circadian rhythmic regulation of melatonin secretion is blunted in PD, as shown by the decreased amplitude of the melatonin rhythm and the reduced 24-h secreted melatonin level [49]. Breen et al. proposed that the degenerative changes of the neural structures controlling pineal function, especially the hypothalamic gray matter volume loss, are responsible for the reduced melatonin, which might be associated with the severity of PD [69]. These findings provide anatomical and physiological evidence of an intrinsic sleep and circadian phenotype [69]. In PD, after DAergic treatment, the secretion of melatonin is profoundly increased [70]. Despite melatonin, the circadian rhythm of cortisol secretion as a sensitive marker of circadian function is also influenced in PD [20]. At approximately 01:00, cortisol begins to rise with a peak at approximately 06:00, then declines with some small fluctuations; this trend also occurs in normal healthy volunteers, but the secretory pattern in PD patients from 18:00 to 01:00 tends to be flatter [18]. This curve indicates that cortisol release retains its daily rhythmic pattern but the total diurnal amount of cortisol secretion is elevated in PD [18]. This disturbed circadian rhythm of hormone secretion results in endocrine imbalance and has been demonstrated to be associated with the impaired alertness and sleep disorders of PD [18, 49].
GI dysfunction in PD has also been associated with circadian rhythm dysregulation [71]. PD patients without GI dysfunction show a higher plasma melatonin concentration than those with GI dysfunction [72]. Researchers have found that the GI tract is the largest organ to produce exogenous melatonin other than the pineal gland, and melatonin may serve important GI barrier functions [72, 73]. This implies a certain relationship between GI dysfunction and circadian rhythm dysregulation in PD [72].
Although increasing evidence has predicted potential correlations between circadian rhythm dysregulation and motor and/or non-motor symptoms fluctuations, the underlying pathological mechanisms are still far from being clearly investigated [74]. It has been hypothesized that circadian rhythm dysregulation impacts PD via the induction of neuroinflammation, impaired protein homeostasis, and redox homeostasis [75, 76]. Circadian rhythm dysregulation can trigger strong neuroinflammation and degeneration of the nigral DAergic neuronal system, exacerbating the motor deficit, as an environmental risk factor for PD development [60, 76]. Interestingly, α-synuclein is rhythmic in various tissues and the synuclein-interacting protein synphilin shows a strong circadian rhythm in the brain [77]. Circadian rhythm dysregulation may contribute to PD with aberrant α-synuclein aggregation [77]. Another study has proposed that circadian rhythm disturbs the redox homeostasis resulting in PD [78].
Changes of Imaging and Electroencephalogram in Parkinson’s Disease Patients with Circadian Rhythm Dysregulation
While the current diagnosis of PD mainly depends on clinicians' subjective judgment of clinical symptoms, imaging and the EEG may provide assistance in the early diagnosis and detection of PD pathology [79]. Structural and functional magnetic resonance imaging (MRI), positron emission tomography (PET)/single photon emission computed tomography (SPECT), and cardiac uptake of 123I-labeled meta-iodobenzylguanidine (MIBG) scintigrams are commonly used for PD diagnosis [80, 81]. The circadian rhythm modulates the neuronal activity in specific areas and widespread networks across the brain. Imaging and the EEG can present some of these abnormalities that are directly related to the ongoing neurodegeneration in the brain of patients with PD [82].
In structural imaging, alterations of gray matter and white matter in PD are usually identified [83]. White matter changes are usually associated with circadian autonomic dysfunctions, including HR and BP variability [84]. Circadian rhythm dysregulation of melatonin levels is associated with significant hypothalamic gray matter volume loss and the severity of PD, which may provide anatomical and physiological evidence for circadian rhythm dysfunction in PD [69]. The reduced thalamic volume in PD patients with RBD suggests a pathophysiologic role of the thalamus the underlying mechanism [85]. Sleep impairment in PD patients has also been determined in association with widespread white matter disintegration [86]. Specifically, PD patients with nocturnal hallucinations often exhibit a prominently reduced basal ganglia volume [87]. PD patients with sleep impairment display smaller cortical thickness in more extensive areas, including the bilateral frontoparietal and lateral temporal regions [86]. Reduced gray matter volume has been detected in PD patients with EDS and RLS [83]. Despite structural imaging, functional MRI is widely used to measure the functional changes in patients suffering from sleep impairment by estimating the abnormal patterns of brain connectivity [88]. As a whole, during the basic process of circadian rhythm patterns, basal ganglia dysfunction can lead to a more complex signal in task-relevant areas, such as the planum temporale and the inferior parietal lobule, and in the sequential activation of brain areas, circadian rhythm onset may result in high activity in the saliency network and widespread motor areas, and the caudate nucleus in patients with PD [82].
Combined with molecular imaging including PET and SPECT, these methods are often used to investigate the striatal DAergic function by measuring the extent of neuronal loss in PD [83]. A more severe deafferentation in the caudate of PD patients with RBD compared to those without RBD has been reported [80, 89], and the putamen DAergic function is more severely damaged in PD patients with RBD than those without RBD [80, 89]. PD patients with RBD exhibit more severe striatal DAergic deficiency, indicating a strong association between the presynaptic DAergic defect and RBD [83]. Molecular imaging reveals negative correlations between metabolic activity and DAergic function of the caudate and the severity of EDS [83]. Other than the DAergic system, cholinergic deficiency has been detected in PD patients with RBD, and the iron level is altered in PD patients with RLS [83]. Interestingly, cardiac uptake in 123I-labeled MIBG scintigrams, which measures the function of cardiac sympathetic neurons, has revealed a significant correlation between the systemic BP circadian patterns and cardiac 123I-MIBG uptake in patients with PD [90]. Furthermore, cardiac uptake of 123I-labeled MIBG is decreased in PD patients with clinical RBD compared to those with subclinical RBD and without sleep disorders [91, 92].
Neuroelectrophysiological examination is becoming another important technique for detecting the sleep disorders in PD [93, 94]. Polysomnography can be used to monitor the whole-night EEG followed by multiscale entropy (MSE) analysis [93]. PD patients usually show longer sleep latency and a higher spontaneous EEG arousal index [93]. During non-REM (NREM) sleep, the stage-specific MSE is increased in PD [93]. Cycling alternating pattern is a sensitive marker of the early NREM sleep instability of sleep microstructure altered in PD [94]. EEG α and σ activity are often increased during NREM sleep at an early stage of PD [95]. Disruption of REM sleep homeostasis has also been reported in PD with high θ/α (7.8 Hz –10.5 Hz) frequency during 23.00 to 01.40 at night [96]. Moreover, local field potentials of subthalamic nucleus activity are significantly increased for β power values during REM sleep.
While neuroimaging provides spatial evidence about the structural and functional basis of circadian rhythm dysregulation, neuroelectrophysiological examination is usually a supplement for neuroimaging measurement, considering its high temporal resolution [97, 98]. Both are useful tools to determine the changes of PD-related circadian rhythm dysregulation, and may serve as potential biomarkers to help the early diagnosis of PD.
Altered Circadian Rhythm Genes in Animal Models of Parkinson’s Disease
Increasing lines of evidence have revealed the altered expression of circadian rhythm genes in various animal models of PD (as summarized in Table 1), including 6-hydroxydopamine (6-OHDA) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned rodents, and transgenic animal models [99, 100]. For example, decreased Bmal1 and increased RORα have been reported in the brain of 6-OHDA model mice [101]. Repeated Mn2+ administration produces abnormal circadian rhythm gene expression in the brain, together with non-motor symptoms and PD-like motor impairments in mice [102].
Table 1.
Altered circadian rhythm genes in animal models and patients with Parkinson's disease.
| PD models | Samples | Methods | Results and conclusions | References |
|---|---|---|---|---|
| Rats induced by 6-OHDA | Striatum | RT-qPCR; WB | Bmal1, Per2↓, Rorα↑. A link between attenuated antioxidative response and circadian dysregulations in PD. | Wang et al. [101] |
| Rats induced by 6-OHDA treated with levodopa | Striatum, SCN, Plasma | RT- qPCR; ELISA; HPLC | After levodopa treatment, Bmal1↓, peak of Per2 delayed, cortisol secretion↑, melatonin↓ | Li et al. [163] |
| RIPD rats | Substantia nigra | RT-qPCR; WB | Bmal1, Clock, NPAS2, Per1 and Per2, Rev-erbα and DBP↓. Chronic low-grade neuroinflammation aggravates circadian dysregulation in RIPD rats. | Li et al. [110] |
| Mul1A6 and park1 mutants Drosophila | Brain | RT-qPCR; IHC; WB | Mul1 and park mutations disrupt Per, Tim, and Clock normal circadian rhythmic expression during the day. ATG5↓, autophagy involved in circadian dysregulation | Doktór et al. [27] |
| MPTP mouse | SCN | Bioluminescence, RT-PCR | Bmal1, Cry1, Rev-erbα↓, activation of AMPK causes circadian dysfunction | Hayashi et al. [152] |
| Rats injected with Mn2+ | Hypothalamus | RT-qPCR; IHC | Bmal1, Clock, NPAS2, Cry1, Per1 and Per2↓, Nr1d1 and DBP↑. Mn2+ administration produces abnormal circadian rhythm gene expression | Li et al. [102] |
| ASO transgenic mice | SCN | IHC | Per2 expression is not altered in the SCN of ASO mice, weakening of circadian output is a core feature of PD | Kudo et al. [99] |
| 17 PD patients | PBL | RT-qPCR | Bmal1, Bmal2↓.The relative Bmal1 level correlates positively with PD severity |
Cai et al. [132] Ding et al. [133] |
| 239 PD patients | PBL | ELISA, RT-qPCR | Serum cortisol level↑, circulating melatonin level↓ | Breen et al. [6] |
| 206 PD patients | PBL | MSP and sequencing | Methylation only detected in the CRY1 and NPAS2 promoters. NPAS2 hypomethylation detected in PD vs control | Lin et al. [134] |
| 480 PD patients | PBL | PCR-RFLP | Polymorphism of Tef rs738499 is associated with depression symptoms in PD | Hua et al. [57] |
| 1394 PD patients | PBL | Illumina GoldenGate chips | Genetic polymorphisms in Bmal1 and Per1genes contribute to PD | Gu et al. [136] |
| 646 PD patients | PBL | Competitive allele-specific PCR | CLOCK 3111T/C variant can be an independent risk factor for motor fluctuation and sleep disorders in PD | Lou et al. [138] |
The animal models of PD as well as the number of patients with PD, the tissues, methods of examination, and the conclusions in each study are displayed
The MPTP marmoset model of PD shows a significant sleep disturbance remarkably similar to PD patients manifesting with sleep-onset insomnia and disturbances in the circadian rhythm [103]. Circadian rhythm alterations in MPTP-induced DAergic nigro-striatal system lesions in non-human primates have been evaluated by in vivo PET and post-mortem TH and DA transporter quantification [104]. Interestingly, in the light-dark cycle, MPTP-treated non-human primates show rest-wake locomotor rhythms, although DA-depleted non-human primates exhibit low amplitude, decreased stability, and increased fragmentation [104]. When the circadian system is exposed to constant light, controls are less influenced whereas in DA-depleted non-human primates, locomotor rhythms are severely disturbed or completely abolished together with unaltered hormonal rhythms [104]. Expression of the circadian rhythm in MPTP monkeys requires environmental timing cues [104]. In other words, the central circadian rhythm in the SCN remains complete in PD primates with DA lesions, but in the absence of regulation by light, circadian rhythmic processes of striatal and DAergic functions that control locomotor output are unable to drive [104]. The circadian rhythm system of the sleep-wake disturbances in PD is more profoundly affected than previously thought.
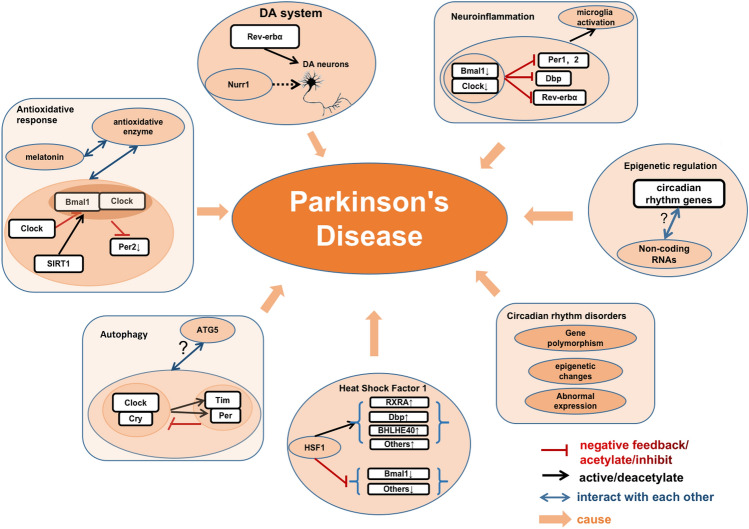
In addition, the relationship between circadian rhythm genes and PD pathology has become a hot research topic (Fig. 2). Based on recent discoveries of circadian rhythm gene networks associated with PD, a clear linkage has been found between the antioxidative response and circadian rhythm gene networks [101, 105]. For example, the melatonin rhythm modulates the daily rhythmic expression and activity of several antioxidant enzymes; at night, melatonin promotes the activity of glutathione peroxidase, a free radical scavenger, and increases the levels of oxidized glutathione [105]. Per2 is decreased in mice injected with 6-OHDA [101], whereas Cry1 binds to the Clock/Bmal1 complex and inhibits Clock/Bmal1-dependent transcription of Per2, therefore attenuating the antioxidative response in PD [106]. Silent information regulator 1 (SIRT1), a highly conserved nicotinamide adenine dinucleotide (+)-dependent class III deacetylase, deacetylates Bmal1 and Per2 and exerts protective effects against PD [101, 107]. The SIRT1 activator resveratrol reverses the 6-OHDA-induced damage of antioxidative activity in PD models by decreasing the acetylation of Bmal1 [101]. Interestingly, light entrainment including impaired masking, entrainment, and re-entrainment are affected in the SCN of human mutant A53T-SNCA transgenic mice [108]. This is associated with reduced vesicular glutamate transporter 2 immunoreactivity in the SCN, indicating affected glutamatergic signaling of retinal ganglion cells, which contributes to the input into the circadian system [108]. In mice overexpressing α-synuclein, Per2 expression is not altered, but the firing rate of SCN neurons is reduced, implying that weakened circadian output is a critical characteristic of PD [99]. The increased frequency of Tregs expressing Helios and NRP-1 is associated with the severity of PD [109]. Previous studies have reported that chronic low-grade neuroinflammation induced by lipopolysaccharides potentiates the neurotoxicity of rotenone and disrupts circadian rhythm gene or protein expression [110]. BMAL1 plays an important role in the survival of DAergic neurons and maintain normal function of the DAergic signaling pathway through regulating microglia-mediated neuroinflammation [111]. Rev-erbα has also been reported to regulate neuroinflammation [112].
Fig. 2.
Circadian rhythm genes and Parkinson’s disease. Circadian rhythm genes participate in PD pathology via various pathways. The Clock/Cry complex activates Tim and Per expression to form complexes and inhibits Clock/Cry complex activity. This network interacts with ATG5 to regulate autophagy in PD. Clock acetylates Bmal1, and inhibits the Bmal1/Clock-dependent transcription of Per2. SIRT1 deacetylates Per2 and Bmal1. This network along with melatonin interacts with antioxidant enzymes yielding an antioxidative response in PD. Bmal1 and Clock are lower in PD and the Bmal1/Clock complex-dependent transcription of Per1, Per2, Dbp, and Rev-erbα is inhibited. This network activates microglia-dependent neuroinflammation in PD. Rev-erbα competitively cooperates with Nurr1 to control DA gene transcription and the development and function of DA neurons. Some HSF1 targets that are associate with circadian rhythm genes (RXRA, Bhlhe40, and DBP) are upregulated, while some others (Bmal1) are inhibited. Non-coding RNAs undertake epigenetic regulation of the circadian rhythm system, but the mechanism is not clear. Circadian rhythm alterations, including abnormal expression of circadian rhythm genes, epigenetic changes, and gene polymorphisms, participate in the pathogenesis of PD.
Adult mammals undergo diurnal cycles of autophagy, which plays a key role in the cellular metabolic cycle [113]. In the absence of rhythmic autophagy, the accumulation and aggregation of misfolded proteins may lead to neurodegeneration [113–116]. Circadian rhythm dysregulation during cognitive loss and aging has been tied to the induction of autophagy [114]. Rev-erbα links the circadian and DA systems and competitively cooperates with Nurr1, which control the DAergic neuron-associated gene transcription, and further the optimal development and function of DAergic neurons [117–121]. Nur77, a member of the nuclear steroid receptor subgroup together with Nurr1 and NOR-1 participating in DAergic neuron loss and dyskinesia induced by levodopa in PD [122], displays an obvious circadian rhythm with an elevated mRNA level at night [123].
It has been reported that heat shock factor 1 (HSF1) participates in neurodegeneration [124, 125]. Some of the HSF1 targets are associated with alterations of the circadian rhythm, evidenced by upregulated Bhlhe40 and DBP and downregulated Bmal1 [124]. Non-coding RNAs, which control the development of neuronal stem cells and neuronal differentiation [126, 127], undertake epigenetic regulation of the brain clock system at the transcriptional and post-transcriptional levels [128, 129]. Although the mechanisms underlying the interplay between circadian rhythm genes and PD pathology are still largely unknown, increasing evidence from clinical or experimental studies has revealed the interactions between circadian rhythm and PD related genes [130]. Mutations in the PARK2 gene coding for parkin is a crucial pathogenic gene for the disruption of mitophagy-mediated mitochondrial quality control [131]. Using fibroblasts from PD patients carrying PARK2 mutations, Pacelli et al. revealed that the dramatic impact of metabolic fluxes on cell circadian rhythm and circadian rhythm genes relies on mutations in parkin [130]. Drosophila with mutations in genes encoding mitochondrial ligases MUL1 and PARKIN, a commonly used model for research on PD, show an increasing level of total Per protein, accompanied by elevated total activity, shorter sleep, higher levels of free radicals, and inhibited autophagy [27].
Increasing evidence has indicated that the expressions of circadian rhythm genes are abnormal in various PD models, and may predict the mechanisms underlying PD [27, 74, 99]. It is likely that the network of circadian rhythm genes may play a role in the pathogenic mechanism of PD. All these findings indicate a potential role of the circadian rhythm gene network in PD pathogenesis. The exact modulating mechanisms between the key molecules such as Nurr1, α-synuclein, and circadian rhythm genes needs further investigation.
Altered Circadian Rhythm Genes in Patients with Parkinson's Disease
More and more clinical studies have investigated the alteration of circadian rhythm gene expression in peripheral blood lymphocytes (PBLs) from patients with PD [57, 132–138] (Table 1). Several studies have found abnormal expression of circadian rhythm genes in PBLs from PD patients. Bmal1 and Bmal2 are significantly decreased in PD, while the relative Bmal1 level is positively correlated with PD severity [132, 133]. Most circadian rhythm gene promoters are devoid of methylation, except for CRY1 and NPAS2 [134]. However, compared with healthy controls, epigenetic changes of hypomethylation in the NPAS2 promoter have been found in the early stage of disease in PBLs from PD patients [134, 135]. This finding provides a potential biomarker for discerning PD patients from healthy subjects [134, 135]. The negative feedback loop of circadian rhythm genes prompting epigenetic changes of NPAS2 expression may be the main cause of abnormal Bmal1 and Bmal2 levels in PD patients’ leukocytes [134]. Gene polymorphisms in the promoter region of the circadian rhythm genes Bmal1 and Per1 may play a role in the development of PD [136]. The variation of Bmal1 rs900147 is more robust in tremor-dominant patients than postural-instability and gait-difficulty cases, while the association of the PER1 rs2253820 variant is stronger in postural-instability and gait-disturbance cases than tremor-dominant cases [136]. The polymorphism of Tef rs738499 is often associated with the depression symptoms and sleep disturbances in PD [57, 137]. The Clock 3111T/C variant can be regarded as an independent risk factor for non-motor sleep impairment and motor fluctuation in PD [138]. The abnormal expression of circadian rhythm genes, epigenetic changes, and gene polymorphisms may provide a new perspective for future study of PD pathogenesis and serve as potential biomarkers for PD diagnosis.
The possible mechanisms of RBD neuropathology associated with PD have not been clearly investigated. It is likely that RBD arises from the same pathogenic mechanisms that underlie synucleinopathies, with disease processes beginning in the caudal brain stem where REM sleep atonia is controlled [139]. Interestingly, Liu et al. recently found that sleep-wake brain states and motor behaviors are co-regulated by glutamic acid decarboxylase 2 neurons in the substantia nigra pars reticulata [111]. It is well accepted that early basal ganglia network dysfunction during RBD contributes to the development of PD [140]. In addition, sleep is proposed to help remove the aggregates of neurotoxic α-synuclein from the brain via the lymphatic system [141, 142]. REM sleep disorders, especially RBD, circadian rhythms, and circadian rhythm gene dysfunctions are proposed to disturb the glymphatic flow and are linked with Lewy body aggregates and substantia nigra DAergic cell loss [142]. Furthermore, RBD is associated with altered expression of clock genes and delayed melatonin secretion, and circadian rhythm dysregulation is a part of RBD [143]. Weissova et al. assessed the expression levels of circadian rhythm genes in PBLs by real-time quantitative PCR and analyzed 24-h melatonin profiles in peripheral blood serum by radioimmunoassay in 10 RBD patients and 9 controls [143]. In the RBD patients, Per2, Bmal1, and Rev-erbα circadian rhythms disappear, the amplitude of Per3 diminished, and melatonin secretion was delayed [143]. It is proposed that alterations in the expression of circadian rhythm genes and melatonin levels in patients with RBD might be a potential biomarker in the early stage of synucleinopathies including PD [143]. The intimate relationship between circadian rhythm disorders and PD with RBD pathology should be further explored.
Potential Impact of Pharmacological and Non-pharmacological Therapies on the Circadian Rhythm
The recent therapies for PD, including levodopa, dopamine agonists, and monoamine oxidase-B inhibitors, are useful initial therapies. However the efficacy decreases over time due to medication tolerance [144]. Then the subsequent therapy is started through increasing the dose of the initial therapy or adding new kinds of treatment including catechol-O-methyltransferase inhibitors, istradefylline, and amantadine. Levodopa carbidopa enteral suspension infusion and unilateral or bilateral deep brain stimulation as advanced therapy are further added for long-duration PD patients [144]. Much more interestingly, therapeutic approaches targeting circadian rhythm system may hold efficacy and promise. Circadian rhythm dysregulation, as a potential contributor to the development and progression of PD, has been proposed as a target for either pharmacological or non-pharmacological therapy against PD [13, 18, 49, 127, 145, 146]. Among PD patients, especially those with daytime sleepiness and impaired circadian melatonin secretion [49], exogenous supplementation of melatonin is considered to be therapeutic for impaired alertness and poor sleep [49]. However, so far, the outcome from randomized controlled clinical trials of melatonin supplements on sleep quality and activity rhythms in patients with PD has yielded inconsistent results [13]. Melatonin administration successfully improves self-report measures of sleep as evaluated by Pittsburgh Sleep Quality Index, but fails to ameliorate sleep quality monitored by polysomnography and motor dysfunction assessed by the United Parkinson’s Disease Rating Scale [147]. In addition, melatonin may be neuroprotective via the MT1 and MT2 high-affinity G protein-coupled melatonin receptors [148]. The discovery of MT1 or MT2 melatonin receptor-selective drugs may improve the efficacy and lead to new therapeutic candidates [148]. The WNT/β-catenin pathway plays an important role in maintaining mitochondrial functions [149]. The activation of peroxisome proliferator-activated γ (PPARγ) can be induced by inhibition of the WNT/β-catenin pathway [150]. The circadian rhythm can directly regulate the WNT/β-catenin pathway and PPARγ involved in the reprogramming of cellular energy metabolism, resulting in down-regulation of the classical WNT/β-catenin pathway and upregulation of PPARγ [150, 151]. The WNT/β-catenin pathway and PPARγ could serve as potential therapeutic targets against PD [151]. Activation of adenosine 5′-monophosphate-activated protein kinase (AMPK) results in circadian dysfunction, suggesting that adenosine 5′-triphosphate (ATP) might be a novel therapeutic strategy in PD [152]. It is well-accepted that DA regulates the circadian rhythm system both directly and indirectly [153]. Interestingly, researchers have found that sodium can activate neurons in the SCN by regulating the circadian rhythm system and output via an excitatory GABAergic pathway [154]. It seems that pharmacological therapies alleviating various symptoms of circadian rhythm dysregulation need to be appraised [153].
Non-pharmacological therapies also play a vital role in PD treatment. Results from recent clinical trials suggest that timed light therapy may be a feasible intervention for improving the sleep-wake cycle, reducing daytime sleepiness, and increasing daily physical activity for PD [127]. It seems that the combination of timed light therapy with timed melatonin supplements may be more effective [13]. Daily time-dependent physical exercise at moderate intensity has differential effects on the circadian melatonin rhythm [146]. Morning exercise may increase parasympathetic activity, while evening exercise may enhance sympathetic activity during sleep [146]. The specific characteristics of the autonomic nervous system could be responsible; furthermore, evening exercise may shift the offset phase of the nocturnal melatonin rise [146]. Thus, the sleep-wake cycle is regulated primarily by physical exercise [146], which gives timed physical exercise the potential to treat circadian rhythm dysregulation in PD [49]. Reduction in caloric intake and fasting extends the life span in animal models [71]. The underlying mechanism has been attributed the strong antioxidant capacity of melatonin which is produced by the GI tract [71, 155]. Fasting in animals increases the level of melatonin produced by the GI tract [155]. Recently, researchers have founded that intake of melatonin before going to bed might achieve the same effect as fasting [156]. More and more patients taking advantage of the antioxidant capacity of melatonin are taking melatonin daily to treat age-related PD [71].
It is worth noting that almost all DAergic anti-PD medications impact sleep [157]. A ‘sleep attack’ which describes suddenly and unintendedly falling asleep, may place patients at risk during daily activities such as eating and driving [157, 158]. PD patients treated with DA receptor agonists alone or in combination with levodopa, have a higher incidence of sleep attacks than patients treated with other agents [157]. To date, sleep attacks have been occasionally reported in PD patients treated with the DA receptor agonists pramipexole, ropinirole, piribedil, and pergolide [158–162]. The potential impact of medications on the circadian rhythm system in PD have not been fully investigated. Anti-PD medications may also disturb the circadian rhythm. PD patients receiving the classical anti-PD drug levodopa usually suffer from severe circadian dysfunction [163]. In contrast, melatonin administration can restore the daily rhythms of serotonin metabolism and the expression of clock genes in PD animal models [164].
Conclusions
This review summarizes recent research progress on the non-motor features of PD with special focus on circadian rhythm dysregulation and altered circadian rhythm genes. The circadian rhythm gene network maintains the generation and regulation of the circadian rhythm. Circadian rhythm dysregulation, especially sleep impairment, is an early symptom of PD. Findings from imaging and EEG are useful supplements for the diagnosis of PD associated with circadian rhythm dysregulation. Many studies have confirmed the abnormal expression of circadian rhythm genes in cell and animal models of PD, and occasionally in patients with PD, indicating a possible involvement of circadian rhythm genes in disease development. Among so many etiologies and mechanisms of PD, these findings of circadian rhythm dysregulation may provide a new perspective for further study of its pathogenesis. The alterations of circadian rhythm genes reported in PD patients may become a new biomarker for its diagnosis and evaluation of its severity. Moreover, the impacts of pharmacological and non-pharmacological therapies for PD on the circadian rhythm system may provide a novel and prognostic target for its management.
Acknowledgements
This review was supported by the National Nature Science Foundation of China (81771521), Key Research and Development Plan of Liaoning Science and Technology Department (2018225051), Guangdong Provincial Key R&D Program (2018B030337001), and the National Key Research and Development Program of China (2016YFC1306600).
Abbreviations
- PD
Parkinson’s disease
- DA
Dopamine
- SCN
Suprachiasmatic nucleus
- Clock
Circadian locomotor output cycles kaput
- Bmal1
Brain and muscle Arnt-like protein 1
- Per
Period
- Cry
Cryptochrome
- NPAS2
Neuronal PAS domain protein 2
- ROR
Retinal related orphan receptor
- RREs
ROR response elements
- NR1D1
Nuclear receptor subfamily 1 group D member 1
- Bhlhe
Basic helix-loop-helix family member
- Tim
Timeless
- DBP
D-box-binding protein
- REM
Rapid eye movement
- RBD
Rapid eye movement sleep behavior disorder
- BP
Blood pressure
- CBT
Core-body temperature
- HRV
Heart rate variability
- GI
Gastrointestinal
- MRI
Magnetic resonance imaging
- PET
Positron emission tomography
- SPECT
Single photon emission computed tomography
- NURR1
Nuclear receptor-related 1 protein
- TH
Tyrosine hydroxylase
- EDS
Excessive daytime sleepiness
- RLS
Restless leg syndrome
- MIBG
Meta-iodobenzylguanidine
- PSG
Polysomnograph
- MSE
Multiscale entropy
- NREM
Non-REM
- CAP
Cycling alternating pattern
- 6-OHDA
6-Hydroxydopamine
- MPTP
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- Mn
Manganese
- SIRT1
Silent information regulator 1
- HSF1
Heat shock factor 1
- RT-qPCR
Real-time quantitative polymerase chain reaction
- HPLC
High-performance liquid chromatography
- WB
Western blotting
- RIPD
Rotenone-induced PD
- LPS
Lipopolysaccharides
- ROT
Rotenone
- ATG5
Autophagy-related gene 5
- AMPK
Adenosine 5′-monophosphate (AMP)-activated protein kinase
- ATP
Adenosine 5′-triphosphate
- ASO
Alpha-synuclein overexpressing
- IHC
Immunohistochemical staining
- ELISA
Enzyme linked immunosorbent assay
- MSP
Methylation-specific PCR
- PCR-RFLP
Polymerase chain reaction-restriction fragment length polymorphism
- PPARγ
Peroxisome proliferator-activated γ
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Erkkinen MG, Kim MO, Geschwind MD. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2018;10:a033118. doi: 10.1101/cshperspect.a033118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li TB, Yang ZF, Li S, Cheng C, Shen BR, Le WD. Alterations of NURR1 and cytokines in the peripheral blood mononuclear cells: Combined biomarkers for Parkinson's disease. Front Aging Neurosci. 2018;10:392. doi: 10.3389/fnagi.2018.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung SJ, Armasu SM, Anderson KJ, Biernacka JM, Lesnick TG, Rider DN, et al. Genetic susceptibility loci, environmental exposures, and Parkinson's disease: A case-control study of gene-environment interactions. Parkinsonism Relat Disord. 2013;19:595–599. doi: 10.1016/j.parkreldis.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li TB, Le WD. Biomarkers for Parkinson's disease: How good are they? Neurosci Bull. 2020;36:183–194. doi: 10.1007/s12264-019-00433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickson DW. Parkinson's disease and Parkinsonism: Neuropathology. Cold Spring Harb Perspect Med. 2012;2:a009258. doi: 10.1101/cshperspect.a009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breen DP, Vuono R, Nawarathna U, Fisher K, Shneerson JM, Reddy AB, et al. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol. 2014;71:589–595. doi: 10.1001/jamaneurol.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radhakrishnan DM, Goyal V. Parkinson's disease: A review. Neurol India. 2018;66:S26–S35. doi: 10.4103/0028-3886.226451. [DOI] [PubMed] [Google Scholar]
- 8.Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci. 2017;18:435–450. doi: 10.1038/nrn.2017.62. [DOI] [PubMed] [Google Scholar]
- 9.Schrag A, Horsfall L, Walters K, Noyce A, Petersen I. Prediagnostic presentations of Parkinson's disease in primary care: A case-control study. Lancet Neurol. 2015;14:57–64. doi: 10.1016/S1474-4422(14)70287-X. [DOI] [PubMed] [Google Scholar]
- 10.Ma JF, Hou MM, Tang HD, Gao X, Liang L, Zhu LF, et al. REM sleep behavior disorder was associated with Parkinson's disease: A community-based study. BMC Neurol. 2016;16:123. doi: 10.1186/s12883-016-0640-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trenkwalder C. Sleep dysfunction in Parkinson's disease. Clin Neurosci N Y N Y. 1998;5:107–114. [PubMed] [Google Scholar]
- 12.Zhang F, Niu L, Liu X, Liu Y, Li S, Yu H, et al. Rapid eye movement sleep behavior disorder and neurodegenerative diseases: An update. Aging Dis. 2020;11:315–326. doi: 10.14336/AD.2019.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hood S, Amir S. Neurodegeneration and the circadian clock. Front Aging Neurosci. 2017;9:170. doi: 10.3389/fnagi.2017.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahsan Ejaz A, Sekhon IS, Munjal S. Characteristic findings on 24-h ambulatory blood pressure monitoring in a series of patients with Parkinson's disease. Eur J Intern Med. 2006;17:417–420. doi: 10.1016/j.ejim.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 15.Kallio M, Haapaniemi T, Turkka J, Suominen K, Tolonen U, Sotaniemi K, et al. Heart rate variability in patients with untreated Parkinson's disease. Eur J Neurol. 2000;7:667–672. doi: 10.1046/j.1468-1331.2000.00127.x. [DOI] [PubMed] [Google Scholar]
- 16.Pursiainen V, Haapaniemi TH, Korpelainen JT, Huikuri HV, Sotaniemi KA, Myllylä VV. Circadian heart rate variability in Parkinson's disease. J Neurol. 2002;249:1535–1540. doi: 10.1007/s00415-002-0884-0. [DOI] [PubMed] [Google Scholar]
- 17.Zhong G, Bolitho S, Grunstein R, Naismith SL, Lewis SJ. The relationship between thermoregulation and REM sleep behaviour disorder in Parkinson's disease. PLoS ONE. 2013;8:e72661. doi: 10.1371/journal.pone.0072661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartmann A, Veldhuis JD, Deuschle M, Standhardt H, Heuser I. Twenty-four hour cortisol release profiles in patients with Alzheimer's and Parkinson's disease compared to normal controls: Ultradian secretory pulsatility and diurnal variation. Neurobiol Aging. 1997;18:285–289. doi: 10.1016/s0197-4580(97)80309-0. [DOI] [PubMed] [Google Scholar]
- 19.Fasano A, Visanji NP, Liu LWC, Lang AE, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson's disease. Lancet Neurol. 2015;14:625–639. doi: 10.1016/S1474-4422(15)00007-1. [DOI] [PubMed] [Google Scholar]
- 20.Li SY, Wang YL, Wang F, Hu LF, Liu CF. A new perspective for Parkinson's disease: Circadian rhythm. Neurosci Bull. 2017;33:62–72. doi: 10.1007/s12264-016-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patke A, Young MW, Axelrod S. Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol. 2020;21:67–84. doi: 10.1038/s41580-019-0179-2. [DOI] [PubMed] [Google Scholar]
- 22.Dardente H, Cermakian N. Molecular circadian rhythms in central and peripheral clocks in mammals. Chronobiol Int. 2007;24:195–213. doi: 10.1080/07420520701283693. [DOI] [PubMed] [Google Scholar]
- 23.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Logan RW, McClung CA. Rhythms of life: Circadian disruption and brain disorders across the lifespan. Nat Rev Neurosci. 2019;20:49–65. doi: 10.1038/s41583-018-0088-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto T, Nakahata Y, Soma H, Akashi M, Mamine T, Takumi T. Transcriptional oscillation of canonical clock genes in mouse peripheral tissues. BMC Mol Biol. 2004;5:18. doi: 10.1186/1471-2199-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo H, Brewer JM, Champhekar A, Harris RB, Bittman EL. Differential control of peripheral circadian rhythms by suprachiasmatic-dependent neural signals. PNAS. 2005;102:3111–3116. doi: 10.1073/pnas.0409734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doktór B, Damulewicz M, Pyza E. Effects of MUL1 and PARKIN on the circadian clock, brain and behaviour in Drosophila Parkinson's disease models. BMC Neurosci. 2019;20:24. doi: 10.1186/s12868-019-0506-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee C, Bae K, Edery I. PER and TIM inhibit the DNA binding activity of a Drosophila CLOCK-CYC/dBMAL1 heterodimer without disrupting formation of the heterodimer: A basis for circadian transcription. Mol Cell Biol. 1999;19:5316–5325. doi: 10.1128/mcb.19.8.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Videnovic A, Willis GL. Circadian system - A novel diagnostic and therapeutic target in Parkinson's disease? Mov Disord. 2016;31:260–269. doi: 10.1002/mds.26509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: Implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM. Posttranslational mechanisms regulate the mammalian circadian clock. Cell. 2001;107:855–867. doi: 10.1016/s0092-8674(01)00610-9. [DOI] [PubMed] [Google Scholar]
- 32.Yujnovsky I, Hirayama J, Doi M, Borrelli E, Sassone-Corsi P. Signaling mediated by the dopamine D2 receptor potentiates circadian regulation by CLOCK: BMAL1. PNAS. 2006;103:6386–6391. doi: 10.1073/pnas.0510691103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reinke H, Asher G. Crosstalk between metabolism and circadian clocks. Nat Rev Mol Cell Biol. 2019;20:227–241. doi: 10.1038/s41580-018-0096-9. [DOI] [PubMed] [Google Scholar]
- 34.Akashi M, Takumi T. The orphan nuclear receptor RORalpha regulates circadian transcription of the mammalian core-clock Bmal1. Nat Struct Mol Biol. 2005;12:441–448. doi: 10.1038/nsmb925. [DOI] [PubMed] [Google Scholar]
- 35.Bunney BG, Li JZ, Walsh DM, Stein R, Vawter MP, Cartagena P, et al. Circadian dysregulation of clock genes: Clues to rapid treatments in major depressive disorder. Mol Psychiatry. 2015;20:48–55. doi: 10.1038/mp.2014.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Videnovic A, Lazar AS, Barker RA, Overeem S. 'The clocks that time us'——circadian rhythms in neurodegenerative disorders. Nat Rev Neurol. 2014;10:683–693. doi: 10.1038/nrneurol.2014.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gros P, Videnovic A. Overview of sleep and circadian rhythm disorders in Parkinson disease. Clin Geriatr Med. 2020;36:119–130. doi: 10.1016/j.cger.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin H, Zhang JR, Shen Y, Liu CF. Clinical significance of REM sleep behavior disorders and other non-motor symptoms of Parkinsonism. Neurosci Bull. 2017;33:576–584. doi: 10.1007/s12264-017-0164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin CY, Yu RL, Wu RM, Tan CH. Effect of ALDH2 on sleep disturbances in patients with Parkinson's disease. Sci Rep. 2019;9:18950. doi: 10.1038/s41598-019-55427-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnaldi D, Latimier A, Leu-Semenescu S, Vidailhet M, Arnulf I. Loss of REM sleep features across nighttime in REM sleep behavior disorder. Sleep Med. 2016;17:134–137. doi: 10.1016/j.sleep.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez CL, Jaimchariyatam N, Budur K. Rapid eye movement sleep behavior disorder: A review of the literature and update on current concepts. Chest. 2017;152:650–662. doi: 10.1016/j.chest.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 42.Kim Y, Kim YE, Park EO, Shin CW, Kim HJ, Jeon B. REM sleep behavior disorder portends poor prognosis in Parkinson's disease: A systematic review. J Clin Neurosci. 2018;47:6–13. doi: 10.1016/j.jocn.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 43.Yan YY, Lei K, Li YY, Liu XF, Chang Y. The correlation between possible RBD and cognitive function in Parkinson's disease patients in China. Ann Clin Transl Neurol. 2019;6:848–853. doi: 10.1002/acn3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schrempf W, Brandt MD, Storch A, Reichmann H. Sleep disorders in Parkinson's disease. J Parkinsons Dis. 2014;4:211–221. doi: 10.3233/JPD-130301. [DOI] [PubMed] [Google Scholar]
- 45.Fereshtehnejad SM, Shafieesabet M, Shahidi GA, Delbari A, Lökk J. Restless legs syndrome in patients with Parkinson's disease: A comparative study on prevalence, clinical characteristics, quality of life and nutritional status. Acta Neurol Scand. 2015;131:211–218. doi: 10.1111/ane.12307. [DOI] [PubMed] [Google Scholar]
- 46.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J, et al. Restless legs syndrome: Diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–119. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 47.Jung JS, Lee HJ, Cho CH, Kang SG, Yoon HK, Park YM, et al. Association between restless legs syndrome and CLOCK and NPAS2 gene polymorphisms in schizophrenia. Chronobiol Int. 2014;31:838–844. doi: 10.3109/07420528.2014.914034. [DOI] [PubMed] [Google Scholar]
- 48.Whittom S, Dumont M, Petit D, Desautels A, Adam B, Lavigne G, et al. Effects of melatonin and bright light administration on motor and sensory symptoms of RLS. Sleep Med. 2010;11:351–355. doi: 10.1016/j.sleep.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 49.Videnovic A, Noble C, Reid KJ, Peng J, Turek FW, Marconi A, et al. Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol. 2014;71:463–469. doi: 10.1001/jamaneurol.2013.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker WH, 2nd, Walton JC, DeVries AC, Nelson RJ. Circadian rhythm disruption and mental health. Transl Psychiatry. 2020;10:28. doi: 10.1038/s41398-020-0694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldstein AN, Walker MP. The role of sleep in emotional brain function. Annu Rev Clin Psychol. 2014;10:679–708. doi: 10.1146/annurev-clinpsy-032813-153716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Armitage R. Sleep and circadian rhythms in mood disorders. Acta Psychiatr Scand Suppl 2007: 104–115. [DOI] [PubMed]
- 53.Vandekerckhove M, Cluydts R. The emotional brain and sleep: An intimate relationship. Sleep Med Rev. 2010;14:219–226. doi: 10.1016/j.smrv.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 54.Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson's disease: Dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8:464–474. doi: 10.1016/S1474-4422(09)70068-7. [DOI] [PubMed] [Google Scholar]
- 55.Brown RG, Landau S, Hindle JV, Playfer J, Samuel M, Wilson KC, et al. Depression and anxiety related subtypes in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2011;82:803–809. doi: 10.1136/jnnp.2010.213652. [DOI] [PubMed] [Google Scholar]
- 56.Kronfeld-Schor N, Einat H. Circadian rhythms and depression: Human psychopathology and animal models. Neuropharmacology. 2012;62:101–114. doi: 10.1016/j.neuropharm.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 57.Hua P, Liu WG, Kuo SH, Zhao YY, Chen L, Zhang N, et al. Association of Tef polymorphism with depression in Parkinson disease. Mov Disord. 2012;27:1694–1697. doi: 10.1002/mds.25195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bedrosian TA, Nelson RJ. Sundowning syndrome in aging and dementia: Research in mouse models. Exp Neurol. 2013;243:67–73. doi: 10.1016/j.expneurol.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 59.Kim J, Jang S, Choe HK, Chung S, Son GH, Kim K. Implications of circadian rhythm in dopamine and mood regulation. Mol Cells. 2017;40:450–456. doi: 10.14348/molcells.2017.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lauretti E, Di Meco A, Merali S, Praticò D. Circadian rhythm dysfunction: A novel environmental risk factor for Parkinson’s disease. Mol Psychiatry. 2017;22:280–286. doi: 10.1038/mp.2016.47. [DOI] [PubMed] [Google Scholar]
- 61.Chung S, Lee EJ, Yun S, Choe HK, Park SB, Son HJ, et al. Impact of circadian nuclear receptor REV-ERBα on midbrain dopamine production and mood regulation. Cell. 2014;157:858–868. doi: 10.1016/j.cell.2014.03.039. [DOI] [PubMed] [Google Scholar]
- 62.Vallelonga F, di Stefano C, Merola A, Romagnolo A, Sobrero G, Milazzo V, et al. Blood pressure circadian rhythm alterations in alpha-synucleinopathies. J Neurol. 2019;266:1141–1152. doi: 10.1007/s00415-019-09244-w. [DOI] [PubMed] [Google Scholar]
- 63.Kanegusuku H, Silva-Batista C, Peçanha T, Silva-Junior N, Queiroz A, Costa L, et al. Patients with Parkinson disease present high ambulatory blood pressure variability. Clin Physiol Funct Imaging. 2017;37:530–535. doi: 10.1111/cpf.12338. [DOI] [PubMed] [Google Scholar]
- 64.Raupach AK, Ehgoetz Martens KA, Memarian N, Zhong G, Matar E, Halliday GM, et al. Assessing the role of nocturnal core body temperature dysregulation as a biomarker of neurodegeneration. J Sleep Res. 2020;29:e12939. doi: 10.1111/jsr.12939. [DOI] [PubMed] [Google Scholar]
- 65.Iranzo A, Molinuevo JL, Santamaría J, Serradell M, Martí MJ, Valldeoriola F, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: A descriptive study. Lancet Neurol. 2006;5:572–577. doi: 10.1016/S1474-4422(06)70476-8. [DOI] [PubMed] [Google Scholar]
- 66.Heart rate variability. standards of measurement, physiological interpretation, and clinical use. task force of the European society of cardiology and the North American society of pacing and electrophysiology. Eur Heart J 1996, 17: 354–381. [PubMed]
- 67.Harnod D, Wen SH, Chen SY, Harnod T. The association of heart rate variability with parkinsonian motor symptom duration. Yonsei Med J. 2014;55:1297–1302. doi: 10.3349/ymj.2014.55.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haapaniemi TH, Pursiainen V, Korpelainen JT, Huikuri HV, Sotaniemi KA, Myllylä VV. Ambulatory ECG and analysis of heart rate variability in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2001;70:305–310. doi: 10.1136/jnnp.70.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Breen DP, Nombela C, Vuono R, Jones PS, Fisher K, Burn DJ, et al. Hypothalamic volume loss is associated with reduced melatonin output in Parkinson's disease. Mov Disord. 2016;31:1062–1066. doi: 10.1002/mds.26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bolitho SJ, Naismith SL, Rajaratnam SM, Grunstein RR, Hodges JR, Terpening Z, et al. Disturbances in melatonin secretion and circadian sleep-wake regulation in Parkinson disease. Sleep Med. 2014;15:342–347. doi: 10.1016/j.sleep.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 71.Bubenik GA, Konturek SJ. Melatonin and aging: Prospects for human treatment. J Physiol Pharmacol. 2011;62:13–19. [PubMed] [Google Scholar]
- 72.Li LY, Zhao ZX, Ma JJ, Zheng JH, Huang S, Hu SY, et al. Elevated plasma melatonin levels are correlated with the non-motor symptoms in Parkinson's disease: A cross-sectional study. Front Neurosci. 2020;14:505. doi: 10.3389/fnins.2020.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sommansson A, Saudi WS, Nylander O, Sjöblom M. Melatonin inhibits alcohol-induced increases in duodenal mucosal permeability in rats in vivo. Am J Physiol Gastrointest Liver Physiol. 2013;305:G95–G105. doi: 10.1152/ajpgi.00074.2013. [DOI] [PubMed] [Google Scholar]
- 74.De Lazzari F, Bisaglia M, Zordan MA, Sandrelli F. Circadian rhythm abnormalities in Parkinson's disease from humans to flies and back. Int J Mol Sci. 2018;19:E3911. doi: 10.3390/ijms19123911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leng Y, Goldman SM, Cawthon PM, Stone KL, Ancoli-Israel S, Yaffe K. Excessive daytime sleepiness, objective napping and 11-year risk of Parkinson's disease in older men. Int J Epidemiol. 2018;47:1679–1686. doi: 10.1093/ije/dyy098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leng Y, Musiek ES, Hu K, Cappuccio FP, Yaffe K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 2019;18:307–318. doi: 10.1016/S1474-4422(18)30461-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hastings MH, Goedert M. Circadian clocks and neurodegenerative diseases: Time to aggregate? Curr Opin Neurobiol. 2013;23:880–887. doi: 10.1016/j.conb.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Musiek ES, Lim MM, Yang GR, Bauer AQ, Qi L, Lee Y, et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest. 2013;123:5389–5400. doi: 10.1172/JCI70317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lotankar S, Prabhavalkar KS, Bhatt LK. Biomarkers for Parkinson's disease: Recent advancement. Neurosci Bull. 2017;33:585–597. doi: 10.1007/s12264-017-0183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Orimo S. New development of diagnosis and treatment for Parkinson's disease. Rinsho Shinkeigaku. 2017;57:259–273. doi: 10.5692/clinicalneurol.cn-000969. [DOI] [PubMed] [Google Scholar]
- 81.Bourgouin PA, Rahayel S, Gaubert M, Arnaldi D, Hu M, Heidbreder A, et al. Neuroimaging of rapid eye movement sleep behavior disorder. Int Rev Neurobiol. 2019;144:185–210. doi: 10.1016/bs.irn.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 82.Vikene K, Skeie GO, Specht K. Abnormal phasic activity in saliency network, motor areas, and basal Ganglia in Parkinson's disease during rhythm perception. Hum Brain Mapp. 2019;40:916–927. doi: 10.1002/hbm.24421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yousaf T, Pagano G, Wilson H, Politis M. Neuroimaging of sleep disturbances in movement disorders. Front Neurol. 2018;9:767. doi: 10.3389/fneur.2018.00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oh YS, Kim JS, Yang DW, Koo JS, Kim YI, Jung HO, et al. Nighttime blood pressure and white matter hyperintensities in patients with Parkinson disease. Chronobiol Int. 2013;30:811–817. doi: 10.3109/07420528.2013.766618. [DOI] [PubMed] [Google Scholar]
- 85.Salsone M, Cerasa A, Arabia G, Morelli M, Gambardella A, Mumoli L, et al. Reduced thalamic volume in Parkinson disease with REM sleep behavior disorder: Volumetric study. Parkinsonism Relat Disord. 2014;20:1004–1008. doi: 10.1016/j.parkreldis.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 86.Chung SJ, Choi YH, Kwon H, Park YH, Yun HJ, Yoo HS, et al. Sleep disturbance may alter white matter and resting state functional connectivities in Parkinson's disease. Sleep 2017, 40. 10.1093/sleep/zsx009. [DOI] [PubMed]
- 87.Radziunas A, Deltuva VP, Tamasauskas A, Gleizniene R, Pranckeviciene A, Petrikonis K, et al. Brain MRI morphometric analysis in Parkinson's disease patients with sleep disturbances. BMC Neurol. 2018;18:88. doi: 10.1186/s12883-018-1092-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Filippi M, Elisabetta S, Piramide N, Agosta F. Functional MRI in idiopathic Parkinson's disease. Int Rev Neurobiol. 2018;141:439–467. doi: 10.1016/bs.irn.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 89.Arnaldi D, Famà F, de Carli F, Morbelli S, Ferrara M, Picco A, et al. The role of the serotonergic system in REM sleep behavior disorder. Sleep. 2015;38:1505–1509. doi: 10.5665/sleep.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Manabe Y, Fujii D, Kono S, Sakai Y, Tanaka T, Narai H, et al. Systemic blood pressure profile correlates with cardiac 123I-MIBG uptake in patients with Parkinson's disease. J Neurol Sci. 2011;307:153–156. doi: 10.1016/j.jns.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 91.Kashihara K, Imamura T, Shinya T. Cardiac 123I-MIBG uptake is reduced more markedly in patients with REM sleep behavior disorder than in those with early stage Parkinson's disease. Parkinsonism Relat Disord. 2010;16:252–255. doi: 10.1016/j.parkreldis.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 92.Nomura T, Inoue Y, Högl B, Uemura Y, Kitayama M, Abe T, et al. Relationship between (123)I-MIBG scintigrams and REM sleep behavior disorder in Parkinson's disease. Parkinsonism Relat Disord. 2010;16:683–685. doi: 10.1016/j.parkreldis.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 93.Chung CC, Kang JH, Yuan RY, Wu DA, Chen CC, Chi NF, et al. Multiscale entropy analysis of electroencephalography during sleep in patients with Parkinson disease. Clin EEG Neurosci. 2013;44:221–226. doi: 10.1177/1550059412475066. [DOI] [PubMed] [Google Scholar]
- 94.Priano L, Bigoni M, Albani G, Sellitti L, Giacomotti E, Picconi R, et al. Sleep microstructure in Parkinson's disease: Cycling alternating pattern (CAP) as a sensitive marker of early NREM sleep instability. Sleep Med. 2019;61:57–62. doi: 10.1016/j.sleep.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 95.Margis R, Schönwald SV, Carvalho DZ, Gerhardt GJ, Rieder CR. NREM sleep alpha and Sigma activity in Parkinson's disease: Evidence for conflicting electrophysiological activity? Clin Neurophysiol. 2015;126:951–958. doi: 10.1016/j.clinph.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 96.Wetter TC, Brunner H, Högl B, Yassouridis A, Trenkwalder C, Friess E. Increased alpha activity in REM sleep in de novo patients with Parkinson's disease. Mov Disord. 2001;16:928–933. doi: 10.1002/mds.1163. [DOI] [PubMed] [Google Scholar]
- 97.Jankovic J. Parkinson's disease: Clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 98.Shokrollahi M, Krishnan S. A review of sleep disorder diagnosis by electromyogram signal analysis. Crit Rev Biomed Eng. 2015;43:1–20. doi: 10.1615/critrevbiomedeng.2015012037. [DOI] [PubMed] [Google Scholar]
- 99.Kudo T, Loh DH, Truong D, Wu YF, Colwell CS. Circadian dysfunction in a mouse model of Parkinson's disease. Exp Neurol. 2011;232:66–75. doi: 10.1016/j.expneurol.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 100.Le WD, Sayana P, Jankovic J. Animal models of Parkinson's disease: A gateway to therapeutics? Neurotherapeutics. 2014;11:92–110. doi: 10.1007/s13311-013-0234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang YL, Lv D, Liu WW, Li SY, Chen J, Shen Y, et al. Disruption of the circadian clock alters antioxidative defense via the SIRT1-BMAL1 pathway in 6-OHDA-induced models of Parkinson's disease. Oxid Med Cell Longev. 2018;2018:4854732. doi: 10.1155/2018/4854732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li H, Fan XM, Luo Y, Song S, Liu J, Fan QY. Repeated manganese administration produced abnormal expression of circadian clock genes in the hypothalamus and liver of rats. Neurotoxicology. 2017;62:39–45. doi: 10.1016/j.neuro.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 103.Choudhury GR, Daadi MM. Charting the onset of Parkinson-like motor and non-motor symptoms in nonhuman primate model of Parkinson's disease. PLoS One. 2018;13:e0202770. doi: 10.1371/journal.pone.0202770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fifel K, Vezoli J, Dzahini K, Claustrat B, Leviel V, Kennedy H, et al. Alteration of daily and circadian rhythms following dopamine depletion in MPTP treated non-human Primates. PLoS One. 2014;9:e86240. doi: 10.1371/journal.pone.0086240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baydas G, Gursu MF, Yilmaz S, Canpolat S, Yasar A, Cikim G, et al. Daily rhythm of glutathione peroxidase activity, lipid peroxidation and glutathione levels in tissues of pinealectomized rats. Neurosci Lett. 2002;323:195–198. doi: 10.1016/s0304-3940(02)00144-1. [DOI] [PubMed] [Google Scholar]
- 106.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 108.Pfeffer M, Zimmermann Z, Gispert S, Auburger G, Korf HW, von Gall C. Impaired photic entrainment of spontaneous locomotor activity in mice overexpressing human mutant α-synuclein. Int J Mol Sci. 2018;19:E1651. doi: 10.3390/ijms19061651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Daneshvar Kakhaki R, Kouchaki E, Dadgostar E, Behnam M, Tamtaji OR, Nikoueinejad H, et al. The correlation of helios and neuropilin-1 frequencies with parkinson disease severity. Clin Neurol Neurosurg. 2020;192:105833. doi: 10.1016/j.clineuro.2020.105833. [DOI] [PubMed] [Google Scholar]
- 110.Li H, Song S, Wang Y, Huang C, Zhang F, Liu J, et al. Correction to: Low-grade inflammation aggravates rotenone neurotoxicity and disrupts circadian clock gene expression in rats. Neurotox Res. 2019;35:999–1000. doi: 10.1007/s12640-018-9982-3. [DOI] [PubMed] [Google Scholar]
- 111.Liu WW, Wei SZ, Huang GD, Liu LB, Gu C, Shen Y, et al. BMAL1 regulation of microglia-mediated neuroinflammation in MPTP-induced Parkinson's disease mouse model. FASEB J. 2020;34:6570–6581. doi: 10.1096/fj.201901565RR. [DOI] [PubMed] [Google Scholar]
- 112.Griffin P, Dimitry JM, Sheehan PW, Lananna BV, Guo C, Robinette ML, et al. Circadian clock protein Rev-erbα regulates neuroinflammation. Proc Natl Acad Sci USA. 2019;116:5102–5107. doi: 10.1073/pnas.1812405116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sachdeva UM, Thompson CB. Diurnal rhythms of autophagy: Implications for cell biology and human disease. Autophagy. 2008;4:581–589. doi: 10.4161/auto.6141. [DOI] [PubMed] [Google Scholar]
- 114.Maiese K. Moving to the rhythm with clock (circadian) genes, autophagy, mTOR, and SIRT1 in degenerative disease and cancer. Curr Neurovasc Res. 2017;14:299–304. doi: 10.2174/1567202614666170718092010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moors TE, Hoozemans JJ, Ingrassia A, Beccari T, Parnetti L, Chartier-Harlin MC, et al. Therapeutic potential of autophagy-enhancing agents in Parkinson's disease. Mol Neurodegener. 2017;12:11. doi: 10.1186/s13024-017-0154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guo F, Liu X, Cai H, Le W. Autophagy in neurodegenerative diseases: Pathogenesis and therapy. Brain Pathol. 2018;28:3–13. doi: 10.1111/bpa.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim J, Jang S, Choi M, Chung S, Choe Y, Choe HK, et al. Abrogation of the circadian nuclear receptor REV-ERBα exacerbates 6-hydroxydopamine-induced dopaminergic neurodegeneration. Mol Cells. 2018;41:742–752. doi: 10.14348/molcells.2018.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Heng X, Jin G, Zhang X, Yang DH, Zhu MZ, Fu SJ, et al. Nurr1 regulates Top IIβ and functions in axon genesis of mesencephalic dopaminergic neurons. Mol Neurodegener. 2012;7:4. doi: 10.1186/1750-1326-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chu YP, Le WD, Kompoliti K, Jankovic J, Mufson EJ, Kordower JH. Nurr1 in Parkinson's disease and related disorders. J Comp Neurol. 2006;494:495–514. doi: 10.1002/cne.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Le W, Pan T, Huang M, Xu P, Xie W, Zhu W, et al. Decreased NURR1 gene expression in patients with Parkinson's disease. J Neurol Sci. 2008;273:29–33. doi: 10.1016/j.jns.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jankovic J, Chen S, Le WD. The role of Nurr1 in the development of dopaminergic neurons and Parkinson's disease. Prog Neurobiol. 2005;77:128–138. doi: 10.1016/j.pneurobio.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 122.Rouillard C, Baillargeon J, Paquet B, St-Hilaire M, Maheux J, Lévesque C, et al. Genetic disruption of the nuclear receptor Nur77 (Nr4a1) in rat reduces dopamine cell loss and l-Dopa-induced dyskinesia in experimental Parkinson's disease. Exp Neurol. 2018;304:143–153. doi: 10.1016/j.expneurol.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 123.Humphries A, Weller J, Klein D, Baler R, Carter DA. NGFI-B (Nurr77/Nr4a1) orphan nuclear receptor in rat pinealocytes: Circadian expression involves an adrenergic-cyclic AMP mechanism. J Neurochem. 2004;91:946–955. doi: 10.1111/j.1471-4159.2004.02777.x. [DOI] [PubMed] [Google Scholar]
- 124.Kovács D, Sigmond T, Hotzi B, Bohár B, Fazekas D, Deák V, et al. HSF1Base: A comprehensive database of HSF1 (heat shock factor 1) target genes. Int J Mol Sci. 2019;20:E5815. doi: 10.3390/ijms20225815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Du YL, Wang F, Zou J, Le WD, Dong Q, Wang ZY, et al. Histone deacetylase 6 regulates cytotoxic α-synuclein accumulation through induction of the heat shock response. Neurobiol Aging. 2014;35:2316–2328. doi: 10.1016/j.neurobiolaging.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 126.Maiese K. Novel treatment strategies for the nervous system: Circadian clock genes, non-coding RNAs, and forkhead transcription factors. Curr Neurovascular Res. 2018;15:81–91. doi: 10.2174/1567202615666180319151244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Videnovic A, Klerman EB, Wang W, Marconi A, Kuhta T, Zee PC. Timed light therapy for sleep and daytime sleepiness associated with parkinson disease: A randomized clinical trial. JAMA Neurol. 2017;74:411–418. doi: 10.1001/jamaneurol.2016.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bhadra U, Patra P, Pal-Bhadra M. Cardinal epigenetic role of non-coding regulatory RNAs in circadian rhythm. Mol Neurobiol. 2018;55:3564–3576. doi: 10.1007/s12035-017-0573-8. [DOI] [PubMed] [Google Scholar]
- 129.Mehta N, Cheng HY. Micro-managing the circadian clock: The role of microRNAs in biological timekeeping. J Mol Biol. 2013;425:3609–3624. doi: 10.1016/j.jmb.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 130.Pacelli C, Rotundo G, Lecce L, Menga M, Bidollari E, Scrima R, et al. Parkin mutation affects clock gene-dependent energy metabolism. Int J Mol Sci. 2019;20:E2772. doi: 10.3390/ijms20112772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Okuzumi A, Hatano T, Ueno SI, Ogawa T, Saiki S, Mori A, et al. Metabolomics-based identification of metabolic alterations in PARK2. Ann Clin Transl Neurol. 2019;6:525–536. doi: 10.1002/acn3.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cai Y, Liu S, Sothern RB, Xu S, Chan P. Expression of clock genes Per1 and Bmal1 in total leukocytes in health and Parkinson's disease. Eur J Neurol. 2010;17:550–554. doi: 10.1111/j.1468-1331.2009.02848.x. [DOI] [PubMed] [Google Scholar]
- 133.Ding H, Liu S, Yuan YP, Lin QL, Chan P, Cai YN. Decreased expression of Bmal2 in patients with Parkinson's disease. Neurosci Lett. 2011;499:186–188. doi: 10.1016/j.neulet.2011.05.058. [DOI] [PubMed] [Google Scholar]
- 134.Lin Q, Ding H, Zheng Z, Gu Z, Ma J, Chen L, et al. Promoter methylation analysis of seven clock genes in Parkinson's disease. Neurosci Lett. 2012;507:147–150. doi: 10.1016/j.neulet.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 135.Mao W, Zhao CS, Ding H, Liang K, Xue JH, Chan P, et al. Pyrosequencing analysis of methylation levels of clock genes in leukocytes from Parkinson's disease patients. Neurosci Lett. 2018;668:115–119. doi: 10.1016/j.neulet.2018.01.027. [DOI] [PubMed] [Google Scholar]
- 136.Gu ZQ, Wang BB, Zhang YB, Ding H, Zhang YL, Yu J, et al. Association of ARNTL and PER1 genes with Parkinson's disease: A case-control study of Han Chinese. Sci Rep. 2015;5:15891. doi: 10.1038/srep15891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hua P, Liu WG, Chen DH, Zhao YY, Chen L, Zhang N, et al. Cry1 and Tef gene polymorphisms are associated with major depressive disorder in the Chinese population. J Affect Disord. 2014;157:100–103. doi: 10.1016/j.jad.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lou F, Li M, Luo XG, Ren Y. CLOCK 3111T/C variant correlates with motor fluctuation and sleep disorders in Chinese patients with Parkinson's disease. Parkinsons Dis. 2018;2018:4670380. doi: 10.1155/2018/4670380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McKenna D, Peever J. Degeneration of rapid eye movement sleep circuitry underlies rapid eye movement sleep behavior disorder. Mov Disord. 2017;32:636–644. doi: 10.1002/mds.27003. [DOI] [PubMed] [Google Scholar]
- 140.Rolinski M, Griffanti L, Piccini P, Roussakis AA, Szewczyk-Krolikowski K, Menke RA, et al. Basal Ganglia dysfunction in idiopathic REM sleep behaviour disorder parallels that in early Parkinson's disease. Brain. 2016;139:2224–2234. doi: 10.1093/brain/aww124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Videnovic A, Golombek D. Circadian dysregulation in Parkinson's disease. Neurobiol Sleep Circadian Rhythms. 2017;2:53–58. doi: 10.1016/j.nbscr.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sundaram S, Hughes RL, Peterson E, Müller-Oehring EM, Brontë-Stewart HM, Poston KL, et al. Establishing a framework for neuropathological correlates and glymphatic system functioning in Parkinson's disease. Neurosci Biobehav Rev. 2019;103:305–315. doi: 10.1016/j.neubiorev.2019.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Weissová K, Škrabalová J, Skálová K, Červená K, Bendová Z, Miletínová E, et al. Circadian rhythms of melatonin and peripheral clock gene expression in idiopathic REM sleep behavior disorder. Sleep Med. 2018;52:1–6. doi: 10.1016/j.sleep.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 144.Armstrong MJ, Okun MS. Diagnosis and treatment of parkinson disease: A review. JAMA. 2020;323:548–560. doi: 10.1001/jama.2019.22360. [DOI] [PubMed] [Google Scholar]
- 145.Yamanaka Y, Hashimoto S, Masubuchi S, Natsubori A, Nishide SY, Honma S, et al. Differential regulation of circadian melatonin rhythm and sleep-wake cycle by bright lights and nonphotic time cues in humans. Am J Physiol Regul Integr Comp Physiol. 2014;307:R546–R557. doi: 10.1152/ajpregu.00087.2014. [DOI] [PubMed] [Google Scholar]
- 146.Yamanaka Y, Hashimoto S, Takasu NN, Tanahashi Y, Nishide SY, Honma S, et al. Morning and evening physical exercise differentially regulate the autonomic nervous system during nocturnal sleep in humans. Am J Physiol Regul Integr Comp Physiol. 2015;309:R1112–R1121. doi: 10.1152/ajpregu.00127.2015. [DOI] [PubMed] [Google Scholar]
- 147.Medeiros CAM, Carvalhedo de Bruin PF, Lopes LA, Magalhães MC, de Lourdes Seabra M, Sales de Bruin VM. Effect of exogenous melatonin on sleep and motor dysfunction in Parkinson's disease. J Neurol 2007, 254: 459–464. [DOI] [PubMed]
- 148.Liu JB, Clough SJ, Hutchinson AJ, Adamah-Biassi EB, Popovska-Gorevski M, Dubocovich ML. MT1 and MT2 melatonin receptors: A therapeutic perspective. Annu Rev Pharmacol Toxicol. 2016;56:361–383. doi: 10.1146/annurev-pharmtox-010814-124742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mishra A, Singh S, Tiwari V, Chaturvedi S, Wahajuddin M, Shukla S. Dopamine receptor activation mitigates mitochondrial dysfunction and oxidative stress to enhance dopaminergic neurogenesis in 6-OHDA lesioned rats: A role of Wnt signalling. Neurochem Int. 2019;129:104463. doi: 10.1016/j.neuint.2019.104463. [DOI] [PubMed] [Google Scholar]
- 150.Vallée A, Lecarpentier Y, Guillevin R, Vallée JN. Opposite interplay between the canonical WNT/β-catenin pathway and PPAR gamma: A potential therapeutic target in gliomas. Neurosci Bull. 2018;34:573–588. doi: 10.1007/s12264-018-0219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vallée A, Lecarpentier Y, Vallée JN. Circadian rhythms and energy metabolism reprogramming in Parkinson's disease. Curr Issues Mol Biol. 2019;31:21–44. doi: 10.21775/cimb.031.021. [DOI] [PubMed] [Google Scholar]
- 152.Hayashi A, Matsunaga N, Okazaki H, Kakimoto K, Kimura Y, Azuma H, et al. A disruption mechanism of the molecular clock in a MPTP mouse model of Parkinson's disease. Neuromolecular Med. 2013;15:238–251. doi: 10.1007/s12017-012-8214-x. [DOI] [PubMed] [Google Scholar]
- 153.Korshunov KS, Blakemore LJ, Trombley PQ. Dopamine: A modulator of circadian rhythms in the central nervous system. Front Cell Neurosci. 2017;11:91. doi: 10.3389/fncel.2017.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gizowski C, Bourque CW. Sodium regulates clock time and output via an excitatory GABAergic pathway. Nature. 2020;583:421–424. doi: 10.1038/s41586-020-2471-x. [DOI] [PubMed] [Google Scholar]
- 155.Bubenik GA, Ball RO, Pang SF. The effect of food deprivation on brain and gastrointestinal tissue levels of tryptophan, serotonin, 5-hydroxyindoleacetic acid, and melatonin. J Pineal Res. 1992;12:7–16. doi: 10.1111/j.1600-079x.1992.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 156.Rasmussen DD, Mitton DR, Larsen SA, Yellon SM. Aging-dependent changes in the effect of daily melatonin supplementation on rat metabolic and behavioral responses. J Pineal Res. 2001;31:89–94. doi: 10.1034/j.1600-079x.2001.310113.x. [DOI] [PubMed] [Google Scholar]
- 157.Zesiewicz TA, Hauser RA. Sleep attacks and dopamine agonists for Parkinson's disease: What is currently known? CNS Drugs. 2003;17:593–600. doi: 10.2165/00023210-200317080-00004. [DOI] [PubMed] [Google Scholar]
- 158.Plowman BK, Boggie DT, Morreale AP, Schaefer MG, Delattre ML, Chan H. Sleep attacks in patients receiving dopamine-receptor agonists. Am J Health Syst Pharm. 2005;62:537–540. doi: 10.1093/ajhp/62.5.537. [DOI] [PubMed] [Google Scholar]
- 159.Ryan M, Slevin JT, Wells A. Non-ergot dopamine agonist-induced sleep attacks. Pharmacotherapy. 2000;20:724–726. doi: 10.1592/phco.20.7.724.35181. [DOI] [PubMed] [Google Scholar]
- 160.Tan EK. Piribedil-induced sleep attacks in Parkinson's disease. Fundam Clin Pharmacol. 2003;17:117–119. doi: 10.1046/j.1472-8206.2003.00122.x. [DOI] [PubMed] [Google Scholar]
- 161.Frucht S, Rogers JD, Greene PE, Gordon MF, Fahn S. Falling asleep at the wheel: Motor vehicle mishaps in persons taking pramipexole and ropinirole. Neurology. 1999;52:1908–1910. doi: 10.1212/wnl.52.9.1908. [DOI] [PubMed] [Google Scholar]
- 162.Chaudhuri KR, Pal S, Brefel-Courbon C. ‘Sleep attacks’ or ‘unintended sleep episodes’ occur with dopamine agonists: Is this a class effect? Drug Saf. 2002;25:473–483. doi: 10.2165/00002018-200225070-00001. [DOI] [PubMed] [Google Scholar]
- 163.Li SY, Wang YL, Liu WW, Lyu DJ, Wang F, Mao CJ, et al. Long-term levodopa treatment accelerates the circadian rhythm dysfunction in a 6-hydroxydopamine rat model of Parkinson's disease. Chin Med J. 2017;130:1085–1092. doi: 10.4103/0366-6999.204920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mattam U, Jagota A. Daily rhythms of serotonin metabolism and the expression of clock genes in suprachiasmatic nucleus of rotenone-induced Parkinson's disease male Wistar rat model and effect of melatonin administration. Biogerontology. 2015;16:109–123. doi: 10.1007/s10522-014-9541-0. [DOI] [PubMed] [Google Scholar]