The projections from hypothalamic magnocellular neuroendocrine cells (MNCs) to the posterior pituitary (PPi) are traditionally defined as the hypothalamo-neurohypophysial system (HNS), which releases oxytocin (OXT) and arginine vasopressin (AVP) into the blood stream to regulate peripheral function [1]. Recently, accumulating evidence has confirmed that OXT and AVP are not only involved in peripheral regulation [2], but also contribute to various central actions [3, 4], such as memory and social and stress-related behaviors [5]. However, peripherally released OXT and AVP rarely cross the blood-brain barrier and return to the central nervous system. So, whether and how they exert central effects remain to be elucidated. It is supposed that MNCs projecting to the PPi also have collaterals projecting centrally, and these might be responsible for the central actions of OXT and AVP. But there has been no cell-type-specific dissection of the hypothalamic MNC projections, due to the limited methods and techniques for imaging and manipulation. Recently, in Neuron [6], researchers at Zhejiang University and Huazhong University of Science and Technology in China report the skillful use of multiple viruses and advanced techniques to the realize cell-type specific labeling and manipulation of hypothalamic MNCs. This is the first report of the three-dimensional reconstruction and cell-type specific functional dissection of the HNS in coordinating both peripheral and central actions, which provide technical references and new insights into the MNC-related mechanisms underlying central behaviors and psychiatric diseases (Fig. 1).
Fig. 1.
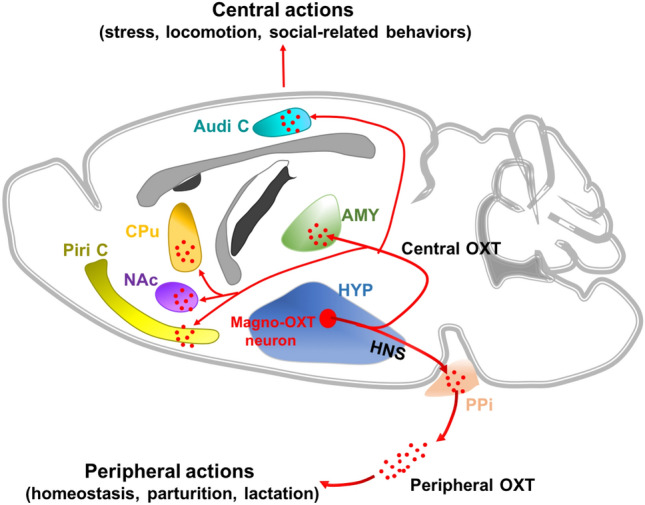
Magnocellular oxytocin neurons coordinate both peripheral and central actions. The projections from hypothalamic magnocellular oxytocin cells (Magno-OXT neurons) to the posterior pituitary (PPi) were traditionally defined as the hypothalamo-neurohypophysial system (HNS); they release oxytocin (OXT) into the peripheral blood stream to regulate peripheral function. Magno-OXT neurons also project to extra-hypothalamic nuclei, including the amygdala (AMY), caudate-putamen (CPu), nucleus accumbens (NAc), piriform cortex (Piri C), and auditory cortex (Audi C), to regulate central function.
Despite the pivotal importance of the HNS in neuroendocrine regulation, knowledge on its general structure is poor due to the limited labeling efficiency of traditional tracers and the low resolution of imaging techniques [7, 8]. To fully reconstruct the architecture of the HNS and selectively label the PPi-projecting MNC ensemble in Sprague-Dawley rats, the authors applied the advanced retrograde viral tracer Retro-GFP, which was injected into the PPi to selectively label the cell ensemble directly innervating the neurohypophysis as GFP+ cells. To assess the neuroendocrine nature of the labeled cells, Fluoro-Gold (FG) was injected intraperitoneally to label the neuroendocrine neurons as FG+ cells. And the results showed that 100% of the GFP+ cells in the hypothalamic supraoptic (SON) and paraventricular (PVN) nuclei were co-labeled with FG, which demonstrated that the labeled GFP+ cells were neuroendocrine neurons. Moreover, the authors used a well-established marker for parvocellular neuroendocrine cells (PNCs), thyrotropin-releasing hormone (TRH), to label the PNCs and found no co-localization of GFP+ and TRH+ cells in the PVN, which further identified the GFP+ cells as MNCs rather than PNCs [9]. Above all, the retrograde viral tracing with Retro-GFP proved to be a fine choice to label the HNS with higher labeling efficiency and specificity for the first time, and it is impressive that the authors cleverly used viral and immune markers to exclude the interference of non-neuroendocrine neurons and PNCs. Although this method has made advances, it is still not quite perfect. For example, all neurons in the SON project to the neurohypophysis [10], but only 60% of the FG+ cells in the SON were co-labeled with GFP. This result indicates that although Retro-GFP has higher efficiency, it still cannot achieve 100% labeling of PPi-projecting MNCs due to defective viral infection and retrograde transport, which might affect the integrity of the HNS structure. Moreover, the method of labeling neuroendocrine neurons by intraperitoneally-injected FG is not generally acknowledged and its efficiency needs to be adequately verified.
Besides efficient viral tracers, advanced imaging techniques with higher resolution are also crucial for a clear visualization of the HNS. Then, with the above established method for selective labeling, the authors further used advanced fluorescent micro-optical sectioning tomography (fMOST) to make a 3D reconstruction of the HNS at high resolution. The 3D overview and measurement of the HNS showed that labeled MNCs were distributed in >8 hypothalamic nuclei, and they were divided into three MNC ensembles: a principal MNC nucleus with abundant GFP+ cells, an accessory MNC nucleus with densely-packed GFP+ cells, and a scattered MNC system with scattered GFP+ cells. However, it is noteworthy that GFP+ fibers were observed outside the hypothalamus, such as in the piriform cortex, amygdala, and nucleus accumbens, and they likely represent central collaterals of the PPi-projecting MNCs. Subsequently, the authors delineated the morphology of MNCs by single-cell reconstruction and provided more clear illustrations of the axons from the PPi-projecting MNCs. Moreover, the authors further designed two dual-viral strategies in both anterograde and retrograde directions, and verified that PPi-projecting MNCs really sent collaterals to extra-hypothalamic areas. In previous studies, knowledge of the general structure of the HNS was poor, and MNCs were traditionally considered to send unipolar axons to the neurohypophysis [11]. Here, the authors inspiringly revealed central collaterals from the PPi-projecting MNCs for the first time, which is pivotal in revealing the previously unrecognized complexity of the HNS and provides structural evidence for PPi-projecting MNCs in coordinating both peripheral and central actions. Moreover, the reconstructed atlas needs continuing improvement with more data to summarize the collaterals of PPi-projecting MNCs from different hypothalamic subregions and distinguish their cell subsets in the future, which will benefit the functional dissection of MNC-related circuits.
After the structural imaging of MNC projections, the authors investigated their function in coordinating both peripheral and central actions. In this study, a majority of the PPi-projecting MNCs were shown to be magnocellular OXT (Magno-OXT) neurons through immunostaining with OXT antibody. Moreover, Magno-OXT neurons projected collaterals to multiple extra-hypothalamic regions, including the amygdala, caudate-putamen, and nucleus accumbens, reported for the first time in this study. Then, the authors generated an OXT-Cre rat line, and found that chemogenetic manipulation of Magno-OXT neurons coordinated both peripheral and central OXT-mediated actions. Particularly, the promotion of locomotion induced by chemogenetic activation of Magno-OXT neurons was reversed by infusing an OXT receptor antagonist into the caudate-putamen, further verifying the central release of OXT by Magno-OXT neurons. Although previous studies have already reported the role of central OXT in various social and stress-related behaviors, they did not specify the underlying related circuits. Here, the authors specifically demonstrated for the first time that Magno-OXT neurons release central OXT, and they are involved in the circuits mediating OXT-related central actions, breaking new ground in dissecting the oxytocinergic circuit mechanisms underlying OXT-related central behaviors and mental disorders. However, the wide distribution of PPi-projecting MNCs enhances the difficulty in area-specific labeling and manipulation of the Magno-OXT neuronal ensembles in different hypothalamic nuclei. Nevertheless, a dual-viral strategy by infusing Retro-DIO-Flp into the PPi and AAV-fDIO-hM3d/hM4d-EGFP into different hypothalamic nuclei in OXT-Cre rats might be a feasible method to overcome the difficulty. Moreover, other than OXT, MNCs also release other peptides, such as AVP, which has also been reported to be involved in central behaviors [12]. Therefore, future studies on the function of MNCs should also pay attention to Magno-AVP neurons or any other peptide-releasing magnocellular neurons. Although the functional dissection was not exhaustive, it at least offered technical references for future MNC-related studies.
In conclusion, this study not only provides advanced techniques and an efficient strategy for mapping the connectivity of the HNS, it also offers new insights for exploring the circuit mechanisms underlying MNC-related central behaviors and mental diseases, such as stress, autism, and anxiety-related disorders [13]. Although mapping of the HNS has been reported in earlier studies, the poor efficiency of traditional retrograde tracers and imaging instruments limited the structural dissection. Now, combing advanced retrograde viral tracers with high-resolution imaging fMOST, this study provides a full and clear reconstruction of the HNS architecture for the first time. Moreover, the authors first realized the specific labeling of Magno-OXT neurons in OXT-Cre rats, which is of great importance for further function dissection. Certainly, the present study is just the beginning for MNC-related circuit investigation and many valuable issues still need further research. First, MNCs are distributed in various hypothalamic nuclei, and it is important to segregate their anatomical and functional characteristics. Second, MNCs primarily release OXT and AVP, and it would be valuable to distinguish the roles of Magno-OXT neurons and Magno-AVP neurons in behaviors. Above all, this study used state-of-the-art techniques in the tracing and manipulation of MNCs, providing valuable references for further illumination of neuroendocrine networks.
Acknowledgments
This Highlight was supported by the National Natural Science Foundation of China (81830035 and 82003729).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Hashimoto H, Matsuura T, Ueta Y. Fluorescent visualization of oxytocin in the hypothalamo-neurohypophysial system. Front Neurosci. 2014;8:213. doi: 10.3389/fnins.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antunes-Rodrigues J, de Castro M, Elias LL, Valenca MM, McCann SM. Neuroendocrine control of body fluid metabolism. Physiol Rev. 2004;84:169–208. doi: 10.1152/physrev.00017.2003. [DOI] [PubMed] [Google Scholar]
- 3.Raggenbass M. Vasopressin- and oxytocin-induced activity in the central nervous system: electrophysiological studies using in-vitro systems. Prog Neurobiol. 2001;64:307–326. doi: 10.1016/S0301-0082(00)00064-2. [DOI] [PubMed] [Google Scholar]
- 4.Hasan MT, Althammer F, Silva da Gouveia M, Goyon S, Eliava M, Lefevre A, et al. A fear memory engram and its plasticity in the hypothalamic oxytocin system. Neuron 2019, 103: 133–146 e138. [DOI] [PubMed]
- 5.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 6.Zhang B, Qiu L, Xiao W, Ni H, Chen L, Wang F, et al. Reconstruction of the hypothalamo-neurohypophysial system and functional dissection of magnocellular oxytocin neurons in the brain. Neuron. 2021;109(331–346):e7. doi: 10.1016/j.neuron.2020.10.032. [DOI] [PubMed] [Google Scholar]
- 7.Taniguchi Y, Yoshida M, Ishikawa K, Suzuki M, Kurosumi K. The distribution of vasopressin- or oxytocin-neurons projecting to the posterior pituitary as revealed by a combination of retrograde transport of horseradish peroxidase and immunohistochemistry. Arch Histol Cytol. 1988;51:83–89. doi: 10.1679/aohc.51.83. [DOI] [PubMed] [Google Scholar]
- 8.Fisher AW, Price PG, Burford GD, Lederis K. A 3-dimensional reconstruction of the hypothalamo-neurohypophysial system of the rat. The neurons projecting to the neuro/intermediate lobe and those containing vasopressin and somatostatin. Cell Tissue Res 1979, 204: 343–354. [DOI] [PubMed]
- 9.Eliava M, Melchior M, Knobloch-Bollmann HS, Wahis J, da Silva Gouveia M, Tang Y, et al. A New population of parvocellular oxytocin neurons controlling magnocellular neuron activity and inflammatory pain processing. Neuron. 2016;89:1291–1304. doi: 10.1016/j.neuron.2016.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong WE, Warach S, Hatton GI, McNeill TH. Subnuclei in the rat hypothalamic paraventricular nucleus: a cytoarchitectural, horseradish peroxidase and immunocytochemical analysis. Neuroscience. 1980;5:1931–1958. doi: 10.1016/0306-4522(80)90040-8. [DOI] [PubMed] [Google Scholar]
- 12.Brunnlieb C, Nave G, Camerer CF, Schosser S, Vogt B, Munte TF, et al. Vasopressin increases human risky cooperative behavior. Proc Natl Acad Sci U S A. 2016;113:2051–2056. doi: 10.1073/pnas.1518825113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang R, Zhang HF, Han JS, Han SP. Genes related to oxytocin and arginine-vasopressin pathways: associations with autism spectrum disorders. Neurosci Bull. 2017;33:238–246. doi: 10.1007/s12264-017-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


