Abstract
β-Glucan from Saccharomyces cerevisiae has been described to be effective antioxidants, but the specific antioxidation mechanism of β-glucan is unclear. The objectives of this research were to determine whether the β-glucan from Saccharomyces cerevisiae could regulate oxidative stress through the Dectin-1/Nrf2/HO-1 signaling pathway in lipopolysaccharides (LPS)-stimulated RAW264.7 cells. In this study, we examined the effects of β-glucan on the enzyme activity or production of oxidative stress indicators in LPS-stimulated RAW264.7 cells by biochemical analysis and the protein expression of key factors of Dectin-1/Nrf2/HO-1 signaling pathway by immunofluorescence and western blot. The biochemical analysis results showed that β-glucan increased the LPS-induced downregulation of enzyme activity of intracellular heme oxygenase (HO), superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) while decreasing the production of reactive oxygen species (ROS) and malondialdehyde (MDA). Furthermore, immunofluorescence results showed that β-glucan can activate the nuclear factor erythroid 2-related factor 2 (Nrf2). The antioxidant mechanism study indicated that β-glucan activated dendritic-cell-associated C-type lectin 1 (Dectin-1) receptors mediated Nrf2/HO-1 signaling pathway, thereby downregulating the production of ROS and thus produced the antioxidant effects in LPS-stimulated RAW 264.7 cells. In conclusion, these results indicate that β-glucan potently alleviated oxidative stress via Dectin-1/Nrf2/HO-1 in LPS-stimulated RAW 264.7 cells.
Keywords: β-Glucan, Oxidative stress, Dectin-1, Nrf2, HO-1
Introduction
Multiple diseases and pathological states caused by LPS from Gram-negative bacteria are accompanied by oxidative stress. Oxidative stress is fundamental to the maintenance of cell metabolism and vitality. The evidence suggests that excessive intracellular reactive oxygen species (ROS) is the most important factors for the modification or degradation of DNA, protein, and carbohydrates, resulting in tissue oxidative damage (Park et al. 2017). Imbalanced generation of ROS contributes to local inflammatory pathologies induced by LPS (Mills et al. 2016; Plotnikov et al. 2013). Macrophages act as central regulators of organ development, disease progression, and tissue restitution following injury. Because of these properties, macrophages form an integral part of the immune system and play a key role in many physiological processes that help maintain the overall internal environment balance (Rehman et al. 2021). Macrophages activated by LPS produce and release large quantities of ROS into the interstitial space, causing an oxidative stress on the surrounding tissue (Shen et al. 2021). Numerous animal models of sepsis induced by LPS have similar clinical features, such as increased level of ROS, decreased antioxidant capacity, and mitochondrial oxidative damage (Rocha et al. 2012; Supinski et al. 2020). In addition, the decline of antioxidative enzymes and glutathione content caused by LPS contributes to the impairment of endogenous antioxidant defense and the subsequent increase in ROS generation (Liu et al. 2019; Sener et al. 2005b). In order to eliminate oxidative stress, living cells activate effective antioxidant defense mechanisms by inducing oxidases, including superoxide dismutase (SOD) and catalase (CAT) to eliminate ROS.
There is a growing body of research that recognizes the importance of the nuclear factor erythroid 2-related factor 2 (Nrf2) in regulating antioxidative genes under stress response (Ding et al. 2020; Zhang et al. 2015). In response to the oxidative challenge, the antioxidant defense system is strictly regulated by Nrf2. Indeed, the synthesis of SOD, glutathione peroxidase (GSH-Px), and CAT is controlled by Nrf2 and responsible for the removal of free radicals at the site of their production. Since superoxide free radicals are harmful products generated in physiological processes, it can be effectively targeted for clearance by antioxidant defense mechanisms (Shen et al. 2019). Upon activation, Nrf2 can be transported to the nucleus and binds to the antioxidant response element (ARE), leading to the gene expression of heme oxygenase-1 (HO-1) (Kensler et al. 2007). Research has also shown that mitochondria-targeted antioxidants alleviate LPS-induced oxidative stress in intestinal epithelial cells, triggering the nuclear translocation of the nuclear factor Nrf2, which, in turn, stimulates the expression of its downstream antioxidant genes (Zhang et al. 2020). HO-1 is a metabolic enzyme responsible for the degradation of heme, including biliverdin, carbon monoxide, and free iron. Its metabolites have shown effective protection against oxidative stress and inflammation regulated by the Nrf2 signaling pathway (Zhang et al. 2019). The vital role of HO-1 in adaptation to oxidative stress has been demonstrated in HO-1-deficient animal models (Liu et al. 2018). HO-1 has remarkable antioxidant properties and has potent protective actions against ROS-induced oxidative damage both in vitro and in vivo (Croft et al. 2017; Datla et al. 2007; Jiang et al. 2006). Moreover, increasing number of therapeutic agents exert their antioxidant and anti-inflammatory effects by inducing HO-1 expression (Lee et al. 2012; Lin et al. 2003). The modulation of HO-1 activity may represent a potential therapeutic target for the treatment of diseases associated with oxidative stress.
Yeast polysaccharides play an important role in enhancing antioxidant and immune response capacity in sheep and other ruminants (Celi et al. 2010; Sandvik et al. 2007; Tsoni and Brown 2008). β-Glucan from Saccharomyces cerevisiae cell walls is a polysaccharide with antioxidant effect and potent stimulators of nonspecific defense mechanisms in animals (Babincova et al. 2002; Deng et al. 2017; Wilson et al. 2015). The evidence indicated that β-glucan from Saccharomyces cerevisiae attenuates isoprenaline-induced myocardial injury in rats by reducing MDA, enhancing SOD, CAT, and GSH-Px (Cetin 2019). The antioxidant activity of β-glucan extract was significantly higher than that of yeast cell walls, significantly reducing the production of MDA and NO (Bacha et al. 2017). β-Glucan can protect organ from LPS-induced oxidative injury by means of significantly increasing GSH levels and reducing MDA levels and MPO activity (Sener et al. 2005a). Dectin-1 is a newly discovered pattern recognition receptor, which is expressed on the surface of immune cells, such as macrophages and dendritic cells, and responsible for triggering the immune response and conducting signaling pathways (Drummond and Brown 2011; Taylor et al. 2007). Dectin-1 can recognize β-glucan (Sahasrabudhe et al. 2016), and once activated by β-glucan, it could induce innate immune responses such as phagocytosis and inflammation (Su et al. 2020). Recently, studies have pointed out that β-glucan can regulate HO-1 activity through Nrf2-dependent manner in oral keratinocytes infected by Candida albicans (Ishida et al. 2018). For this reason, β-glucan from Saccharomyces cerevisiae has attracted attention due to its natural antioxidative substances, which are known as antioxidants able to protect living cells against the attack of ROS (Babincova et al. 2002; Hino et al. 2012). Hence, therapeutic intervention via suppression of ROS generation or enhancement of endogenous antioxidant enzymes might be a potential target to combat oxidative injury.
Therefore, the present study aimed to investigate the antioxidant molecular mechanisms of β-glucan via Dectin-1/Nrf2/HO-1 signaling pathway on RAW264.7 cells in vitro. This study will provide a theoretical foundation for β-glucan treatment for oxidative stress-related diseases and improve the clinical application value of β-glucan.
Materials and methods
Chemical reagents and antibodies
β-Glucan, laminarin, and LPS were purchased from Sigma (Munich, Germany); ML385 was from Medchem Express (NJ, USA); tin protoporphyrin IX dichloride (SnPP) was purchased from Santa Cruz Biotechnology (TX, USA); antibodies to Dectin-1, Nrf2 and β-actin were from Abcam (Cambridge, UK); antibody to HO-1 was from Gene Tex (Southern California, USA); HRP-conjugated goat anti-rabbit IgGs secondary antibody was from Sungene Biotech (Tianjin, China); the microplate test kits for SOD, CAT, GSH-Px, and malondialdehyde (MDA) were from Nanjing Jiancheng Bioengineering Institute (Nanjing, China); the ROS and MTT kit were from Beyotime Institute of Biotechnology (Shanghai, China); DMEM was from Gibco (Grand Island, USA); fetal bovine serum (FBS) was from Excell Biology Company (Shanghai, China); and penicillin-streptomycin was from TransGen Biotech (Beijing, China).
Cell culture and treatment
The RAW264.7 murine macrophages were purchased from Procell Life Science and Technology Co., Ltd (Wuhan, China) and cultured in high-glucose DMEM medium containing 10% FBS and 1% penicillin-streptomycin at 37 °C in a 5% CO2 incubator.
Cytotoxicity assay
The cells were seeded in 96-well plates with 1 × 104 cells/well and 100 μL medium/well for 8 h, and then treated with β-glucan (0, 5, 10, 20, 50, 100, and 200 μg/mL) with or without LPS (1 μg/mL) for 16 h, followed by treatment with 50 μL/well MTT solution for 4 h. The supernatant was removed, then the 96-well plate was treated by 150 μL/well formazan resolved with DMSO. The optical density of each well was measured at 490 nm on a microplate reader.
Measurement of the oxidation and antioxidation markers
RAW264.7 cells were seeded in a 6-well plate at a density of 1 × 106 cells/well, incubated for 24 h, treated with β-glucan for 1 h, and then exposed to LPS (1 μg/mL) for 12 h. The activities of the intracellular SOD, CAT, and GSH-Px, as well as the content of the intracellular MDA in cells were detected using commercial kits according to the manufacturer’s instructions. HO-1 activity was detected to follow the assay of previous research (Srisook and Cha 2004).
Reactive oxygen species detection
The production of cellular ROS was measured with ROS detection kits (Beyotime Institute, Shanghai, China) according to the instructions. Cells were observed with fluorescence microscope (Olympus, IX71, Tokyo, Japan). The intracellular fluorescence intensity was detected by excitation at 488 nm and emission of 525 nm by fluorescence microplate reader (Synergy H4, BioTek, USA).
Western blot
Total cellular proteins were extracted using protein extraction kits (Thermo Fisher Scientific, Shanghai, China) according to the manufacturer’s instructions. The protein samples (20 μg) were separated on a 10% SDS-polyacrylamide gel and transferred to polyvinylidene fluoride (PVDF) membrane. The membranes were blocked for 3 h at room temperature with 5% BSA in Tris-buffered saline with Tween 20 (TBST) solution, incubated with specific antibodies to Dectin-1, Nrf2, HO-1, and β-actin, and then hybridized with a secondary antibody for 45 min. Immunoreactive bands were detected with an ECL solution (Thermo Fisher Scientific, Shanghai, China). The images were analyzed by the Image-Pro Plus 6.0 software (Media Cybernetics, MD, USA).
Immunofluorescence assays
RAW264.7 cells were seeded on coverslip at a density of 1 × 105 cells/well in 6-well plates and then incubated for 24 h. After being treated with 20 μg/mL β-glucan for 6 h, the cells were fixed with 4% paraformaldehyde for 20 min and subsequently permeabilized with 0.1% Triton X-100, and blocked with 5% BSA for 1 h at room temperature. After this, the cells were incubated with the primary antibody Nrf2 at 4 °C overnight, next incubated with a FITC-conjugated secondary antibody for 1 h and counterstained with DAPI for 10 min (Beyotime, China). Fluorescence images were photographed using a fluorescence microscope (Zeiss, AX10, Jena, Germany).
Statistical analyses
The statistical analysis was conducted with the t-test and one-way analysis of variance by SPSS 19.0 statistical software (SPSS Inc., IBM, USA). Quantitative data are presented as means ± SEM. All the experiments were repeated independently at least three times.
Results
Cytotoxicity of β-glucan on RAW264.7 cells
RAW264.7 cells were treated with β-glucan (0, 5, 10, 20, 50, 100, and 200 μg/mL) for 16 h. As shown in Fig. 1, β-glucan was not cytotoxic up to 20 μg/mL, while treatment with 50 μg/mL or above caused significant cytotoxicity. Furthermore, LPS-stimulated macrophage activity is unaffected by β-glucan. Thus, the non-cytotoxic concentrations of β-glucan (5, 10, and 20 μg/mL) were selected for further study.
Fig. 1.
Effects of β-glucan on the cell viability of RAW264.7 cells cultured with various concentrations of β-glucan (0, 5, 10, 20, 50, 100, and 200 μg/mL) for 16 h. Cell viability was determined by MTT assay. a Cytotoxicity of β-glucan on RAW264.7 cells. b The effect of β-glucan on LPS-stimulated RAW264.7 cells’ activity. RAW264.7 cells were pretreated with β-glucan (5, 10, 20 μg/mL) for 1 h, stimulated with LPS for 16 h, and cell viability was detected by MTT assay. Data are presented as mean ± SEM (n = 3). *p < 0.05 and **p < 0.01
The effects of β-glucan on oxidation and antioxidation markers in LPS-stimulated RAW 264.7 cells
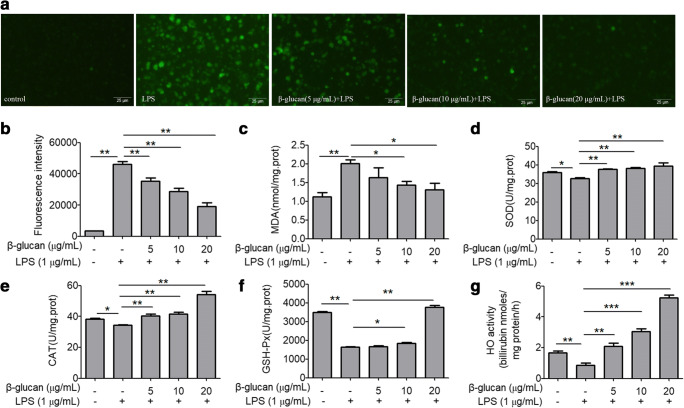
The histogram in Fig. 2 indicates that LPS significantly induces the production of ROS and MDA and inhibits the enzyme activity of the SOD, CAT, GSH-Px, and HO-1. However, after pretreatment with β-glucan, the production of MDA and ROS was significantly reduced, while the activity of antioxidant enzymes HO-1, SOD, CAT, and GSH-Px was significantly increased in a concentration-dependent manner. Further, in order to study the molecular mechanism of antioxidant function of β-glucan, we detected the protein expression levels of Dectin-1, Nrf2, and HO-1 by western blot. The results presented in Fig. 3a show that LPS significantly inhibited the protein expression of Nrf2 and HO-1, compared with the control group, while β-glucan significantly enhanced the levels of these three proteins compared with the LPS treatment group. The above data clearly reveals that β-glucan has an antioxidant function, and this antioxidative effect may be related to Dectin-1 expression. Interestingly, the Nrf2, HO-1, and Dectin-1 protein expression showed the same trend, so we speculated that Dectin-1 might regulate Nrf2/HO-1 signaling pathway.
Fig. 2.
Effects of β-glucan on ROS and antioxidation and oxidation markers in LPS-stimulated RAW264.7 cells. a The production of ROS. b The mean ROS fluorescence intensity. c The MDA levels and d–g SOD, CAT, GSH-Px, and HO activity. *p < 0.05 and **p < 0.01
Fig. 3.
Effects of β-glucan on the protein expression levels of Dectin-1, Nrf2, and HO-1 in LPS-stimulated RAW264.7 cells. a Western blot analysis for Dectin-1, Nrf2, and HO-1 expression in the β-glucan and/or LPS-treated RAW264.7 cells. b Western blot analysis for Nrf2 and HO-1 expression in the laminarin-treated RAW264.7 cells subsequent to β-glucan and/or LPS. c Western blot analysis for Nrf2 and HO-1 expression in the ML385-treated RAW264.7 cells subsequent to β-glucan and/or LPS. d The mean ROS fluorescence intensity. *p < 0.05 and **p < 0.01
β-Glucan regulates Nrf2/HO-1 signaling pathway via Dectin-1 in LPS-stimulated RAW264.7 cells
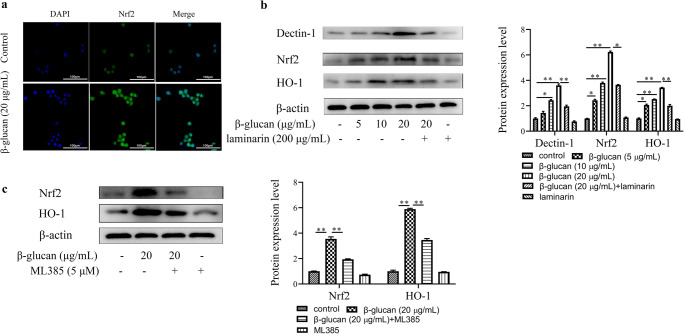
It is apparent from Fig. 3b that LPS significantly inhibited the protein expression of Nrf2 and HO-1 in LPS treatment group compared with the control group, and β-glucan reverses this inhibitory state in β-glucan + LPS treatment group; compared with β-glucan + LPS treatment group, the protein expression of Nrf2 and HO-1 significantly decreased in the laminarin (Dectin-1 inhibitor) + β-glucan + LPS treatment group. What’s more, the protein expression levels of HO-1 significantly decreased in β-glucan + ML385 (Nrf2 inhibitor) + LPS treatment group (Fig. 3c). Compared with the control group, LPS significantly increased the production of ROS, β-glucan decreased ROS production by LPS-stimulated cells, but after the laminarin, ML385, or SnPP (HO-1 inhibitor) pretreatment, the production of ROS was significantly increased compared with β-glucan + LPS treatment group (Fig. 3d). As hypothesized, our experiments show that β-glucan adjusts the Nrf2/HO-1 signaling pathway through Dectin-1 to achieve an antioxidant effect.
β-Glucan regulates Nrf2/HO-1 by Dectin-1 in RAW264.7 cells
In order to verify the hypothesis that β-glucan could regulate oxidative stress through the receptor Dectin-1 mediated Nrf2/HO-1 signaling pathway, we used β-glucan to treat RAW264.7 cells alone and detected the protein expression levels of Dectin-1, Nrf2, and HO-1. The immunofluorescence results showed that Nrf2 transferred to the nucleus after β-glucan treated RAW264.7 cells for 6 h compared with the control group in Fig. 4a. As we can see in Fig. 4b, there is a clear increasing trend of Dectin-1, Nrf2, and HO-1 expression in β-glucan treatment group compared with control group, but Dectin-1, Nrf2, and HO-1 expression significantly decreased in the laminarin + β-glucan treatment group than that in the 20 μg/mL β-glucan treatment group. The protein levels of Nrf2 and HO-1 displayed a clear decline in the ML385 + β-glucan treatment group than in the 20 μg/mL β-glucan treatment group (Fig. 4c). The most striking result to emerge from the data is that β-glucan activates the Nrf2/HO-1 signaling pathway through its receptor Dectin-1, thereby regulating cellular antioxidant function. These data fully support our hypothesis that β-glucan can mediate Nrf2/HO-1 signaling pathway through Dectin-1 to regulate oxidative stress in LPS-induced RAW264.7 cells.
Fig. 4.
Dectin-1 regulated the Nrf2/HO-1 signaling pathway in β-glucan-stimulated RAW264.7 cells. a The immunofluorescence of Nrf2 in β-glucan-stimulated RAW264.7 cells. b Western blot analysis for Dectin-1, Nrf2, and HO-1 expression in the laminarin-treated RAW264.7 cells subsequent to β-glucan. c Western blot analysis for Nrf2 and HO-1 expression in the ML385-treated RAW264.7 cells subsequent to β-glucan. *p < 0.05 and **p < 0.01
Discussion
Alternatives to nonantimicrobial feed additives that enhance health and performance of animals are continuously being evaluated as methods to strengthen antioxidant activity. According to several studies, β-glucan naturally have excellent antioxidant capacity (Friedman 2016; Shi et al. 2016; Yuan et al. 2019). β-Glucan in yeast is an edible that contains flavonoids, tannins, and other compounds which have excellent antioxidative capacities. The broad clinical applications have indicated that natural polysaccharides can profoundly affect the immune system and show potential as immunomodulators (Wang et al. 2013). One of the more important findings to emerge from this study is that β-glucan has no effect on cell viability within 5–20 μg/mL, while the cell viability significantly decreased in 50, 100, and 200 μg/mL, therefore the β-glucan concentrations used in this study were selected to be 5, 10, and 20 μg/mL. Furthermore, LPS-induced cell viabilities were not affected by β-glucan (5, 10, and 20 μg/mL), thus the dosage was chosen optimally in all the following experiments.
In recent years, several studies have focused on the physiological balance of ROS as a key factor causing oxidative stress in the organisms. On the other hand, it is well known that CAT and SOD reduce the oxidation potential of superoxide free radicals in cells, and GSH-Px can prevent them from interacting with key cell components by removing reactive oxygen intermediates (Goc et al. 2017; Pan and Lin 2019). HO-1 is a rate-limiting enzyme that catalyzes the degradation of heme and has antioxidant effects. Recently, nuclear HO-1 was shown to interact with Nrf2 and set an adaptive reprogramming that enhances antioxidant defenses in oxidative stress (Biswas et al. 2014). Clinical anti-tumor drug experiments showed that cinnamaldehyde (CA) and zinc protoporphyrin (ZnPP) inhibit the expression of antioxidant HO-1 and produce ROS to synergistically enhance oxidative stress, leading to apoptosis (Noh et al. 2019). All of the four enzymes above are recognized as antioxidant enzymes. In this study, our data confirmed that LPS can induce oxidative stress in RAW264.7 cells by enhancing the production of cellular ROS and MDA, the oxidative damage markers of lipids, as well as reducing the enzyme activity of CAT, SOD, and GSH-Px. LPS increased oxidative injury by generating ROS, resulting in lipid peroxidation (Circu and Aw 2010), that supports our outcomes. Furthermore, the single most striking observation to emerge from the data comparison was that β-glucan suppressed the production of ROS and MDA and increased the activity of SOD, CAT, HO-1, and GSH-Px in LPS-stimulated RAW264.7 cells. Thus, β-glucan could be considered as a potential candidate of antioxidant agents, but the molecular regulation mechanism needs further study. Dectin-1 is recognized as the most important β-glucan receptor expressed in macrophages, which can detect innately non-self-structures and initiate the immune responses (Brown and Gordon 2005; Goodridge et al. 2009). Dectin-1 activates Syk in macrophages and is important for Dectin-1-mediated reactive oxygen production, but not for phagocytosis (Underhill et al. 2005). The generating of ROS through TLR2/Dectin-1 is essential for efficient immune response against Mycobacteria (Romero et al. 2016). Laminarin significantly inhibited Dectin-1 expression on the surface of non-opsonized zymosan activated murine mast cells and that intracellular ROS production depends on the expression of Dectin-1 in response to zymosan (Yang and Marshall 2009). In this study, the results illustrated that β-glucan enhanced Dectin-1 protein expression, indicating that the antioxidant function of β-glucan may be associated with Dectin-1 receptor.
Nrf2 is a key transcription factor that plays an important role in the maintenance of oxidative balance by regulating the activity of a variety of antioxidant enzymes in the cellular defense system, such as SOD, GSH-Px, and CAT (Amaral et al. 2019; Motohashi and Yamamoto 2004). Moreover, it has been suggested that Nrf2 signaling pathway regulated antioxidant gene expression, reduced ROS production, and contributed to anti-inflammation (Kobayashi et al. 2016). Our data showed that β-glucan enhanced Nrf2 protein expression reduced by LPS, but this elevating effect was reversed by pretreatment of laminarin, indicating that the activation of Nrf2 can be regulated by Dectin-1. These results show that β-glucan can protect RAW264.7 cells from LPS-induced oxidative stress through the Dectin-1/Nrf2 signaling pathway.
In the process of oxidative stress response, HO-1 is a key enzyme that enhances antioxidant activity (Liang et al. 2013). Many studies have been published on the expression enhancement of HO-1, as a protective mechanism for cells in oxidative stress (Chung et al. 2008; Ryter et al. 2006). In this study, HO-1 located at downstream antioxidant of the Nrf2 signaling pathway was investigated. Interestingly, we found that the protein expression levels of HO-1 in β-glucan + LPS treatment groups were obviously higher than those of LPS treatment group, while such increased trend was evidently inhibited by either laminarin or ML385, implying that HO-1 could be a downstream target of Dectin-1/Nrf2 signaling pathway. These results further confirmed that β-glucan could exert antioxidant function in LPS-stimulated RAW264.7 cells.
To verify that β-glucan regulates the Nrf2/HO-1 signaling pathway through the receptor Dectin-1 in LPS-stimulated RAW264.7 cells, we applied laminarin, ML385, and SnPP to detect the production of ROS. Our findings revealed that all of these three inhibitors weakened the repression of β-glucan on ROS production in LPS-treated cells, meaning that β-glucan regulated the ROS production through Dectin-1/Nrf2/HO-1 signaling pathway, which offered strong support for our hypothesis.
In order to determine the molecular mechanism of Dectin-1 regulating the Nrf2/HO-1 signaling pathway, we used β-glucan to treat RAW264.7 cells alone. The results of immunofluorescence and western blot clearly show that β-glucan could activate its receptor Dectin-1, enhance the expression of Nrf2 and HO-1, and mediate the activation of Nrf2/HO-1 signaling pathway. These data are in the lines of earlier literature reporting that Dectin-1 was expressed on RAW264.7 cells at a high level by β-glucan activation (Lee and Johnson 2004). These results further strengthened our hypothesis that Dectin-1 could regulate the Nrf2/HO-1 signaling pathway.
Taken together, the current research demonstrates that β-glucan exerts antioxidative protective effect via Dectin-1/Nrf2/HO-1 signaling pathway in LPS-stimulated RAW264.7 cells, the mechanism of which is attributed to triggering the Dectin-1 expression, activating the Nrf2/HO-1 signaling pathway for decreasing ROS and alleviating oxidative stress. This study may provide new natural compounds for the treatment of oxidative stress-related diseases.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32060774), China Agriculture Research System (CARS-36), and Inner Mongolia Autonomous Region Science and Technology Plan Project (2020GG0036).
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Amaral JH, Rizzi ES, Alves-Lopes R, Pinheiro LC, Tostes RC, Tanus-Santos JE. Antioxidant and antihypertensive responses to oral nitrite involves activation of the Nrf2 pathway. Free Radic Biol Med. 2019;141:261–268. doi: 10.1016/j.freeradbiomed.2019.06.028. [DOI] [PubMed] [Google Scholar]
- Babincova M, Bacova Z, Machova E, Kogan G. Antioxidant properties of carboxymethyl glucan: comparative analysis. Journal of medicinal food. 2002;5:79–83. doi: 10.1089/109662002760178159. [DOI] [PubMed] [Google Scholar]
- Bacha U, Nasir M, Iqbal S, Anjum AA. Nutraceutical, anti-inflammatory, and immune modulatory effects of beta-glucan isolated from yeast. Biomed Res Int. 2017;2017:8972678–8972614. doi: 10.1155/2017/8972678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas C, Shah N, Muthu M, la P, Fernando AP, Sengupta S, Yang G, Dennery PA. Nuclear heme oxygenase-1 (HO-1) modulates subcellular distribution and activation of Nrf2, impacting metabolic and anti-oxidant defenses. J Biol Chem. 2014;289:26882–26894. doi: 10.1074/jbc.M114.567685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD, Gordon S. Immune recognition of fungal beta-glucans. Cell Microbiol. 2005;7:471–479. doi: 10.1111/j.1462-5822.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- Celi P, Di Trana A, Claps S. Effects of plane of nutrition on oxidative stress in goats during the peripartum period. Veterinary journal. 2010;184:95–99. doi: 10.1016/j.tvjl.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Cetin E. Pretreatment with beta-glucan attenuates isoprenaline-induced myocardial injury in rats. Experimental physiology. 2019;104:505–513. doi: 10.1113/EP086739. [DOI] [PubMed] [Google Scholar]
- Chung SW, Liu X, Macias AA, Baron RM, Perrella MA. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. The Journal of clinical investigation. 2008;118:239–247. doi: 10.1172/JCI32730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft KD, Zhang D, Jiang R, Ayer A, Shengule S, Payne RJ, Ward NC, Stocker R. Structural requirements of flavonoids to induce heme oxygenase-1 expression. Free Radic Biol Med. 2017;113:165–175. doi: 10.1016/j.freeradbiomed.2017.09.030. [DOI] [PubMed] [Google Scholar]
- Datla SR, Dusting GJ, Mori TA, Taylor CJ, Croft KD, Jiang F. Induction of heme oxygenase-1 in vivo suppresses NADPH oxidase derived oxidative stress. Hypertension. 2007;50:636–642. doi: 10.1161/HYPERTENSIONAHA.107.092296. [DOI] [PubMed] [Google Scholar]
- Deng S, Yu K, Jiang W, Li Y, Wang S, Deng Z, Yao Y, Zhang B, Liu G, Liu Y, Lian Z. Over-expression of Toll-like receptor 2 up-regulates heme oxygenase-1 expression and decreases oxidative injury in dairy goats. Journal of animal science and biotechnology. 2017;8:3. doi: 10.1186/s40104-016-0136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, et al. Melatonin prevents LPS-induced epithelial-mesenchymal transition in human alveolar epithelial cells via the GSK-3beta/Nrf2 pathway. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2020;132:110827. doi: 10.1016/j.biopha.2020.110827. [DOI] [PubMed] [Google Scholar]
- Drummond RA, Brown GD. The role of Dectin-1 in the host defence against fungal infections. Curr Opin Microbiol. 2011;14:392–399. doi: 10.1016/j.mib.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Friedman M (2016) Mushroom polysaccharides: chemistry and antiobesity, antidiabetes, anticancer, and antibiotic properties in cells, rodents, and humans. Foods 5. 10.3390/foods5040080 [DOI] [PMC free article] [PubMed]
- Goc Z, Szaroma W, Kapusta E, Dziubek K. Protective effects of melatonin on the activity of SOD, CAT, GSH-Px and GSH content in organs of mice after administration of SNP. The Chinese journal of physiology. 2017;60:1–10. doi: 10.4077/CJP.2017.BAF435. [DOI] [PubMed] [Google Scholar]
- Goodridge HS, Wolf AJ, Underhill DM. Beta-glucan recognition by the innate immune system. Immunol Rev. 2009;230:38–50. doi: 10.1111/j.1600-065X.2009.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hino S, Kito A, Yokoshima R, Sugino R, Oshima K, Morita T, Okajima T, Nadano D, Uchida K, Matsuda T. Discharge of solubilized and Dectin-1-reactive beta-glucan from macrophage cells phagocytizing insoluble beta-glucan particles: involvement of reactive oxygen species (ROS)-driven degradation. Biochemical and biophysical research communications. 2012;421:329–334. doi: 10.1016/j.bbrc.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Ishida Y et al (2018) Candida albicans beta-glucan-containing particles increase HO-1 expression in oral keratinocytes via a reactive oxygen species/p38 mitogen-activated protein kinase/Nrf2 pathway. Infect Immun 86. 10.1128/IAI.00575-17 [DOI] [PMC free article] [PubMed]
- Jiang F, Roberts SJ, Datla S, Dusting GJ. NO modulates NADPH oxidase function via heme oxygenase-1 in human endothelial cells. Hypertension. 2006;48:950–957. doi: 10.1161/01.HYP.0000242336.58387.1f. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annual review of pharmacology and toxicology. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H, Tanaka N, Moriguchi T, Motohashi H, Nakayama K, Yamamoto M. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nature communications. 2016;7:11624. doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Johnson JA. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J Biochem Mol Biol. 2004;37:139–143. doi: 10.5483/bmbrep.2004.37.2.139. [DOI] [PubMed] [Google Scholar]
- Lee SE, Jeong SI, Yang H, Jeong SH, Jang YP, Park CS, Kim J, Park YS. Extract of Salvia miltiorrhiza (Danshen) induces Nrf2-mediated heme oxygenase-1 expression as a cytoprotective action in RAW 264.7 macrophages. Journal of ethnopharmacology. 2012;139:541–548. doi: 10.1016/j.jep.2011.11.046. [DOI] [PubMed] [Google Scholar]
- Liang L, Gao C, Luo M, Wang W, Zhao C, Zu Y, Efferth T, Fu Y. Dihydroquercetin (DHQ) induced HO-1 and NQO1 expression against oxidative stress through the Nrf2-dependent antioxidant pathway. J Agric Food Chem. 2013;61:2755–2761. doi: 10.1021/jf304768p. [DOI] [PubMed] [Google Scholar]
- Lin HY, Juan SH, Shen SC, Hsu FL, Chen YC. Inhibition of lipopolysaccharide-induced nitric oxide production by flavonoids in RAW264.7 macrophages involves heme oxygenase-1. Biochemical pharmacology. 2003;66:1821–1832. doi: 10.1016/s0006-2952(03)00422-2. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhu Q, Zhang M, Yin T, Xu R, Xiao W, Wu J, Deng B, Gao X, Gong W, Lu G, Ding Y. Isoliquiritigenin ameliorates acute pancreatitis in mice via inhibition of oxidative stress and modulation of the Nrf2/HO-1 pathway. Oxid Med Cell Longev. 2018;2018:7161592–7161512. doi: 10.1155/2018/7161592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yang W, Sun X, Xie L, Yang Y, Sang M, Jiao R. SS31 ameliorates sepsis-induced heart injury by inhibiting oxidative stress and inflammation. Inflammation. 2019;42:2170–2180. doi: 10.1007/s10753-019-01081-3. [DOI] [PubMed] [Google Scholar]
- Mills EL, Kelly B, Logan A, Costa ASH, Varma M, Bryant CE, Tourlomousis P, Däbritz JHM, Gottlieb E, Latorre I, Corr SC, McManus G, Ryan D, Jacobs HT, Szibor M, Xavier RJ, Braun T, Frezza C, Murphy MP, O’Neill LA. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. 2016;167:457-470 e413. doi: 10.1016/j.cell.2016.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends in molecular medicine. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Noh J, Jung E, Lee J, Hyun H, Hong S, Lee D. Engineered polymeric micelles for combinational oxidation anticancer therapy through concurrent HO-1 inhibition and ROS generation. Biomacromolecules. 2019;20:1109–1117. doi: 10.1021/acs.biomac.8b01802. [DOI] [PubMed] [Google Scholar]
- Pan Y, Lin Z. Anti-aging effect of Ganoderma (Lingzhi) with health and fitness. Advances in experimental medicine and biology. 2019;1182:299–309. doi: 10.1007/978-981-32-9421-9_13. [DOI] [PubMed] [Google Scholar]
- Park JY, Kwon YW, Lee SC, Park SD, Lee JH. Herbal formula SC-E1 suppresses lipopolysaccharide-stimulated inflammatory responses through activation of Nrf2/HO-1 signaling pathway in RAW 264.7 macrophages. BMC complementary and alternative medicine. 2017;17:374. doi: 10.1186/s12906-017-1874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikov EY, Morosanova MA, Pevzner IB, Zorova LD, Manskikh VN, Pulkova NV, Galkina SI, Skulachev VP, Zorov DB. Protective effect of mitochondria-targeted antioxidants in an acute bacterial infection. Proc Natl Acad Sci U S A. 2013;110:E3100–E3108. doi: 10.1073/pnas.1307096110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman A, Pacher P, Hasko G. Role of macrophages in the endocrine system. Trends in endocrinology and metabolism: TEM. 2021;32:238–256. doi: 10.1016/j.tem.2020.12.001. [DOI] [PubMed] [Google Scholar]
- Rocha M, Herance R, Rovira S, Hernandez-Mijares A, Victor VM. Mitochondrial dysfunction and antioxidant therapy in sepsis. Infectious disorders drug targets. 2012;12:161–178. doi: 10.2174/187152612800100189. [DOI] [PubMed] [Google Scholar]
- Romero M, Basile J, Corra Feo L, López B, Ritacco V, Alemán MJCM. Reactive oxygen species production by human dendritic cells involves TLR2 and dectin-1 and is essential for efficient immune response against. Mycobacteria. 2016;18:875–886. doi: 10.1111/cmi.12562. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiological reviews. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- Sahasrabudhe NM, Dokter-Fokkens J, de Vos P. Particulate beta-glucans synergistically activate TLR4 and Dectin-1 in human dendritic cells. Molecular nutrition & food research. 2016;60:2514–2522. doi: 10.1002/mnfr.201600356. [DOI] [PubMed] [Google Scholar]
- Sandvik A, Wang YY, Morton HC, Aasen AO, Wang JE, Johansen FE. Oral and systemic administration of beta-glucan protects against lipopolysaccharide-induced shock and organ injury in rats. Clin Exp Immunol. 2007;148:168–177. doi: 10.1111/j.1365-2249.2006.03320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sener G, Toklu H, Ercan F, Erkanli G. Protective effect of beta-glucan against oxidative organ injury in a rat model of sepsis. International immunopharmacology. 2005;5:1387–1396. doi: 10.1016/j.intimp.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Sener G, et al. Melatonin protects against oxidative organ injury in a rat model of sepsis. Surgery today. 2005;35:52–59. doi: 10.1007/s00595-004-2879-1. [DOI] [PubMed] [Google Scholar]
- Shen BY, Zhao C, Wang Y, Peng Y, Cheng J, Li Z, Wu L, Jin M, Feng H. Aucubin inhibited lipid accumulation and oxidative stress via Nrf2/HO-1 and AMPK signalling pathways. J Cell Mol Med. 2019;23:4063–4075. doi: 10.1111/jcmm.14293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Jia Y, Wang X, Zhang J, Liu K, Wang J, Cai W, Li J, Li S, Zhao M, Wang Y, Hu D. Exosomes from adipose-derived stem cells alleviate the inflammation and oxidative stress via regulating Nrf2/HO-1 axis in macrophages. Free Radic Biol Med. 2021;165:54–66. doi: 10.1016/j.freeradbiomed.2021.01.023. [DOI] [PubMed] [Google Scholar]
- Shi L, Lin Q, Yang T, Nie Y, Li X, Liu B, Shen J, Liang Y, Tang Y, Luo F. Oral administration of Lentinus edodes beta-glucans ameliorates DSS-induced ulcerative colitis in mice via MAPK-Elk-1 and MAPK-PPARgamma pathways. Food Funct. 2016;7:4614–4627. doi: 10.1039/c6fo01043a. [DOI] [PubMed] [Google Scholar]
- Srisook K, Cha YN. Biphasic induction of heme oxygenase-1 expression in macrophages stimulated with lipopolysaccharide. Biochemical pharmacology. 2004;68:1709–1720. doi: 10.1016/j.bcp.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Su CH, Tseng YT, Lo KY, Lai MN, Ng LT (2020) Differences in anti-inflammatory properties of water soluble and insoluble bioactive polysaccharides in lipopolysaccharide-stimulated RAW264.7 macrophages. Glycoconjugate journal. 10.1007/s10719-020-09934-y [DOI] [PubMed]
- Supinski GS, Schroder EA, Callahan LA. Mitochondria and critical illness. Chest. 2020;157:310–322. doi: 10.1016/j.chest.2019.08.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PR, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–38. doi: 10.1038/Ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoni SV, Brown GD. beta-Glucans and Dectin-1. Ann Ny Acad Sci. 2008;1143:45–60. doi: 10.1196/annals.1443.019. [DOI] [PubMed] [Google Scholar]
- Underhill DM, Rossnagle E, Lowell CA, Simmons RM. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood. 2005;106:2543–2550. doi: 10.1182/blood-2005-03-1239%JBlood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Meng X, Yang R, Qin T, Li Y, Zhang L, Fei C, Zhen W, Zhang K, Wang X, Hu Y, Xue F. Cordyceps militaris polysaccharides can improve the immune efficacy of Newcastle disease vaccine in chicken. Int J Biol Macromol. 2013;59:178–183. doi: 10.1016/j.ijbiomac.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Wilson W, Lowman D, Antony SP, Puthumana J, Singh ISB, Philip R. Immune gene expression profile of Penaeus monodon in response to marine yeast glucan application and white spot syndrome virus challenge. Fish & Shellfish Immunology. 2015;43:346–356. doi: 10.1016/j.fsi.2014.12.032. [DOI] [PubMed] [Google Scholar]
- Yang Z, Marshall JS. Zymosan treatment of mouse mast cells enhances dectin-1 expression and induces dectin-1-dependent reactive oxygen species (ROS) generation. Immunobiology. 2009;214:321–330. doi: 10.1016/j.imbio.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Yuan H, Lan P, He Y, Li C, Ma X (2019) Effect of the modifications on the physicochemical and biological properties of beta-glucan-a critical review. Molecules 25. 10.3390/molecules25010057 [DOI] [PMC free article] [PubMed]
- Zhang H, Davies KJA, Forman HJ. Oxidative stress response and Nrf2 signaling in aging. Free Radic Biol Med. 2015;88:314–336. doi: 10.1016/j.freeradbiomed.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ding M, Zhu P, Huang H, Zhuang Q, Shen J, Cai Y, Zhao M, He Q. New insights into the Nrf-2/HO-1 signaling axis and its application in pediatric respiratory diseases. Oxid Med Cell Longev. 2019;2019:3214196–3214199. doi: 10.1155/2019/3214196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhou Q, Li Y, Zhang Y, Wu Y. MitoQ modulates lipopolysaccharide-induced intestinal barrier dysfunction via regulating Nrf2 signaling. Mediators of inflammation. 2020;2020:3276148–3276149. doi: 10.1155/2020/3276148. [DOI] [PMC free article] [PubMed] [Google Scholar]