Abstract
The usefulness of magnetic resonance imaging (MRI) in predicting gait ability in stroke patients remains unclear. Therefore, MRI evaluations have not yet been standardized in stroke rehabilitation. We performed a systematic review to consolidate evidence regarding the use of MRIs in predicting gait ability of stroke patients. The Medline, Cumulative Index to Nursing and Allied Health Literature, and SCOPUS databases were comprehensively searched. We included all literature published from each source’s earliest date to August 2020. We included 19 studies: 8 were classified as structure- or function-based MRI studies and 11 as neural tract integrity-based MRI studies. Most structure- or function-based MRI studies indicated that damage to motor-related areas (primary motor cortex, corona radiata, internal capsule, and basal ganglia) or insula was related to poor gait recovery. In neural tract integrity-based MRI studies, integrity of the corticospinal tract was related to gait ability. Some studies reported predictive value of the corticoreticular pathway. All included studies had some concerns, at least one, based on the Cochrane risk of bias instrument. This review suggests that MRIs are useful in predicting gait ability of stroke patients. However, we cannot make definitive conclusion regarding the predictive value, due to the lack of quantitative evaluations.
Subject terms: Health care, Medical research, Neurology
Introduction
Gait ability is important for mobility and maintaining general health1. Stroke patients usually have residual disabilities; in particular, many stroke survivors experience a gait disability because of lower limb hemiparalysis, resulting in movement restrictions in daily life2–4. The patients with post-stroke hemiparesis frequently present with asymmetric gait patterns5. The asymmetric gait patterns are characterized increased or decreased swing time and stance time, (i.e., temporal asymmetry) and increased or decreased step length (i.e., spatial asymmetry)6–8. The altered gait pattern leads to decreased walking velocity5. Acquiring functional gait ability is considered a principal goal of rehabilitation, because gait affects a patient’s or family’s quality of life9,10. Even in cases where an individual is not expected to acquire functional gait ability, a rehabilitation program focused on substitutional locomotion, such as using a wheelchair or modifying the individual’s environment, can be meaningful for expanding an individual’s mobility.
Predicting an individual’s gait ability from the early phase after stroke onset is crucial for setting realistic rehabilitative goals and/or arranging a rehabilitation program. Previous studies reported that the initial motor and functional impairment level had predictive value for gait ability, as did specific evaluation tools, like the revised version of the Ability for Basic Movement Scale II11,12. Developments in the field of neuroscience have been gradually clarifying the complex regulation of the neural network for gait13–17, and anatomically determining the extent of damage to the gait-related neural network might have extreme value in predicting gait ability. Brain imaging, including structural imaging and functional imaging, has been widely used in clinical situations for disease diagnoses, lesion identification, or understanding recovery mechanisms18–20. In magnetic resonance imaging (MRI), in particular, diffusion-weighted imaging (DWI) has been used for early detection of ischemic brain lesions18 and diffusion tensor imaging (DTI) has been applied to describe neural tracts in recent years21–23. In fact, MRIs have already been used to predict a patient’s functional outcomes24 or ability to perform activities of daily living25. Stinear and Ward26 stated in a systematic review that imaging may help clinicians to identify each patient’s potential for recovery, set realistic rehabilitation goals, and select therapy techniques on the basis of residual connections between key elements of the central nervous system.
Skilled clinicians who empirically understand the usefulness of brain imaging have already been applying it in routine patient evaluations, including to predict gait ability. However, no systematic review has evaluated the usefulness of MRIs in predicting the gait ability of stroke patients; as a result, MRI evaluations have not been standardized in the field of stroke rehabilitation. Therefore, this systematic review aimed to consolidate evidence regarding the use of MRIs in predicting the stroke patient’s gait ability including the degree of gait independence, gait speed, or gait endurance.
Methods
A systematic review of the literature was performed according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines27. This review was registered in PROSPERO (ID: CRD 42020206355).
Selection criteria
Studies were included in this systematic review if they met following criteria: (1) the patients were diagnosed with hemorrhagic or ischemic strokes; (2) the patients had a conventional MRI (T1-weighted imaging, T2-weighted imaging, or fluid-attenuated inversion recovery), functional MRI, DWI, or DTI; (3) gait ability outcomes were assessed; (4) the MRI was applied in predicting gait ability; (5) the study was a cohort study or case–control study; and (6) the article was written in English.
Studies were excluded if: (1) the study included patients with subarachnoid hemorrhages; (2) the study was a case study or cross-sectional study; or (3) the study was a review article.
Search strategy and study selection
The Medline, Cumulative Index to Nursing and Allied Health Literature, and SCOPUS electronic databases were comprehensively searched. The search terms “patient”, “exposure” , and “outcome” were combined with the “AND” operator. “Patient” was defined as a stroke patient. “Exposure” was defined as an MRI evaluation. “Outcome” was defined as gait performance prediction. For each concept, we combined synonyms and Medical Subject Heading terms with the “OR” operator. There were no limits with regard to dates. The searches were performed on August 31, 2020. An example of the search strategy used in the Medline database is shown in Supplementary File 1.
The articles identified through database searching were summarized into spreadsheet that were created using Microsoft Excel 2019. After excluding duplicates, two reviewers (TI and TM) independently screened each article based on the title and abstract using predetermined eligibility criteria in order to determine relevant manuscripts for full-text review. Subsequently, full-text copies of articles that were not excluded based on the title or abstract were retrieved, and the inclusion and exclusion criteria were reapplied to these studies to determine suitability for final inclusion. Any disagreements during the article screening and selection were resolved through discussion, and decisions were made by a third person if the two reviewers could not reach a consensus.
Data extraction
Predesigned spreadsheets that were created using Microsoft Excel 2019 were used to extract data on participants, exposures, outcome measurements, and results. Two reviewers (TI and TM) discussed and decided on the extraction data, and a third person confirmed.
Risk of bias evaluation in individual studies
To evaluate the risk of bias in each study, two researchers (TI and TM) independently applied the tool to assess risk of bias in cohort studies (the Cochrane risk of bias instrument). The articles were evaluated using predetermined criteria (Supplementary File 2).
Results
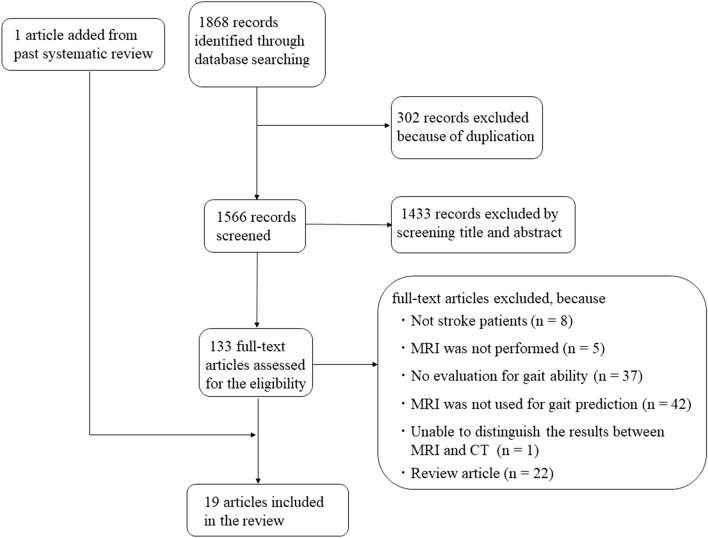
The combined database search identified 1868 studies (Fig. 1). After adjusting for duplicates, 1566 studies were considered. Out of these, 1433 studies did not meet the selection criteria after a review of the titles and abstracts. The complete texts of the remaining 133 studies were examined in detail, and 115 studies did not meet the inclusion criteria. One article was added from a past systematic review. Finally, 19 studies fulfilled the inclusion criteria and were included in the analysis. Critical information regarding the included studies is summarized in Table 1, including data on the study population, type of MRI evaluation, key analysis, and predictive outcomes. The average age of participants in all the studies ranged from 52.1 to 71.5 years. In addition, the stroke phase at baseline ranged from within 3 days to an average of 212 days after onset. The included studies were divided broadly into two research categories: (1) structure- or function-based MRI studies, which focused on affected brain structures or imaging findings; and (2) neural tract integrity-based MRI studies, which focused on neural tract integrity using DTI methodology.
Figure 1.
Flow diagram of included and excluded studies. CT computed tomography, MRI magnetic resonance imaging.
Table 1.
Summary of included studies.
| Study: author, journal, year (retrospective or prospective) | Participants | MRI evaluation | Analysis | Predictive outcome | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Size (n) | Age, years | Sex, M/F, n | Stroke type, n | Stroke location | Lesion side, R/L, n | Stroke phase at baseline | Function at baseline | Tesla | Contents | Evaluation days from onset | Outcome scale | Evaluation days from onset | ||
| Lee et al., Brain Behav, 201729 (retrospective) | 30 | 55.0 ± 13.7 | 17/13 |
Ischemic: 10 Hemorrhagic: 20 |
Supratentorial | 15/15 | Acute (within 14 days after onset) |
FMA-UE: 20.1 ± 18.5 FMA-LE: 14.0 ± 8.1 FMA-S: 10.5 ± 8.4 |
3 |
T1WI T2WI |
Within 14 days after onset |
Overlay of lesions Subtraction analysis Voxel-based lesion symptom mapping |
FAC | Initial assessment (within 14 days), 1, 3, and 6 months after onset |
| Kim et al., Neuroreport, 201839 (not mentioned) | 48 |
Group A: 64.0 ± 12.7 Group B: 63.9 ± 12.4 Group C: 67.9 ± 12.3 |
34/14 |
Ischemic: 40 Hemorrhagic: 8 |
Supratentorial | 29/19 | Acute to subacute (within 6 weeks after onset) |
FMA (Group A: 45.4 ± 20.4, Group B: 32.4 ± 19.9, Group C: 17.2 ± 13.8) |
3 | DTI | Within 6 weeks after onset | 3 groups comparison | FAC | Baseline (within 1 week after DTI) and at 2 years after onset |
| Jones et al., Hum Brain Mapp, 201631 (prospective) | 50 | 64.6 ± 15.0 | 28/22 |
Ischemic: 41 Hemorrhagic: 9 |
Supratentorial and infratentorial | 25/25 | Acute to subacute (median 16 days, range: 3–42 days) | – | 1.5 |
T1WI T2WI FLAIR |
Median 52 days after onset (range 17–74 days) |
Overlay of lesions Multiple regression analysis |
FAC Gait speed MRMI |
At entry into the study and the end of 6 weeks of intervention phase |
| Yeo et al., J Stroke Cerebrovasc Dis, 202041 (retrospective) | 9 | 59.3 ± 12.4 | 7/2 |
Ischemic: 0 Hemorrhagic: 9 |
Infratentorial | – | Acute to subacute (15.3 ± 6.6 days) | – | 1.5 |
T2WI DTI |
15.3 ± 6.6 days after onset and 41.2 ± 21.6 days after onset | 2 groups comparison | FAC | 15.3 ± 6.6 days after onset and 41.2 ± 21.6 days after onset |
| Miyai et al., Stroke, 200028 (not mentioned) | 94 |
IC and Pt: 58, Th: 63, IC, Pt, and Th: 60 |
IC and Pt :22/33Th:11/13 IC, Pt, and Th: 4/11 |
Ischemic: 0 Hemorrhagic: 94 |
Supratentorial |
IC and Pt :25/30 Th:11/13 IC, Pt, and Th: 5/10 |
Chronic (106 days after onset) |
SIAS (UE + LE) IC and Pt: 10 Th: 11 IC, Pt, and Th: 10 FIM IC and Pt: 86 Th: 87 IC, Pt, and Th: 84 |
1.0 |
T1WI T2WI |
2, 4, and 6 months after onset | 3 groups comparison | FIM (mobility) and the probability of ambulation without physical assistance | On admission and discharge |
| Moon et al., Neuroradiology, 201732 (retrospective) | 102 | 65.77 ± 13.85 | 60/42 |
Ischemic: 15 Hemorrhagic: 39 |
Supratentorial and infratentorial | 52/41 both: 9 | Subacute (≤ 90 days after onset) (mean 26.8 ± 19.2 days) | FIM: 57.58 ± 24.95 | – |
T1WI FLAIR |
– |
2 groups comparison Overlay of lesions Voxel-based lesion symptom mapping analysis Multivariate logistic regression analysis |
FAC Gait speed |
Baseline (≤ 90 days after onset) and after the 4-week rehabilitation |
| Jang et al., Ann Neurol, 200836 (prospective) | 25 | 61.6 ± 9.92 | 11/14 |
Ischemic: 25 Hemorrhagic: 0 |
Infratentorial | 15/10 | Acute to subacute (15.28 ± 6.88 days after onset,range 5–30 days) |
MBC: 0.12 ± 0.33 MI: 18.96 ± 14.05 |
1.5 |
T2WI DTI |
15.28 ± 6.88 days after onset (range 5–30 days) | 2 groups comparison | FAC | At onset and 6 months after onset |
| Kim et al., NeuroRehabilitation, 201338 (retrospective) | 37 | 57.4 ± 15.2 | 28/9 |
Ischemic: 37 Hemorrhagic: 0 |
Supratentorial | 16/21 | Acute to subacute (5–30 days after onset) |
MI: 7.30 ± 11.15 MBC: 1.05 ± 0.23 |
1.5 |
T2WI DTI |
19.2 ± 7.5 days after onset | 3 groups comparison | FAC | At onset and 6 months after onset |
| Soulard et al., Neurology, 202042 (prospective) | 29 | 52.14 ± 9.84 | 21/8 |
Ischemic: 29 Hemorrhagic: 0 |
Supratentorial | 10/19 | Acute (14 days after onset) | NIHSS: 13.90 ± 4.72 | 3 |
T1WI FLAIR DTI |
1 month after onset | Correlation analysis | Walking score (the sum of Barthel Index (gait subscore and stairs subscore) | 1, 1.5, 3, 5, 7, 13, and 25 months after onset |
| Baillieul et al., Hum Mov Sci, 201930 (prospective) | 33 | 63.9 ± 12.9 | 21/12 |
Ischemic: 33 Hemorrhagic: 0 |
Supratentorial | 14/19 | Acute (2.9 ± 2.7 days after onset) |
NIHSS Score 0: 6 Score 1–4: 18 Score 5–15: 8 Score 16–20: 1 |
1.5 |
T1WI T2WI FLAIR |
2.9 ± 2.7 days after onset |
Overlay of lesions Voxel-based lesion symptom mapping analysis |
Rivermead Mobility Index Gait speed Walking actibity |
At 3 months after onset |
| Sagnier et al., Stroke, 202046 (prospective) | 207 | 66 ± 13 | 138/69 |
Ischemic: 207 Hemorrhagic: 0 |
Supratentorial | 97/98 both: 12 | Acute (within 24 to 72 h after onset) | NIHSS: median 3 (IQR: 2–6) | 3 |
DWI FLAIR DTI |
Within 24 to 72 h after onset |
Multiple regression analysis Path analysis Tract-based spatial statistics analysis |
Gait speed | 1 year after onset |
| Jang et al., Int J Neurosci, 201337 (not mentioned) | 21 | 62.66 ± 8.58 | 6/15 |
Ischemic: 0 Hemorrhagic: 21 |
Supratentorial | 12/9 | Acute to subacute (16.66 ± 5.71 days after onset, range 7–30 days) |
MI: 5.80 ± 7.92 MBC: 1.00 ± 0.00 |
1.5 |
T2WI DTI |
16.66 ± 5.71 days after onset (range 7–30 days) |
2 groups comparison Correlation analysis |
FAC | At onset and 6 months after onset |
| Jang et al., Somatosens Mot Res, 201644 (retrospective) | 31 | 64.76 ± 10.76 | 12/19 |
Ischemic: 31 Hemorrhagic: 0 |
Infratentorial | 20/11 | Acute to subacute (12.71 ± 4.63 days after onset, range 7–28 days) | – | 1.5 |
T2WI DTI |
12.71 ± 4.63 days after onset (range 7–28 days) | Correlation analysis | FAC | At onset and 6 months after onset |
| Imura et al., J Phys Ther Sci, 201540 (not mentioned) | 25 | 71.5 ± 11.0 | 14/11 |
Ischemic: 16 Hemorrhagic: 9 |
– | – | Acute (within 10 days after onset) | – | 3 | DTI | Within 10 days after onset | Correlation analysis |
Barthel Index (gait subscore) Functional Independence Measure (gait subscore) |
1 month after onset |
| Burke et al., Stroke, 201447 (prospective) | 33 | 61 ± 14 | – | – | – | – | Chronic (212 ± 104 days after onset) |
mRS: 0.18 ± 0.46 Barthel Index: 81 ± 18 |
1.5 |
T1WI fMRI |
212 ± 104 days after onset | Multivariate analysis |
Gait speed Gait endurance |
At baseline and 12 weeks after baseline |
| Lam et al., Neurorehabil Neural Repair, 201033 (prospective) | 52 | 66.8 | 34/18 |
Ischemic: 52 Hemorrhagic: 0 |
Supratentorial and infratentorial | 20/32 | Chronic (at least 6 months after onset) | NIHSS: 4.08 |
1.5 (n = 20) 3 (n = 32) |
T1WI fMRI (n = 20) |
Within 2 weeks of the start and end of the training | General linear model |
Gait speed Gait endurance |
Before and after training period (6 months, n = 20; 3 months, n = 32) |
| Loos et al., Int J Stroke, 201834 (prospective) | 200 | 66.8 ± 11.4 | 112/88 |
Ischemic: 200 Hemorrhagic: 0 |
– | – | – | NIHSS: 1 (range 0–7) | 1.5 |
T1WI T2W2 FLAIR DWI |
Median 7 days after onset (range: 0–142 days) | Multiple regression analysis |
Timed Up and Go test Stroke impact scale (mobility domain) |
3 years after onset |
| Smith et al., Neurorehabil Neural Repair, 201745 (not mentioned) | 41 | Median 72 (range 43–96) | 17/24 |
Ischemic: 35 Hemorrhagic: 6 |
Supratentorial and infratentorial | 20/21 | Acute (within 3 days after onset) | NIHSS: median 8 (range 1–21) | 1.5 |
T1WI DWI DTI |
7 to 14 days after onset |
Logistic regression analysis Classification and regression tree analysis |
FAC | Baseline (within 3 days after onset), 6 weeks, and 12 weeks after onset |
| Cho et al., Neurosci Lett, 200735 (prospective) | 40 | 53.35 ± 9.93 | 21/19 |
Ischemic: 0 Hemorrhagic: 40 |
Supratentorial | 13/27 | Acute to subacute (22.45 ± 8.04 days after onset, range 7–30 days) |
MI-UE: 0.0 MI-LE: 1.0 FAC: 0 |
1.5 |
T2WI DTI |
22.45 ± 8.04 days after onset (range 7–30 days) | 4 groups comparison | FAC | At onset and 6 months after onset |
DTI diffusion tensor imaging, DWI diffusion weighted imaging, FAC functional ambulation category, FIM functional independence measure, FLAIR fluid-attenuated inversion-recovery, fMRI functional magnetic resonance imaging, FMA Fugl–Meyer Assessment, FMA-S Fugl–Meyer Assessment sensory subscore, IC internal capsule, IQR interquartile range, LE lower extremity, MI Motricity Index, MBC modified Brunnstrom classification, MRI magnetic resonance imaging, MRMI modified Rivermead mobility index, mRS modified Rankin scale, NIHSS National Institutes of Health stroke scale, Pt putamen, SIAS stroke impairment assessment set, Th thalamus, T1WI T1-weighted imaging, T2WI T2-weighted imaging, UE upper extremity.
Table 2 summarizes important information from the eight structure- or function-based MRI studies, including the participants’ stroke types, MRI contents, key structure or imaging findings, and main results. All studies showed the usefulness of key structure or imaging findings in predicting gait ability. In particular, most studies indicated that damage to motor-related areas (e.g., primary motor cortex, corona radiata, internal capsule, and basal ganglia) or insula were related to poor gait recovery. Table 3 summarizes considerable information from the 11 neural tract integrity-based MRI studies, including the patients’ stroke types, MRI contents, imaging parameters, analyzed tracts, and main results. All studies included DTI-related results, and most studies showed the usefulness of a tract integrity analysis in predicting gait ability. In particular, integrity of the corticospinal tract (CST) was related to gait ability. Some studies reported the predictive value of the corticoreticular pathway (CRP).
Table 2.
Summary of structure- or function-based MRI studies.
| Study: author, journal, year | Stroke type, n | MRI contents | Key structures or imaging findings | Main results |
|---|---|---|---|---|
| Lee et al., Brain Behav, 201729 |
Ischemic: 10 Hemorrhagic: 20 |
T1WI T2WI |
Corona radiata IC Globus pallidus Putamen Cingulum Primary motor cortex Caudate nucleus |
Corona radiata, internal capsule, globus pallidus, putamen, and cingulum, primary motor cortex, and caudate nucleus were related with poor gait recovery |
| Jones et al., Hum Brain Mapp, 201631 |
Ischemic: 41 Hemorrhagic: 9 |
T1WI T2WI FLAIR |
CST Putamen Insula External capsule and neighboring white matter |
CST damage independently predicted response to therapy for FAC and MRMI, but not for walk speed Walk speed response to rehabilitation was affected by damage involving the putamen, insula, external capsule and neighboring white matter but not the CST |
| Miyai et al., Stroke, 200028 |
Ischemic: 0 Hemorrhagic: 94 |
T1WI T2WI |
IC Putamen Thalamus |
The patients who had all 3 lesions (IC, Pt, and Th) showed greater improvement of FIM mobility scores and the probability of ambulation without physical assistance on discharge compared with the patients who had lesions in IC and Pt or Th only All patients who had all 3 lesions (IC, Pt, and Th) showed intact anterior ventral nucleus and damage in the posterior half of the internal capsule |
| Moon et al., Neuroradiology, 201732 |
Ischemic: 15 Hemorrhagic: 39 |
T1WI FLAIR |
Insula IC |
Damage to the insula and internal capsule affected gait velocity change |
| Baillieul et al., Hum Mov Sci, 201930 |
Ischemic: 33 Hemorrhagic: 0 |
T1WI T2WI FLAIR |
Putamen (posterior part) IC (posterior limb) Corona radiata (anterior part) |
Lower level of walking activity were related to lesions of the posterior part of putamen, posterior limb of internal capsule, and anterior part of corona radiata |
| Burke et al., Stroke, 201447 | – |
T1WI fMRI |
Primary sensorimotor cortex | Treatment-related gains in gait velocity were related to activation volume in ipsilesional foot primary sensorimotor cortex at baseline |
| Lam et al., Neurorehabil Neural Repair, 201033 |
Ischemic: 52 Hemorrhagic: 0 |
T1WI fMRI (n = 20) |
Subcortical lesion Left-sided lesion |
10 m walk velocity improved more in the patients with subcortical rather than in the patients with cortical lesions Improvements in 6 min walk velocity were greater in the patients with left-sided lesions |
| Loos et al., Int J Stroke, 201834 |
Ischemic: 200 Hemorrhagic: 0 |
T1WI T2W2 FLAIR DWI |
Total cerebral small vessel disease burden |
Total cerebral small vessel disease burden was not associated with gait impairment in all stroke patients, nor in lacunar stroke In non-lacunar stroke patients, total cerebral small vessel disease burden was associated with lower stroke impact scale (mobility domain) |
CST corticospinal tract, DWI diffusion weighted imaging, FAC functional ambulation category, FIM functional independence measure, FLAIR fluid-attenuated inversion-recovery, fMRI functional magnetic resonance imaging, IC internal capsule, MRI magnetic resonance imaging, MRMI modified Rivermead mobility index, Pt putamen, Th thalamus, T1WI T1-weighted imaging, T2WI T2-weighted imaging.
Table 3.
Summary of neural tract integrity-based MRI studies.
| Study: author, journal, year | Stroke type, n | MRI contents | Imaging parameter | Analyzed tract | Main results |
|---|---|---|---|---|---|
| Kim et al., Neuroreport, 201839 |
Ischemic: 40 Hemorrhagic: 8 |
DTI | Visual | CST | The FAC scores in the group A (CST was preserved around the lesion area) and the group B (CST was similar to group A, except that the fiber originated from cortex other than primary motor cortex) tended to be higher than that of group C (CST was interrupted or not shown) |
| Yeo et al., J Stroke Cerebrovasc Dis, 202041 |
Ischemic: 0 Hemorrhagic: 9 |
T2WI DTI |
FA MD Visual |
CST CRP Medial VST Lateral VST |
CST and VST did not play essential role in independent gait Intact CRP was related to the gait function |
| Jang et al., Ann Neurol, 200836 |
Ischemic: 25 Hemorrhagic: 0 |
T2WI DTI |
Visual | CST | FAC score improvement were significantly higher in DTT type A (the CST was preserved) than DTT type B (the CST was interrupted) |
| Kim et al., NeuroRehabilitation, 201338 |
Ischemic: 37 Hemorrhagic: 0 |
T2WI DTI |
Infarct volume FA ratio Visual |
CST |
FAC scores in group A (integrity of the CST was preserved around the infarct) were significantly higher than group B (integrity of CST was discontinuous) and group C (the upper end of the CST did not reach the infarct) There were positive correlation between FA ratio and FAC scores (r = 0.5, p = 0.002) There were negative correlation between infarct volume and FAC scores (r = − 0.361, p = 0.028) |
| Soulard et al., Neurology, 202042 |
Ischemic: 29 Hemorrhagic: 0 |
T1WI FLAIR DTI |
FA value Lesion volume |
CST CRP |
Walking score were correlated with lesion volume Walking score significantly correlated with FA values from ipsilesional CST, contralesional CST, ipsilesional CRP, and bilateral cerebellar peduncles Walking recovery was predicted by FA values from ipsilesional CST, ipsilesional CRP, and contralesional superior cerebellar peduncle |
| Sagnier et al., Stroke, 202046 |
Ischemic: 207 Hemorrhagic: 0 |
DWI FLAIR DTI |
Axial diffusivity FA MD Radial diffusivity |
NAWM | NAWM FA was associated with gait speed (β = − 0.31, p < 0.001) |
| Jang et al., Int J Neurosci, 201337 |
Ischemic: 0 Hemorrhagic: 21 |
T2WI DTI |
FA ratio Tract length Number of fibers Visual |
CST |
FA ratio, fiber number ratio, and tract length ratio were positively correlated with FAC (r = 0.455, p = 0.038; r = 0.602, p = 0.004; r = 0.6, p = 0.004, respectively) FAC score in DTT type A (the CST was preserved around the hematoma) was higher than those in DTT type B (the CST was interrupted) |
| Jang et al., Somatosens Mot Res, 201644 |
Ischemic: 31 Hemorrhagic: 0 |
T2WI DTI |
FA ratio Infarct size Number of fibers Size of the CST area |
CST |
Fiber number ratio and CST area ration were positively correlated with FAC (r = 0.50, p = 0.004; r = 0.50, p = 0.004, respectively) There was no significant correlation between the FA ratio and FAC |
| Imura et al., J Phys Ther Sci, 201540 |
Ischemic: 16 Hemorrhagic: 9 |
DTI |
FA Number of fibers ADC |
CST |
There was positive correlation between the FA value of affected CST and gait parameters (gait item of Barthel Index and gait item of FIM) There was no significant correlation between other DTI parameters (ADC and number of fibers) and gait parameters (gait item of Barthel Index and gait item of FIM) |
| Smith et al., Neurorehabil Neural Repair, 201745 |
Ischemic: 35 Hemorrhagic: 6 |
T1WI DWI DTI |
FA ratio Lesion load |
CST | MRI parameters were not found to have predictive value and not included in CART analysis |
| Cho et al., Neurosci Lett, 200735 |
Ischemic: 0 Hemorrhagic: 40 |
T2WI DTI |
Visual | CST | Distribution of FAC were affected by classification defined by the integrity of CST |
ADC apparent diffusion coefficient, CART classification and regression tree, CST corticospinal tract, CRP corticoreticular pathway, DTI diffusion tensor imaging, DWI diffusion-weighted imaging, FA fractional anisotropy, FAC functional ambulation category, FIM functional independence measure, FLAIR fluid-attenuated inversion-recovery, MD mean diffusivity, MRI magnetic resonance imaging, NAWM normal-appearing white matter, T1WI T1-weighted imaging, T2WI T2-weighted imaging, VST vestibulospinal tract.
Table 4 summarizes the risk of bias evaluation of the included studies. In brief, two articles had five items rated as probably no (PN) or definitely no (DN). Five articles had four items rated as PN or DN. Six articles had three items rated as PN or DN. With similar rules, 1 or 5 articles had 2 or 1 items rated as PN or DN.
Table 4.
Risk of bias evaluation of included studies in the systematic review.
| Lee et al. 2017 | Kim et al. 2018 | Jones et al. 2016 | Yeo et al. 2020 | Miyai et al. 2000 | Moon et al. 2017 | Jang et al. 2008 | Kim et al. 2013 | Soulard et al. 2020 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Was selection of exposed and non-exposed cohorts drawn from the same population? | PY | DY | PY | PY | DY | PY | DY | DY | PY | |
| 2. Can we be confident in the assessment of exposure? | DY | PY | DY | PN | PN | PY | DY | PY | PN | |
| 3. Can we be confident that the outcome of interest was not present at start of study? | DY | DY | DY | DY | DY | DY | DY | DY | DY | |
| 4. Did the study match exposed and unexposed for all variables that are associated with the outcome of interest or did the statistical analysis adjust for these prognostic variables? | DN | PY | PY | DN | PY | PY | DY | DY | DN | |
| 5. Can we be confident in the assessment of the presence or absence of prognostic factors? | PN | PN | DN | DN | PY | PN | PY | PY | PY | |
| 6. Can we be confident in the assessment of outcome? | PN | PN | PN | PN | PY | PN | PY | PY | PY | |
| 7. Was the follow up of cohorts adequate? | DY | DY | DY | DY | DY | DY | DY | DY | DY | |
| 8. Were co-interventions similar between groups? | PY | PN | PN | PN | PY | PN | PN | PN | DN |
| Baillieul et al. 2019 | Sagnier et al. 2020 | Jang et al. 2013 | Jang et al. 2016 | Imura et al. 2015 | Burke et al. 2014 | Lam et al. 2010 | Loos et al. 2018 | Smith et al. 2017 | Cho et al. 2007 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Was selection of exposed and non-exposed cohorts drawn from the same population? | PY | PY | DY | PY | PY | PY | PY | PY | PY | DY |
| 2. Can we be confident in the assessment of exposure? | DY | PN | PN | PY | PY | PN | PY | PY | PN | PN |
| 3. Can we be confident that the outcome of interest was not present at start of study? | DY | DY | DY | DY | DY | DY | DY | DY | DY | DY |
| 4. Did the study match exposed and unexposed for all variables that are associated with the outcome of interest or did the statistical analysis adjust for these prognostic variables? | DN | PY | DY | DN | DN | PY | PN | PY | PY | DY |
| 5. Can we be confident in the assessment of the presence or absence of prognostic factors? | PN | PN | PN | DN | PY | PN | PN | PY | PY | PN |
| 6. Can we be confident in the assessment of outcome? | PN | PN | PN | PY | PY | PN | PN | PY | PY | PN |
| 7. Was the follow up of cohorts adequate? | DY | DY | DY | DY | DY | PN | DY | PY | DY | DY |
| 8. Were co-interventions similar between groups? | PN | PN | PN | PN | PN | DN | DN | PN | PY | PN |
DY definitely yes (low risk of bias), PY probably yes, PN probably no, DN definitely no (high risk of bias), N/A: not applicable.
Discussion
The present systematic review aimed to evaluate the usefulness of MRI in predicting the gait ability of stroke patients. Out of the 19 studies that met our criteria, eight were classified as structure- or function-based MRI studies and 11 as neural tract integrity-based MRI studies. All included studies had some concerns, at least one, based on the Cochrane risk of bias instrument.
The eight structure- or function-based MRI studies showed that MRIs are useful in predicting gait ability. Overall, most studies revealed that the patients who had damage to their motor-related structures—that is to say, component structures of the CST (primary motor cortex, corona radiata, and internal capsule) or basal ganglia (caudate nucleus, putamen, and globus pallidus)—showed poor gait recovery28–30. Interestingly, Jones et al.31 reported that CST damage independently predicted the response to therapy for general mobility ability, defined using the functional ambulation category and the modified Rivermead mobility index, but not walk speed. Alternatively, they showed that the walk speed response to rehabilitation was affected by damage involving the putamen, insula, external capsule, and neighboring white matter, but not the CST. Moon et al.32 investigated the predictors of gait velocity change and the association between a lesion location and a change in the gait function. As a result, they concluded that damage to the insula, in addition to the internal capsule, affected the gait velocity change after rehabilitation. Moreover, it has already been suggested that improvements in 6-min walk velocity were greater in those patients with left-sided lesions33. In short, from the perspective of structure- or function-based MRI studies, damage to CST-related structures was associated with fundamental gait ability, defined using a functional ambulation category or modified Rivermead mobility index31, while improvement of more applicative gait ability (e.g., gait velocity) seemed to be present in those patients with an intact basal ganglia, insula, or external capsule and left-sided lesions31,33. In addition, it has already been suggested that the total cerebral small vessel disease burden in non-lacunar stroke patients is associated with gait impairment34, indicating that such findings should be carefully observed adding to damage to motor-related structures.
Out of the 11 neural tract integrity-based MRI studies, 10 showed usability of MRIs in predicting gait ability. Several previous studies suggested that patients whose CST was visually preserved showed better walking recovery compared to those whose CST was interrupted or not shown, regardless of differences in brain infarctions or hemorrhaging35–39. Additionally, a significant correlation was observed between the fractional anisotropy value of CST and gait recovery37,38,40. Interestingly, in contrast, Yeo et al.41 showed that neither the CST nor vestibulospinal tract played an important role in independent gait, but an intact CRP was related to gait function in 9 patients with pontine hemorrhage. They demonstrated the important relationship exists between the CRP, not the CST, and gait ability although the lack of relationship between the CST and walking ability might be affected by the limited sample size. Soulard et al.42 also suggested the importance of CRP for gait prediction. The corticoreticulospinal tract, which consists of the CRP and the reticulospinal tract, is a well-known neural network for walking and proximal muscle regulation43. No consensus was obtained regarding the predictive value of fiber number-related parameters37,40,44. The findings from the neural tract integrity-based MRI studies were summarized that the CST integrity evaluated by DTI were basically thought as a useful predictor. Remarkably, even those patients who was not described the CST by DTI, clinician need to bear in mind that there might be still possibility for regaining walking ability if the CRP was not destruction. Smith et al.45 performed a classification and regression tree analysis with various variables such as physical functions, neurophysiological findings using transcranial magnetic stimulation, and MRI information to identify the factors that predict time to independent walk. As a result, TMS and MRI measures did not have predictive value.
Regarding the risk of bias evaluation of included studies, thirteen of nineteen articles were rated as PN or DN in more than three items. In particular, the items that evaluate the assessment of the presence or absence of prognostic factors and the concerning of co-interventions between groups were rated as PN or DN in many articles. Moreover, none of the included articles investigated the additional value into other predictors or the competitive advantage of the use of MRIs in predicting gait ability of stroke patients. With these consideration in mind, it is expected that further studies will be performed to consolidate strong evidence.
This study has certain limitations. First, we might have missed some relevant studies because our search strategy consisted of selected words and databases. Second, we only included studies that were published in the English language; therefore, we have to consider relevant language biases and the limited generalizability of the present results. Third, we could not apply a quantitative analysis in this review, because the included studies were heterogeneous. Fourth, most included studies, even those showing usefulness of MRIs for gait prediction, did not investigate the additional value into other basic predictors or the competitive advantage throughout comparison with other clinical basic variables. Despite these limitations, to our knowledge, this is the first report to consolidate evidence regarding the usefulness of MRIs in predicting the gait ability of stroke patients.
In conclusion, the present systematic review suggests that MRIs are useful in predicting the gait ability of stroke patients. We were able to suggest important findings for predicting gait ability from an MRI. However, we cannot make definitive conclusions regarding the predictive value and effects of gait prediction using MRI findings, due to the lack of quantitative evaluations. Therefore, more high-quality studies are needed related to gait prediction using MRIs, including verification of their predictive accuracy.
Supplementary Information
Acknowledgements
We thank Chiemi Katayama and Yuka Yamada for assistance with literature collection.
Author contributions
Conceptualization: T.I., supervision: T.I. and R.T., literature search: T.I. and T.M., literature collection: T.I., T.M., Y.I., and R.T., methodology: T.I., T.M., and R.T., acquisition of data: T.I., T.M., and Y.I., interpretation of data: T.I., T.M., and R.T., writing-original draft: T.I., writing-review and editing: T.I., T.M., Y.I., and R.T.
Funding
The present study was supported, in part, by the Japan Society for the Promotion of Sciences (JSPS) KAKENHI Grant number 20K19309.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-93717-4.
References
- 1.Jang SH. The recovery of walking in stroke patients: A review. Int. J. Rehabil. Res. 2010;33:285–289. doi: 10.1097/MRR.0b013e32833f0500. [DOI] [PubMed] [Google Scholar]
- 2.Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Recovery of walking function in stroke patients: The Copenhagen Stroke Study. Arch. Phys. Med. Rehabil. 1995;76:27–32. doi: 10.1016/S0003-9993(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 3.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995;26:982–989. doi: 10.1161/01.STR.26.6.982. [DOI] [PubMed] [Google Scholar]
- 4.Mayo NE, Wood-Dauphinee S, Côté R, Durcan L, Carlton J. Activity, participation, and quality of life 6 months poststroke. Arch. Phys. Med. Rehabil. 2002;83:1035–1042. doi: 10.1053/apmr.2002.33984. [DOI] [PubMed] [Google Scholar]
- 5.Guzik A, et al. Relationships between walking velocity and distance and the symmetry of temporospatial parameters in chronic post-stroke subjects. Acta Bioeng. Biomech. 2017;19:147–154. [PubMed] [Google Scholar]
- 6.Hsu AL, Tang PF, Jan MH. Analysis of impairments influencing gait velocity and asymmetry of hemiplegic patients after mild to moderate stroke. Arch. Phys. Med. Rehabil. 2003;84:1185–1193. doi: 10.1016/S0003-9993(03)00030-3. [DOI] [PubMed] [Google Scholar]
- 7.Patterson KK, Gage WH, Brooks D, Black SE, McIlroy WE. Changes in gait symmetry and velocity after stroke: A cross-sectional study from weeks to years after stroke. Neurorehabil. Neural Repair. 2010;24:783–790. doi: 10.1177/1545968310372091. [DOI] [PubMed] [Google Scholar]
- 8.Roth EJ, Merbitz C, Mroczek K, Dugan SA, Suh WW. Hemiplegic gait. Relationships between walking speed and other temporal parameters. Am. J. Phys. Med. Rehabil. 1997;76:128–133. doi: 10.1097/00002060-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Price R, Choy NL. Investigating the relationship of the functional gait assessment to spatiotemporal parameters of gait and quality of life in individuals with stroke. J. Geriatr. Phys. Ther. 2019;42:256–264. doi: 10.1519/JPT.0000000000000173. [DOI] [PubMed] [Google Scholar]
- 10.Menon B, Salini P, Habeeba K, Conjeevaram J, Munisusmitha K. Female caregivers and stroke severity determines caregiver stress in stroke patients. Ann. Indian Acad. Neurol. 2017;20:418–424. doi: 10.4103/aian.AIAN_203_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masiero S, Avesani R, Armani M, Verena P, Ermani M. Predictive factors for ambulation in stroke patients in the rehabilitation setting: A multivariate analysis. Clin. Neurol. Neurosurg. 2007;109:763–769. doi: 10.1016/j.clineuro.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Uwatoko H, et al. Prediction of independent gait in acute stroke patients with hemiplegia using the Ability for Basic Movement Scale II Score. Eur. Neurol. 2020;83:49–55. doi: 10.1159/000506421. [DOI] [PubMed] [Google Scholar]
- 13.MacKay-Lyons M. Central pattern generation of locomotion: A review of the evidence. Phys. Ther. 2002;82:69–83. doi: 10.1093/ptj/82.1.69. [DOI] [PubMed] [Google Scholar]
- 14.Jahn K, et al. Imaging human supraspinal locomotor centers in brainstem and cerebellum. Neuroimage. 2008;39:786–792. doi: 10.1016/j.neuroimage.2007.09.047. [DOI] [PubMed] [Google Scholar]
- 15.Takakusaki K. Forebrain control of locomotor behaviors. Brain Res. Rev. 2008;57:192–198. doi: 10.1016/j.brainresrev.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 16.Kawashima N, Nakazawa K, Akai M. Characteristics of the locomotor-like muscle activity during orthotic gait in paraplegic persons. Neurol. Res. 2008;30:36–45. doi: 10.1179/016164107X235482. [DOI] [PubMed] [Google Scholar]
- 17.Yokoyama H, Ogawa T, Shinya M, Kawashima N, Nakazawa K. Speed dependency in α-motoneuron activity and locomotor modules in human locomotion: Indirect evidence for phylogenetically conserved spinal circuits. Proc. Biol. Sci. 2017;284:20170290. doi: 10.1098/rspb.2017.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warach S. Use of diffusion and perfusion magnetic resonance imaging as a tool in acute stroke clinical trials. Curr. Control Trials Cardiovasc. Med. 2001;2:38–44. doi: 10.1186/CVM-2-1-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atchaneeyasakul K, Shang T, Haussen D, Ortiz G, Yavagal D. Impact of MRI selection on triage of endovascular therapy in acute ischemic stroke: The MRI in Acute Management of Ischemic Stroke (MIAMIS) registry. Interv. Neurol. 2020;8:135–143. doi: 10.1159/000490580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vicentini JE, et al. Subacute functional connectivity correlates with cognitive recovery six months after stroke. NeuroImage Clin. 2020;29:102538. doi: 10.1016/j.nicl.2020.102538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang SH. Prediction of motor outcome for hemiparetic stroke patients using diffusion tensor imaging: A review. NeuroRehabilitation. 2010;27:367–372. doi: 10.3233/NRE-2010-0621. [DOI] [PubMed] [Google Scholar]
- 22.Kumar P, Kathuria P, Nair P, Prasad K. Prediction of upper limb motor recovery after subacute ischemic stroke using diffusion tensor imaging: A systematic review and meta-analysis. J. Stroke. 2016;18:50–59. doi: 10.5853/jos.2015.01186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin JF, Guo ZT, Zhang YP, Chen YY. Prediction of motor recovery after ischemic stroke using diffusion tensor imaging: A meta-analysis. World J. Emerg. Med. 2017;8:99–105. doi: 10.5847/wjem.j.1920-8642.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schiemanck SK, Kwakkel G, Post MW, Prevo AJH. Predictive value of ischemic lesion volume assessed with magnetic resonance imaging for neurological deficits and functional outcome poststroke: A critical review of the literature. Neurorehabil. Neural Repair. 2006;20:492–502. doi: 10.1177/1545968306289298. [DOI] [PubMed] [Google Scholar]
- 25.Veerbeek JM, Kwakkel G, van Wegen EE, Ket JC, Heymans MW. Early predation of outcome of activities of daily living after stroke: A systematic review. Stroke. 2011;42:1482–1488. doi: 10.1161/STROKEAHA.110.604090. [DOI] [PubMed] [Google Scholar]
- 26.Stinear CM, Ward NS. How useful if imaging in predicting outcomes in stroke rehabilitation? Int. J. Stroke. 2013;8:33–37. doi: 10.1111/j.1747-4949.2012.00970.x. [DOI] [PubMed] [Google Scholar]
- 27.Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyai I, Suzuki T, Kang J, Volpe BT. Improved functional outcome in patients with hemorrhagic stroke in putamen and thalamus compared with those with stroke restricted to the putamen or thalamus. Stroke. 2000;31:1365–1369. doi: 10.1161/01.STR.31.6.1365. [DOI] [PubMed] [Google Scholar]
- 29.Lee KB, et al. Brain lesions affecting gait recovery in stroke patients. Brain Behav. 2017;7:e00868. doi: 10.1002/brb3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baillieul S, Elsworth-Edelsten C, Saj A, Allali G. Neural substrates of reduced walking activity after supratentorial stroke: A voxel-based lesion symptom mapping study. Hum. Mov. Sci. 2019;67:102517. doi: 10.1016/j.humov.2019.102517. [DOI] [PubMed] [Google Scholar]
- 31.Jones PS, et al. Does stroke location predict walk speed response to gait rehabilitation? Hum. Brain Mapp. 2016;37:689–703. doi: 10.1002/hbm.23059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moon HI, Lee HJ, Yoon SY. Lesion location associated with balance recovery and gait velocity change after rehabilitation in stroke patients. Neuroradiology. 2017;59:609–618. doi: 10.1007/s00234-017-1840-0. [DOI] [PubMed] [Google Scholar]
- 33.Lam JM, et al. Predictors of response to treadmill exercise in stroke survivors. Neurorehabil. Neural Repair. 2010;24:567–574. doi: 10.1177/1545968310364059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loos CM, et al. The relation between total cerebral small vessel disease burden and gait impairment in patients with minor stroke. Int. J. Stroke. 2018;13:518–524. doi: 10.1177/1747493017730780. [DOI] [PubMed] [Google Scholar]
- 35.Cho SH, et al. Motor outcome according to diffusion tensor tractography findings in the early stage of intracerebral haemorrhage. Neurosci. Lett. 2007;421:142–146. doi: 10.1016/j.neulet.2007.04.052. [DOI] [PubMed] [Google Scholar]
- 36.Jang SH, et al. Motor outcome prediction using diffusion tensor tractography in pontine infarct. Ann. Neurol. 2008;64:460–465. doi: 10.1002/ana.21444. [DOI] [PubMed] [Google Scholar]
- 37.Jang SH, Choi BY, Chang CH, Kim SH, Chang MC. Prediction of motor outcome based on diffusion tensor tractography findings in thalamic hemorrhage. Int. J. Neurosci. 2013;123:233–239. doi: 10.3109/00207454.2012.752364. [DOI] [PubMed] [Google Scholar]
- 38.Kim EH, Lee J, Jang SH. Motor outcome prediction using diffusion tensor tractography of the corticospinal tract in large middle cerebral artery territory infarct. NeuroRehabilitation. 2013;32:583–590. doi: 10.3233/NRE-130880. [DOI] [PubMed] [Google Scholar]
- 39.Kim AR, et al. Can the integrity of the corticospinal tract predict the long-term motor outcome in poststroke hemiplegic patients? Neuroreport. 2018;29:453–458. doi: 10.1097/WNR.0000000000000994. [DOI] [PubMed] [Google Scholar]
- 40.Imura T, et al. Prediction of motor outcomes and activities of daily living function using diffusion tensor tractography in acute hemiparetic stroke patients. J. Phys. Ther. Sci. 2015;27:1383–1386. doi: 10.1589/jpts.27.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeo SS, Jang SH, Park GY, Oh S. Effects of injuries to descending motor pathways on restoration of gait in patients with pontine hemorrhage. J. Stroke Cerebrovasc. Dis. 2020;29:104857. doi: 10.1016/j.jstrokecerebrovasdis.2020.104857. [DOI] [PubMed] [Google Scholar]
- 42.Soulard J, et al. Motor tract integrity predicts walking recovery: A diffusion MRI study in subacute stroke. Neurology. 2020;94:e583–e593. doi: 10.1212/WNL.0000000000008755. [DOI] [PubMed] [Google Scholar]
- 43.Matsuyama K, et al. Locomotor role of the corticoreticular-reticulospinal-spinal interneuronal system. Prog. Brain Res. 2004;143:239–249. doi: 10.1016/S0079-6123(03)43024-0. [DOI] [PubMed] [Google Scholar]
- 44.Jang SH, et al. Prediction of motor outcome using remaining corticospinal tract in patients with pontine infarct: Diffusion tensor imaging study. Somatosens. Mot. Res. 2016;33:99–103. doi: 10.1080/08990220.2016.1194821. [DOI] [PubMed] [Google Scholar]
- 45.Smith MC, Barber PA, Stinear CM. The TWIST algorithm predicts time to walking independently after stroke. Neurorehabil. Neural Repair. 2017;31:955–964. doi: 10.1177/1545968317736820. [DOI] [PubMed] [Google Scholar]
- 46.Sagnier S, et al. Normal-appearing white matter integrity is a predictor of outcome after ischemic stroke. Stroke. 2020;51:449–456. doi: 10.1161/STROKEAHA.119.026886. [DOI] [PubMed] [Google Scholar]
- 47.Burke E, Dobkin BH, Noser EA, Enney LA, Cramer SC. Predictors and biomarkers of treatment gains in a clinical stroke trial targeting the lower extremity. Stroke. 2014;45:2379–2384. doi: 10.1161/STROKEAHA.114.005436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.