Abstract
Long-term recurrent stress is a common cause of neuropsychiatric disorders. Animal models are widely used to study the pathogenesis of stress-related psychiatric disorders. The zebrafish (Danio rerio) is emerging as a powerful tool to study chronic stress and its mechanisms. Here, we developed a prolonged 11-week chronic unpredictable stress (PCUS) model in zebrafish to more fully mimic chronic stress in human populations. We also examined behavioral and neurochemical alterations in zebrafish, and attempted to modulate these states by 3-week treatment with an antidepressant fluoxetine, a neuroprotective omega-3 polyunsaturated fatty acid eicosapentaenoic acid (EPA), a pro-inflammatory endotoxin lipopolysaccharide (LPS), and their combinations. Overall, PCUS induced severe anxiety and elevated norepinephrine levels, whereas fluoxetine (alone or combined with other agents) corrected most of these behavioral deficits. While EPA and LPS alone had little effects on the zebrafish PCUS-induced anxiety behavior, both fluoxetine (alone or in combination) and EPA restored norepinephrine levels, whereas LPS + EPA increased dopamine levels. As these data support the validity of PCUS as an effective tool to study stress-related pathologies in zebrafish, further research is needed into the ability of various conventional and novel treatments to modulate behavioral and neurochemical biomarkers of chronic stress in this model organism.
Subject terms: Neurochemistry, Neuroscience, Emotion, Stress and resilience, Pharmacology
Introduction
Stress potently modulates behavior and physiology, including the neuroendocrine and the immune systems1,2 both implicated in psychiatric illnesses, such as anxiety, depression and post-traumatic stress disorder (PTSD)3,4. Widespread and severely debilitating, affective disorders represent an urgent unsolved medical problem5,6, whose therapy is also complicated due to heterogenic nature, determined by multiple genetic, environmental, and other risk factors7,8. Animal models are widely used to study affective pathogenesis, typically involving stress as a common pathogenetic factor9,10. One of the most commonly used stress models is chronic unpredictable stress (CUS)11,12, which exposes an animal (usually, a rodent) to varying stressors for several weeks11,13,14, to evoke ‘affective’ (anxiety- and/or depression-like) states15. Behavioral and molecular consequences of CUS typically parallel those observed clinically16.
The zebrafish (Danio rerio) is a relatively novel model species, rapidly becoming widely used to complement rodent data in stress neurobiology research17,18. Zebrafish possess high genetic and physiological homology to humans19, especially in terms of their evolutionarily conserved neurotransmitter systems20,21 and the central nervous system (CNS) morphology22,23. Zebrafish are also used in aquatic CUS protocols adapted from rodent models24,25. For example, we have recently established a 5-week CUS protocol in zebrafish, examining behavioral, neurochemical, neuroinflammatory and transcriptomic changes induced by CUS, as well as their potential correction by 1-week antidepressant treatment26,27.
Importantly, in most clinical cases chronic stress typically lasts longer than 5 weeks, and antidepressant effects take several weeks to occur28,29. To address this translational problem, here we develop a novel, prolonged chronic unpredictable stress (PCUS) model, based on rigorous 11-week CUS protocol (Fig. 1 and Table 1) with a 3-week exposure to a conventional antidepressant, a selective serotonin reuptake inhibitor (SSRI) fluoxetine. The effects of this clinically relevant serotonergic antidepressant in the PCUS model were also compared with those of putative positive and negative neuromodulators, such as a neuroprotective omega-3 polyunsaturated fatty acid (PUFA) eicosapentaenoic acid (EPA) and a pro-inflammatory bacteria-derived lipopolysaccharide (LPS), alone or in combinations with fluoxetine.
Figure 1.

A brief diagram outlining the study experimental design, including the prolonged 11-week chronic unpredictable stress (PCUS) protocol and behavioral testing battery (see Table 1 for details of the PCUS stressors applied in the present study). Abbreviations: NTT the novel tank test, CPA the conditioned place aversion test, SH shoaling test, EPA eicosapentaenoic acid, LPS lipopolysaccharide, HPLC high-performance liquid chromatography.
Table 1.
Summary of the 11-week prolonged chronic unpredictable stress (PCUS) protocol used in the present study (adapted from26,27, with modifications).
| Days | Specific CUS stress procedures |
|---|---|
| 1 | Three 5-min net chasing sessions with 30-min breaks + predator, two Blue marble gouramis (Trichogaster trichopterus) exposure for 24 h in the hometank |
| 2 | Three 1-min air exposures and cooling to 10°C for 30 s + exposure to a different zebrafish strain (green GloFish) for 24 h in the hometank |
| 3 | Net chasing for 20 min + vortexing for 30 s + darkness for 24 h in the hometank |
| 4 | Crowding (10 fish/L) for 6 h + noise (drill sound, 50 db) exposure for 2 h with 30% predator water added into the hometank |
| 5 | Crowding/novelty stress in red 8-L bucket (10 fish/L) for 8 h with alarm pheromone + three 1-min electric shocks with 30-min breaks prior to returning to the hometank |
| 6 | Three 5-min net chasing sessions with 30-min breaks + food deprivation + alarm pheromone exposure for 24 h in the hometank |
| 7 | Vibration (40 Hz) for 2 h + social isolation for 8 h + 30% predator water in the hometank |
| 8 | Three 1-min air exposures + shoaling test for 10 min + 30% shallow water with darkness for 24 h in the hometank |
| 9 | Three 1-min high temperature (35 °C) exposures with 30-min breaks + 3-cup crowding stress + 60% predator water with 35 novel objects (Kinder surprise toys) for 18 h in the hometank |
| 10 | Food deprivation + light–dark box for 8 h + 50% shallow water with bright light (300 lux) for 2 h prior to returning to the hometank |
| 11 | Three 1-min electric shocks + three 5-min net chasing sessions with a 3-min break + darkness for 24 h in the hometank |
| 12 | Three 1-min cooling + noise for 4 h + predator exposure for 24 h in the hometank |
| 13 | Light–dark box for 2 h + three 2-min electric shock sessions + noise for 2 h prior to returning to the hometank |
| 14 | Social isolation for 8 h + extra-bright light (1500 lux) for 2 h + 60% predator water added into the hometank |
| 15 | Food deprivation for 24 h + three 5-min net chasing sessions with 30-min breaks + 50% predator water exposure in the hometank |
| 16 | Three 2-min high temperature exposures + 30 min break + vortexing (10 fish/50-mL tube at 1000 rpm) for 30 s + 0.5 mL/L alarm pheromone exposure for 24 h in the hometank |
| 17 | Darkness for 24 h with noise exposure for 6 h in the hometank |
| 18 | Shallow water for 8 h (60% of normal water level) + shaking for 30 s + 40% predator water with 35 novel objects in the hometank |
| 19 | Three 1-min high temperature exposures, 30 min break + vibration for 4 h + predator exposure for 24 h in the hometank |
| 20 | Crowding stress in white bucket for 8 h + three 1-min electric shocks with 30-min breaks prior to returning to the hometank |
| 21 | Three 10-min net chasing sessions with 40-min breaks + darkness and food deprivation with an alarm pheromone added for 24 h in the hometank |
| 22 | Crowding stress in red bucket with bright-light for 8 h + 60% predator water added in the hometank |
| 23 | Three 1-min cooling sessions with 20-min breaks + predator exposure for 24 h in the hometank |
| 24 | 30% shallow water and bright light for 2 h in the hometank + exposure to 35 novel objects for 24 h in the hometank |
| 25 | Shaking in vortex for 30 s with alarm pheromone added + 30-s cooling session + bright light exposure for 6 h in the hometank |
| 26 | Noise and vibration for 4 h + novel predator, Oscar fish (Astronotus ocellatus) exposure for 8 h prior to returning to the hometank |
| 27 | 3-cup crowding (12 fish/0.5-L cup) for 6 h under bright light + shallow water (50% of the original, normal level) for 16 h in the hometank |
| 28 | Social isolation for 8 h + net chasing for 15 min + 30% predator water added into the hometank |
| 29 | Three 1-min air exposures with 30-min breaks + Astronotus ocellatus for 2.5 h + Trichogaster trichopterus for 2.5 g + alarm pheromone 7 times for 15 min each, prior to returning to the hometank |
| 30 | Shaking in vortex for 30 s + darkness with food deprivation for 24 h in the hometank |
| 31 | Crowding in red bucket for 8 h + exposure to a different zebrafish strain (green GloFish) for 14 h prior to returning to the hometank |
| 32 | Net chasing for 15 min + local hypothermia (22 °C) 2 h + shallow water (40%) exposure for 24 h in the hometank |
| 33 | 3-cup stress for 6 h + 35 novel objects for 24 h in the hometank |
| 34 | Light–dark box for 5 min + three 1-min air exposures with 10-min breaks + predator Trichogaster trichopterus exposure for 24 h in the hometank |
| 35 | Shaking in vortex for 30 s + shallow water (60%) for 18 h with alarm pheromone added 5 times, with 15-min intervals, prior to returning to the hometank |
| 36 | Three 1-min electric shocks + vibration for 4 h + predator Trichogaster trichopterus exposure for 24 h in the hometank |
| 37 | Food deprivation for 24 h + 50% predator water + alarm pheromone 5 times with 20-min intervals prior to returning to the hometank |
| 38 | Shaking in vortex for 30 s + three 1-min high temperature exposures with 30-min breaks + three 5-min net chasing sessions with 30-min breaks + extra-bright light exposure for 20 min in the hometank |
| 39 | Three 1-min electric shocks + three 30-s cooling sessions with 30-min breaks + darkness for 24 h in the hometank |
| 40 | Social isolation for 8 h + food deprivation for 24 h + vibration for 2 h, prior to returning to the hometank |
| 41 | Crowding in red bucket for 6 h + shoaling test for 10 min + extra-bright light for 20 min in the hometank |
| 42 | Three 1-min air exposures with 10-min breaks + Astronotus ocellatus for 2.5 h + Trichogaster trichopterus for 2.5 h, prior to returning to the hometank |
| 43 | Net chasing for 20 min + extra-bright light for 2 h + shallow water (40%) exposure for 24 h, prior to returning to the hometank |
| 44 | Noise exposure for 4 h + mild hypothermia (22 °C) for 2 h and predator (Trichogaster trichopterus) for 24 h in the hometank |
| 45 | 3-cup crowding for 6 h + 60% predator water with novel objects for 18 h in the hometank |
| 46 | Net chasing for 20 min + cooling for 30 s + darkness with food deprivation for 24 h in the hometank |
| 47 | Crowing in white bucket for 8 h + vibration and noise for 6 h, prior to returning to the hometank |
| 48 | Three 1-min electric shocks with 30-min breaks + exposure to a different zebrafish strain (green GloFish) for 24 h in the hometank |
| 49 | Shoaling test for 5 min + social isolation for 15 min + noise for 4 h + net chasing for 20 min, prior to returning to the hometank |
| 50 | Light–dark box for 5 min + 3-cup crowding for 6 h + alarm pheromone for 24 h in the hometank |
| 51 | Three 1-min air exposure with 1-min electric shock 10 min break + 60% predator water with novel objects for 24 h in the hometank |
| 52 | Cooling for 30-s + shaking in vortex with alarm pheromone for 30 s + bright light for 6 h in the hometank |
| 23 | Noise exposure for 2 h + vibration for 2 h + social isolation for 4 h, prior to returning to the hometank |
| 54 | Three 5-min net chasing sessions with 30-min breaks + extra-bright light for 30 min + exposure to Astronotus ocellatus for 2.5 h + Trichogaster trichopterus for 2.5 h, prior to returning to the hometank |
| 55 | Shaking in vortex for 40 s + three 2-min high temperature exposures with 30-min breaks + darkness for 24 h in the hometank |
| 56 | Shallow water (30%) under bright light for 2 h + shoaling test for 10 min + food deprivation for 24 h in the hometank |
| 57 | Light–dark box for 12 h + three 2-min electric shock sessions with 30-min breaks + 15-min net chasing, prior to returning to the hometank |
| 58 | Two-min cooling sessions with a 20-min break + predator (Trichogaster trichopterus) exposure for 24 h in the hometank |
| 59 | Shaking in vortex for 30 s + vibration and noise for 6 h + darkness for 18 h in the hometank |
| 60 | 3-cup crowding for 6 h + 60% predator water with an alarm pheromone and novel objects for 18 h in the hometank |
| 61 | 20 min net chasing + 2.5 h exposure to Trichogaster trichopterus + 2.5 h to Astronotus ocellatus + exposure to a different zebrafish strain (green GloFish) for 24 h in the hometank |
| 62 | Food deprivation for 24 h + social isolation for 8 h + bright light for 2 h, prior to returning to the hometank |
| 63 | Three 1-min electric shock exposures with 30-min breaks + light–dark box for 8 h, prior to returning to the hometank |
| 64 | Three 1-min air exposures with 30-min breaks + 50% shallow water for 12 h + alarm pheromone added into the hometank |
| 65 | Noise and vibration for 6 h + net chasing for 20 min, prior to returning to the hometank |
| 66 | Net chasing for 20 min, a 30-min break + vortexing 30 s + darkness and food deprivation for 24 h with an alarm pheromone in the hometank |
| 67 | Bright light exposure for 2 h + green GloFish exposure with novel objects for 24 h in the hometank |
| 68 | 3-cup crowding for 6 h + 3 1-min high temperature (35 C) + 30% predator water for 18 h |
| 69 | Shaking in vortex with alarm pheromone for 40 s + noise for 4 h + three 30-s cooling sessions with 10-min breaks, prior to returning to the hometank |
| 70 | Noise and vibration for 4 h + Trichogaster trichopterus exposure for 18 h in the hometank |
| 71 | Three 1-min electric shocks with 30-min breaks + two 1-min air exposures + shallow water (30%) exposure for 6 h in the hometank |
| 72 | Crowding in red bucket for 6 h + shoaling test for 10 min + alarm pheromone exposure for 18 h in the hometank |
| 73 | Shallow water (60%) with bright light for 8 h + three 1-min air exposures with 30-min breaks + vibration for 2 h, prior to returning to the hometank |
| 74 | Astronotus ocellatus exposure for 3 h + Trichogaster trichopterus exposure for 3 h + net chasing for 20 min, prior to returning to the hometank |
| 75 | 3-cup crowding for 6 h + noise exposure for 2 h + 60% predator water with novel objects in the hometank |
| 76 | Vibration for 4 h + darkness with an alarm pheromone exposure for 24 h in the hometank |
| 77 | Food deprivation for 24 h + social isolation for 6 h + three 1-min electric shocks with 30-min breaks + vortexing for 30 s, prior to returning to the hometank |
| 78 | Behavioral testing in the novel tank test prior to returning to the hometank |
| 79 | Training zebrafish in the conditioned place aversion test prior to returning to the hometank |
| 80 | Behavioral testing in the conditioned place aversion test prior to returning to the hometank |
| 81 | Behavioral testing in the shoaling test, prior to sacrificing the fish and collecting brain samples one day later |
Results
Behavioral studies
In the novel tank test (NTT), the PCUS protocol produced several significant treatment effects, summarized in detail in Table 2 and Supplementary Table S1. Overall, the EPA, fluoxetine + EPA and fluoxetine + LPS exposure significantly reduced zebrafish swim velocity (p < 0.001 vs. control, Dunn’s test, Fig. 2). Stress, EPA and the LPS exposure reduced time in top of the tank vs. control (p < 0.001 for all groups, Dunn’s test) and vs. fluoxetine-treated groups (p < 0.01 for all groups, Fig. 2, Table 2 and Supplementary Table S1). Stress exposure also increased the latency to enter the top vs. both control (p < 0.05, Dunn’s test) and fluoxetine groups (p < 0.01), whereas LPS increased it only compared to fluoxetine-treated group (p < 0.05, Fig. 2, Table 2 and Supplementary Table S1). Finally, fluoxetine + LPS exposure reduced the number of top entries (p < 0.05 vs. control group, Dunn’s test, Fig. 2, Table 2 and Supplementary Table S1).
Table 2.
Summary of the Kruskal–Wallis test results for behavioral and neurochemical alterations induced by the prolonged chronic unpredictable stress (PCUS) exposure and fluoxetine, EPA or LPS treatments in adult zebrafish brain (see also Figs. 2, 3 and 4 for graphical representation and Supplementary Tables S1–S2 for post-hoc test results). Abbreviations: DOPAC 3,4-dihydroxyphenylacetic acid, 5-HIAA 5-hydroxyindoleacetic acid, NS not significant (p > 0.05).
| Parameters | H | df | p value |
|---|---|---|---|
| The novel tank test | |||
| Velocity, cm/s | 30.84 | 6 | p < 0.0001 |
| Time spent in top, s | 41.81 | 6 | p < 0.0001 |
| Latency to top, s | 32.65 | 6 | p < 0.0001 |
| Number of top entries | 22.11 | 6 | p < 0.01 |
| The shoaling test | |||
| Average inter-fish distance, cm | 162.25 | 6 | p < 0.001 |
| Conditioned place aversion | |||
| Time spent in light, s | 36.86 | 7 | p < 0.0001 |
| Neurochemical analyses | |||
| Norepinephrine, pg/mg | 20.45 | 6 | p < 0.005 |
| Dopamine, pg/mg | 18.70 | 6 | p < 0.005 |
| DOPAC, pg/mg | 7.49 | 6 | NS |
| Serotonin, pg/mg | 34.71 | 6 | p < 0.0001 |
| 5-HIAA, pg/mg | 33.11 | 6 | p < 0.0001 |
| Monoamine metabolism ratios | |||
| 5-HIAA to serotonin ratio | 33.31 | 6 | p < 0.0001 |
| DOPAC to dopamine ratio | 24.76 | 6 | p < 0.0005 |
Figure 2.

Behavioral alterations induced by prolonged chronic unpredictable stress (PCUS) exposure and fluoxetine, EPA, or LPS treatment in adult zebrafish tested in the novel tank test. Data are presented as median and Q1, Q3 (n = 12–22 per group). *p < 0.05, **p < 0.01, ***p < 0.001 vs. control, post-hoc Dunn’s test for significant Kruskal–Wallis data. Graphs were constructed using the ggplot2 R package30 (also see Table 2 and Supplementary Table S1 for statistical details). Groups: C control, S PCUS, F fluoxetine, E eicosapentaenoic acid (EPA), L lipopolysaccharide (LPS).
Conditioned place aversion (CPA) protocol was efficient in inducing avoidance learning in the zebrafish, as assessed by time spent in light in intact (non-learning) fish (p < 0.01 vs. control group, Dunn’s test, Fig. 3, Table 2 and Supplementary Table S1). While no behavioral alterations were observed in experimental vs. control groups (p > 0.05), the PCUS group spent more time in light than intact fish (p < 0.0001, Dunn’s test), as well as fluoxetine-treated (p < 0.05), fluoxetine + EPA (p < 0.001) and fluoxetine + LPS groups (p < 0.05, Fig. 3, Table 2 and Supplementary Table S1).
Figure 3.
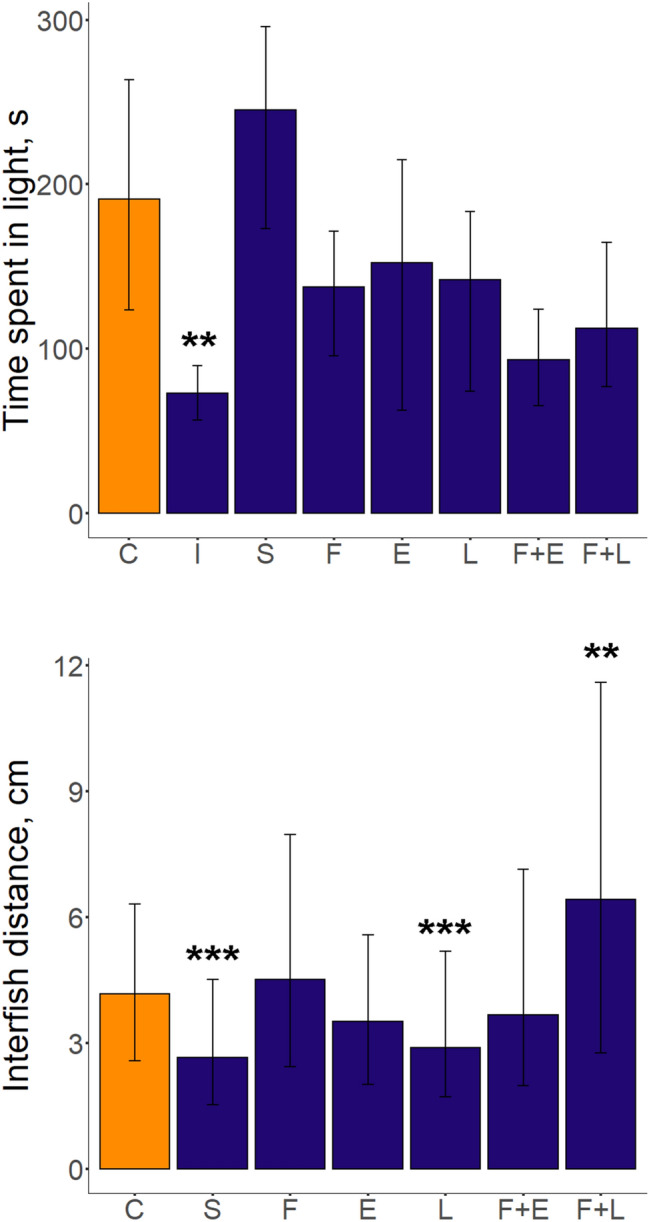
Behavioral alterations induced by prolonged chronic unpredictable stress (PCUS) exposure and fluoxetine, EPA, or LPS treatment in adult zebrafish tested in the shoaling test (ST; inter-fish distance) and conditioned place avoidance (CPA, time spent in the light compartment). Data are presented as median and Q1, Q3 (total number of still images: 198–541 per group for ST (calculated as distances between each zebrafish in each captured still image, thus representing the number of images taken), n = 12–22 per group for CPA). *p < 0.05, **p < 0.01, ***p < 0.001 vs. control, post-hoc Dunn’s test for significant Kruskal–Wallis data. Graphs were constructed using the ggplot2 R package30, also see Table 2 and Supplementary Table S1 for statistical details). Groups: C control, S PCUS, I intact (no learning), F fluoxetine, E eicosapentaenoic acid (EPA), L lipopolysaccharide (LPS).
In the shoaling test (ST), PCUS and LPS exposure both reduced average inter-fish distance (p < 0.0001 vs. control group, Dunn’s test, Fig. 3, Table 2 and Supplementary Table S1). Furthermore, PCUS exposure decreased inter-fish distance vs. all other groups, except for LPS (p < 0.01, Dunn’s test, Fig. 3, Table 2 and Supplementary Table S1). In contrast, fluoxetine + LPS exposure significantly increased the average inter-fish distance in this test, compared to every other group (p < 0.05), fluoxetine reduced the shoal cohesion vs. EPA and LPS (p < 0.0001), whereas fluoxetine + EPA increased the inter-fish distance compared to LPS (p < 0.01, Dunn’s test, Fig. 3, Table 2 and Supplementary Table S1). No other behavioral effects were observed for all endpoints in the NTT, CPA and ST tests between different experimental groups used in this study (p > 0.05, NS, Fig. 3, Table 2, Supplementary Table S1).
Neurochemical analyses
Significant treatment effects on zebrafish neurochemical parameters are presented in Table 2 and Supplementary Table S2. Overall, PCUS and LPS both increased levels of norepinephrine in zebrafish brain (p < 0.05 and p < 0.01 vs. control group, respectively, Dunn’s test, Fig. 4, Table 2 and Supplementary Table S2). Chronic fluoxetine also lowered the 5-hydroxyindoleacetic acid (5HIAA)/serotonin ratio (p < 0.01 vs. control) that reflects serotonin turnover, whereas fluoxetine + EPA increased the 5HIAA levels (p < 0.05 vs. control), without altering serotonin turnover. Moreover, both LPS and EPA increased dopamine levels (p < 0.05 vs. control group, Dunn’s test, Fig. 4, Table 2 and Supplementary Table S2).
Figure 4.
Neurochemical alterations induced by the prolonged chronic unpredictable stress (PCUS) exposure and fluoxetine, EPA, or LPS treatment in adult zebrafish brain assessed using HPLC. Data are presented as median and Q1, Q3 (n = 12 per group). *p < 0.05, **p < 0.01 vs. control, post-hoc Dunn’s test for significant Kruskal–Wallis data. Graphs were constructed using the ggplot2 R package30, also see Table 2 and Supplementary Table S2 for statistical details. Groups: C control, S PCUS, F fluoxetine, E eicosapentaenoic acid (EPA), L lipopolysaccharide (LPS).
Likewise, we found pronounced neurochemical differences between the experimental groups, since both fluoxetine and fluoxetine + EPA groups show reduced 5HIAA levels compared to the stress fish (p < 0.05 and p < 0.0001, respectively), whereas fluoxetine + EPA fish display lowered 5HIAA vs. the LPS group (p < 0.001, Dunn’s test, Fig. 4, Table 2 and Supplementary Table S2). Interestingly, fluoxetine + LPS markedly reduced serotonin levels in zebrafish brain vs. PCUS, fluoxetine alone, fluoxetine + LPS (p < 0.01) and LPS groups (p < 0.001, Dunn’s test, Fig. 4, Table 2 and Supplementary Table S2). Furthermore, fluoxetine + LPS also elevated serotonin turnover vs. fluoxetine, EPA, LPS and fluoxetine + EPA groups (p < 0.0001, p < 0.01, p < 0.05 and p < 0.001, respectively, Dunn’s test). Finally, fluoxetine + LPS increased dopamine turnover (assessed here as the 3,4-dihydroxyphenylacetic acid (DOPAC)/dopamine ratio) vs. EPA and LPS groups (p < 0.01, Dunn’s test, Fig. 4, Table 2 and Supplementary Table S2), with no other differences observed between the groups (p > 0.05, NS).
Discussion
The present study for the first time applied clinically relevant PCUS model in adult zebrafish, evaluating a wide range of behavioral and neurochemical alterations evoked by stress. The 11-week PCUS protocol developed here aimed to achieve more pronounced and stable behavioral and neurochemical alterations that may better fit the existing clinical data. Moreover, the present study also explored potential treatments of PCUS-evoked behavioral and neurochemical deficits, including both conventional (an SSRI fluoxetine) and novel putative (EPA) treatments, as well as the combination of PCUS with a pro-inflammatory agent LPS.
In general, our behavioral analyses revealed overt NTT anxiety induced by chronic stress exposure, as well as its efficient recovery by fluoxetine in the fluoxetine, fluoxetine + LPS and fluoxetine + EPA groups, but not by EPA or LPS. Fluoxetine alone increased top exploration vs. chronic stress or EPA and LPS exposure (but not fluoxetine + EPA and fluoxetine + LPS). Notably, other anxiety-related NTT endpoints were less sensitive to experimental manipulations.
Interestingly, PCUS stress alone did not alter locomotor endpoints, such as velocity and time spent mobile, whereas EPA, fluoxetine + EPA and fluoxetine + LPS showed hypolocomotion by reducing zebrafish velocity. While shorter CUS batteries affected zebrafish locomotion, these effects are conflicting, and include both hyper- and hypocolomotor27 phenotypes, likely representing psychomotor agitation or motor retardation, commonly reported in clinical affective disorders31. In contrast, elevated anxiety in zebrafish remains a strikingly consistent finding across multiple CUS studies25–27, including here, hence collectively reinforcing its high clinical and translational value as a hallmark affective phenotype evoked in zebrafish by chronic stress. Because anxiety is commonly seen in clinical patients exposed to chronic stress32, as well as in chronically stressed rodent models33,34, the later also emphasizes the value of elevated anxiety response as an evolutionarily conserved, ‘core’ affective phenotype across taxa that is nearly 400 million years old.
Likewise, PCUS reduced the ST average inter-fish distance in zebrafish, indicating increased anxiety and/or increased sociality35,36, whereas fluoxetine, EPA, and their combination all recovered this index. Although LPS alone did not worsen zebrafish group behavior (compared to PCUS fish), LPS + fluoxetine did (vs. any other group tested), suggesting some complex interplay between these compounds, likely involving inflammatory pathways.
Notably, PCUS somewhat increased time spent in light CPA area (indicating increased learning capabilities) compared to all other experimental groups, except the controls. As the CPA protocol is based on the avoidance stress response, alterations in baseline stress reactivity (e.g., baseline anxiety) may confound the phenotype observed here. For example, stressed fish may be more sensitive to stressful stimuli, and hence be more sensitive to conditional avoidance (and learn faster) than control fish. In line with this notion, all low-stress groups (fluoxetine, fluoxetine + EPA and fluoxetine + LPS) learned worse than the PCUS fish, as indicated by shorter time spent in the light compartment. However, other studies report poorer learning in zebrafish in a closely related inhibitory avoidance learning paradigm37, which suggests possible conceptual/construct differences between the tests. These effects may also correlate with increased density of dendritic spines observed in chronically stressed zebrafish that was recovered to the control levels following fluoxetine treatment26. Finally, CPA itself was successfully modeled here, as indicated by the light conditioning in control group vs. intact, experimentally naïve fish, thereby confirming the validity of the method used here.
Chronic fluoxetine treatment reversed most of PCUS behavioral effects and efficiently counteracted the effects of PCUS + LPS exposure, in line with anxiolytic38, antidepressant39 and anti-inflammatory40 effects observed for SSRIs clinically, hence supporting the overall translational validity of the PCUS model developed here. This finding also parallels fluoxetine effects in other zebrafish chronic stress studies, including the modulation of NTT anxiety and shoaling, observed after 5-week CUS26,27. Interestingly, while fluoxetine and LPS alone did not alter zebrafish locomotion, their combination produced hypolocomotion here. Furthermore, while fluoxetine normalized the ST inter-fish distance, and LPS did not affect this endpoint compared to stress group, their combination increased it, supporting complex interactions between the two compounds likely associated with altered inflammatory pathways in the brain.
Intriguingly, EPA alone did not recover zebrafish NTT anxiety-related behavior, whereas fluoxetine + EPA was close to both control and stressed groups, highlighting a weaker effect of the drug combination than that of fluoxetine alone. These results are at odds with some clinical studies on positive effects of EPA alone in depression41,42, and with the fact that fluoxetine + EPA is more efficient (vs. them alone) in such therapy43. The exact nature of these observed discrepancies in zebrafish is unclear. One possible explanation concerns overt metabolic differences between zebrafish and humans, and the fact that some EPA effects in human studies may be metabolic- and dietary-related41,42. However, EPA counteracted stress effects in ST, suggesting its correction of social deficits in stressed fish. Although fluoxetine efficiently counteracted PCUS + LPS-induced anxiety, given jointly with LPS, it produced hypolocomotion and shoal disruption not observed in either treatment group. Thus, further studies are needed to better understand the LPS effects in the PCUS zebrafish model developed here.
While disturbed serotonergic system is often observed in zebrafish models of stress27,44, serotonin was unaltered by PCUS here, likely because serotonergic changes may occur earlier then norepinephrinergic, given that all previous fish studies used shorter CUS models, whose neurochemical responses highly depend on the duration of stress27. Similarly, in rats, 6-week CUS decreases norepinephrine in PFC, hippocampus and hypothalamus, and increases dopamine levels in the same brain areas45, whereas 7-day CUS decreases dopamine and serotonin levels in PFC, striatum and hippocampus46. Norepinephrine levels rise in plasma of depressed patients47,48, and correlate with depressive and anxiety symptoms in healthy subjects49. At the same time, we recognize that rodent studies usually focus on various brain areas, whereas our study investigates whole-brain neurochemical changes, thus complicating direct cross-species comparisons.
In summary, our PCUS protocol induced anxiety-like effect that may be rescued with fluoxetine, but not EPA alone, and was not sufficiently worsened by exogenous pro-inflammatory modulation by LPS. While PCUS increased norepinephrine in zebrafish brain, fluoxetine or EPA restored its levels, fluoxetine decreased serotonin metabolism, EPA increased dopamine, and LPS increased both norepinephrine and dopamine levels. Finally, fluoxetine effectively recovered most of PCUS-evoked behavioral and neurochemical alterations alone and in combination with LPS or EPA.
In general, brain monoamines are major factors in chronic stress in fish, mammals50 and humans51. Here, PCUS significantly elevated only norepinephrine levels vs. controls. Given that stress increases the activity of the sympathetic nervous system, this observation parallels rodent data where stress also increases norepinephrine52,53. A widely used SSRI, fluoxetine, decreased 5-HIAA (the main metabolite of serotonin) in zebrafish brain, as well as the ratio of 5-HIAA/serotonin (vs. control group), reflecting lower serotonin turnover, a common biomarker of SSRI antidepressant action in zebrafish models54,55. In the present study, fluoxetine also normalized norepinephrine levels vs. PCUS alone and in combination with other drugs.
LPS, the main component of the membrane of gram-negative bacteria, can trigger inflammation via immune and non-immune mechanisms in vivo56, promoting the release of pro-inflammatory cytokines interleukin (IL) IL-1β and tumor necrosis factor-β (TNF-β)57. Proinflammatory cytokines are often associated with various mental illnesses, such as depression58. Although norepinephrine remained elevated (vs. control) by LPS, the latter also increased dopamine levels in the brain, suggesting the ability of LPS to promote zebrafish stress response, paralleling stress-potentiated dopamine activity in clinical and rodent models59,60.
LPS also modulates serotonin metabolism, for example, activating the serotonin transporter (SERT) and, consequently, serotonin reuptake61,62. Similar effect was observed here for LPS + fluoxetine, lowering serotonin levels (vs. chronic stress, fluoxetine, EPA and LPS), and also affecting serotonin turnover. Furthermore, the same combination also elevated dopamine turnover vs. LPS alone, which was interesting, given that both fluoxetine63 and LPS in our study increased brain dopamine levels. Finally, fluoxetine rescued norepinephrine levels even in combination with LPS, proving efficiency of this SSRI to counteract both inflammatory and stress-associated neurochemical alterations.
In general, EPA is a critical PUFA with multiple physiological functions in vivo. Unlike LPS, it exerts pronounced anti-inflammatory properties, beneficial in various psychiatric disorders64,65. The link of omega-3 PUFAs to dopamine and its metabolism has already been demonstrated66. In line with this, zebrafish treated with EPA in the present study had higher levels of dopamine and DOPAC/dopamine ratios here, and EPA effectively recovered norepinephrine levels. However, EPA evoked similar effects only in combination with fluoxetine, which not only normalized norepinephrine (elevated by PCUS), but also lowered 5-HIAA levels, and, like fluoxetine alone, decreased the 5-HIAA/serotonin ratio compared to control, thus promoting beneficial monoaminergic effects of fluoxetine. Collectively, our neurochemical results support the possibility that the combination of EPA + fluoxetine is more effective in the treatment of stress-related pathologies than these agents alone67.
However, there were also some conceptual and methodological limitations of the present study. For example, neurochemical analyses used here utilized whole-brain samples in contrast to the region-specific study of neurochemical changes and thus may mask neurochemical differences that are specific for brain areas. Furthermore, the zebrafish, like many other model organisms, exhibits pronounced intraspecies differences68,69, including sex differences70, that may influence stress and pharmacologically induced phenotypes. Although studying intraspecies variation was beyond the scope of the present study, this aspect of PUCS-evoked phenotypes clearly merits further scrutiny. Likewise, complementing our whole-brain HPLC analyses here, assessing more nuanced (brain region-specific) profiles of PCUS- and treatment-evoked neurochemical responses is also warranted.
Moreover, while most zebrafish CUS studies have shown anxiogenic phenotype44,71,72, as we did here as well, some reports failed to induce pronounced anxiety73, likely due to a short-lived nature of the affective syndrome they attempted to evoke. Given problems with data reliability and replicability in the field74, the choice of prolonged stress protocol, such as PUCS developed here, seems to be justified. Finally, while chronic stress models are widely interpreted as models of affective disorders, including depression, it is still unclear whether there is a clear-cut depression-like phenotype in the zebrafish75. Given the constantly evolving, and sometimes opposite, behavioral patterns evoked by chronic stress in many neurobehavioral domains (except anxiety)26,27, further studies are needed to better understand the exact interplay between these factors in CUS, necessitating further protocols with differing numbers of stressors, their severity, and modeling duration.
Methods
Animals and housing
Adult mature (5–7 months old) wild-type short-fin zebrafish of both sexes (approximately 50:50 ratio) were received from a local distributor (Tropic Aquarium, Ltd., St. Petersburg, Russia). Prior to testing, the fish were kept for at least 3 weeks under standard conditions in large 110-L plastic covered containers, with water temperature 27 ± 0.5 °C, pH 7.4, lighting (950–960 lux), adherence to day and night regimen, feeding twice a day with special feed pellets Neon Micro Granules for fish 1–2 cm in size (Dajana Pet, Bohuňovice, Czech Republic)76. Zebrafish were housed in the ZebTec Active Blue Stands with Water Treatment Unit (Tecniplast, West Chester, USA). All fish were from the same population, and were randomly divided into experimental groups using an online random number generator. The strain selection for the present study was based on population validity considerations and their relevance for the present study77. Specifically, while genetically controlled inbred zebrafish strains may offer reproducible and more reliable systems for neurogenetics research, modeling CNS disorders (such as in the present study) involves mimicking ‘real’ human maladies that affect genetically heterogenous clinical populations. Thus, using outbred populations of zebrafish can represent a more populationally valid and translationally relevant approach for the purpose of this study. This selection also considered overt strain-specific peculiarities of zebrafish behaviors in different tests78 that can be mitigated by using wild-type outbred fish, and also paralleled recent rodent findings (noting no higher phenotypic trait variability in outbred (vs. inbred) mice and concluding that outbred strains may be better subjects for most biomedical experiments79).
All animals tested were included in final analyses, without removing outliers. All experiments were performed as planned, and all analyses and endpoints assessed were included without omission. Animal experiments were approved by the Institutional IACUC and fully adhered to National and Institutional guidelines and regulations. The study experimental design and its description here, as well as data analysis and presenting, adhered to the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines for reporting animal research and the PREPARE (Planning Research and Experimental Procedures on Animals: Recommendations for Excellence) guidelines for planning animal research and testing.
Prolonged chronic unpredictable stress (PCUS)
The experimental fish were kept in 4-L hometanks (20 fish/tank) and subjected to various stressors daily for the 11 weeks, similar to26,27, including crowding (10 fish/L) in 20-L buckets for 6 h, 1-min air exposure (by lifting the fish from their tanks by the net and leaving in the air), net chasing, cooling (to 10 °C), mild hypothermia (22 °C) and hyperthermia (35 °C) in 5-L jars, 3-cup crowding stress (12 fish/0.5-L cup), shoaling (placing 4–5 fish per NTT), predator (12-cm Oscar fish, Astronotus ocellatus, and 7-8-cm Blue marble gourami, Trichogaster trichopterus) or predator water exposure (water collected after 7-day housing from predator hometanks), exposure to a different (green GloFish) zebrafish strain, to novel objects (35 plastic 'Kinder surprise' toys), light–dark box (see above), shallow water (30–60% of the normal water level), 300-lux bright and 1500-lux extra-bright light (produced by two 60-wt light bulbs placed 20 or 2 cm above the water surface, respectively), electric shock (0.1 V/cm), noise (a 50-db drill sound from an online YouTube drilling video), shaking/vortexing for 30 s (10 fish/50-ml tube, at 1000 rmp), alarm pheromone exposure (0.5 mL/L), social isolation (1 fish/90-mL plastic cup), food deprivation and darkness (see Table 1 for details).
Control fish were housed similarly to the experimental cohort but remained experimentally naïve for the entire study duration. On Day 57, the stressed fish cohort was divided into six groups: the group continued to be exposed only to chronic stress, or groups exposed to chronic fluoxetine, eicosapentaenoic acid (EPA), lipopolysaccharide (LPS), fluoxetine + EPA), and fluoxetine + LPS for the final 3 weeks. Since LPS was injected intraperitoneally once a week, another group was added, where saline solution was injected intraperitoneally (injection control). As no behavioral alterations were observed between the groups, this additional control was excluded from further analyses (NS, data not shown).
Fluoxetine (Biocom Ltd., Stavropol, Russia) is a commonly used antidepressant clinically80–82 and has been tested extensively in various animal models, including rodents83–86 and zebrafish25,26,87–89. The duration of treatment, concentration and route of administration were selected based on the previous studies in stress-related models26,50. The test fish were kept in hometanks with 0.1 mg/L fluoxetine, and the water was changed daily, as in 27.
LPS from Escherichia coli O55:B5 (Sigma Aldrich, St. Louis, MO, USA), was chosen here for its ability to induce inflammation90. EPA was used here for its anti-inflammatory properties91. 10 μL LPS solution was injected intraperitoneally (once a week) and contained 3 μg LPS. During the injection procedure, the animals were briefly immobilized by a wet net on a wet sponge, and quickly injected through the net using a 26G needle without anesthesia, as described previously92. The dose were chosen based on zebrafish93,94 and rodent95–99 studies, and adjusted for chronic exposure. EPA (Tokyo Chemical Industry Co. Ltd., Tokyo, Japan) was fed to the fish with Neon Micro Granules (100 mg of EPA mixed with 7 g of standard fish fit used in the study, 10.5 g of gelatin, and 35 ml of water, as in 100). The EPA doses were adapted and extrapolated from rodent studies101–105. Mortalities observed due to the PCUS exposure and pharmacological manipulations are reported in Supplementary Table S3.
Behavioral testing
Following an 11-week PCUS protocol, zebrafish behavioral and cognitive phenotypes were assessed in the novel tank test (NTT), shoaling test (ST), and the conditioned place aversion (CPA) test. Behavioral assays were organized in the order of increasing stress intensity, aiming to reduce the effect of the preceding testing17. Prior to testing, the fish were kept for 2 h in a testing room for acclimation, and were returned to the holding room after testing. Behavioral testing was performed between 11.00 and 17.00 h and was recorded with a SJ4000 action camera (SJCAM, Ltd., Shenzhen, China) at 60 frames/s. Experimenters were blinded to the treatments during behavioral testing and neurochemical analyses, including statistical and video analyses, and used individual codes for fish/groups identification. Manual analysis of behavioral data was performed by two highly-trained observers (blinded to the groups) with inter- and intra-rater reliability of > 0.85, as assessed by Spearman correlation as part of the laboratory’s standard operating procedure (SOP).
The NTT apparatus was an acrylic rectangular tank (20 height × 20 length × 5 width, cm), filled with water up to a 19-cm height, and divided into two equal virtual horizontal portions. The back and lateral sides of the tank were pasted with a white PVC envelope to increase contrast during behavioral recording. Each fish was recorded separately, immediately after being taken from the hometank, by a SJ4000 action camera for 5 min, assessinging velocity (cm/s), the number of top entries, time spent in top (s), and the latency to enter the top (s) of the tank106, using Noldus EthoVision XT11.5 software (Noldus IT, Wageningen, Netherlands).
The ST apparatus was similar to that of the NTT. During the testing, the fish from each group were placed in the tank in groups of 4–6, immediately after being taken from the hometank, and (after a 10-min acclimation to the apparatus) their shoals were photographed using a SJ4000 action camera every 60 s for 10 min. Each photo (total number: 198–541 photos per group) was next calibrated to the size of the tank, manually measuring the average inter-fish distance (cm)107,108.
The CPA apparatus was a plastic rectangular tank (12 long, 25 wide, 14 high cm) divided into two equal-sized black and light compartments filled with water. In the dark part, two metal plates were attached opposite to each other, connected to a custom-made current generator (0.1 V/cm). On Day 1 of the CPA test, the fish were trained by placing shoals of 10 fish in a CPA tank for 4 h with the generator turned on, supplying current to the dark section of the apparatus. On Day 2, each fish was recorded separately for 5 min with the current generator turned off, immediately after being taken from the hometank. In this assay, the time spent in the 'preferred' light zone (s) vs. 'punished' dark zone was assessed using Noldus EthoVision XT11.5 software.
Neurochemical analyses
One day after the last behavioral experiment (shoaling test on Day 81 of PUCS protocol), the fish (n = 12 per group) were sacrificed in ice water followed by decapitation, their brains dissected on ice, frozen in liquid nitrogen, and stored at − 80 °C. Monoamines in adult zebrafish brain were assayed using the high-performance liquid chromatography (HPLC)109,110, and were chosen here as key stress biomarkers in various mental disorders in humans, as well as in rodent and zebrafish models55,111. The study examined norepinephrine, as well as serotonin, dopamine and their respective metabolites 5-HIAA and DOPAC.
All samples were weighed and placed in test tubes with ice-cold 10 μL of 0.1 M perchloric acid solution (Sigma Aldrich, St. Louis, MO, USA) per 1 mg of brain weight, with 100 ng/mL 3,4-dihydroxybenzylamine (DHBA, internal standard) to preserve neurochemical analytes54. For homogenization, the samples were sonicated for 10 s using a Qsonica sonicator (Qsonica, LLC, Newtown, CT, USA). The resulting homogenized product was centrifuged for 10 min (at 14,000 rpm in 4 °C), the supernatant was filtered through a Durapore-PVDF centrifuge filter with a pore size of 0.22 μm (Merck Millipore, Billerica, MA, USA)54. HPLC was performed on an HTEC-500 chromatograph (Eicom, San Diego, CA, USA) with a WE-3 G carbon electrode using an applied potential of + 650 mV with a CA-5ODS column. The mobile phase consisted of 0.1 M phosphate buffer, 400 mg/L sodium octyl sulfonate, 50 mg/L ethylenediamine tetra-acetic acid (EDTA), and 17% methanol. The required pH value (4.5) was adjusted with phosphoric acid. All reagents were purchased from Sigma Aldrich. Concentration data were normalized using individual DHBA concentrations in the samples, and are reported as pg/mg brain tissue weight. We also computed the 5-HIAA/serotonin and DOPAC/dopamine ratios (that reflect the metabolism/turnover of the corresponding monoamines in the brain), similar to54.
Statistical analyses and data handling
Statistical analysis was performed using the Statistics 10 and GraphPad Prism 7 (GraphPad, San Diego, CA). Behavioral data were analyzed using the Kruskal–Wallis (KW) test, followed by Dunn's post-hoc test for pairwise group comparisons. Results are presented as median and interquartile range. The sample size was determined based on previous behavioral studies in zebrafish26,54,55,109,112. Initially, we started with n = 22 per group in all groups at the beginning of the PCUS protocol, although n’s decreased in some groups to 21 for control (due to injections) and PCUS, to 12–16 for the LPS, EPA and fluoxetine + LPS on the last day of the study (Supplementary Table 3S). All fish tested were included in the final analysis without attrition or exclusion, and all planned analyzes were presented here. All experimenters were unaware of the treatment groups during behavioral testing, neurochemical and genomic analyzes (including statistics and video analysis), using individual codes to identify fish/groups.
Ethical confirmation statements
Animal experiments were approved by IACUC of St. Petersburg State University and fully adhered to the National and Institutional guidelines and regulations on animal experimentation, as well as the 3Rs principles of humane animal experimentation.
Supplementary Information
Acknowledgements
This research was supported solely by the Russian Science Foundation (RSF) grant 19‐15‐00053. K.A.D. is supported by the Special Rector's Productivity Fellowship for SPSU PhD Students, and the lab is supported by St. Petersburg State University state budgetary funds (project ID 73026081). A.V.K. is the Chair of the International Zebrafish Neuroscience Research Consortium (ZNRC) and President of the International Stress and Behavior Society (ISBS, www.stress-and-behavior.com) that coordinated this collaborative multi-laboratory project. The consortium provided a collaborative idea exchange platform for this study, it is not considered as affiliation and did not fund the study. A.V.K. lab is supported by the Southwest University (SWU) Zebrafish Platform Construction Fund (Chongqing, China). The authors thank Professor Raul R. Gainetdinov (Institute of Translational Biomedicine, St. Petersburg State University, St. Petersburg, Russia) for his generous assistance with the HPLC studies in his laboratory. The funders had no role in the design, analyses, and interpretation of the submitted study, or decision to publish.
Author contributions
K.A.D. (Investigation) (Methodology) (Project administration) (Validation) (Visualization) (Writing—original draft) (Writing—review and editing), T.O.K. (Visualization) (Investigation) (Methodology) (Formal analysis) (Writing—Original Draft) (Writing—review and editing), D.S.G. (Investigation) (Methodology) (Formal analysis) (Writing—review and editing) (Writing—Original Draft), N.A.K. (Investigation) (Methodology) (Writing—review and editing), N.P.I. (Investigation) (Writing—review and editing), K.A.D. (Investigation) (Writing—review and editing), N.A.L. (Investigation) (Writing—review and editing), T.S. (Investigation) (Writing—review and editing), E.V.P. (Investigation) (Writing—review and editing), M.S.d.A. (Investigation) (Writing—review and editing), M.S. (Investigation) (Writing—review and editing, Y.V.C. (Investigation) (Writing—review and editing), I.M.K. (Investigation) (Writing—review and editing), D.V.S. (Investigation) (Writing—review and editing), K.N.Z. (Investigation) (Writing—review and editing), M.S.M. (Methodology) (Writing—review and editing), E.V.E. (Methodology) (Writing—review and editing), and A.V.K. (Conceptualization) (Funding acquisition) (Methodology) (Project administration) (Resources) (Supervision) (Validation) (Writing—review and editing).
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding authors upon reasonable requests.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Konstantin A. Demin, Tatiana O. Kolesnikova and David S. Galstyan.
Contributor Information
Konstantin A. Demin, Email: deminkasci@gmail.com
Allan V. Kalueff, Email: avkalueff@gmail.com
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-92422-6.
References
- 1.Golovatscka V, Ennes H, Mayer EA, Bradesi S. Chronic stress-induced changes in pro-inflammatory cytokines and spinal glia markers in the rat: a time course study. Neuroimmunomodulation. 2012;19:367–376. doi: 10.1159/000342092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang P, et al. Changes in proinflammatory cytokines and white matter in chronically stressed rats. Neuropsychiatr. Dis. Treat. 2015;11:597–607. doi: 10.2147/NDT.S78131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chrousos GP. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009;5:374. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 4.Barden N. Implication of the hypothalamic–pituitary–adrenal axis in the physiopathology of depression. J. Psychiatry Neurosci. 2004;29:185–193. [PMC free article] [PubMed] [Google Scholar]
- 5.Bale TL, et al. The critical importance of basic animal research for neuropsychiatric disorders. Neuropsychopharmacology. 2019;44:1349–1353. doi: 10.1038/s41386-019-0405-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meier SM, et al. Genetic variants associated with anxiety and stress-related disorders: a genome-wide association study and mouse-model study. JAMA Psychiat. 2019;76:924–932. doi: 10.1001/jamapsychiatry.2019.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandi C, Richter-Levin G. From high anxiety trait to depression: a neurocognitive hypothesis. Trends Neurosci. 2009;32:312–320. doi: 10.1016/j.tins.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scharf SH, Schmidt MV. Animal models of stress vulnerability and resilience in translational research. Curr. Psychiatry Rep. 2012;14:159–165. doi: 10.1007/s11920-012-0256-0. [DOI] [PubMed] [Google Scholar]
- 10.Czéh B, Fuchs E, Wiborg O, Simon M. Animal models of major depression and their clinical implications. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;64:293–310. doi: 10.1016/j.pnpbp.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Otabi H, Goto T, Okayama T, Kohari D, Toyoda A. The acute social defeat stress and nest-building test paradigm: a potential new method to screen drugs for depressive-like symptoms. Behav. Proc. 2017;135:71–75. doi: 10.1016/j.beproc.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Monteiro S, et al. An efficient chronic unpredictable stress protocol to induce stress-related responses in C57BL/6 mice. Front. Psychiatry. 2015;6:6. doi: 10.3389/fpsyt.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 14.Katz RJ. Animal model of depression: pharmacological sensitivity of a hedonic deficit. Pharmacol. Biochem. Behav. 1982;16(6):965–968. doi: 10.1016/0091-3057(82)90053-3. [DOI] [PubMed] [Google Scholar]
- 15.Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav. Brain Res. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 16.Hill MN, Hellemans KG, Verma P, Gorzalka BB, Weinberg J. Neurobiology of chronic mild stress: parallels to major depression. Neurosci. Biobehav. Rev. 2012;36:2085–2117. doi: 10.1016/j.neubiorev.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart AM, Braubach O, Spitsbergen J, Gerlai R, Kalueff AV. Zebrafish models for translational neuroscience research: from tank to bedside. Trends Neurosci. 2014;37:264–278. doi: 10.1016/j.tins.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalueff, A. V., Echevarria, D. J. & Stewart, A. M. Gaining translational momentum: more zebrafish models for neuroscience research. Prog. Neuropsychopharmacol. Biol. Psychiatry.55, 1–6 (2014). [DOI] [PubMed]
- 19.Howe K, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rico E, et al. Zebrafish neurotransmitter systems as potential pharmacological and toxicological targets. Neurotoxicol. Teratol. 2011;33:608–617. doi: 10.1016/j.ntt.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Panula P, et al. Modulatory neurotransmitter systems and behavior: towards zebrafish models of neurodegenerative diseases. Zebrafish. 2006;3:235–247. doi: 10.1089/zeb.2006.3.235. [DOI] [PubMed] [Google Scholar]
- 22.Panula P, et al. The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurobiol. Dis. 2010;40:46–57. doi: 10.1016/j.nbd.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Wulliman MF, Rupp B, Reichert H. Neuroanatomy of the zebrafish brain: a topological atlas. Birkhäuser; 2012. [Google Scholar]
- 24.Rambo CL, et al. Gender differences in aggression and cortisol levels in zebrafish subjected to unpredictable chronic stress. Physiol. Behav. 2017;171:50–54. doi: 10.1016/j.physbeh.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 25.Marcon M, et al. Prevention of unpredictable chronic stress-related phenomena in zebrafish exposed to bromazepam, fluoxetine and nortriptyline. Psychopharmacology. 2016;233:3815–3824. doi: 10.1007/s00213-016-4408-5. [DOI] [PubMed] [Google Scholar]
- 26.Song C, et al. Modeling consequences of prolonged strong unpredictable stress in zebrafish: complex effects on behavior and physiology. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018;81:384–394. doi: 10.1016/j.pnpbp.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 27.Demin KA, et al. Understanding complex dynamics of behavioral, neurochemical and transcriptomic changes induced by prolonged chronic unpredictable stress in zebrafish. Sci. Rep. 2020;10:1–20. doi: 10.1038/s41598-020-75855-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harmer CJ, Goodwin GM, Cowen PJ. Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant drug action. Br. J. Psychiatry. 2009;195:102–108. doi: 10.1192/bjp.bp.108.051193. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell AJ. Two-week delay in onset of action of antidepressants: new evidence. Br. J. Psychiatry. 2006;188:105–106. doi: 10.1192/bjp.bp.105.011692. [DOI] [PubMed] [Google Scholar]
- 30.Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer; 2016. [Google Scholar]
- 31.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). (American Psychiatric Publishing, 2013).
- 32.Patriquin MA, Mathew SJ. The neurobiological mechanisms of generalized anxiety disorder and chronic stress. Chronic Stress. 2017;1:1–10. doi: 10.1177/2470547017703993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Normandeau CP, et al. A key role for neurotensin in chronic-stress-induced anxiety-like behavior in rats. Neuropsychopharmacology. 2018;43:285–293. doi: 10.1038/npp.2017.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y-L, et al. Microglial activation mediates chronic mild stress-induced depressive-and anxiety-like behavior in adult rats. J. Neuroinflamm. 2018;15:1–14. doi: 10.1186/s12974-017-1027-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller NY, Gerlai R. Shoaling in zebrafish: what we don’t know. Rev. Neurosci. 2011;22:17–25. doi: 10.1515/rns.2011.004. [DOI] [PubMed] [Google Scholar]
- 36.Pham M, et al. Zebrafish Protocols for Neurobehavioral Research. Springer; 2012. pp. 231–246. [Google Scholar]
- 37.Manuel R, et al. Unpredictable chronic stress decreases inhibitory avoidance learning in Tuebingen long-fin zebrafish: stronger effects in the resting phase than in the active phase. J. Exp. Biol. 2014;217:3919–3928. doi: 10.1242/jeb.109736. [DOI] [PubMed] [Google Scholar]
- 38.Baldwin D, Woods R, Lawson R, Taylor D. Efficacy of drug treatments for generalised anxiety disorder: systematic review and meta-analysis. BMJ. 2011;342:d1199. doi: 10.1136/bmj.d1199. [DOI] [PubMed] [Google Scholar]
- 39.Greenberg, R. P., Bornstein, R. F., Zborowski, M. J., Fisher, S. & Greenberg, M. D. A Meta-analysis of fluoxetine outcome in the treatment of depression. J. Nerv. Ment. Dis. 182, 547–551 (1994). [DOI] [PubMed]
- 40.Sacre S, Medghalchi M, Gregory B, Brennan F, Williams R. Fluoxetine and citalopram exhibit potent antiinflammatory activity in human and murine models of rheumatoid arthritis and inhibit toll-like receptors. Arthritis Rheum. 2010;62:683–693. doi: 10.1002/art.27304. [DOI] [PubMed] [Google Scholar]
- 41.Mozaffari-Khosravi H, Yassini-Ardakani M, Karamati M, Shariati-Bafghi S-E. Eicosapentaenoic acid versus docosahexaenoic acid in mild-to-moderate depression: a randomized, double-blind, placebo-controlled trial. Eur. Neuropsychopharmacol. 2013;23:636–644. doi: 10.1016/j.euroneuro.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Sublette ME, Ellis SP, Geant AL, Mann JJ. Meta-analysis: effects of eicosapentaenoic acid in clinical trials in depression. J. Clin. Psychiatry. 2011;72:1577. doi: 10.4088/JCP.10m06634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jazayeri S, et al. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust. N. Z. J. Psychiatry. 2008;42:192–198. doi: 10.1080/00048670701827275. [DOI] [PubMed] [Google Scholar]
- 44.Fulcher N, Tran S, Shams S, Chatterjee D, Gerlai R. Neurochemical and behavioral responses to unpredictable chronic mild stress following developmental isolation: the zebrafish as a model for major depression. Zebrafish. 2017;14:23–34. doi: 10.1089/zeb.2016.1295. [DOI] [PubMed] [Google Scholar]
- 45.Jankovic M, Spasojevic N, Ferizovic H, Stefanovic B, Dronjak S. Inhibition of the fatty acid amide hydrolase changes behaviors and brain catecholamines in a sex-specific manner in rats exposed to chronic unpredictable stress. Physiol. Behav. 2020;227:113174. doi: 10.1016/j.physbeh.2020.113174. [DOI] [PubMed] [Google Scholar]
- 46.Ahmad A, Rasheed N, Banu N, Palit G. Alterations in monoamine levels and oxidative systems in frontal cortex, striatum, and hippocampus of the rat brain during chronic unpredictable stress. Stress. 2010;13:356–365. doi: 10.3109/10253891003667862. [DOI] [PubMed] [Google Scholar]
- 47.Veith RC, et al. Sympathetic nervous system activity in major depression: basal and desipramine-induced alterations in plasma norepinephrine kinetics. Arch. Gen. Psychiatry. 1994;51:411–422. doi: 10.1001/archpsyc.1994.03950050071008. [DOI] [PubMed] [Google Scholar]
- 48.Roy A, Pickar D, De Jong J, Karoum F, Linnoila M. Norepinephrine and its metabolites in cerebrospinal fluid, plasma, and urine: relationship to hypothalamic-pituitary-adrenal axis function in depression. Arch. Gen. Psychiatry. 1988;45:849–857. doi: 10.1001/archpsyc.1988.01800330081010. [DOI] [PubMed] [Google Scholar]
- 49.Hughes JW, Watkins L, Blumenthal JA, Kuhn C, Sherwood A. Depression and anxiety symptoms are related to increased 24-hour urinary norepinephrine excretion among healthy middle-aged women. J. Psychosom. Res. 2004;57:353–358. doi: 10.1016/S0022-3999(04)00064-9. [DOI] [PubMed] [Google Scholar]
- 50.Demin, K. A. et al. Understanding neurobehavioral effects of acute and chronic stress in zebrafish. Stress 24 (1), 1–18 (2020). [DOI] [PubMed]
- 51.Charney DS. Monoamine dysfunction and the pathophysiology and treatment of depression. J. Clin. Psychiatry. 1998;59(Suppl 14):11–14. [PubMed] [Google Scholar]
- 52.Schmidt KT, et al. Stress-induced alterations of norepinephrine release in the bed nucleus of the stria terminalis of mice. ACS Chem. Neurosci. 2019;10:1908–1914. doi: 10.1021/acschemneuro.8b00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Finlay JM, Zigmond MJ, Abercrombie ED. Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: effects of diazepam. Neuroscience. 1995;64:619–628. doi: 10.1016/0306-4522(94)00331-x. [DOI] [PubMed] [Google Scholar]
- 54.Demin KA, et al. The zebrafish tail immobilization (ZTI) test as a new tool to assess stress-related behavior and a potential screen for drugs affecting despair-like states. J. Neurosci. Methods. 2020;337:108637. doi: 10.1016/j.jneumeth.2020.108637. [DOI] [PubMed] [Google Scholar]
- 55.Meshalkina DA, et al. The effects of chronic amitriptyline on zebrafish behavior and monoamine neurochemistry. Neurochem. Res. 2018;43:1191–1199. doi: 10.1007/s11064-018-2536-5. [DOI] [PubMed] [Google Scholar]
- 56.Yucel G, et al. Lipopolysaccharides induced inflammatory responses and electrophysiological dysfunctions in human-induced pluripotent stem cell derived cardiomyocytes. Sci. Rep. 2017;7:2935. doi: 10.1038/s41598-017-03147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Connor TJ, Harkin A, Kelly JP, Leonard BE. Olfactory bulbectomy provokes a suppression of interleukin-1beta and tumour necrosis factor-alpha production in response to an in vivo challenge with lipopolysaccharide: effect of chronic desipramine treatment. NeuroImmunoModulation. 2000;7:27–35. doi: 10.1159/000026417. [DOI] [PubMed] [Google Scholar]
- 58.van Heesch F, et al. Systemic tumor necrosis factor-alpha decreases brain stimulation reward and increases metabolites of serotonin and dopamine in the nucleus accumbens of mice. Behav. Brain Res. 2013;253:191–195. doi: 10.1016/j.bbr.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 59.Wand GS, et al. Association of amphetamine-induced striatal dopamine release and cortisol responses to psychological stress. Neuropsychopharmacology. 2007;32:2310–2320. doi: 10.1038/sj.npp.1301373. [DOI] [PubMed] [Google Scholar]
- 60.Saszik SM, Smith CM. The impact of stress on social behavior in adult zebrafish (Danio rerio) Behav. Pharmacol. 2018;29:53–59. doi: 10.1097/FBP.0000000000000338. [DOI] [PubMed] [Google Scholar]
- 61.Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31:2121–2131. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]
- 62.Korte-Bouws GAH, et al. Bacterial lipopolysaccharide increases serotonin metabolism in both medial prefrontal cortex and nucleus accumbens in male wild type rats. Pharmaceuticals (Basel) 2018;11(3):66–78. doi: 10.3390/ph11030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kobayashi K, Haneda E, Higuchi M, Suhara T, Suzuki H. Chronic fluoxetine selectively upregulates dopamine D(1)-like receptors in the hippocampus. Neuropsychopharmacology. 2012;37:1500–1508. doi: 10.1038/npp.2011.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chapkin RS, Kim W, Lupton JR, McMurray DN. Dietary docosahexaenoic and eicosapentaenoic acid: emerging mediators of inflammation. Prostaglandins Leukot Essent Fatty Acids. 2009;81:187–191. doi: 10.1016/j.plefa.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Augimeri G, et al. N-eicosapentaenoyl dopamine, a conjugate of dopamine and eicosapentaenoic acid (EPA), exerts anti-inflammatory properties in mouse and human macrophages. Nutrients. 2019;11(9):2247–2260. doi: 10.3390/nu11092247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zimmer L, et al. Chronic n-3 polyunsaturated fatty acid deficiency alters dopamine vesicle density in the rat frontal cortex. Neurosci Lett. 2000;284:25–28. doi: 10.1016/S0304-3940(00)00950-2. [DOI] [PubMed] [Google Scholar]
- 67.Jazayeri S, et al. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry. 2008;42:192–198. doi: 10.1080/00048670701827275. [DOI] [PubMed] [Google Scholar]
- 68.Demin KA, et al. Cross-species analyses of intra-species behavioral differences in mammals and fish. Neuroscience. 2020;429:33–45. doi: 10.1016/j.neuroscience.2019.12.035. [DOI] [PubMed] [Google Scholar]
- 69.Demin KA, et al. The role of intraspecies variation in fish neurobehavioral and neuropharmacological phenotypes in aquatic models. Aquat. Toxicol. 2019;210:44–55. doi: 10.1016/j.aquatox.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 70.Genario R, de Abreu MS, Giacomini AC, Demin KA, Kalueff AV. Sex differences in behavior and neuropharmacology of zebrafish. Eur. J. Neurosci. 2019;52(1):2586–2603. doi: 10.1111/ejn.14438. [DOI] [PubMed] [Google Scholar]
- 71.Piato ÂL, et al. Unpredictable chronic stress model in zebrafish (Danio rerio): behavioral and physiological responses. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35:561–567. doi: 10.1016/j.pnpbp.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 72.Chakravarty S, et al. Chronic unpredictable stress (CUS)-induced anxiety and related mood disorders in a zebrafish model: altered brain proteome profile implicates mitochondrial dysfunction. PLoS One. 2013;8:e63302. doi: 10.1371/journal.pone.0063302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang V, Butler AA, Lubin FD. Telencephalon transcriptome analysis of chronically stressed adult zebrafish. Sci. Rep. 2019;9:1–9. doi: 10.1038/s41598-018-37761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gerlai R. Reproducibility and replicability in zebrafish behavioral neuroscience research. Pharmacol. Biochem. Behav. 2019;178:30–38. doi: 10.1016/j.pbb.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 75.de Abreu MS, et al. Zebrafish models: do we have valid paradigms for depression? J. Pharmacol. Toxicol. Methods. 2018;94:16–22. doi: 10.1016/j.vascn.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 76.Watts SA, Powell M, D'Abramo LR. Fundamental approaches to the study of zebrafi sh nutrition. ILAR J. 2012;53:144–160. doi: 10.1093/ilar.53.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Serikuly N, et al. Effects of acute and chronic arecoline in adult zebrafish: Anxiolytic-like activity, elevated brain monoamines and the potential role of microglia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2020;104:109977. doi: 10.1016/j.pnpbp.2020.109977. [DOI] [PubMed] [Google Scholar]
- 78.Audira G, Siregar P, Strungaru S-A, Huang J-C, Hsiao C-D. Which zebrafish strains are more suitable to perform behavioral studies? A comprehensive comparison by phenomic approach. Biology. 2020;9:200. doi: 10.3390/biology9080200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tuttle AH, Philip VM, Chesler EJ, Mogil JS. Comparing phenotypic variation between inbred and outbred mice. Nat. Methods. 2018;15:994–996. doi: 10.1038/s41592-018-0224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cooper GL. The safety of fluoxetine-an update. Br. J. Psychiatry. 1988;153:77–86. doi: 10.1192/S000712500029733X. [DOI] [PubMed] [Google Scholar]
- 81.Bergstrom R, Lemberger L, Farid N, Wolen R. Clinical pharmacology and pharmacokinetics of fluoxetine: a review. Br. J. Psychiatry. 1988;153:47–50. doi: 10.1192/S0007125000297286. [DOI] [PubMed] [Google Scholar]
- 82.Chouinard G. A double-blind controlled clinical trial of fluoxetine and amitriptyline in the treatment of outpatients with major depressive disorder. J. Clin. Psychiatry. 1985;46:32–37. [PubMed] [Google Scholar]
- 83.Liu X-L, et al. Fluoxetine regulates mTOR signalling in a region-dependent manner in depression-like mice. Sci. Rep. 2015;5:1–11. doi: 10.1038/srep16024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nollet M, et al. Activation of orexin neurons in dorsomedial/perifornical hypothalamus and antidepressant reversal in a rodent model of depression. Neuropharmacology. 2011;61:336–346. doi: 10.1016/j.neuropharm.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 85.Christiansen S, Olesen MV, Wörtwein G, Woldbye DPD. Fluoxetine reverts chronic restraint stress-induced depression-like behaviour and increases neuropeptide Y and galanin expression in mice. Behav. Brain Res. 2011;216:585–591. doi: 10.1016/j.bbr.2010.08.044. [DOI] [PubMed] [Google Scholar]
- 86.Hodes GE, Hill-Smith TE, Lucki I. Fluoxetine treatment induces dose dependent alterations in depression associated behavior and neural plasticity in female mice. Neurosci. Lett. 2010;484:12–16. doi: 10.1016/j.neulet.2010.07.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abreu MS, Giacomini AC, Kalueff AV, Barcellos LJ. The smell of “anxiety”: behavioral modulation by experimental anosmia in zebrafish. Physiol. Behav. 2016;157:67–71. doi: 10.1016/j.physbeh.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 88.Egan RJ, et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009;205:38–44. doi: 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wong RY, Oxendine SE, Godwin J. Behavioral and neurogenomic transcriptome changes in wild-derived zebrafish with fluoxetine treatment. BMC Genom. 2013;14:348. doi: 10.1186/1471-2164-14-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shen Y, Connor TJ, Nolan Y, Kelly JP, Leonard BE. Differential effect of chronic antidepressant treatments on lipopolysaccharide-induced depressive-like behavioural symptoms in the rat. Life Sci. 1999;65:1773–1786. doi: 10.1016/S0024-3205(99)00430-0. [DOI] [PubMed] [Google Scholar]
- 91.Cheng CL, et al. Transgenic expression of omega-3 PUFA synthesis genes improves zebrafish survival during Vibrio vulnificus infection. J. Biomed. Sci. 2015;22:103. doi: 10.1186/s12929-015-0208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Costa FV, et al. Understanding nociception-related phenotypes in adult zebrafish: Behavioral and pharmacological characterization using a new acetic acid model. Behav. Brain Res. 2019;359:570–578. doi: 10.1016/j.bbr.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 93.Forn-Cuní G, Varela M, Pereiro P, Novoa B, Figueras A. Conserved gene regulation during acute inflammation between zebrafish and mammals. Sci. Rep. 2017;7:1–9. doi: 10.1038/srep41905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Preux Charles A-S, Bise T, Baier F, Sallin P, Jaźwińska A. Preconditioning boosts regenerative programmes in the adult zebrafish heart. Open Biol. 2016;6:160101. doi: 10.1098/rsob.160101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Casaril AM, et al. Depression-and anxiogenic-like behaviors induced by lipopolysaccharide in mice are reversed by a selenium-containing indolyl compound: behavioral, neurochemical and computational insights involving the serotonergic system. J. Psychiatr. Res. 2019;115:1–12. doi: 10.1016/j.jpsychires.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 96.Chang D, et al. Effect of ketamine combined with DHA on lipopolysaccharide-induced depression-like behavior in rats. Int. Immunopharmacol. 2019;75:105788. doi: 10.1016/j.intimp.2019.105788. [DOI] [PubMed] [Google Scholar]
- 97.Mello BSF, et al. Sex influences in behavior and brain inflammatory and oxidative alterations in mice submitted to lipopolysaccharide-induced inflammatory model of depression. J. Neuroimmunol. 2018;320:133–142. doi: 10.1016/j.jneuroim.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 98.Ji M-H, et al. Overinhibition mediated by parvalbumin interneurons might contribute to depression-like behavior and working memory impairment induced by lipopolysaccharide challenge. Behav. Brain Res. 2020;383:112509. doi: 10.1016/j.bbr.2020.112509. [DOI] [PubMed] [Google Scholar]
- 99.Liang M, et al. Postnatal lipopolysaccharide exposure impairs adult neurogenesis and causes depression-like behaviors through astrocytes activation triggering GABAA receptor downregulation. Neuroscience. 2019;422:21–31. doi: 10.1016/j.neuroscience.2019.10.025. [DOI] [PubMed] [Google Scholar]
- 100.Grossman L, et al. Effects of piracetam on behavior and memory in adult zebrafish. Brain Res. Bull. 2011;85:58–63. doi: 10.1016/j.brainresbull.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 101.Oshima Y, et al. Effects of eicosapentaenoic acid and docosahexaenoic acid on anxiety-like behavior in socially isolated rats. Biosci. Biotechnol. Biochem. 2018;82:716–723. doi: 10.1080/09168451.2017.1403888. [DOI] [PubMed] [Google Scholar]
- 102.Nishimura M, et al. Eicosapentaenoic acid stimulates nitric oxide production and decreases cardiac noradrenaline in diabetic rats. Clin. Exp. Pharmacol. Physiol. 2000;27:618–624. doi: 10.1046/j.1440-1681.2000.03311.x. [DOI] [PubMed] [Google Scholar]
- 103.Nobre MEP, et al. Eicosapentaenoic acid and docosahexaenoic acid exert anti-inflammatory and antinociceptive effects in rodents at low doses. Nutr. Res. 2013;33:422–433. doi: 10.1016/j.nutres.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 104.Pérez-Matute P, Pérez-Echarri N, Martínez JA, Marti A, Moreno-Aliaga MJ. Eicosapentaenoic acid actions on adiposity and insulin resistance in control and high-fat-fed rats: role of apoptosis, adiponectin and tumour necrosis factor-α. Br. J. Nutr. 2007;97:389–398. doi: 10.1017/S0007114507207627. [DOI] [PubMed] [Google Scholar]
- 105.Horrobin DF, Lonergan PE, Martin DS, Lynch MA. Neuroprotective effect of eicosapentaenoic acid in hippocampus of rats exposed to γ-irradiation. J. Biol. Chem. 2002;277:20804–20811. doi: 10.1074/jbc.M202387200. [DOI] [PubMed] [Google Scholar]
- 106.Stewart A, et al. Modeling anxiety using adult zebrafish: a conceptual review. Neuropharmacology. 2012;62:135–143. doi: 10.1016/j.neuropharm.2011.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miller N, Gerlai R. From schooling to shoaling: patterns of collective motion in zebrafish (Danio rerio) PLoS ONE. 2012;7:e48865. doi: 10.1371/journal.pone.0048865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Miller NY, Gerlai R. Shoaling in zebrafish: what we don't know. Rev. Neurosci. 2011;22:17–25. doi: 10.1515/rns.2011.004. [DOI] [PubMed] [Google Scholar]
- 109.Demin KA, et al. Acute effects of amitriptyline on adult zebrafish: Potential relevance to antidepressant drug screening and modeling human toxidromes. Neurotoxicol. Teratol. 2017;62:27–33. doi: 10.1016/j.ntt.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 110.Wang D, et al. Behavioral and physiological effects of acute and chronic kava exposure in adult zebrafish. Neurotoxicol. Teratol. 2020;79:106881. doi: 10.1016/j.ntt.2020.106881. [DOI] [PubMed] [Google Scholar]
- 111.Zabegalov KN, et al. Understanding zebrafish aggressive behavior. Behav. Proc. 2019;158:200–210. doi: 10.1016/j.beproc.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 112.Wang J, et al. High-glucose/high-cholesterol diet in zebrafish evokes diabetic and affective pathogenesis: The role of peripheral and central inflammation, microglia and apoptosis. Prog. Neuro Psychopharmacol. Biol. Psychiatry. 2020;96:109752. doi: 10.1016/j.pnpbp.2019.109752. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding authors upon reasonable requests.



