Abstract
Plasmodium falciparum is the most lethal malaria parasite. The present study investigates the interaction capabilities of select plant derivatives, iso-mukaadial acetate (IMA) and ursolic acid acetate (UAA), against P. falciparum Hsp70-1 (PfHsp70-1) using in vitro approaches. PfHsp70-1 facilitates protein folding in the parasite and is deemed a prospective antimalarial drug target. Recombinant PfHsp70-1 protein was expressed in E. coli BL21 cells and homogeneously purified by affinity chromatography. The interaction between the compounds and PfHsp70-1 was evaluated using malate dehydrogenase (MDH), and luciferase aggregation assay, ATPase activity assay, and Fourier transform infrared (FTIR). PfHsp70-1 prevented the heat-induced aggregation of MDH and luciferase. However, the PfHsp70-1 chaperone role was inhibited by IMA or UAA, leading to both MDH and luciferase’s thermal aggregation. The basal ATPase activity of PfHsp70-1 (0.121 nmol/min/mg) was closer to UAA (0.131 nmol/min/mg) (p = 0.0675) at 5 mM compound concentration, suggesting that UAA has no effect on PfHsp70-1 ATPase activity. However, ATPase activity inhibition was similar between IMA (0.068 nmol/min/mg) (p < 0.0001) and polymyxin B (0.083 nmol/min/mg) (p < 0.0001). The lesser the Pi values, the lesser ATP hydrolysis observed due to compound binding to the ATPase domain. FTIR spectra analysis of IMA and UAA resulted in PfHsp70-1 structural alteration for β-sheets shifting the amide I band from 1637 cm−1 to 1639 cm−1, and for α-helix from 1650 cm−1 to 1652 cm−1, therefore depicting secondary structural changes with an increase in secondary structure percentage suggesting that these compounds interact with PfHsp70-1.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12192-021-01212-6.
Keywords: Plasmodium falciparum, Heat shock protein, Iso-mukaadial acetate, Ursolic acid acetate, Polymyxin B
Introduction
Heat shock proteins (Hsp) are classified as the centre of cellular homeostasis, and their main functions include protein folding and trafficking, protein degradation, protein aggregation, and misfold prevention. These functions are carried out by Hsp forming a multi-protein complex, such as the Hsp90-Hsp70 and Hsp70-Hsp40-NEF, which are specific to different cellular compartments (endoplasmic reticulum (ER), cytoplasm, mitochondrion, and nucleus) (Botha et al. 2007; Doyle et al. 2019). Hsp70 is housekeeping molecular chaperones that are conserved across different animal species. The P. falciparum, parasite genome encodes six Hsp70s, namely, PfHsp70-1, PfHsp70-2, PfHsp70-3, PfHsp70-x, PfHsp70-y and PfHsp70-z. Of these known Hsp70s, PfHsp70-1 is a well-characterized essential protein and ubiquitous protein vital for parasite survival. PfHsp70-1 is known to be expressed throughout the parasite erythrocytic stage and plays a crucial role in protein quality control, thus imperative for developing malaria parasites (Shonhai et al. 2007; Shonhai 2014). However, there are also differences in structural and specific features between species that have been identified as functional domains, including the ATPase and substrate-binding domain and the linker region. These domains have been identified as drug candidates aimed at reducing the optimum functionally of those targeted proteins. The heat shock protein of Plasmodium species has been proposed as potential drug targets because of their regulated role by nucleotides, the structural difference across species, and formation of a multi-protein complex, such as the PfHsp90-PfHsp70 and PfHsp70-PfHsp40-NEF (Zininga et al. 2014). Small inhibitor molecules that have been reported to bind PfHsp70 include polymyxin B (PMB) and (-)-epigallocatechin-3-gallate (EGCG), both of which attach to the N-terminal ATPase domain. Therefore, it mimics the binding mechanism of ATP/ADP (Zininga et al. 2017).
The currently used antimalarial drugs such as chloroquine and artemisinin-combination therapies (ACTs) are becoming ineffective due to the emergence of drug resistance. The functional evolution of P. falciparum, such as the upregulation of thermotolerant heat shock proteins facilitating the refolding of heat-denatured plasmodium proteins, is an adaptive stress response, which is a type of hormetic response (Daniyan et al. 2019; Mattson 2008). As a result of P. falciparum infection, the human body temperature increases, denaturing newly produced plasmodium proteins and halting parasite proliferation. However, the parasite has developed mechanisms to adapt to the changing environment via hormetic response pathways that involve transcriptional factors, facilitating expression of vitagenes such as heat shock proteins that are hermetic stress resistance (Calabrese et al. 2010, 2011). Vitagenes are type of genes responsible for maintaining cellular proteostasis and redox during pathogenesis of infections, thus coordinating mechanisms of stress resistance (Calabrese et al. 2010; Siracusa et al. 2020).
Recent studies are exploring the synthesis of quinoline-inspired hybrids as potential plasmodial inhibitors. Quinoline-pyrimidine hybrids have been reported as potent antimalarial compounds that specifically target PfHsp70-1 and PfHsp70-z (Kayamba et al. 2021). A multi-target drug approach in malaria treatment was recently reviewed as an ideal drug design strategy to counter the occurrence of drug-resistant parasites (Makhoba et al. 2020). However, medicinal plants are still preferred compared to synthesized drugs, as they generally do not harbor “drug” actions or adverse effects. In various ethnobotanical studies, plants have exhibited the excellent potential to fight against infections and may be used to develop new drugs to treat malaria and other diseases (Cock et al. 2019; Bekono et al. 2020). Warbugia salutaris and Mimusops caffra are radical scavenging plants with anti-plasmodium treatment due to their active phytocompounds isolated, which includes iso-mukaadial acetate (IMA) and ursolic acid acetate (UAA), respectively (Simelane et al. 2013; Nyaba et al. 2018; Ayeni et al. 2019). Previous studies have reported that both compounds have in vivo and in vitro anti-plasmodial activities (Simelane et al. 2013; Nyaba et al. 2018). IMA and UAA have been shown to bind and inhibit P. falciparum protein, such as PfHGXPRT (Opoku et al. 2019). Therefore, the current study explored whether these compounds would be able to inhibit other P. falciparum proteins, which include PfHsp70-1. P. falciparum Hsps are seen in the parasite’s cellular functional networks, and their organized expression is essential in the formation of proteins. Therefore, because of their role as the “watchman” for aggregation-prone protein, Hsps are possible plant-derived drug targets (Matambo et al. 2004; Stephens et al. 2011; Miller and Fort 2018). PfHsp70-1 is a thermotolerant molecular chaperone found in the cytosol and nucleus. It is also responsible for protein refolding by interacting with PfHsp70-z—which functions as substrate holdase, contributing to the cytoprotection of P. falciparum within the host. PfHsp70-1 also facilitates translocation of the newly synthesized protein to the mitochondria through the Translocase of the outer membrane (TOM) complex and the Translocase of the inner membrane (Tim23) complex that is associated with PfHsp70-3 (Przyborski et al. 2015) and also helps promote further host cell invasion (Shonhai et al. 2007; Misra and Ramachandran 2009). PfHsp70-1 was chosen as the target due to its protein-protein interactions with other P. falciparum Hsp. In the current study, we sought to understand IMA and UAA anti-plasmodium properties by using PfHsp70-1 as a possible target and investigating these study compounds’ possible mechanism of action.
Materials and methods
Materials
Unless otherwise stated, all the chemicals used, including the solvents, were of analytical grade from Inqaba Biotechnical Industries (South Africa), Separations Scientific SA (Pty) Ltd, and Sigma-Aldrich Co., Ltd (Steinheim, Germany).
Recombinant PfHsp70-1 protein expression
The chemically prepared competent E. coli BL21 (DE3) cells were transformed with the pQE30/PfHsp70-1 plasmid construct previously reported by Matambo et al. (2004). A single transformed colony of cells was inoculated into 50 ml of 2xYT (0.5% w/v sodium chloride, 1% w/v yeast, 1.5 % w/v agar, and 1.6% w/v tryptone) broth supplemented with 100 μg/ml kanamycin in a 150 ml conical flask, which was then incubated in a shaking incubator (Merck) at 162 rpm overnight at 37°C. The 50 ml overnight inoculum was sub-cultured into a 500 ml conical flask with 150 ml 2xYT broth containing 100 μg/ml kanamycin and incubated at 37°C, shaking at 200 rpm until the culture reached an optical density (OD) between 0.5 and 0.6 Abs (600 nm). Protein expression was then induced by adding 1 mM isopropyl-β-D-1-thiogalactopyranoside (IPTG); post-induction samples were harvested at 3 and 24 h centrifugation at 5000g for 30 min at 4°C and stored at −80°C. PfHsp70-1 protein expression profile was analysed using a pellet sample of untransformed cells and post-induction cell cultures centrifuged at 5000g for 30 min at 4°C. A 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of these pellets was run at 150V, 25mA for an hour and a half. Gels stained using Coomassie brilliant blue and visualized with ChemiDoc Imaging system (Bio-Rad, USA). Western blot analysis (ran at 100V, 3A for 30 min with the Trans-blot Turbo system (Bio-Rad, Inc., U.S.A) was used to confirm protein expression using monoclonal anti-polyHistidine−peroxidase conjugated antibody (1:2000) produced in mouse on PVDF membrane block with 1 mg/ml BSA.
Purification of expressed PfHsp70-1 protein
Purification of PfHsp70-1 proteins was conducted based on native purification protocol, with minor modifications (Pooe et al. 2017; Opoku et al. 2019). The harvested E. coli BL21 cells harboring the expressed PfHsp70-1 proteins, stored at −80°C were retrieved and allowed to thaw at 4°C, and 0.1% (v/v) poly-(ethyleneimine) (PEI) added for nucleic acids precipitation. The lysis buffer (10 mM Tris-HCl, pH 7.4, 300 mM NaCl, 10 mM imidazole, 1mM phenylmethylsulphonyl fluoride (PMSF), and 1 mg/ml lysozyme ) was used to resuspend the cell pellet on ice, followed by a mild sonification (4× cycles of 30 s with 60% power and 15 s incubation on ice between each cycle). The clear cell lysate was obtained by centrifugation at 12,000g for 30 min at 4°C, loaded onto a Nickel-charged nitrilotriacetic acid resin (Ni-NTA) (Thermo Scientific) equilibrated with the binding buffer (10 mM Tris-HCl, pH 7.4, 300 mM NaCl, 30 mM imidazole) and allowed the His6-PfHsp70-1 to bind to Ni-NTA at 4°C for 4 h on a shaking platform. Non-specific proteins were washed out using a stepwise wash with only an increased imidazole concentration by 5 mM increments (wash buffer: 10 mM Tris-HCl, pH 7.4, 300 mM NaCl, 30–50 mM imidazole). The bound proteins were eluted using the native elution buffer (10 mM Tris-HCl, pH 7.4, 300 mM NaCl, 500 mM imidazole). The eluents were subjected to buffer exchange using a pre-soaked Snake-Skin tubing 7000 MWCO (Thermo Scientific) in a dialysis buffer (10 mM Tris-HCl, pH 7.4, 300 mM NaCl, 10 mM Imidazole, 10 % (v/v) glycerol, and 1mM PMSF) and kept in the storage buffer (10 mM Tris-HCl, pH 7.4, 100 mM NaCl, 10 % (v/v) glycerol and 1 mM PMSF) for downstream assays. A 12% SDS-PAGE and Western blot analysis were done as described above to analyse and confirm PfHsp70-1 purity.
Investigating the in vitro suppression of PfHsp70-1 chaperone activity
According to previously conducted studies, PfHsp70-1 has been shown to be functionally stable when subjected to heat below 60 0C (Misra and Ramachandran 2009). PfHsp70-1 has been shown to prevent heat-induced aggregation of both malate dehydrogenase (MDH) from porcine heart and luciferase (Zininga et al. 2015). Thus, we sought to investigate whether the addition of 10 mM IMA and 10 mM UAA may be used to disrupt the thermal aggregation of MDH and luciferase. Briefly, samples containing MDH and luciferase respectively were incubated in assay buffer (20 mM HEPES-KOH; pH 7.5, 50 mM NaCl) and left to equilibrate at 45°C for 10 min before adding PfHsp70-1. PMB instead of compounds and Bovine serum albumin (BSA) instead of PfHsp70-1 were used as controls. The heat-induced suppression was measured after 25 min for MDH at 360 nm and luciferase at 340 nm. Absorbance readings were measured using the BioTek Synergy HT microplate reader controlled by a computer installed Gen5 software.
ATPase activity of purified PfHsp70-1 protein
The steady-state basal activity of PfHsp70-1 was determined by using the previously described protocol by Matambo et al. (2004) and Mabate et al. (2018). The assays were conducted in buffer HDKM (10 mM HEPES-KOH; pH 7.5, 0.5 mM dithiothreitol (DTT), 100 mM KCl, 2 mM MgCl2) with 1 mM PfHsp70-1 being incubated in the absence and presence of varying compound concentrations of 1–5 mM IMA, UAA and PMB (positive-control), at 37°C for 5 min. The assay reactions were then initiated by adding constant ATP concentration (5 mM) and allowed to occur for 4 h at 37°C. After every hour, 50 μl of each reaction mixture was collected, and 50 μl of both 10% SDS added to stop the reaction. An additional 50 μl of both 1.25% ammonium molybdate and 9% ascorbic acid were used for color formation, and the released inorganic phosphate (measured as Pi released/min/mg protein) was quantified spectrophotometrically at an absorbance of 660 nm (A660) using the UV-1800 spectrophotometer (Shimadzu, Japan).
Fourier transform infrared analysis
The dialysed 0.5 mM PfHsp70-1 protein was incubated with 1mM study compounds (IMA and UAA) prepared in 100% dimethyl sulfoxide (DMSO). The protein-ligand complex was allowed to form in an hour of incubation at room temperature (≈22°C). For positive control, 1mM PMB was used in place of study compounds. FT-IR measurements were done using IRAffinity-1S (Shimadzu, Japan) at room temperature (≈22°C). A background scan of the dialysis buffer was done before sample readings. The IR spectral signals readings (the vibration frequencies in the amide A and B, I, II, and III) of PfHsp70-1 alone and in solution with polymyxin B, IMA and UAA separately were done, between 400 and 4000 cm−1 at a resolution of 2 cm−1 and with 64 scans. The FT-IR spectral data were analysed with OriginPro 8.5 software.
Statistical analysis
The mean and the standard deviation errors of the three experimental replicates were calculated, and the data was analysed using GraphPad Prism version 8.4.3. The observed data were subjected to ordinary one-way and two-way analysis of variance (ANOVA). The p-value ≤ of 0.05 was considered statistically significant.
Results
The expression and purification of PfHs70-1 recombinant protein
Recombinant PfHsp70-1 was over-expressed in E. coli BL21 (DE3) cells and successfully purified using nickel-affinity chromatography. The expression profile of the PfHsp70-1 protein (74 kDa) was resolved with a 12% SDS PAGE (Fig. 1). Lane CL, control depicts untransformed cells; lane 0, pre-induction; lane 3 and 24 represent 1 and 5-hour post-IPTG induction samples. Western Blot was used to confirm successful protein expression and purification, horseradish peroxidase-conjugated polyhistidine antibodies, were used to confirm the N-terminal-His6-tagged PfHsp70-1 protein. Minimal protein was detected during the wash steps, showing high binding specificity of the purification beads used in this study (Fig. 1, lane W). Homogenously purified PfHsp70-1 was identified in the elution fraction (Fig. 1, lane E) and subsequently confirmed by western blot analysis (Fig. 1, lower panel).
Fig. 1.
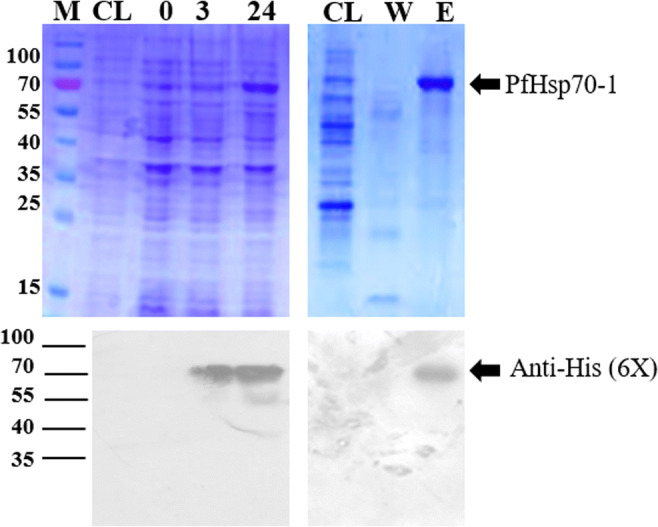
Recombinant PfHsp70-1 expression and purification analysis. PfHsp70-1 was over-expressed in E. coli BL21 (DE3) cells and successfully purified using nickel-affinity chromatography. Lane M, prestained dual colour protein marker; lane CL, control depicts untransformed cells; lane 0, pre-IPTG induction; lane 3 and 24 represent, 3 and 24 h post-IPTG induction samples. Lane W represent the unbound flow through, whereas lane E represents the elution fraction containing the purified protein. Western blot analysis was used to confirm the presence expression and purification of PfHsp70-1 using monoclonal anti-polyHistidine−peroxidase conjugated antibody (1:2000) produced in mouse on PVDF membrane block with 1 mg/ml BSA (lower panel)
Iso-mukaadial acetate and ursolic acid acetate suppress chaperone activity of PfHsp70-1
Both substrate/model proteins (MDH and luciferase) aggregated when PfHsp70-1 was incubated with either IMA, UAA, or PMB, as shown in Figs. 2 and 3, respectively. The aggregation of both model proteins formed insoluble particles due to study compounds interacting with PfHsp70-1 and rendering it inactive to bind MDH or luciferase, which increased the cloudiness of the assay sample. The purified PfHsp70-1 alone was able to prevent both MDH and luciferase aggregation. However, incubation with BSA led to substrate aggregation. In the presence of MDH or luciferase, an increase in PfHsp70-1 concentration (5 μM or 10 μM) was seen to decrease the degree of aggregation (absorbance). The aggregation assay was repeated in the presence or absence of IMA or UAA
Fig. 2.
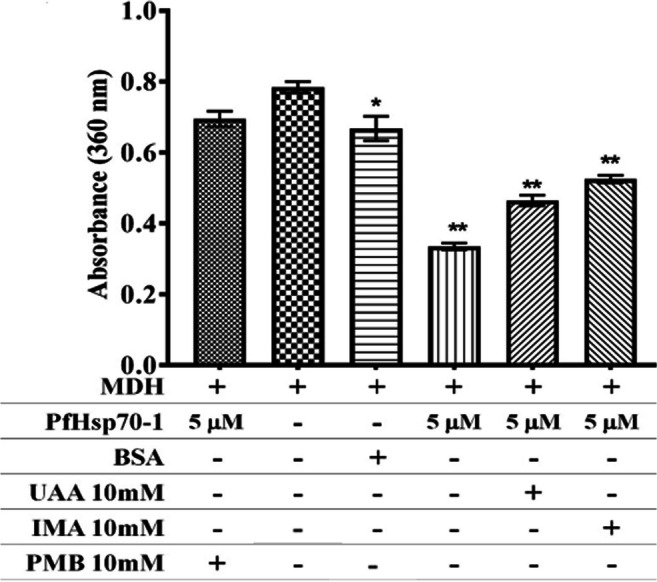
MDH thermal aggregation. MDH denaturation was monitored under heating conditions (45°C for 25 min) when incubated PfHsp70-1, in the presence or absence study compounds (10 mM IMA and 10 mM UAA), 10 mM polymyxin B (positive control) and 2 mg/mL BSA. One-way ANOVA with p = 0.0489* and p = 0.0001** was used as statistical analysis comparing to MDH only column to others
Fig. 3.
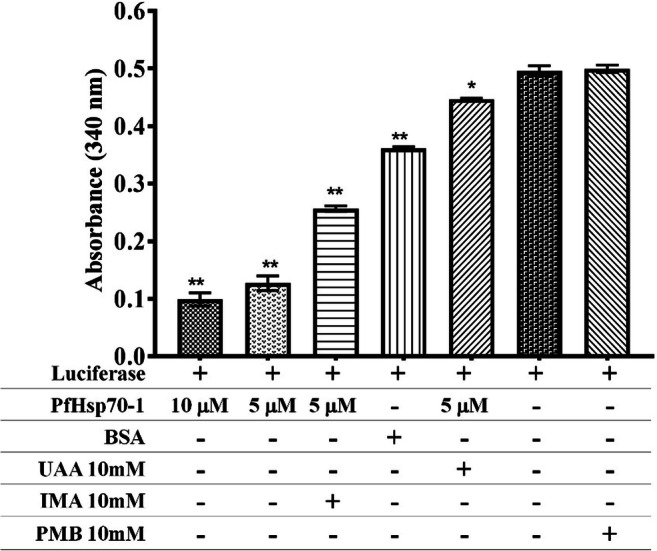
Thermal induced aggregation of luciferase. Luciferase denaturation was monitored under heating conditions (45°C for 25 min) when incubated along PfHsp70-1 with and without study compounds (10 mM IMA and 10 mM UAA), 10 mM polymyxin B (positive control) and 2 mg/mL BSA in place of PfHsp70-1. One-way ANOVA with p = 0.0019* and p = 0.0001** was used as statistical analysis comparing to luciferase only column to others
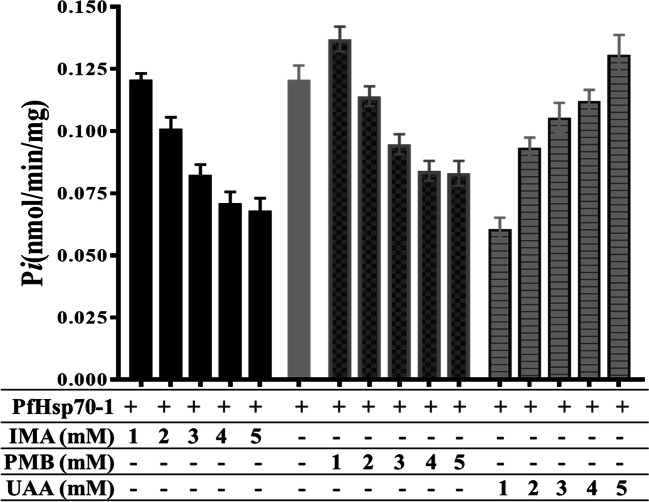
Basal ATPase functional activity of PfHsp70-1
As expected PfHsp70-1 alone had a higher Pi value (0.121 nmol/min/mg), but in the presence of 5mM PMB, PfHsp70-1 had Pi value of 0.083 nmol/min/mg. In the presence of study compounds such as 5 mM IMA, PfHsp70-1 had a Pi value of 0.068 nmol/min/mg (Fig. 4). Whereas, in the presence of 5mM UAA, the Pi value increased to 0.131 nmol/min/mg closer to that of free-PfHsp70-1 (Fig. 4). The basal ATPase activity of the purified PfHs70-1 protein decreased when incubated with IMA and increased when incubated with UAA.
Fig. 4.
The basal PfHsp70-1 ATPase activity analysis without compounds and with study compounds (IMA, UAA) and PMB at varying compound concentrations (1–5 mM), both activities incubated at 37°C for 4 h with constant 5 mM ATP and 1 mM PfHsp70-1 concentration. The inorganic phosphate from the phosphomolybdate complex formed during the assay was measured at 660 nm in a direct colorimetric assay to determine the ATPase activity. Two-way ANOVA with p = 0.0012 was used as the statistical analysis between columns of the same concentration
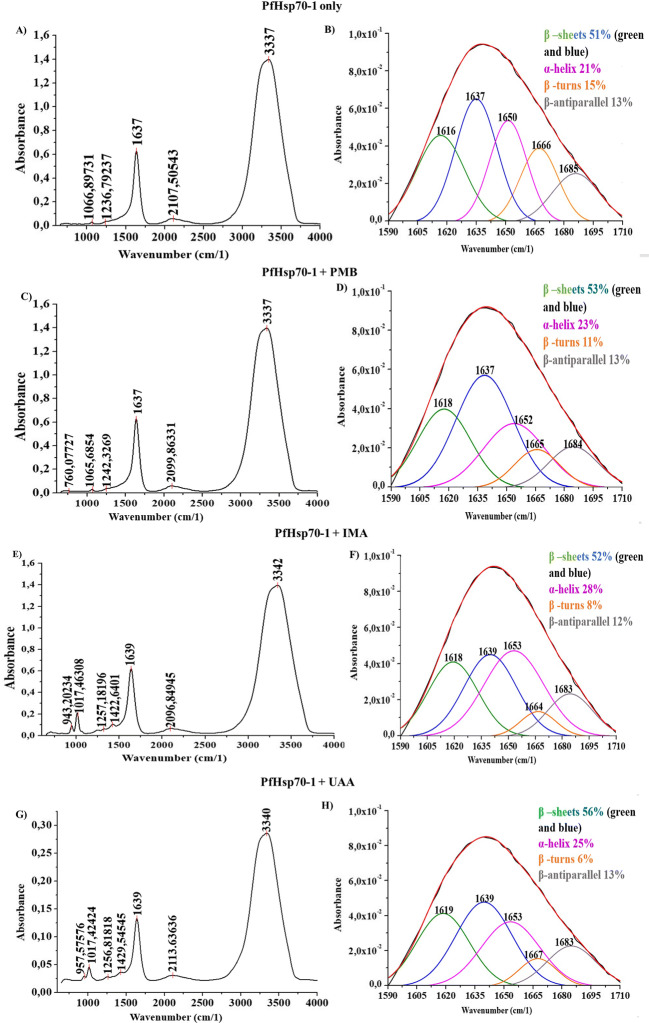
Interaction of iso-mukaadial acetate, ursolic acid acetate with PfHsp70-1 is associated with conformational changes
The incubation of iso-mukaadial acetate, ursolic acid acetate, or polymyxin B with PfHsp70-1 resulted in spectral signals shifting and the amide’s vibration frequencies at varying wavenumbers (cm−1) (Fig. 5). In Fig. 5A and Fig. 5B, the FTIR spectra of PfHsp70-1 amide I band was deconvoluted for secondary structure determination with and without study compounds. Fig. 5 FTIR spectra of PfHsp70-1-compound interaction showing PfHsp70-1 incubated with polymyxin B, iso-mukaadial acetate, and ursolic acid acetate. Spectrum images labelled as (A) represents amide band peaks between 400 and 4000 cm−1 (Fig. 5A, C, E) and fitted curve of amide band I at 1600–1700 cm−1 for secondary structure distribution (Fig. 5B, D, F and H).
Fig. 5.
FTIR spectra of PfHsp70-1-compound interaction. The image shows FTIR analysis for: native PfHsp70-1 only, PfHsp70-1 incubated with 1 mM polymyxin B, PfHsp70-1 incubated with 1 mM iso-mukaadial acetate and 1 mM ursolic acid acetate. Spectrum images labelled as fitted curve of amide band I at 1600–1700 cm−1 (B, D, F and H), whereas amide band peaks between 400 and 4000 cm−1 (A, C, E and G) representing secondary structure distribution
Discussion
PfHsp70-1 is a heat-stable protein localised on the cytosol of infected erythrocytes and is responsible for the malaria-causing parasite’s survival and pathogenicity development. As a molecular chaperone, it facilitates protein folding machinery of the parasite-produced proteins implicated in host cell invasion and immune system evasion. Thus, contributing to malaria prevalence (Shonhai et al. 2008; Misra and Ramachandran 2009). PfHsp70-1 plays a central role in regulating protein homeostasis due to its association with other chaperones and co-chaperones (Kampinga and Craig 2010). Because of its capabilities, PfHsp70-1 was chosen in this study as a possible target for investigating the anti-plasmodium activity of both IMA and UAA. Both IMA and UAA have been validated as potential drug candidates in a study by Opoku et al. (2019). Our findings are in line with previous study.
PfHsp70-1 chaperone activity
According to previously conducted studies, PfHsp70-1 has been shown to be functionally stable when subjected to heat (Shonhai et al. 2008; Zininga et al. 2017), and it is part of the coordinated cellular hormetic stress response pathways that disrupts proteotoxicity of aggregated Plasmodium proteins (Mattson 2008). Both malate dehydrogenase (MDH) and luciferase aggregation assay are turbidimetric assays, where the aggregation of both model proteins forms insoluble particles, and absorbance is read as a degree of aggregation. The presence of study compounds (IMA and UAA) resulted in the heat-induced aggregation of both MDH and luciferase similar to that of PMB. It is apparent that the aggregation of model substrate/proteins was caused by the binding of IMA and UAA to PfHsp70-1, thus suppressing chaperone function activity towards refolding model substrates to their native conformation state and preventing them from forming insoluble aggregated substances. Since Hsp70 are encoded by vitagenes as a means of preventing proteotoxicity and cellular redox imbalances during disease stages (Siracusa et al. 2020), the inhibition of PfHsp70-1 chaperone activity by both IMA and UAA presents the possibility of disrupting the cellular hormetic resistance pathway, which underlines the refolding of aggregated proteins and thus, causing Plasmodium proteins to remain aggregated, rendering them unfunctional and leading to parasite death. In the presence of PfHsp70-1 without the addition of any compounds, the degree of aggregation measured as absorbance decreased compared to when MDH and luciferase were incubated alone in their respective assay buffers. Both model proteins also aggregated in response to heat when incubated with non-chaperone Bovine serum albumin (BSA) protein. These results are similar to those obtained by Shonhai et al. (2008), Xu et al. (2012), and Zininga et al. (2017). Therefore, evidently showing that the function of PfHsp70-1 as a heat-stable chaperone, which suppresses protein aggregation, was affected by the presence of both IMA and UAA.
Basal ATPase activity
The purified PfHsp70-1 protein’s functional activities were done to assess whether the extracted protein can perform downstream in vitro processes or functions. The N-terminal of PfHsp70-1 has an active ATPase domain or nucleotide-binding domain (NBD) that binds to ATP, causing substantial conformational changes, and hydrolysis of the bound ATP releasing ADP and Pi (inorganic phosphate) also results in re-arrangement of protein’s secondary motif structures in the C-terminal substrate-binding domain via allosteric communication between the two domains during the Hsp70 functional cycle. Determination of basal ATPase activity of proteins was based on a colorimetric assay, which measures Pi bound to acidified ammonium molybdate forming phosphomolybdate complex at 37°C (Kyaw et al. 1985; Mabate et al. 2018). The basal ATPase activity of PfHsp70-1 has been previously established in a study by Matambo et al. (2004). From this study, the free-PfHsp70-1 alone had a higher Pi value (0.121 nmol/min/mg) compared to when bound to study compounds, thus implicating a higher affinity for ATP. However, in the presence of 5mM PMB (as positive control), PfHsp70-1 had a declined Pi value of 0.083 nmol/min/mg that was almost two times lesser than that of free-PfHsp70-1, suggesting a lower affinity for ATP as a result of already occupied ATPase domain by PMB as it was also observed in a study by Zininga et al. (2017). In the presence of study compounds such as 5 mM IMA, PfHsp70-1 had a Pi value of 0.068 nmol/min/mg, which was lesser than that of the free-PfHsp70-1, thus, suggesting a lower affinity for ATP. This was similar to PMB. However, with 5mM UAA, the Pi value increased to 0.131 nmol/min/mg closer to free-PfHsp70-1, thus indicating a higher ATP affinity and that UAA does not bind more effectively to the PfHsp70-1 N-terminal ATPase domain.
Furthermore, the ATPase activity with increasing PMB concentration (1–5 mM) exhibited a decrease in Pi, and this was a similar trend observed in PfHsp70-1 with IMA, suggesting that the ATPase activity was interrupted by IMA as seen with PMB. Since PMB binds to the same cleft as ATP on the N-terminal ATPase domain as determined by Zininga et al. (2017), IMA could also have attached to the same cleft to hinder ATP from binding, thus resulting in competitive inhibition. PfHsp70-1 with UAA showed an increased ATPase activity as concentration was increased (1–5 mM), which was the opposite effects as compared to that of both PMB and IMA, suggesting that UAA does not bind to the N-terminal ATPase domain and that ATP binding was not inhibited similar to that in the free-PfHsp70-1 state. The UAA-PfHsp70-1 ATPase activity, which was closer to than that of free-PfHsp70-1 but higher than that of polymyxin B-PfHsp70-1 mixture at 5 mM compound concentration, demonstrated that even though UAA did not directly bind to the N-terminal ATPase domain, it might, however, have bound to other parts PfHsp70-1 such as the C-terminal Substrate binding domain. Because, according to PfHsp70-1 chaperone activity, UAA promoted heat-induced aggregation of both substrate proteins (luciferase and malate dehydrogenase) by inhibiting PfHsp70-1 chaperone functional activity, and this inhibition was possibly facilitated through the C-terminal domain interactions. For a stronger or increased ATPase activity, all the domains of PfHsp70-1 play a significant role due to their allosteric communication. Therefore, changes or removal of one domain impacts the other domains as established by Misra and Ramachandran (2009) when the N-terminal binding domain of PfHsp70-1 was subjected to ATPase activity alone without the C-terminal attached, and the resulted activity was less as compared to when PfHsp70-1 domains complete.
FTIR spectral analysis
The interactions of iso-mukaadial acetate, ursolic acid acetate, and polymyxin B towards PfHsp70-1 were separately investigated using FTIR vibration spectroscopy. To determine the effects on the structure of PfHsp70-1 upon binding, spectral signals shifting and the variation frequency in the amide I band dimension of PfHsp70-1 at 1600–1700 cm−1 for proteins, amide II band (CN stretch and NH bending at 1460–1590 cm−1 for proteins), amide III (CN stretch, NH bending between 1229 and 1350 cm−1 for aromatic groups) and amide A and B (OH and NH stretch between 3000 and 3500 cm−1 for phenols) were monitored using the IR affinity-1S FTIR spectrophotometer (Kong and Yu 2007; Hands et al. 2016). The secondary structure of proteins denoted by the amide I band as α-helix (1660–1650 cm−1), β-sheet (1637–1614 cm−1), β-turn (1678–1670 cm−1), and a β-antiparallel (1691–1680 cm−1). These characteristics of the deconvolved amide I band region correspond to those observed in a study by Alhazmi (2019). The FTIR was used to identify and measure metal ions’ binding interaction to Bovine albumin serum (BSA).
In order to determine spectral band shifting, the raw FTIR data of transmittance over wavenumber (cm−1) were converted to absorbance over wavenumber (cm−1). In all FTIR spectra readings observed on Fig. 5, amide band A, due to the phenol groups such as that of the three Tryptophan found on the surface of PfHsp70-1, are the most prominent indicted between 3000 and 3500 cm−1. From the analysis done, the vibration frequency of amide band A characterized by OH and NH bond stretching was observed at 3337 cm−1 for unbound PfHsp70-1 (Fig. 5B), and the same spectra signal band was observed when PfHsp70-1 was incubated with polymyxin B (Fig. 5D). However, with iso-mukaadial acetate (Fig. 5E), band shifting of amide A was to 3342 cm−1 and to 3340 cm−1 with ursolic acid acetate (Fig. 5G). The band shifting to a higher vibration frequency was due to the interaction of IMA and UAA with PfHsp70-1, particularly binding through the phenol (OH) group.
No spectral shits were observed with the amide A and I of the PMB-PfHsp70-1 complex. The amide III spectral band of PfHsp70-1 (for aromatic groups) was noted at 1236 cm−1 and shifted to 1242 cm−1 with polymyxin B, to 1256 cm−1 with UAA, and with IMA to 1257 cm−1 as a result of the interactions of study compounds with the CN and NH groups of the PfHsp70-1. The secondary structure of proteins denoted by the amide I band as α-helix (1660–1650 cm−1), β-sheet (1637–1614 cm−1), β-turn (1678–1670 cm−1), and a β-antiparallel (1691–1680 cm−1) (Litvinov et al. 2012; Alhazmi 2019). The amide I band region of unbound PfHsp70-1 that was examined further had spectral readings at 1616 cm−1, and 1637 cm−1 for β-sheet strands,1650 cm−1 for α-helix, 1666 cm−1 for β-turns, and 1685 cm−1 for β-antiparallel due to the C=O stretches as depicted in Fig. 5B. It was observed that the spectral vibrations of the amide I band within the β-sheets region shifted from 1616 cm−1 to 1618 cm−1 and also remained at 1637 cm−1 with PMB (Fig. 5D). However, with IMA the spectral vibration moved to 1618 cm−1 and 1639 cm−1 and with UAA to 1619 cm−1 and 1639 cm−1. The spectral shifts are accompanied by the increase in the β-sheets percentage, which shows the amount of secondary structures present. Additionally, band shifting towards the higher vibration frequency of α-helix (1660–1650 cm−1) could have depicted PfHsp70-1 structural changes also noted by the increase in α-helix percentages from 21% in unbound PfHsp70-1 to 23% with PMB, 28% with IMA, and 25% with UAA (Fig. 5; SI). Thus, the percentage increase suggests that PfHsp70-1 conformational changes induced by both IMA and UAA through destabilised hydrogen bonds between the polypeptide stands, mainly through the β-turns C=O groups (of which its percentage had decreased) that contributes to either β-sheet or α-helix.
Conclusion
PfHsp70-1 is observed as the centre of cellular proteostasis associated with other Plasmodium proteins, facilitating their folding, and contributing to survival. In our current study, the binding effects of anti-plasmodium compounds iso-mukaadial acetate and ursolic acid acetate towards PfHsp70-1 functionality were investigated. With the use of FTIR spectroscopy, it was established that both IMA and UAA interact with the target study protein PfHsp70-1 as observed with the decreased absorption strength and the shift in spectral wavenumbers (cm−1) along with increased α-helix and β-sheet distribution percentage indicating structural changes. It was further noted that both compounds hinder the chaperone function of PfHsp70-1. However, the ATPase function was notably inhibited by IMA and UAA was less effective. Throughout the study, IMA behaviour or effects were similar to those of polymyxin B, a known PfHsp70-1 inhibitor. Altogether, our findings confirm that both IMA and UAA could bind to the PfHsp70-1 and disrupt its downstream chaperone function. Thus, contributing to the existing knowledge regarding the in vitro and in vivo anti-plasmodium activities observed in previous studies, which formed the basis of this study. Even though IMA and UAA bind PfHsp70-1, further studies are needed to evaluate both compounds with PfHsp70-1 and its co-chaperones.
Supplementary Information
(PDF 411 kb)
Author contribution
Writing of the original draft; N.S, O.J.P., M.B.C.S.; data analysis; N.S.,O.J.P., M.B.C.S.; investigation; N.S., O.J.P., M.B.C.S.; supervision; M.B.C.S.,; funding acquisition; M.B.C.S. All the authors read and approved the final manuscript.
Funding
The authors would like to appreciate University of Johannesburg research fund (URC) (2021URC00229).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alhazmi HA (2019) FT-IR spectroscopy for the identification of binding sites and measurements of the binding interactions of important metal ions with bovine serum albumin. Sci Pharm. 10.3390/scipharm87010005
- Ayeni G, Pooe OJ, Singh M et al (2019) Cytotoxic and antioxidant activities of selected South African medicinal plants. Pharmacogn J:11. 10.5530/PJ.2019.11.234
- Bekono BD, Ntie-Kang F, Onguéné PA, et al (2020) The potential of anti-malarial compounds derived from African medicinal plants: a review of pharmacological evaluations from 2013 to 2019. Malar. J. [DOI] [PMC free article] [PubMed]
- Botha M, Pesce E-R, Blatch GL. The Hsp40 proteins of Plasmodium falciparum and other apicomplexa: regulating chaperone power in the parasite and the host. Int J Biochem Cell Biol. 2007;39:1781–1803. doi: 10.1016/j.biocel.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Dinkova-Kostova AT, et al. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxidants Redox Signal. 2010;13:1763–1811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Cuzzocrea S, et al. Hormesis, cellular stress response and vitagenes as critical determinants in aging and longevity. Mol Aspects Med. 2011;32:279–304. doi: 10.1016/j.mam.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Cock IE, Selesho MI, van Vuuren SF. A review of the traditional use of southern African medicinal plants for the treatment of malaria. J Ethnopharmacol. 2019;245:112176. doi: 10.1016/J.JEP.2019.112176. [DOI] [PubMed] [Google Scholar]
- Daniyan MO, Przyborski JM, Shonhai A. Partners in mischief: functional networks of heat shock proteins of Plasmodium falciparum and their influence on parasite virulence. Biomolecules. 2019;9:295. doi: 10.3390/biom9070295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle SM, Hoskins JR, Kravats AN, et al. Intermolecular interactions between Hsp90 and Hsp70. J Mol Biol. 2019;431:2729–2746. doi: 10.1016/J.JMB.2019.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hands JR, Clemens G, Stables R et al (2016) Brain tumour differentiation: rapid stratified serum diagnostics via attenuated total reflection Fourier-transform infrared spectroscopy. J Neurooncol. 10.1007/s11060-016-2060-x [DOI] [PMC free article] [PubMed]
- Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol. 2010;11:579–592. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayamba F, Malimabe T, Kehinde I, et al. European Journal of Medicinal Chemistry Design and synthesis of quinoline-pyrimidine inspired hybrids as potential plasmodial inhibitors. Eur J Med Chem. 2021;217:113330. doi: 10.1016/j.ejmech.2021.113330. [DOI] [PubMed] [Google Scholar]
- Kong J, Yu S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim Biophys Sin (Shanghai) 2007;39:549–559. doi: 10.1111/j.1745-7270.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- Kyaw A, Maung-U K, Toe T (1985) Determination of inorganic phosphate with molybdate and Triton X-100 without reduction. Anal Biochem. 10.1016/0003-2697(85)90354-9 [DOI] [PubMed]
- Litvinov RI, Faizullin DA, Zuev YF, Weisel JW (2012) The α-helix to β-sheet transition in stretched and compressed hydrated fibrin clots. Biophys J. 10.1016/j.bpj.2012.07.046 [DOI] [PMC free article] [PubMed]
- Mabate B, Zininga T, Ramatsui L et al (2018) Structural and biochemical characterization of Plasmodium falciparum Hsp70-x reveals functional versatility of its C-terminal EEVN motif. Proteins Struct Funct Bioinforma. 10.1002/prot.25600 [DOI] [PMC free article] [PubMed]
- Makhoba XH, Viegas C, Mosa RA, et al. Potential impact of the multi-target drug approach in the treatment of some complex diseases. Drug Des Devel Ther. 2020;14:3235–3249. doi: 10.2147/DDDT.S257494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matambo TS, Odunuga OO, Boshoff A, Blatch GL (2004) Overproduction, purification, and characterization of the Plasmodium falciparum heat shock protein 70. Protein Expr Purif. 10.1016/j.pep.2003.09.010 [DOI] [PubMed]
- Mattson MP. Hormesis defined. Ageing Res Rev. 2008;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DJ, Fort PE (2018) Heat shock proteins regulatory role in neurodevelopment. Front. Neurosci. [DOI] [PMC free article] [PubMed]
- Misra G, Ramachandran R (2009) Hsp70-1 from Plasmodium falciparum: protein stability, domain analysis and chaperone activity. Biophys Chem. 10.1016/j.bpc.2009.03.006 [DOI] [PubMed]
- Nyaba ZN, Murambiwa P, Opoku AR, et al. Isolation, characterization, and biological evaluation of a potent anti-malarial drimane sesquiterpene from Warburgia salutaris stem bark. Malar J. 2018;17:1–8. doi: 10.1186/s12936-018-2439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opoku F, Govender PP, Pooe OJ, Simelane MBC (2019) Evaluating iso-mukaadial acetate and ursolic acid acetate as plasmodium falciparum hypoxanthineguanine-xanthine phosphoribosyltransferase inhibitors. Biomolecules. 10.3390/biom9120861 [DOI] [PMC free article] [PubMed]
- Pooe OJ, Köllisch G, Heine H, Shonhai A. Plasmodium falciparum heat shock protein 70 lacks immune modulatory activity. Protein Pept Lett. 2017;24:503–510. doi: 10.2174/0929866524666170214141909. [DOI] [PubMed] [Google Scholar]
- Przyborski JM, Diehl M, Blatch GL. Plasmodial HSP70s are functionally adapted to the malaria parasite life cycle. Front Mol Biosci. 2015;2:34. doi: 10.3389/fmolb.2015.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonhai A (2014) The role of Hsp70s in the development and pathogenicity of plasmodium species. In: Heat Shock Proteins of Malaria
- Shonhai A, Boshoff A, Blatch GL (2007) The structural and functional diversity of Hsp70 proteins from Plasmodium falciparum. Protein Sci. 10.1110/ps.072918107 [DOI] [PMC free article] [PubMed]
- Shonhai A, Botha M, de Beer T et al (2008) Structure-function study of a Plasmodium falciparum Hsp70 using three dimensional modelling and in vitro analyses. Protein Pept Lett. 10.2174/092986608786071067 [DOI] [PubMed]
- Simelane M, Shonhai A, Shode F, et al. Anti-plasmodial activity of some zulu medicinal plants and of some triterpenes isolated from them. Molecules. 2013;18:12313–12323. doi: 10.3390/molecules181012313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siracusa R, Scuto M, Fusco R, et al. Anti-inflammatory and anti-oxidant activity of hidrox® in rotenone-induced Parkinson’s disease in mice. Antioxidants. 2020;9:1–19. doi: 10.3390/antiox9090824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens LL, Shonhai A, Blatch GL. Co-expression of the Plasmodium falciparum molecular chaperone, PfHsp70, improves the heterologous production of the antimalarial drug target GTP cyclohydrolase I, PfGCHI. Protein Expr Purif. 2011;77:159–165. doi: 10.1016/j.pep.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Xu X, Sarbeng EB, Vorvis C, et al. Unique peptide substrate binding properties of 110-kDa heat-shock protein (Hsp110) determine its distinct chaperone activity. J Biol Chem. 2012;287:5661. doi: 10.1074/JBC.M111.275057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zininga T, Shonhai A, Zininga T, Shonhai A. Are heat shock proteins druggable candidates? Am J Biochem Biotechnol. 2014;10:208–210. doi: 10.3844/ajbbsp.2014.208.210. [DOI] [Google Scholar]
- Zininga T, Makumire S, Gitau GW, et al. Plasmodium falciparum hop (PfHop) interacts with the Hsp70 chaperone in a nucleotide-dependent fashion and exhibits ligand selectivity. PLoS One. 2015;10:e0135326. doi: 10.1371/journal.pone.0135326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zininga T, Pooe OJ, Makhado PB et al (2017) Polymyxin B inhibits the chaperone activity of Plasmodium falciparum Hsp70. Cell Stress Chaperones. 10.1007/s12192-017-0797-6 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 411 kb)