Abstract
Nerve agents are used in civil wars and terrorist attacks, posing a threat to public safety. Acute exposure to nerve agents such as soman (GD) causes serious brain damage, leading to death due to intense seizures induced by acetylcholinesterase inhibition and neuronal injury resulting from increased excitatory amino-acid levels and neuroinflammation. However, data on the anticonvulsant and neuroprotective efficacies of currently-used countermeasures are limited. Here, we evaluated the potential effects of transient receptor vanilloid 4 (TRPV4) in the treatment of soman-induced status epilepticus (SE) and secondary brain injury. We demonstrated that TRPV4 expression was markedly up-regulated in rat hippocampus after soman-induced seizures. Administration of the TRPV4 antagonist GSK2193874 prior to soman exposure significantly decreased the mortality rate in rats and reduced SE intensity. TRPV4-knockout mice also showed lower incidence of seizures and higher survival rates than wild-type mice following soman exposure. Further in vivo and in vitro experiments demonstrated that blocking TRPV4 prevented NMDA receptor-mediated glutamate excitotoxicity. The protein levels of the NLRP3 inflammasome complex and its downstream cytokines IL-1β and IL-18 increased in soman-exposed rat hippocampus. However, TRPV4 inhibition or deletion markedly reversed the activation of the NLRP3 inflammasome pathway. In conclusion, our study suggests that the blockade of TRPV4 protects against soman exposure and reduces brain injury following SE by decreasing NMDA receptor-mediated excitotoxicity and NLRP3-mediated neuroinflammation. To our knowledge, this is the first study regarding the “dual-switch” function of TRPV4 in the treatment of soman intoxication.
Electronic supplementary material
The online version of this article (10.1007/s12264-021-00662-3) contains supplementary material, which is available to authorized users.
Keywords: Nerve agents, Soman, TRPV4, NMDA receptor, NLRP3 inflammasome
Introduction
With the disturbing effects of sarin attacks against civilians in Syria in August 2013, important questions regarding the level of preparedness and the adequacy of existing medical responses against exposure to such nerve agents have been raised [1]. The appearance of Novichok, or “newcomer”, which was used in the attack on a Russian dissident and his daughter in 2018 highlights the importance of protection against the potentially lethal effects of nerve agents [2]. The primary effect of exposure to organophosphorus nerve agents is a decrease in acetylcholinesterase (AChE) activity and a concurrent rise in acetylcholine (ACh) levels, which are considered to be the decisive events that initiate epileptiform activity. The over-elevation of ACh levels mainly activates muscarinic receptors, and, in the cholinergic phase, the administration of receptor antagonists can halt the development of seizures and status epilepticus (SE), but only when they are administered as pretreatments or shortly after the onset of seizures (within 5 min). The anticonvulsant potential of most anticholinergics, such as atropine, is gradually lost when the administration is delayed beyond 20 min, and they completely lose their anticonvulsant potential when administered 40 min after seizure onset [3]. These narrow time windows limit their application. Nevertheless, some anticholinergics such as biperiden and procyclidine can exert anticonvulsant activity even with a 40-min delay in treatment; this is because they have anti-NMDA (N-methyl-D-aspartate) properties to some extent [4], suggesting that seizures are sustained and reinforced by glutamatergic rather than cholinergic hyperexcitation [5, 6]. As seizure activity progresses, non-cholinergic excitatory activity gradually takes control over the seizure independent of the initiating cholinergic drive [3]. This phenomenon is critical for the development of drugs that target the non-cholinergic phase. Several studies have demonstrated that in the case of nerve agent seizures, intense activation of NMDA receptors (NMDARs) would allow additional entry of Ca2+ into neurons, leading to neurotoxicity and subsequent neuropathology [7, 8]. Braitman and Sparenborg (1989) first reported that pretreatment with the NMDA antagonist MK-801 terminates nerve agent-induced SE. Meanwhile, after a period of epileptiform activity, a lower dose of MK-801 is still able to terminate SE [9]. All this evidence indicates that the NMDAR has predominant control over nerve agent-induced seizure activity at the later stages [10–12].
Several studies have reported that exposure to nerve agents is associated with a cellular inflammatory response in the form of astrocytic and microglial activation [13]. Neuroinflammatory genes, such as IL-1β, TNF-α, and IL-6, are widely described to be implicated in nerve agent-induced seizures [14–17]. Unfortunately, since most studies are limited to the downstream factors of the inflammatory response, the biochemical processes remain unclear and the inflammatory mechanism induced by nerve agents needs to be further clarified. Here we attempted, at least to some extent, to identify the intracellular signaling mechanisms of inflammatory responses following soman-induced seizures and key factors in the process.
Transient receptor potential vanilloid 4 (TRPV4), a member of the TRPV ion channel family, is sensitive to various types of stimuli, including hypotonic environments, mechanical forces, arachidonic acid metabolites, and exogenous chemical ligands [18, 19]. Activation of TRPV4 induces Ca2+ influx, thus increasing the intracellular free Ca2+ concentration ([Ca2+]i). It has been reported that TRPV4 plays a pivotal role in central nervous system disorders and injuries such as Alzheimer’s disease [20], ischemic stroke [21], traumatic brain injury [22], and seizures [23]. An interesting finding is that the activation of TRPV4 during cerebral ischemia injury increases NMDAR function, which indicates that closing TRPV4 may be neuroprotective against NMDAR-mediated glutamate excitotoxicity [24]. Furthermore, a study on febrile seizures suggests that TRPV4 channels and NMDARs both play a role in hyperthermia-induced seizures [25]; these results indicate that there may be an association between TRPV4 and NMDARs during epilepsy, and this is supported by a study showing that the activation of TRPV4 depolarizes the resting membrane potential of cultured hippocampal neurons and relieves the blockade of NMDARs by Mg2+ [26]. Recent research has pointed out that the TRPV4 agonist GSK1016790A increases the expression levels of inflammasome components of the NLRP3 pathway and downstream pro-inflammatory cytokines. The increased protein levels of NLRP3-related pro-inflammatory cytokines are blocked by the use of a TRPV4 antagonist, which markedly increases the number of surviving cells in a pilocarpine model of temporal lobe epilepsy in mice [27]. Furthermore, in a previous study, we conducted preliminary assessment of the expression changes of various TRP subtypes (TRPM2, TRPV1, TRPV4, TRPM7, TRPC3, and TRPC6) in a variety of models organophosphorus compound poisoning (including soman), and found that the expression of TRPV4 was significantly increased in all of them.
Taking into account the involvement of NMDAR-mediated excitotoxicity and neuroinflammation in soman-induced seizures and secondary neuronal injury, with the possible triggering of NMDAR function by TRPV4 activation, we hypothesized that TRPV4 could be a key target involved in the seizures and neuronal injury caused by soman exposure. Here, we used a wide range of methods to determine the effects of soman on the function of TRPV4 channels. This included behavioral studies in rats and corresponding histopathology, as well as immunofluorescence, biochemistry, electrophysiological recordings, and Ca2+ imaging in vitro.
Materials and Methods
Chemicals and Agents
Soman (pinacolyl methylphosphonofluoridate) was obtained from the Institute of Nuclear, Biological, and Chemical Defence (Beijing, China), and was only used for the purposes of the current study. Soman was diluted in cold saline. HI-6 was synthesized by the Institute of Pharmacology and Toxicology, Academy of Military Medical Science (Beijing, China) and was dissolved in saline. GSK2193874 (GSK) was from Selleck Chemicals (S8367, Houston, USA). GSK2193874 was dissolved in DMSO (Sinopharm, Shanghai, China), then diluted with Neurobasal medium immediately prior to use in cells (0.05% DMSO) or with sterilized water for use in rats (1% DMSO).
Animals and Experimental Groups
Adult male Sprague-Dawley rats (7–8 weeks old, 180–220 g) and adult male C57BL/6J mice (6–8 weeks old, 18–20 g) were from Vital River Laboratory Animal Technology Co., Ltd (Beijing, China). TRPV4-knockout (TRPV4-KO) mice were bred in-house from heterozygous breeding pairs originally derived from a colony generated by GemPharmatech Co., Ltd (Nanjing, China). All animals were treated humanely and maintained in a temperature-controlled room (22 ± 2°C) with a 12-h light/dark cycle (lights on at 07:00 and off at 19:00), with food and water available ad libitum. All animal experiments were conducted in accordance with national legislation and approved by the Institutional Animal Care and Use Committee (IACUC number: IACUC-2018-075, IACUC-2018-098, National Beijing Center for Animal Drug Safety Evaluation and Research, Beijing, China).
Soman was diluted in cold saline and administered via a single subcutaneous injection (160 μg/kg, 1.4 × LD50). To block peripheral effects and increase the survival rate of rats, the oxime HI-6 (125 mg/kg, i.p.) was administered 30 min prior to soman exposure. HI-6 is a bispyridinium oxime that reactivates inhibited AChE primarily in the periphery to control the peripheral effects of soman and prevent death from respiratory suppression [3, 28]. Also, HI-6 does not prevent the brain injury caused by soman poisoning because it is unable to cross the blood-brain barrier [29]. The rats were randomly divided into three groups: (1) Control: (HI-6 125 mg/kg, i.p., 1% DMSO, i.v., n = 10); (2) Soman: HI-6 (125 mg/kg, i.p.) and solvent (1% DMSO, i.v.) administered 30 min prior to soman (160 μg/kg, s.c.), n = 42; (3) GSK2193874 + soman: TRPV4 antagonist GSK2193874 (1 mg/kg, i.v.) and HI-6 (125 mg/kg, i.p.), administered 30 min prior to soman (160 μg/kg, s.c.), n = 39. To evaluate TRPV4-KO intervention, the mice were divided into three groups (n = 10 per group): (1) WT (wild-type): WT mice treated with HI-6 (125 mg/kg, i.p.); (2) WT + soman: WT mice treated with HI-6 (125 mg/kg, i.p.) 30 min prior to soman (125 μg/kg, s.c.); (3) TRPV4-KO + soman: TRPV4-KO mice treated with HI-6 (125 mg/kg, i.p.) 30 min prior to soman (125 μg/kg, s.c.). The mortality rate was calculated as the number of deceased rats or mice (24 h after soman exposure) divided by the total number of rats or mice.
NMDA was diluted in cold 0.9% NaCl and administered via a single intraperitoneal injection (100 mg/kg). Memantine, an NMDA antagonist, was used as a positive control. The rats were divided randomly into three groups (n = 20 per group): (1) NMDA: rats treated with solvent (1% DMSO) 30 min before NMDA (100 mg/kg, i.p.); (2) Memantine + NMDA: rats treated with memantine (20 mg/kg, i.p.) and solvent (1% DMSO) 30 min before NMDA (100 mg/kg, i.p.); (3) GSK2193874 + NMDA: rats treated with the TRPV4 antagonist GSK2193874 (1 mg/kg, i.v.) 30 min before NMDA (100 mg/kg, i.p.). The mortality rate was calculated as the number of deceased rats (24 h after NMDA exposure) divided by the total number of rats.
Behavioral Assessment
Seizures were classified according to the Racine scale with the following minor modifications: stage 0, no abnormality; stage 1, facial movements, chewing; stage 2, rhythmic head nodding; stage 3, forelimb clonus plus Straub tail, without rearing; stage 4, forelimb clonus, bilateral forelimb clonus plus rearing; stage 5, rearing and falling [30–33].
Histopathological Examination
Rats and mice were anesthetized with chloral hydrate, and then 0.9% NaCl (4°C) was perfused, followed by a fixative solution made up of 4% formaldehyde in 0.1% mol/L phosphate-buffered saline (PBS, pH 7.4). After cervical dislocation, the brain was removed and fixed for 24 h at 4°C (4% formaldehyde in PBS), processed, and embedded in paraffin. The coronal sections of the CA1 hippocampal area were selected and processed for hematoxylin-eosin (H&E) staining. Three sections from each brain were visualized on an inverted microscope (IX71, Olympus, Japan).
Immunofluorescence Analysis
For immunofluorescence staining, paraffin-embedded sections were microwaved in 0.01 mol/L citrate buffer and washed three times with 0.01 mol/L PBS for 15 min each, and subsequently blocked by 5% bovine serum albumin (BSA) in 0.01 mol/L PBS for 30 min at room temperature (22 ± 2°C). Then the sections were incubated with anti-TRPV4 antibody (1:200) at 4°C overnight. After washing three times with PBS for 15 min each, Texas Red-conjugated secondary antibodies (1:1000, ZSGB-BIO) were added and incubated for 60 min in the dark. After washing three times with PBS, sections were treated with Hoechst 33342 (1:2000, H3570, Invitrogen) for 5 min. The positive rate was calculated by dividing the number of positive cells (TRPV4+/Hoechst+) by the total number of cells (Hoechst+).
Primary hippocampal neurons were washed three times in PBS following the appropriate treatment, fixed with 4% paraformaldehyde for 15 min and then washed three times with PBS. The cells were permeated with 0.5% Triton X-100 (solvent in goat serum working reagent) for 10 min on ice and blocked with 1% goat serum working reagent for 1 h. Cells were incubated at room temperature for 5 min with propidium iodide (PI) (1 μg/mL, ab14083, Abcam). After washing three times with PBS, cells were treated with Hoechst 33342 (1:2000, H3570, Invitrogen) for 5 min. The death rate was calculated by dividing the number of dead cells (PI+/Hoechst+) by the total number of cells (Hoechst+).
The positive cells were measured using ImageXpress Micro Confocal (Molecular Devices) and calculated using MetaXpress software (Molecular Devices).
Western Blotting Analysis
Hippocampal tissue was lysed using a membrane protein and cytoplasmic protein extraction kit together with protease and phosphatase inhibitors (KeyGEN Biotech Co., Ltd), and proteins were isolated according to the manufacturer’s instructions. Membrane protein and cytoplasmic protein were quantified using a BCA protein reagent kit (KeyGEN Biotech Co., Ltd). The proteins were separated by 8% or 10% SDS-PAGE and transferred to 0.2 μm PVDF membranes (Bio-Rad). Membranes were blocked with 5% BSA in Tris-buffered saline (TBS) with 0.1% Tween-20 for 2 h at 22 ± 2°C and then incubated at 4°C overnight with the following antibodies: anti-TRPV4 (1:400, PA5-41066, Thermo Fisher), anti-NMDAR2B (1:500, MA1-2014, Thermo Fisher), anti-p-NMDAR2B (1472) (1:500, ab3856, Abcam), anti-NLRP3 (1:400, A14223, Abclonal), anti-ASC (1:500, YT0365, ImmunoWay), anti-caspase-1 (1:400, ab179515, Abcam), anti-IL-1β (1:1000, A16288, Abclonal), and anti-IL-18 (1:500, A16737, Abclonal). After washing with TBS containing 0.1% Tween-20, blots were incubated in horseradish peroxidase-conjugated secondary antibody (1:2000, ZSGB-BIO) for 1 h at 22 ± 2°C. Bands of proteins bound to antibodies were detected using a chemiluminescence detection system (Pro-light HRP Chemiluminescent Kit, TianGen Biotech). Densitometric analysis was performed using Quantity One software (Bio-Rad). These analyses were normalized to β-actin (1:1000, 4970S, CST).
Cell Culture and Treatment
All experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC number: IACUC-2018-107, National Beijing Center for Drug Safety Evaluation and Research, Beijing, China). Primary cultures of rat hippocampal neurons were aseptically obtained from the hippocampus of E17–E18 Wistar rat embryos. The hippocampi were treated with 0.25% trypsin in Ca2+- and Mg2+-free Hank’s balanced salt solution for 15 min in a CO2 incubator, and then washed with 10% fetal bovine serum in Dulbecco’s modified Eagle’s medium to stop trypsin activity. The samples were then transferred to Neurobasal medium (Thermo Fisher) supplemented with B27 (1:50 dilution, Thermo Fisher) and GlutaMAXTM-1 (1:100, Thermo Fisher). The neurons were plated at 4.0–6.0 × 105 cells/well in 96 or 6-well plates, which were pretreated with poly-D-lysine (0.1 mg/mL). The cultures were maintained in a humidified incubator under 5% CO2 at 37°C. Hippocampal neurons were grown for 7 days in culture plates before being used for experiments. First, they were treated with different concentrations of NMDA (50–500 μmol/L) to determine an appropriate dose (Fig. S1). To explore the protective effect of the TRPV4 antagonist GSK2193874 on primary hippocampal neurons against NMDA-induced cytotoxicity, three comparison groups were studied: the control group, in which neurons were treated with solvent (0.01% DMSO); the NMDA group, in which neurons were treated with NMDA (50–500 μmol/L) for 24 h; and the GSK group, in which neurons were treated with GSK2193874 (50–500 nmol/L) for 24 h before NMDA exposure.
Electrophysiological Recording
Whole-cell patch-clamp recording was performed at 22–24°C. Hippocampal neurons were visualized on an inverted microscope (IX71, Olympus, Japan). Inward current (INMDA) was recorded using an EPC-10 amplifier (HEKA Elektronik, Lambrecht/Pfalz, Germany). Neurons were continuously perfused with an external solution of the following composition (in mmol/L): NaCl 140, KCl 4.0, CaCl2 2.0, HEPES 10, and D-glucose 5. The pH was adjusted to 7.4 with NaOH. Glass pipettes (resistance 4–5 MΩ) were pulled from borosilicate glass capillaries on a micropipette puller (P97, Sutter Instruments, USA) and filled with an internal solution of the following composition (in mmol/L): NaCl 10, CsMes 110, MgCl2 2, HEPES 10, EGTA 10, Na2-ATP 2, and Na2-GTP 0.2. The pH was adjusted to 7.2 with CsOH.
When recording INMDA, hippocampal neurons were held at −70 mV. First, NMDA (100 µmol/L) and glycine (10 µmol/L) were applied by local pressure perfusion, followed by NMDA, glycine, and GSK2193874 (0.3 and 3 µmol/L). Strychnine (1 µmol/L), bicuculline (10 µmol/L), NBQX (10 µmol/L), and QX314 (10 mmol/L) were added to the solution to block glycine receptors, GABAA receptors, AMPA receptors, and voltage-gated Na+ channels, respectively. Data were collected by PatchMaster software (HEKA Elektronik) and analyzed with IGOR-Pro (WaveMetrics Inc.).
Cell Viability Assay
Cells were seeded in 96-well plates at 4.0 × 105 cells/mL. NMDA at 100 μmol/L was chosen as the appropriate dose for experiments, based on its toxicity to cells and to ensure a certain cell survival rate. After treatment, 20 μL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (KeyGEN Biotech Co., Ltd) was added to each well, and the cells were incubated for 4 h at 22–24°C. After incubation, MTT was removed and 200 μL DMSO was added to solubilize the formazan crystals that formed in the intact cells. Absorbance was measured at 470 nm with SpectraMax (Molecular Devices). Each group included 8 wells. The results are expressed as the percentage of MTT reduction in the treated cells, assuming taking the absorbance of the control cells as 100%. Cell viability was calculated as OD470 (treatment)/OD470 (control).
Lactate Dehydrogenase Release Assay
Cell death or cytotoxicity is classically evaluated by the quantification of plasma membrane damage. Lactate dehydrogenase (LDH) is a marker of membrane integrity and the amount released into the culture medium was measured using a diagnostic kit (BioVision), according to the manufacturer’s instructions. NMDA at 100 μmol/L was used. Each group included 10 wells.
RNA Silencing
Rat primary hippocampal neurons were transfected with a negative control (NC) siRNA (4390843, Thermo Fisher) or TRPV4-siRNA (s134883, Thermo Fisher) using the Lipofectamine RNAiMAX kit (1295300, Thermo Fisher), following the manufacturer’s instructions. The siRNA duplex was diluted in Opti-MEM I medium without serum and gently mixed with Lipofectamine RNAiMAX. Then, they were mixed in a 1:1 ratio to form the siRNA duplex/Lipofectamine RNAiMAX complexes. The complexes were incubated for 15 min at 22–24°C. The complexes were then added to the wells at 4 × 105 cells/well to a final siRNA concentration of 10 nmol/L. The contents were mixed gently and cells were incubated for 24 h at 37°C in a CO2 incubator.
Calcium Imaging
[Ca2+]i was measured with Fluo-4/AM Direct Calcium Assay kits (Invitrogen). A total of 50 μL of the 2 × Fluo-4 Direct Ca2+ reagent loading solution per well was added to a 96-well plate, 1:1 with Neurobasal medium. The plates were incubated at 37°C for 60 min and then treated with Hoechst 33342. Each group included 6 wells. Fluorescein isothiocyanate and DAPI fluorescence were measured using the ImageXpress Micro Confocal system (Molecular Devices). The mean of the average cell intensity was calculated using MetaXpress software (Molecular Devices).
Statistical Analysis
Data are expressed as the mean ± SD. Fisher’s exact test was used to compare the survival rates between groups. Comparisons among more than two groups were performed using one-way ANOVA followed by Dunnett’s multiple comparisons test. The differences between two treatments for the same sample were compared using the paired t-test. The criterion for statistical significance was P <0.05. Results were analyzed using SPSS 20.0 software (SPSS Inc., Chicago, IL, USA).
Results
Effect of GSK2193874 on Soman-Induced Seizures and Histopathological Lesions in Rats
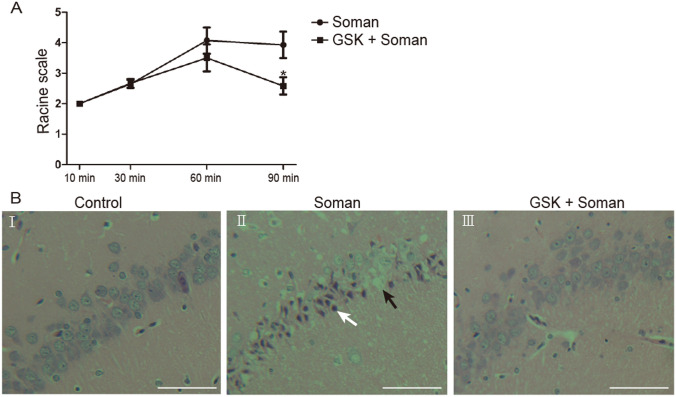
Sprague-Dawley rats were challenged with soman (160 μg/kg, s.c., 1.4 × LD50). To increase the survival rate, all animals were administered HI-6 (125 mg/kg, i.p.) 30 min before soman exposure to block peripheral effects [3] such as respiratory depression. A behavioral seizure score of stage 4 was reached within 30–60 min in the soman group, and SE stopped spontaneously within 6–8 h, while behavioral seizures were significantly suppressed by the TRPV4 antagonist GSK2193874 within 60–90 min (Fig. 1A, P <0.05). The mortality rate in the soman-treated rats was 61.9% (42 in total, 26 deaths), while the administration of GSK2193874 markedly decreased this to 25.6% (39 in total, 10 deaths).
Fig. 1.
GSK2193874 inhibits behavioral seizures and the development of histopathologic lesions induced by soman. Soman group (n = 14): rats were treated with HI-6 (125 mg/kg i.p.) before soman injection (160 μg/kg, s.c.); GSK2193874 (GSK) group (n = 12): rats were additionally treated with the TRPV4 antagonist GSK2193874 (1 mg/kg, i.v.) immediately after HI-6 injection and before soman injection. A Maximum Racine scale scores (means ± SD; *P <0.05 vs soman group, one-way ANOVA). B Development of soman-induced histopathological lesions in the CA1 hippocampal area is attenuated by GSK2193874 (× 200, original magnification, scale bars, 50 μm). B-I Control group retains the typical appearance of normal neurons with a clear edge and uniform size. B-II Soman exposure decreases the volume of neurons and leads to nuclear pyknosis (white arrow) and loss of neurons (black arrow). B-III GSK2193874 reduces the histopathologic lesions caused by soman exposure.
Hippocampal tissue was collected from rats for H&E staining 24 h after soman exposure. Histological examination indicated that the CA1 hippocampal neurons of control rats showed structural integrity with distinct and well-characterized round nucleoli (Fig. 1B-I). Compared with the control group, soman exposure caused severe damage in the rat hippocampi, manifested as loose structure, neuronal loss, and nuclear pyknosis (Fig. 1B-II). GSK2193874 significantly reduced the histopathologic lesions caused by soman exposure (Fig. 1B-III).
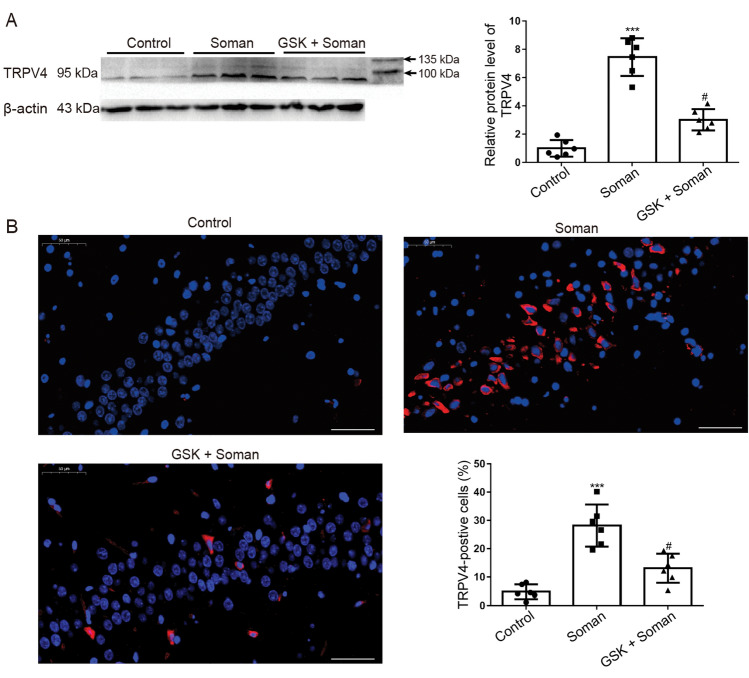
Effect of GSK2193874 on TRPV4 Expression in Rat Hippocampus after Soman Exposure
To investigate the effect of soman exposure (160 μg/kg, s.c.) on the expression levels of TRPV4 in the hippocampus, Western blot (WB) (Fig. 2A) and immunofluorescence analyses (Fig. 2B) were performed. WB analysis showed that the expression of TRPV4 was up-regulated after soman exposure compared to that in the control group (Fig. 2A, P <0.001); however, GSK2193874 treatment abrogated the TRPV4 expression (Fig. 2A, P <0.05). Immunofluorescence analysis showed that the percentage of TRPV4-positive cells markedly increased from 4.83 ± 1.03% to 28.18 ± 3.03% after soman exposure (Fig. 2B, P <0.001); however, GSK2193874 treatment significantly decreased the ratio from 28.18 ± 3.03% to 13.1 ± 2.09% (Fig. 2B, P <0.05). These results indicate that TRPV4 expression in the rat hippocampus is significantly increased after soman exposure and this is reversed by GSK2193874.
Fig. 2.
GSK2193874 inhibits TRPV4 expression in the hippocampus of rats exposed to soman. Protein expression of TRPV4 was assessed by (A) Western blotting and (B) immunofluorescence (red, TRPV4; blue, Hoechst; ×200, original magnification; scale bars, 50 μm). TRPV4+/Hoechst+ cells are TRPV4-positive cells, and Hoechst+ cells are total cells. Soman group: rats were treated with HI-6 (125 mg/kg i.p.) before soman injection (160 μg/kg, s.c.); GSK2193874 (GSK) group: rats were additionally treated with the TRPV4 antagonist GSK2193874 (1 mg/kg, i.v.) immediately after HI-6 injection and before soman injection; Control group: rats received HI-6 and solvent instead of antagonist and soman (mean ± SD, n = 6; ***P <0.001 vs control group, #P <0.05 vs soman group, one-way ANOVA and Dunnett’s multiple comparison test).
Effect of GSK2193874 on NMDA Receptor Function
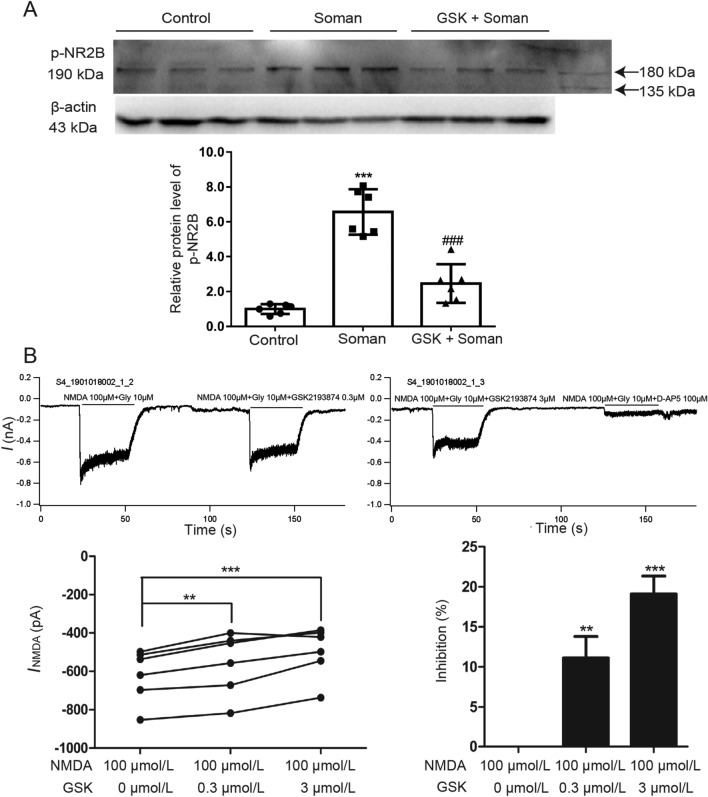
To explore the molecular mechanisms by which the TRPV4 channel might be involved in soman-induced excitotoxicity, we tested the effect of a TRPV4 antagonist on the phosphorylation of the NMDAR 2B subunit (NR2B) protein, a key factor in excitotoxicity [34]. WB analysis showed that NR2B phosphorylation was significantly increased in rat hippocampus after soman exposure compared with the control group, and the phosphorylation was remarkably reversed by GSK2193874 (Fig. 3A, P < 0.001).
Fig. 3.
GSK2193874 inhibits NMDA receptor function. A GSK2193874 inhibits NR2B phosphorylation in the hippocampus of rats exposed to soman. Soman group: rats were treated with HI-6 (125 mg/kg i.p.) before soman injection (160 μg/kg, s.c.); GSK2193874 (GSK) group: rats were additionally treated with the TRPV4 antagonist GSK2193874 (1 mg/kg, i.v.) immediately after HI-6 injection and before soman injection; Control group: rats received HI-6 and solvent instead of the antagonist and soman (mean ± SD, n = 6; ***P <0.001 vs control group, ###P <0.001 vs soman group, one-way ANOVA and Dunnett’s multiple comparison test). B GSK2193874 inhibits INMDA in primary cultured rat hippocampal neurons. In the presence of 0.3 μmol/L and 3 μmol/L GSK2193874, INMDA decreases by 11.13 ± 2.67% and 19.11 ± 2.24%, respectively (n = 6; **P <0.01, ***P <0.001 vs NMDA 100 μmol/L group, paired t-test).
Patch clamp was used to further explore whether TRPV4 inhibition modulates NMDAR function. After application of NMDA (100 µmol/L) and glycine (10 µmol/L) to primary cultured rat hippocampal neurons, an inward current (INMDA) was recorded. According to the results, the current was blocked by the specific NMDAR antagonist D-AP5 (100 µmol/L), confirming its mediation by the NMDAR (Fig. 3B). The NMDA-activated current was markedly decreased by 11.13 ± 2.67% from −619.7 ± 68.15 pA to −557 ± 80.76 pA after application of 0.3 µmol/L GSK2193874 (Fig. 3B, n = 6, paired t-test, P <0.01), and decreased by 19.11 ± 2.24% from −619.7 ± 68.15 pA to −457.7 ± 89.36 pA after application of 3 µmol/L GSK2193874 (Fig. 3B, n = 6, paired t-test, P <0.001). These results imply that blocking TRPV4 inhibits NMDAR function.
Action of GSK2193874 Against NMDA-Induced Excitotoxicity In Vitro and In Vivo
To determine whether TRPV4 is involved in NMDA-mediated excitotoxicity, we further explored the effect of GSK2193874 (1 mg/kg, i.v.) against NMDA-induced (100 mg/kg, i.p.) seizures and death in rats. Memantine, an NMDAR antagonist, was used as a positive control. As shown in Table 1, memantine (20 mg/kg, i.p.) delayed the onset of seizures and completely prevented rat death (Table 1, P <0.05, <0.001), but had no effect on tonic-clonic seizure control. Meanwhile, pretreatment with GSK2193874 delayed the onset of seizures and reduced the tonic-clonic seizures and mortality induced by NMDA (Table 1, P <0.05, <0.001).
Table 1.
Effect of GSK2193874 against NMDA-induced seizures and death in rats
| Tonic-clonic seizures | Latency for seizure onset (s) |
Mortality | |
|---|---|---|---|
| NMDA | 95% (19/20) | 363.5 ± 173.8 | 45% (9/20) |
| Memantine + NMDA | 90% (18/20) | 627.4 ± 224.1 * | 0% (0/20) *** |
| GSK2193874 + NMDA | 65% (13/20) *# | 1096.9 ± 558.9 *# | 10% (2/20) *** |
Data are shown as the mean ± SD, *P < 0.05, ***P <0.001 vs NMDA group; #P <0.05 vs Memantine + NMDA group.
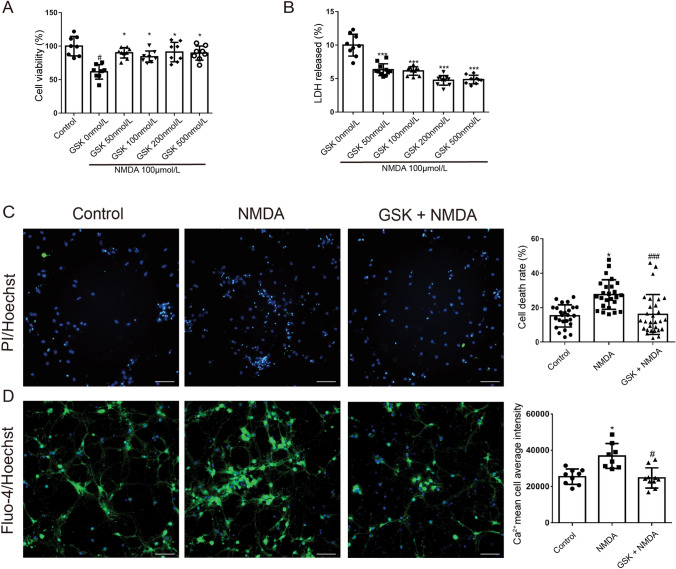
To confirm the neuroprotective properties of GSK2193874 on NMDA-induced excitotoxicity in vitro, its effects on cytotoxicity induced by NMDA in primary cultured rat hippocampal neurons were evaluated by the MTT and LDH release assays. Cells were pretreated with GSK2193874 for 24 h, then exposed to NMDA (100 μmol/L) for a further 24 h (Fig. 4A). GSK2193874 (50, 100, 200, and 500 nmol/L) increased the cell viability from 61.41% to 90.05%, 84.18%, 91.04%, and 89.81%, respectively, compared with the GSK 0 nmol/L group (Fig. 4A, P <0.05) while reducing the LDH release from 10.58% to 6.33%, 6.13%, 4.74%, and 4.86%, respectively (Fig. 4B, P <0.001).
Fig. 4.
NMDA-induced cytotoxicity is prevented by GSK2193874. Effect of GSK2193874 against NMDA-induced cytotoxicity assessed by MTT (A, n = 8) and LDH release (B, n = 10) assays. Neurons were pretreated with GSK2193874 (50, 100, 200, and 500 nmol/L) for 24 h, and then exposed to NMDA (100 μmol/L) for 24 h [means ± SD; #P <0.05 vs control group, *P <0.05, ***P <0.001 vs GSK2193874 (0 nmol/L) group]. C PI+/Hoechst+ cells are dead cells, and the number of Hoechst+ cells indicates the total number of cells (green, PI; blue, Hoechst; original magnification ×200; scale bars, 50 μm; mean ± SD, n = 25; *P <0.05 vs control group, ###P <0.001 vs NMDA group. D Intracellular Ca2+ concentrations measured with Fluo-4 Direct Calcium Assay kits (green, Fluo-4; blue, Hoechst; original magnification ×200; scale bars, 50 μm; mean ± SD, n = 8; *P <0.05 vs control group, #P <0.05 vs NMDA group, one-way ANOVA and Dunnett’s multiple comparison test).
Cell death was also assessed using Hoechst/PI dual staining. This showed that the rate of cell death increased from 19.3% to 27. 9% after NMDA exposure (100 μmol/L) for 24 h (Fig. 4C, P <0.05), whereas pretreatment with GSK2193874 (200 nmol/L) for 24 h reduced the death rate to 16% (Fig. 4C, P <0.001), compared with the NMDA group.
To assess the effect of GSK2193874 on the [Ca2+]i after NMDA exposure, cytoplasmic Ca2+ levels were measured by Ca2+-sensitive Fluo-4/AM staining (Fig. 4D). NMDA treatment (100 μmol/L) increased the [Ca2+]i compared with that of the control group (Fig. 4D, P <0.05). Pretreatment with GSK2193874 (200 nmol/L) for 24 h attenuated the NMDA-induced Ca2+ accumulation compared with that of the NMDA-treated group (Fig. 4D, P <0.05). The above results demonstrated that TRPV4 inhibition attenuates the excitotoxicity induced by NMDA in vitro and in vivo.
Effect of TRPV4 siRNA on Rat Primary Neuron Viability and Intracellular Ca2+ Accumulation in NMDA-Induced Excitotoxicity
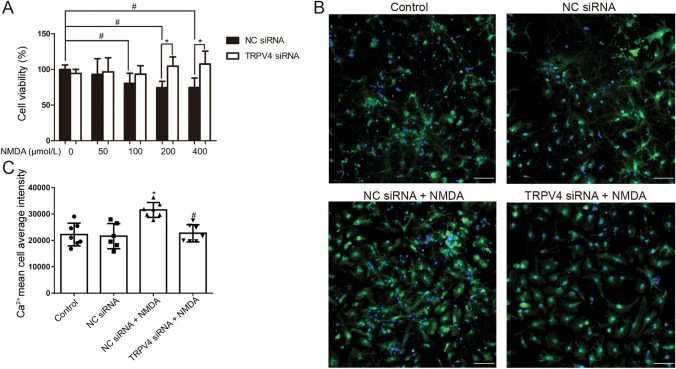
TRPV4 was knocked down using a specific siRNA (10 nmol/L) and transfected cells were cultured for 24 h with or without NMDA. Cell viability was reduced after NMDA treatment (100, 200, and 400 μmol/L) compared with that of the NC siRNA group, while TRPV4 siRNA reversed the decrease in cell viability after NMDA exposure (200 and 400 μmol/L) (Fig. 5A, P <0.05), similar to the effects of GSK2193874.
Fig. 5.
Knock-down of TRPV4 with siRNA-attenuated NMDA-induced cytotoxicity and calcium accumulation in primary cultured hippocampal neurons. Neurons were pretreated with TRPV4 siRNA (10 nmol/L) for 24 h, and then exposed to NMDA (100 μmol/L) for 24 h. A Cell viability evaluated by MTT assays (mean ± SD, n = 6; *P <0.05 vs NC siRNA group, #P <0.05 vs NMDA 0 μmol/L group. B, C Intracellular Ca2+ concentrations measured using Fluo-4 Direct Calcium Assay kits (green, Fluo-4; blue, Hoechst; original magnification ×200; scale bars, 50 μm; mean ± SD, n = 7; *P <0.05 vs negative siRNA group, #P <0.05 vs NMDA group, ANOVA and Dunnett’s multiple comparison test).
Moreover, NMDA treatment (100 μmol/L) increased the [Ca2+]i compared with that in the NC siRNA group, whereas TRPV4 siRNA attenuated the NMDA-induced Ca2+ accumulation (Fig. 5B, C; P <0.05).
Effect of GSK2193874 on Microglia and Astrocyte Activation in Rat Hippocampus after Soman Exposure
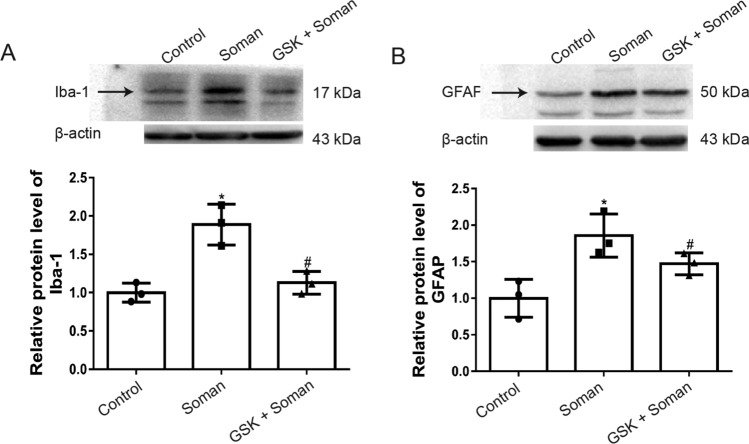
Glial cells are vital for the initiation of neuroinflammatory responses. In the present study, we explored the effects of a TRPV4 antagonist on soman-induced microglial cell and astrocyte activation. Iba-1 was used as a marker for microglial cells and GFAP (glial fibrillary acidic protein) for astrocytes. The expression level of Iba-1 protein was increased in the rat hippocampus after soman exposure, whereas GSK2193874 abrogated the effects of soman exposure on Iba-1 (Fig. 6A, P <0.05). The expression of GFAP was markedly increased after soman exposure (Fig. 6B, P <0.05) and attenuated by GSK2193874. These results indicated that soman exposure leads to the activation of microglia cells and astrocytes, while GSK2193874 inhibits it.
Fig. 6.
Activation of astrocytes and microglial cells by soman is attenuated by GSK2193874. Soman group: rats were treated with HI-6 (125 mg/kg i.p.) before soman injection (160 μg/kg, s.c.); GSK2193874 (GSK) group: rats were additionally treated with the TRPV4 antagonist GSK2193874 (1 mg/kg, i.v.) immediately after HI-6 injection and before soman injection; Control group: rats received HI-6 and solvent instead of the antagonist and soman. Iba-1 and GFAP reflect the activation state of microglial cells and astrocytes, respectively. A, B Protein levels of Iba-1 (A) and GFAP (B) (mean ± SD, n = 3; *P <0.05 vs control group, #P <0.05 vs soman group, one-way ANOVA and Dunnett’s multiple comparison test).
Effect of GSK2193874 on NLRP3 Inflammasome Activation and Downstream Pro-inflammatory Cytokine Production in Rat Hippocampus after Soman Exposure
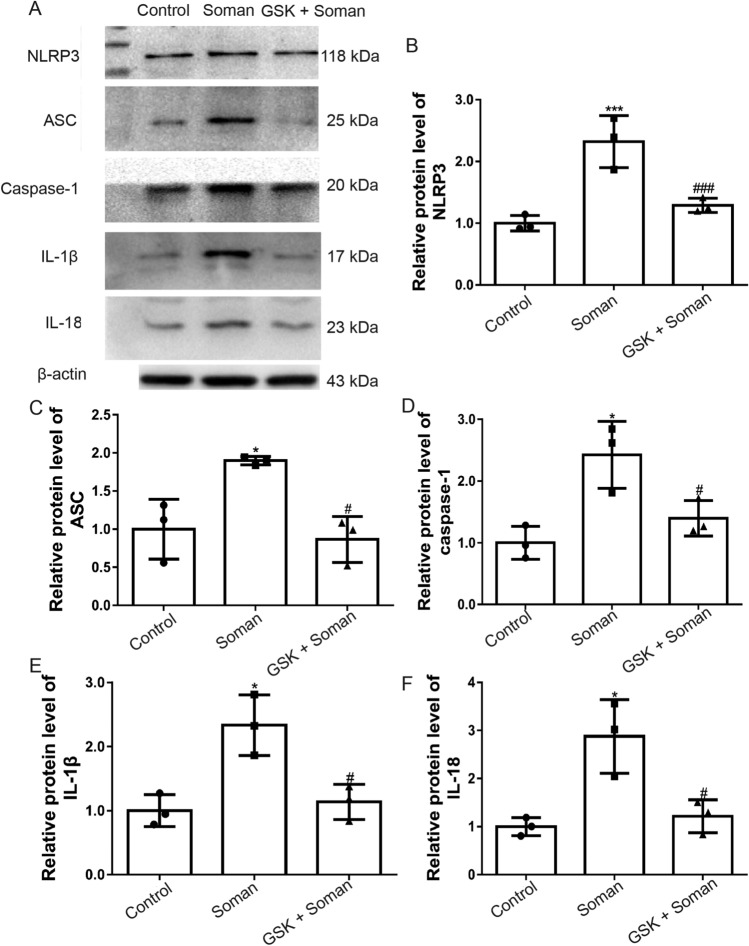
The protein expression levels of NLRP3, ASC, and caspase-1 (Fig. 7A–D) were increased in rat hippocampus after soman exposure compared with the control group (P < 0.05, P < 0.001). Inflammatory cytokines IL-1β (Fig. 7A, E) and IL-18 (Fig. 7A, F) were also markedly increased after soman exposure (P < 0.05). However, pretreatment with GSK2193874 reversed the effect of soman exposure on NLRP3 inflammasome activation and IL-1β and IL-18 cytokine expression (Fig. 7A–F, P < 0.05). These results indicated that soman promotes NLRP3 inflammasome activation and neuroinflammation, and this is reversed by TRPV4 inhibition.
Fig. 7.
GSK2193874 inhibits NLRP3 inflammasome expression and pro-inflammatory cytokine production in rat hippocampus after soman exposure. Soman group: rats were treated with HI-6 (125 mg/kg i.p.) before soman injection (160 μg/kg, s.c.); GSK2193874 (GSK) group: rats were additionally treated with the TRPV4 antagonist GSK2193874 (1 mg/kg, i.v.) immediately after HI-6 injection and before soman injection; Control group: rats received HI-6 and solvent instead of the antagonist and soman. (A–F) Western blots (A) and analysis of NLRP3 (B), ASC (C), caspase-1 (D), IL-1β (E), IL-18 (F), and β-actin protein levels (mean ± SD, n = 3; *P <0.05, ***P <0.001 vs control group, #P <0.05, ###P <0.001 vs soman group, one-way ANOVA and Dunnett’s multiple comparison test).
TRPV4-knockout Protects Against Soman-induced Neurotoxicity by Suppressing NMDAR and NLRP3
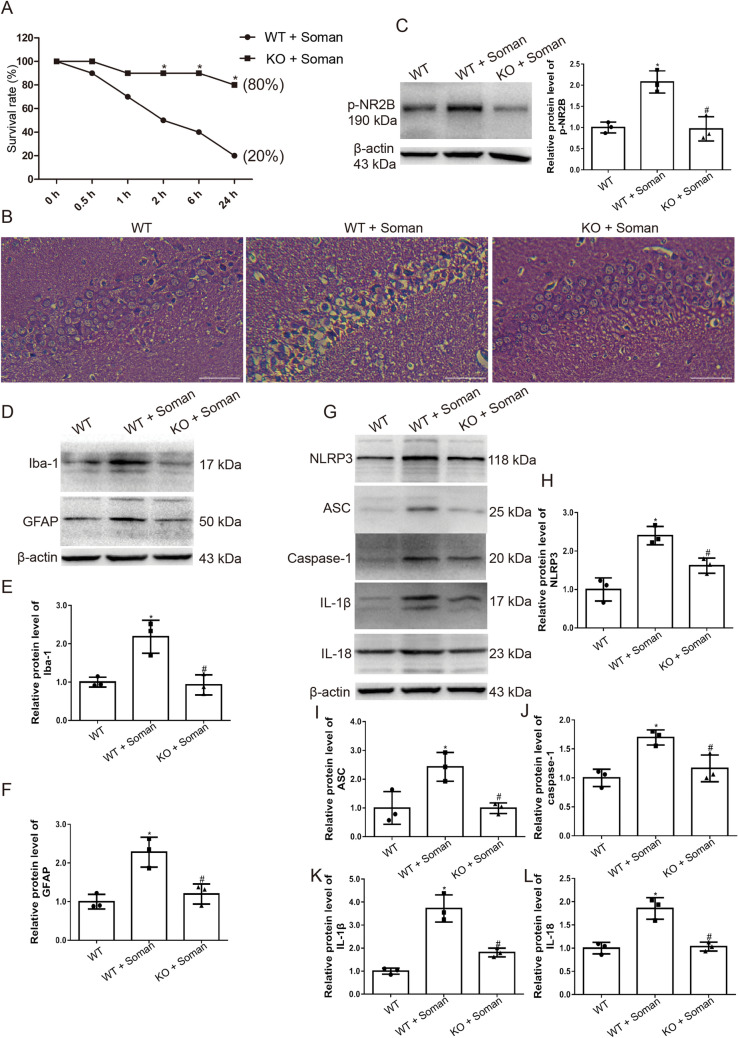
To confirm the role of the TRPV4 channel in soman-induced neurotoxicity, we used TRPV4 knockout mice. Wild-type (WT) and TRPV4-KO mice were administered HI-6 (125 mg/kg i.p.) 30 min prior to soman exposure to block peripheral effects, and then were challenged with soman (125 μg/kg, s.c.). The results showed that, compared with the WT mice, TRPV4-KO mice had fewer seizures, a prolonged seizure latency (Table 2, P <0.05), and a higher survival rate (Fig. 8A, P <0.05). The H&E staining assessment of hippocampal tissue indicated that the neurons of WT control mice were structurally intact with distinct and well-characterized round nucleoli. Soman exposure, however, caused severe pathological injury in the hippocampus of WT mice, with neuronal loss and nuclear pyknosis. Interestingly, assessment of the hippocampal neurons of the TRPV4-KO mice showed reduced histopathological lesions after soman exposure compared with WT mice (Fig. 8B)
Table 2.
Incidence and latency of seizures in WT and TRPV4-KO mice after soman exposure
| Incidence of seizures | Latency for seizure onset (s) | |
|---|---|---|
| WT | 100% (10/10) | 419.7 ± 111.5 |
| TRPV4-KO | 70% (7/10)* | 603.7 ± 212.3* |
Data are shown as the mean ± SD, *P <0.05, vs WT.
Fig. 8.
TRPV4-KO mice are protected against soman exposure. WT mice and TRPV4-KO mice were treated with HI-6 (125 mg/kg i.p.) before soman injection (125 μg/kg, s.c.). A Survival rates calculated as the number of living mice (24 h after soman exposure) divided by the total number of mice (n = 10; *P <0.05 vs WT + soman group. B Histological assessment showing severe injury, neuron loss, and nuclear pyknosis in the WT + soman group; TRPV4-KO reduces the histopathologic lesions in the hippocampus of mice caused by soman exposure (× 200, original magnification, scale bars, 50 μm). C TRPV4-KO inhibits NR2B phosphorylation in the hippocampus of mice exposed to soman, compared to the WT + soman group (mean ± SD, n = 3; *P <0.05 vs WT group, #P <0.05 vs WT + soman group. D, E Iba-1 reflects the activation state of microglial cells. Activation of astrocytes and microglial cells induced by soman is attenuated by TRPV4-KO (mean ± SD, n = 3; *P <0.05 vs WT group, #P <0.05 vs WT + soman group). D, F GFAP reflects the activation of astrocytes. Activation of astrocytes induced by soman is attenuated by TRPV4-KO (mean ± SD, n = 3; *P <0.05 vs WT group, #P <0.05 vs WT + soman group). G–L Western blots (G) and analysis of expression of the NLRP3 inflammasome complex proteins NLRP3 (H), ASC (I), caspase-1 (J), and downstream inflammatory cytokines IL-1β (K), IL-18 (L), and β-actin (mean ± SD, n = 3; *P <0.05 vs WT group, #P <0.05 vs WT + soman group, one-way ANOVA and Dunnett’s multiple comparison test).
To confirm the impact of TRPV4 on soman-induced hyperfunction of NMDARs, NR2B phosphorylation was measured in WT and TRPV4-KO mice by western blot. The results showed that NR2B phosphorylation was higher in the hippocampus of WT mice after soman exposure than in the WT control group, and the phosphorylation was remarkably reversed in the TRPV4-KO group (Fig. 8C, P <0.05).
Then we explored the effects of TRPV4 deletion on soman-induced microglial and astrocyte activation. The protein levels of both Iba-1 and GFAP were increased in WT mice hippocampus after soman exposure, while knocking out TRPV4 abrogated the effects of soman exposure on the activation of microglia and astrocytes (Fig. 8D–F, P <0.05).
The effects of the deletion of TRPV4 on the activation of the NLRP3 inflammasome and the production of downstream pro-inflammatory cytokines in mouse hippocampus after soman exposure were measured by western blot. The expression levels of NLRP3, ASC, and caspase-1 protein were increased in WT hippocampus after soman exposure compared the WT control group (P <0.05) (Fig. 8G–J). Meanwhile, IL-1β and IL-18 were also clearly increased after soman exposure (P <0.05) (Fig. 8G, K, L). However, the soman-induced NLRP3 inflammasome activation and IL-1β and IL-18 production were abrogated in the TRPV4-KO mice (Fig. 8G–L, P <0.05). These results indicated that soman promotes NLRP3 inflammasome activation and neuroinflammation, which can be reversed by deleting TRPV4.
Discussion
Several studies have focused on the role of TRPV4 in modulating nervous system functions due to its widespread expression and Ca2+ permeability. Reports have demonstrated that the neurotoxicity caused by TRPV4 over-activation is an important factor in intracerebral hemorrhage and cerebral ischemic injury [35, 36]. Seizure-related neural activity triggered by an increase in brain temperature is blocked by a TRPV4 antagonist in larval zebrafish [25]. Furthermore, TRPV4 expression is significantly increased in cortical tubers of the tuberous sclerosis complex, a known form of therapy-refractory epilepsy [37]. Taken together, these data imply that TRPV4 is involved in the pathogenesis of epilepsy. In this study, we demonstrated that TRPV4 blockade or deletion was protective against soman-induced SE and neuroinflammation. Its mechanisms of action included a decrease in NMDAR-mediated excitotoxicity and suppression of the NLRP3-mediated neuroinflammatory response.
Our data showed that exposure to soman led to an increase of TRPV4 protein levels in rat hippocampal cells, while treatment with the TRPV4 antagonist GSK2193874 protected the cells against soman poisoning, as reflected by improved survival rates and suppressed behavioral seizures. Unlike anticholinergics, GSK2193874 did not block the onset of soman-induced seizures in rats, but did relieve and terminate seizures, similar to the effect of NMDA antagonists [10]. The reason for this phenomenon is that the seizures after soman exposure are initiated by an increase in ACh levels following the irreversible inhibition of brain AChE by soman. Once initiated, non-cholinergic neurotransmitter systems, especially the NMDARs of the glutamatergic excitatory amino-acid system, are rapidly perturbed by the excitatory activity of the seizure itself, and as it progresses, non-cholinergic excitatory activity gradually gains control over the seizure independent of the initiating cholinergic drive [3]. Activation of NMDARs sustains seizure activity and increases the excessive levels of [Ca2+]i, which launches biochemical reactions that lead to neuronal death. Previous studies have suggested that the NR2B subunit of NMDARs is the major factor in excitotoxicity, with the phosphorylation of tyrosine 1472 (Tyr1472) site of NR2B considered as the vital pathway for excitotoxicity [34, 38]. In our study, soman exposure led to NMDAR activation via up-regulation of NR2B phosphorylation (Tyr1472) in the rat hippocampus, which was reversed by GSK2193874 or TRPV4-KO. Moreover, our in vitro experiments showed that NMDA-induced INMDA was markedly attenuated by a TRPV4 antagonist indicating that TRPV4 inhibition impedes activation of NMDARs and their mediated INMDA. Furthermore, NMDA-induced neurotoxicity was significantly increased in rat primary cultured hippocampal neurons, and pretreatment with GSK2193874 or TRPV4 siRNA reduced the cytotoxicity after NMDA exposure, as reflected in increased cell viability and reduced LDH release. Furthermore, NMDA-induced Ca2+ accumulation was also inhibited by GSK2193874 or TRPV4 siRNA. Therefore, our findings strongly suggest that TRPV4 activation induces NMDAR-mediated excitotoxicity, resulting in seizures and secondary brain injury after soman exposure. This is supported by a previous study using a cerebral ischemia model, which indicated that activation of TRPV4 facilitates and prolongs the glutamate excitotoxicity by potentiating the NMDAR response [24]. In addition, there are limitations in the clinical applications of NMDA antagonists due to their side-effects [39–42]; blocking the TRP channel appears to inhibit NMDAR function by diminishing membrane depolarization and not by directly blocking NMDARs [43, 44], suggesting that TRPV4 is a promising novel target for treatment. However, more evidence is required, and the specific mechanisms of action need to be elucidated in future work.
TRPV4 is strongly expressed in rat hippocampal astrocytes and microglial cells that have been reported to be involved in neuronal injury induced by neuroinflammation. More specifically, TRPV4 is responsible for amyloid β-induced neuronal and astrocytic damage [20] and, in its closed state, inhibits the influx of Ca2+i and decreases the levels of IL-1β and TNF-α in lipopolysaccharide-activated microglial cells [45]. A recent study showed that the TRPV4 agonist GSK1016790A initiates the NLRP3-related inflammatory process by activating astrocytes and microglia [27]. The NLRP3 inflammasome, an integral part of the innate immune system, is a cytoplasmic complex in which NLRP3 interacts with the adaptor protein ASC to enable the recruitment and activation of caspase-1, leading to the maturation of IL-1β and IL-18. In our study, astrocytes and microglial cells were activated in the hippocampus of rats and mice after soman exposure and were attenuated by GSK2193874. Furthermore, blocking or deletion of TRPV4 inhibited the up-regulation of NLRP3, ASC, caspase-1, and the downstream IL-1β and IL-18 protein levels induced by soman. Our results suggest that TRPV4 is involved in the activation of astrocytes and microglia induced by soman and we provide the first evidence that NLRP3 is involved in the inflammatory response after soman exposure, which may be regulated by the TRPV4 channel.
In conclusion, we demonstrated that the “dual-switch” function of TRPV4 mediating Ca2+ influx causes changes in downstream pathways, leading to seizures and secondary brain injury when exposed to soman. Once we turned off the switch by blocking or deleting the TRPV4 channel, a marked protective effect against soman-induced SE and neuroinflammation occurred, via decreased NMDAR-mediated excitotoxicity and reduction in the NLRP3-mediated neuroinflammatory response. The present study provides useful data for future research, particularly for the treatment of casualties caused by exposure to nerve agents or organophosphorus pesticides.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the Special Fund for Military Medical Science (AWS15J007 and BWS16J007) and the National Natural Science Foundation of China (81703505).
Conflict of interest
The authors claim that there are no conflicts of interest.
Contributor Information
Yongan Wang, Email: yonganw@126.com.
Yuan Luo, Email: luoyuan2006@163.com.
References
- 1.Dolgin E. Syrian gas attack reinforces need for better anti-sarin drugs. Nat Med. 2013;19:1194–1195. doi: 10.1038/nm1013-1194. [DOI] [PubMed] [Google Scholar]
- 2.Chai PR, Hayes BD, Erickson TB, Boyer EW. Novichok agents: a historical, current, and toxicological perspective. Toxicol Commun. 2018;2:45–48. doi: 10.1080/24734306.2018.1475151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonough JH, Jr, Shih TM. Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. Neurosci Biobehav Rev. 1997;21:559–579. doi: 10.1016/s0149-7634(96)00050-4. [DOI] [PubMed] [Google Scholar]
- 4.McDonough JH, Jr, Shih TM. A study of the N-methyl-D-aspartate antagonistic properties of anticholinergic drugs. Pharmacol Biochem Behav. 1995;51:249–253. doi: 10.1016/0091-3057(94)00372-p. [DOI] [PubMed] [Google Scholar]
- 5.Shih T, McDonough JH, Jr, Koplovitz I. Anticonvulsants for soman-induced seizure activity. J Biomed Sci. 1999;6:86–96. doi: 10.1007/BF02256439. [DOI] [PubMed] [Google Scholar]
- 6.Skovira JW, McDonough JH, Shih TM. Protection against sarin-induced seizures in rats by direct brain microinjection of scopolamine, midazolam or MK-801. J Mol Neurosci. 2010;40:56–62. doi: 10.1007/s12031-009-9253-0. [DOI] [PubMed] [Google Scholar]
- 7.Olney JW, Collins RC, Sloviter RS. Excitotoxic mechanisms of epileptic brain damage. Adv Neurol. 1986;44:857–877. [PubMed] [Google Scholar]
- 8.Sloviter RS, Dempster DW. "Epileptic" brain damage is replicated qualitatively in the rat hippocampus by central injection of glutamate or aspartate but not by GABA or acetylcholine. Brain Res Bull. 1985;15:39–60. doi: 10.1016/0361-9230(85)90059-0. [DOI] [PubMed] [Google Scholar]
- 9.Braitman DJ, Sparenborg S. MK-801 protects against seizures induced by the cholinesterase inhibitor soman. Brain Res Bull. 1989;23:145–148. doi: 10.1016/0361-9230(89)90173-1. [DOI] [PubMed] [Google Scholar]
- 10.Carpentier P, Foquin-Tarricone A, Bodjarian N, Rondouin G, Lerner-Natoli M, Kamenka JM, et al. Anticonvulsant and antilethal effects of the phencyclidine derivative TCP in soman poisoning. Neurotoxicology. 1994;15:837–851. [PubMed] [Google Scholar]
- 11.Shih TM, Koenig JA, Acon Chen C. Comparative effects of scopolamine and phencynonate on organophosphorus nerve agent-induced seizure activity, neuropathology and lethality. Toxicol Mech Methods. 2019;29:322–333. doi: 10.1080/15376516.2018.1558322. [DOI] [PubMed] [Google Scholar]
- 12.Sparenborg S, Brennecke LH, Jaax NK, Braitman DJ. Dizocilpine (MK-801) arrests status epilepticus and prevents brain damage induced by soman. Neuropharmacology. 1992;31:357–368. doi: 10.1016/0028-3908(92)90068-z. [DOI] [PubMed] [Google Scholar]
- 13.Zimmer LA, Ennis M, Shipley MT. Soman-induced seizures rapidly activate astrocytes and microglia in discrete brain regions. J Comp Neurol. 1997;378:482–492. doi: 10.1002/(sici)1096-9861(19970224)378:4<482::aid-cne4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Ferrara-Bowens TM, Chandler JK, Guignet MA, Irwin JF, Laitipaya K, Palmer DD, et al. Neuropathological and behavioral sequelae in IL-1R1 and IL-1Ra gene knockout mice after soman (GD) exposure. Neurotoxicology. 2017;63:43–56. doi: 10.1016/j.neuro.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Johnson EA, Guignet MA, Dao TL, Hamilton TA, Kan RK. Interleukin-18 expression increases in response to neurovascular damage following soman-induced status epilepticus in rats. J Inflamm (Lond) 2015;12:43. doi: 10.1186/s12950-015-0089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Svensson I, Waara L, Johansson L, Bucht A, Cassel G. Soman-induced interleukin-1 beta mRNA and protein in rat brain. Neurotoxicology. 2001;22:355–362. doi: 10.1016/s0161-813x(01)00022-5. [DOI] [PubMed] [Google Scholar]
- 17.Williams AJ, Berti R, Yao C, Price RA, Velarde LC, Koplovitz I, et al. Central neuro-inflammatory gene response following soman exposure in the rat. Neurosci Lett. 2003;349:147–150. doi: 10.1016/s0304-3940(03)00818-8. [DOI] [PubMed] [Google Scholar]
- 18.Shibasaki K. TRPV4 ion channel as important cell sensors. J Anesth. 2016;30:1014–1019. doi: 10.1007/s00540-016-2225-y. [DOI] [PubMed] [Google Scholar]
- 19.Moore C, Gupta R, Jordt SE, Chen Y, Liedtke WB. Regulation of pain and itch by TRP Channels. Neurosci Bull. 2018;34:120–142. doi: 10.1007/s12264-017-0200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bai JZ, Lipski J. Involvement of TRPV4 channels in Aβ40-induced hippocampal cell death and astrocytic Ca2+ signalling. Neurotoxicology. 2014;41:64–72. doi: 10.1016/j.neuro.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Jie P, Hong Z, Tian Y, Li Y, Lin L, Zhou L, et al. Activation of transient receptor potential vanilloid 4 induces apoptosis in hippocampus through downregulating PI3K/Akt and upregulating p38 MAPK signaling pathways. Cell Death Dis. 2015;6:e1775. doi: 10.1038/cddis.2015.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu KT, Huang TC, Tsai YH, Yang YL. Transient receptor potential vanilloid type 4 channels mediate Na-K-Cl-co-transporter-induced brain edema after traumatic brain injury. J Neurochem. 2017;140:718–727. doi: 10.1111/jnc.13920. [DOI] [PubMed] [Google Scholar]
- 23.Men C, Wang Z, Zhou L, Qi M, An D, Xu W, et al. Transient receptor potential vanilloid 4 is involved in the upregulation of connexin expression following pilocarpine-induced status epilepticus in mice. Brain Res Bull. 2019;152:128–133. doi: 10.1016/j.brainresbull.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Li L, Qu W, Zhou L, Lu Z, Jie P, Chen L, et al. Activation of transient receptor potential vanilloid 4 increases NMDA-activated current in hippocampal pyramidal neurons. Front Cell Neurosci. 2013;7:17. doi: 10.3389/fncel.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunt RF, Hortopan GA, Gillespie A, Baraban SC. A novel zebrafish model of hyperthermia-induced seizures reveals a role for TRPV4 channels and NMDA-type glutamate receptors. Exp Neurol. 2012;237:199–206. doi: 10.1016/j.expneurol.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibasaki K, Suzuki M, Mizuno A, Tominaga M. Effects of body temperature on neural activity in the hippocampus: regulation of resting membrane potentials by transient receptor potential vanilloid 4. J Neurosci. 2007;27:1566–1575. doi: 10.1523/JNEUROSCI.4284-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z, Zhou L, An D, Xu W, Wu C, Sha S, et al. TRPV4-induced inflammatory response is involved in neuronal death in pilocarpine model of temporal lobe epilepsy in mice. Cell Death Dis. 2019;10:386. doi: 10.1038/s41419-019-1612-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang YA, Zhou WX, Li JX, Liu YQ, Yue YJ, Zheng JQ, et al. Anticonvulsant effects of phencynonate hydrochloride and other anticholinergic drugs in soman poisoning: neurochemical mechanisms. Life Sci. 2005;78:210–223. doi: 10.1016/j.lfs.2005.04.071. [DOI] [PubMed] [Google Scholar]
- 29.Yang J, Fan L, Wang F, Luo Y, Sui X, Li W, et al. Rapid-releasing of HI-6 via brain-targeted mesoporous silica nanoparticles for nerve agent detoxification. Nanoscale. 2016;8:9537–9547. doi: 10.1039/c5nr06658a. [DOI] [PubMed] [Google Scholar]
- 30.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 31.Apland JP, Aroniadou-Anderjaska V, Figueiredo TH, Green CE, Swezey R, Yang C, et al. Efficacy of the GluK1/AMPA receptor antagonist LY293558 against seizures and neuropathology in a soman-exposure model without pretreatment and its pharmacokinetics after intramuscular administration. J Pharmacol Exp Ther. 2013;344:133–140. doi: 10.1124/jpet.112.198689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prager EM, Figueiredo TH, Long RP, 2nd, Aroniadou-Anderjaska V, Apland JP, Braga MF. LY293558 prevents soman-induced pathophysiological alterations in the basolateral amygdala and the development of anxiety. Neuropharmacology. 2015;89:11–18. doi: 10.1016/j.neuropharm.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bohnert S, van den Berg RM, Mikler J, Klaassen SD, Joosen MJA. Pharmacokinetics of three oximes in a guinea pig model and efficacy of combined oxime therapy. Toxicol Lett. 2020;324:86–94. doi: 10.1016/j.toxlet.2020.01.013. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Sun W, Han S, Li J, Ding S, Wang W, et al. IGF-1-involved negative feedback of NR2B NMDA subunits protects cultured hippocampal neurons against NMDA-induced excitotoxicity. Mol Neurobiol. 2017;54:684–696. doi: 10.1007/s12035-015-9647-7. [DOI] [PubMed] [Google Scholar]
- 35.Jie P, Lu Z, Hong Z, Li L, Zhou L, Li Y, et al. Activation of transient receptor potential vanilloid 4 is involved in neuronal injury in middle cerebral artery occlusion in mice. Mol Neurobiol. 2016;53:8–17. doi: 10.1007/s12035-014-8992-2. [DOI] [PubMed] [Google Scholar]
- 36.Zhao H, Zhang K, Tang R, Meng H, Zou Y, Wu P, et al. TRPV4 blockade preserves the blood-brain barrier by inhibiting stress fiber formation in a rat model of intracerebral hemorrhage. Front Mol Neurosci. 2018;11:97. doi: 10.3389/fnmol.2018.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X, Yang M, Sun F, Liang C, Wei Y, Wang L, et al. Expression and cellular distribution of transient receptor potential vanilloid 4 in cortical tubers of the tuberous sclerosis complex. Brain Res. 2016;1636:183–192. doi: 10.1016/j.brainres.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Xu XX, Luo JH. Mutations of N-methyl-D-aspartate receptor subunits in epilepsy. Neurosci Bull. 2018;34:549–565. doi: 10.1007/s12264-017-0191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olney JW, Labruyere J, Price MT. Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs. Science. 1989;244:1360–1362. doi: 10.1126/science.2660263. [DOI] [PubMed] [Google Scholar]
- 40.Olney JW, Labruyere J, Wang G, Wozniak DF, Price MT, Sesma MA. NMDA antagonist neurotoxicity: mechanism and prevention. Science. 1991;254:1515–1518. doi: 10.1126/science.1835799. [DOI] [PubMed] [Google Scholar]
- 41.Allen HL, Iversen LL. Phencyclidine, dizocilpine, and cerebrocortical neurons. Science. 1990;247:221. doi: 10.1126/science.2403696. [DOI] [PubMed] [Google Scholar]
- 42.Fix AS, Horn JW, Wightman KA, Johnson CA, Long GG, Storts RW, et al. Neuronal vacuolization and necrosis induced by the noncompetitive N-methyl-D-aspartate (NMDA) antagonist MK(+)801 (dizocilpine maleate): a light and electron microscopic evaluation of the rat retrosplenial cortex. Exp Neurol. 1993;123:204–215. doi: 10.1006/exnr.1993.1153. [DOI] [PubMed] [Google Scholar]
- 43.Chen J, Li Z, Hatcher JT, Chen QH, Chen L, Wurster RD, et al. Deletion of TRPC6 attenuates NMDA receptor-mediated Ca2+ entry and Ca2+-Induced neurotoxicity following cerebral ischemia and oxygen-glucose deprivation. Front Neurosci. 2017;11:138. doi: 10.3389/fnins.2017.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menigoz A, Ahmed T, Sabanov V, Philippaert K, Pinto S, Kerselaers S, et al. TRPM4-dependent post-synaptic depolarization is essential for the induction of NMDA receptor-dependent LTP in CA1 hippocampal neurons. Pflugers Arch. 2016;468:593–607. doi: 10.1007/s00424-015-1764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu M, Liu X, Wang L, Wang Y, Dong F, Wu J, et al. TRPV4 inhibition improved myelination and reduced glia reactivity and inflammation in a cuprizone-induced mouse model of demyelination. Front Cell Neurosci. 2018;12:392. doi: 10.3389/fncel.2018.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.