Abstract
The importance of astrocytes in behavior control is increasingly appreciated, but little is known about the effects of their dynamic activity in regulating learning and memory. In the present study, we constructed AAVs of photoactivatable and photoinactivatable Ras-related C3 botulinum toxin substrate 1 (Rac1) under the mGFAP promoter, which enabled the manipulation of Rac1 activity in astrocytes by optical stimulation in free-moving mice. We found that both up-regulation and down-regulation of astrocytic Rac1 activity in the basolateral amygdala (BLA) attenuated memory acquisition in a fear conditioning mouse model. Meanwhile, neuronal activation in the BLA induced by memory acquisition was inhibited under both the up- and down-regulation of astrocytic Rac1 activity during training. In terms of the impact on fear memory retrieval, we found both up- and down-regulation of BLA astrocytic Rac1 activity impaired memory retrieval of fear conditioning and memory retrieval-induced neuronal activation. Notably, the effect of astrocytic Rac1 on memory retrieval was reversible. Our results demonstrate that the normal activity of astrocytic Rac1 is necessary for the activation of neurons and memory formation. Both activation and inactivation of astrocytic Rac1 activity in the BLA reduced the excitability of neurons, and thereby impaired fear memory acquisition and retrieval.
Keywords: Rac1, Astrocyte, Neuron, BLA, Fear memory
Introduction
Astrocytes are the most numerous glial cells, occupying 25%–50% of brain volume [1]. As believed conventionally, astrocytes are considered to provide structural support for the brain, form the blood-brain barrier, and supply energy for neurons [2]. With the proposal of the ‘tripartite synapse’ theory, many studies focused on their roles in synaptic plasticity, such as secreting signaling molecules to mediate the occurrence, maturation, and deletion of synapses, to maintain their structural plasticity [3, 4]. Besides, astrocytes secrete a variety of glial transmitters, such as glutamate, ATP, and GABA, to regulate the long-term potentiation or depression of synapses and participate in regulating their functional plasticity [5–9]. Furthermore, astrocytes maintain the ion and transmitter homeostasis in synaptic clefts of neurons by recycling potassium ions and glutamate, thus affecting neuronal activity and synaptic plasticity [10]. It has been suggested that the activity of astrocytes is vital for the normal function of neurons and the maintenance of brain homeostasis.
The amygdala is a key area in the control of fear memory, mainly including the basolateral amygdala (BLA) and the central amygdala (CeA) [11]. It is generally believed that the BLA is responsible for receiving information from the cortex, hippocampus, and other brain areas, integrating and then transferring the dominant information to the CeA [11, 12]. The neuronal activity in the BLA is essentially involved in the process of learning and memory. Lesions or functional inhibition of the BLA disrupt the formation and expression of conditional fear memory [13, 14]. Astrocytes play important roles in regulating synaptic plasticity, which is considered to be the basis of learning and memory [15]. Most of the current research is focused on the energy metabolism of astrocytes, the glutamate cycle, and the glial transmitters in learning and memory [16]. The traditional interventions in astrocyte activity are usually irreversible, which may trigger compensatory responses [17, 18]. However, the role of astrocytes in associative learning and memory, such as fear conditioning, is unclear. Whether the real-time structural changes of astrocytes in the BLA regulate fear memory acquisition and expression remains unknown.
Ras-related C3 botulinum toxin substrate 1 (Rac1), is a critical small Rho GTPase for cytoskeletal remodeling [19]. Thus, Rac1 is an important molecule in shaping the morphology of astrocytes. It has been shown that activation of Rac1 in vitro induces astrocytosis, while Rac1 knockout or inhibition of Rac1 activity shrinks astrocytes [20]. Other studies have shown that the skeletal structure of astrocytes changes dynamically during the formation of fear memory, as a result in changes of the activity of Rac1 [21, 22].
Above all, we hypothesized that the activity of BLA astrocytic Rac1 regulates conditioned fear memory formation and retrieval by modulating BLA neuronal functions. To test this hypothesis, we constructed associated adenovirus (AAV) of photoactivatable Rac1 (Rac1-PA) and photoinactivatable Rac1 (Rac1-DN/PA) under the mGFAP promoter to manipulate the structure of astrocytes in real time, to avoid compensatory reactions [23]. By this means, we found that the temporary changes of astrocytic Rac1 activity decreased neuronal activation and impaired fear memory acquisition and retrieval.
Materials and Methods
Animals
Adult C57BL/6 male mice were purchased from Shanghai Laboratory Animal Center, Chinese Academy of Sciences. Animals were housed 4 per cage under a 12-h alternating light/dark cycle, with food and water available ad libitum. Two- to three-month-old mice were used for the experiments. All procedures were approved by the Animal Care and Use Committee of the Shanghai Medical College of Fudan University and were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Preparation of AAVs
The plasmids of Rac1-PA, Rac1-DN/PA, photoinsensitive Rac1 (Rac1-C450A), and pAAV-mGFAP-HA-rM3D(Gs)-IRES-mCherry were from Addgene (Plasmid #22027, #22029, #22028, and #50472). Rac1-PA, Rac1-DN/PA, or Rac1-C450A was inserted into AAV-mGFAP-mCherry as previously described [21]. AAV2/9-mGFAP-Rac1-PA-mCherry, AAV2/9-mGFAP-Rac1-DN/PA-mCherry, and AAV2/9-mGFAP-Rac1-C450A-mCherry were packaged by Obio Technology (Shanghai, China). AAVs at a titer of 2 × 1012 viral genomes/mL were used for the following experiments.
Stereotaxic Surgery
To perform virus injection and optical fiber implantation, mice were anesthetized with 1.5%–2% isoflurane and positioned in a stereotaxic frame (RWD, Shenzhen, China) [24]. AAVs were bilaterally injected into the BLA in a 0.5 μL volume at 0.1 μL/min. The coordinates of the BLA were AP, −1.5 mm; ML, ±3.3 mm; DV, −4.8 mm, referenced to bregma. The needle was left for 5 min after injection and pulled out very slowly to avoid leakage. Ceramic optical fiber cannulas (diameter, 200 μm; numerical aperture, 0.37; Anilab Software & Instruments, Ningbo, China) were bilaterally implanted in the BLA at AP, −1.5 mm; ML, ±3.3 mm; DV, −4.6 mm, referenced to bregma. Dental cement was used to fix the optical fibers on the skull. After surgery, the mice were allowed 3 weeks to recover. The locations of virus expression and the cannulas were checked after behavioral tests. Mice with inaccurate virus expression or fiber implantation were excluded.
Conditioned Fear Memory
For fear conditioning, the mice received 5 trials with conditioned (CS) and unconditioned stimulus (US) pairing in the conditioning chamber (MED Associates, VT, USA). One CS (tone, 2800 Hz, 85 dB, 30 s) was paired with one US (footshock, 0.5 mA, 1 s) and the footshock was delivered at the end of the 30-s tone. There was a 2-min interval between each trial. The contextual and cued memory-retention tests were performed 24 h and 48 h after conditioning, respectively. In the contextual memory-retention test, we placed each mouse in the conditioning chamber for 3 min. In the cued memory-retention test, we placed each mouse in a novel chamber (defined as Pre-cue), with a 3-min tone delivered later (defined as Cue). The freezing percentage was scored manually and double-blinded. To measure memory acquisition, the freezing levels were calculated during each tone period. For the contextual and cued memory-retention tests, the freezing levels were calculated during context and cue presentation.
In Vivo Photostimulation
We connected a 473-nm laser (Shanghai Dream Lasers Technology, Shanghai, China) to a patch cord through a specific omni-directional wheel (Doric Lenses, Quebec, Canada), allowing the fiber to rotate freely. The patch cord was then connected to the implanted ceramic fiber optic cannulas [25]. Sustained 15-mW laser light was delivered. The photostimulation was delivered 5 min before and throughout the conditioning, or 15 min before and during the memory retention tests.
Western Blotting
After 15-min of laser (473 nm) stimulation, mice were anesthetized with 1% pentobarbital sodium. The bilateral BLAs were dissected and the proteins were extracted by RIPA (P0013B, Beyotime Biotechnology, Shanghai, China). The proteins were separated on 12% SDS-PAGE gels and transferred onto nitrocellulose membranes. The membranes were incubated with rabbit anti-cofilin (1:1000, 5175, Cell Signaling Technology, Boston, USA), anti-p-cofilin (1:500, 3313, Cell Signaling Technology), or anti-GAPDH (1:2000, 2118, Cell Signaling Technology) at 4°C for 12 h, and then with anti-rabbit IRDye 800CW secondary antibody (Rockland, PA, USA) at room temperature for 90 min. The Western blot results were analyzed with ImageJ software.
Immunofluorescence
Mice were anesthetized and transcardially perfused with saline followed by 4% paraformaldehyde (PFA, in 0.1 mol/L PB, pH 7.4) [26]. We immersed the brain in 4% PFA at 4°C for 12 h for post-fixation. The brain was then dehydrated in 20% and 30% sucrose. The BLA was cut into 30-μm coronal sections on a cryostat microtome (Leica Instrument Co., Ltd., Germany). We washed the sections in phosphate-buffered saline (0.01 mol/L PBS), and then blocked them with 5% donkey serum (in 0.01 mol/L PBST) at room temperature for 90 min. The sections were incubated with rabbit anti-c-Fos antibody (1:1000, sc-52, Santa Cruz), or mouse anti-NeuN antibody (1:1000, ab104224, Abcam, Cambridge, UK) at 4°C for 24 h. After washing in PBST, the sections were incubated with the secondary antibody Alexa Fluor 488 goat anti-rabbit IgG (1:1000, 111-545-144, Jackson ImmunoResearch, PA, USA), or Alexa Fluor 488 goat anti-mouse IgG (1:1000, 111-545-166, Jackson ImmunoResearch) at room temperature for 90 min. For further imaging and preservation, the sections were mounted with an anti-quenching mounting medium (Thermo Fisher Scientific). Images were captured under a confocal microscope (Nikon A1, Japan).
Cell Counting
To characterize the activation of cells in the BLA induced by fear memory acquisition and retrieval, mice were transcardially perfused 90 min after fear conditioning or the context test [24]. The number of c-Fos+ or NeuN+ neurons in the BLA was calculated automatically by Image-Pro Plus 6.0 software. A threshold for background fluorescence was applied for cell counting. An average number of 3 sections per mouse was calculated to avoid errors. The counting process was performed double-blinded.
Statistical Analyses
Data are presented as the mean ± SEM and plotted by GraphPad Prism v.7 software. Student’s t-test was used to compare two groups. One-way ANOVA, followed by post hoc Bonferroni’s test was used to compare multiple groups. Two-way ANOVA was used to compare two groups at different time points. The differences were calculated with SPSS and the statistical significance was defined as P < 0.05.
Results
Manipulating Rac1 Activity in BLA Astrocytes Suppresses Fear Memory Acquisition
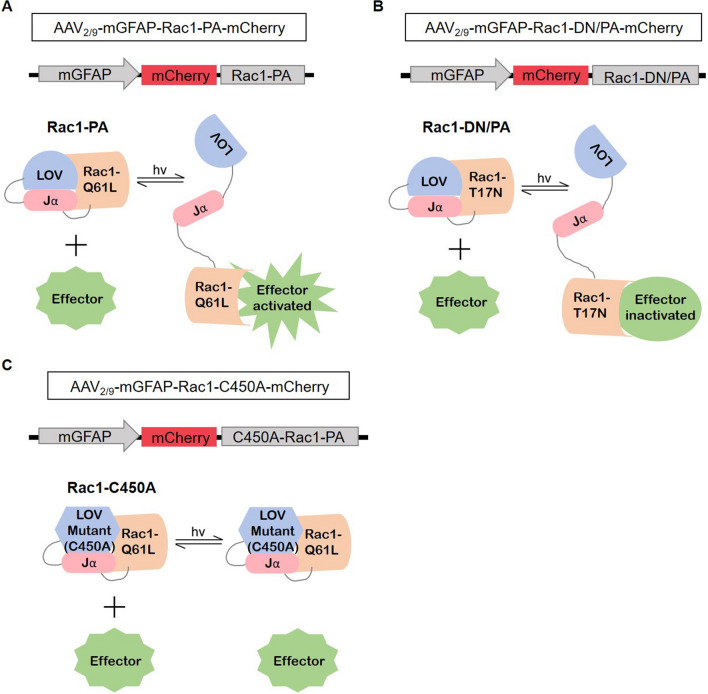
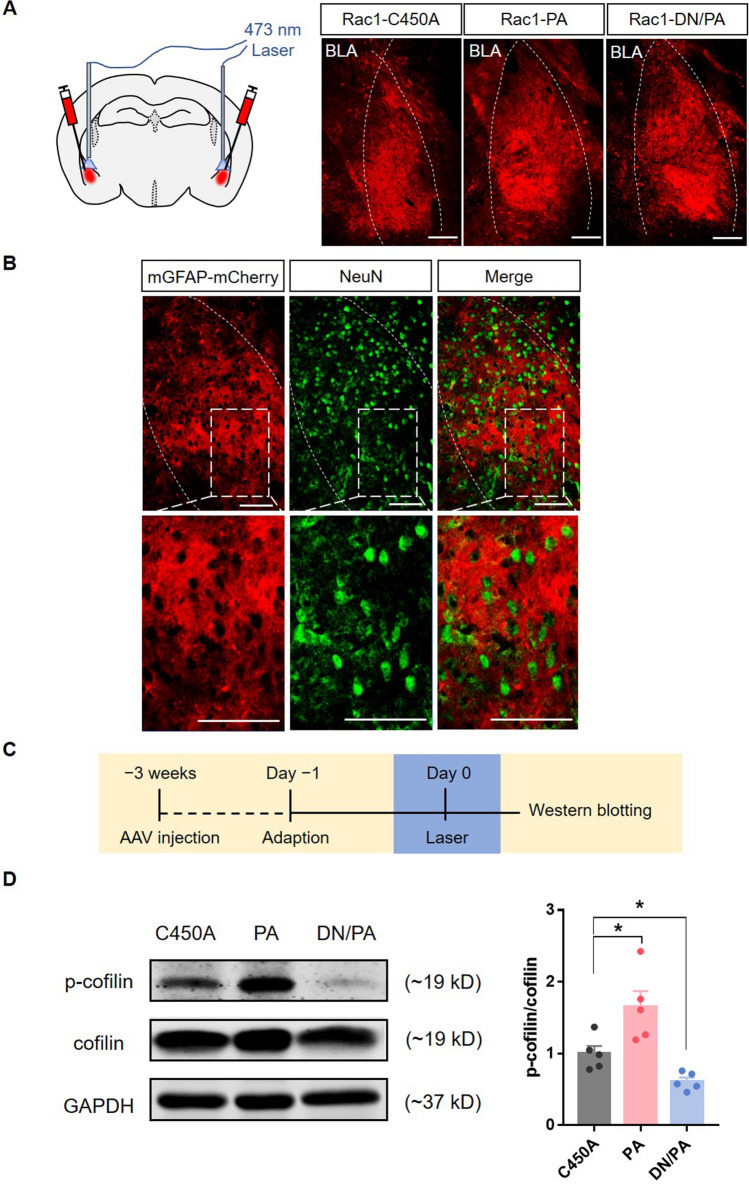
To determine whether the Rac1 activity in BLA astrocytes regulates conditioned fear memory acquisition and retrieval, we constructed AAV-mGFAP-Rac1-PA and AAV-mGFAP-Rac1-DN/PA to test the effects of astrocytic Rac1 activity on behaviors at precise times. Previous studies usually overexpressed or knocked out Rac1, which may cause a compensatory reaction. Here, we connected Rac1-PA or Rac1-DN/PA to the mGFAP promoter to optically up-regulate or down-regulate the activity of Rac1 specifically in astrocytes. In detail, Rac1-PA or Rac1-DN/PA are composed of a constitutively active mutant (Q61L) of Rac1 (Rac1-CA) or a dominant-negative mutant (T17N) of Rac1 (Rac1-DN) and a light oxygen voltage (LOV)-Jα sequence [23]. The compact conformation of the LOV-Jα domain can precludes the combination of effectors with Rac1. Stimulation with 473 nm light unwinds the helix linking LOV to Rac1, which allows the interaction between Rac1 and effectors (Fig. 1A, B). A light-insensitive LOV mutation (C450A) binding to Rac1 (Rac1-C450A) was used as a photo-insensitive control (Fig. 1C). Three weeks after viral injection, we found that only a small proportion (2.49% ± 0.26%) of neurons (NeuN+ cells) were co-labeled with mCherry (Fig. 2A, B), showing that the expression of LOV-Rac1 was restricted to astrocytes. In order to verify the efficiency of the photostimulation of Rac1, we tested the expression levels of phosphorylated cofilin (p-cofilin), a downstream effector of Rac1, and found that, compared to the Rac1-C450A group, photostimulation of Rac1-PA up-regulated p-cofilin, whereas photostimulation of Rac1-DN/PA down-regulated p-cofilin (Fig. 2C, D; F(2,12) = 13.16, P < 0.05). As a result, we succeed in up-regulating (Rac1-PA) or down-regulating (Rac1-DN/PA) the Rac1-activity under 473 nm light stimulation in real-time.
Fig. 1.
Constructing recombinant AAVs of Rac1-PA, Rac1-DN/PA, and Rac1-C450A under the mGFAP promoter. A Cartoon of AAV-mGFAP-Rac1-PA-mCherry design. The photoreactive LOV-Jα domain blocks the binding of effectors to Rac1-Q61L, and laser stimulation (473 nm) causes the unwinding of the LOV-Jα helix, which releases the steric inhibition, and leads to Rac1 and effector activation. B Cartoon of AAV-mGFAP-Rac1-DN/PA-mCherry design. Similar to the above, laser stimulation leads to releasing the steric inhibition of Rac1-T17N, and results in Rac1 and effector inactivation. C Cartoon of AAV-mGFAP-Rac1-C450A-mCherry design. The amino acid substitution in the LOV domain (C450A) leads to the insensitivity to laser stimulation. hv, irradiation.
Fig. 2.
Efficiency tests for Rac1-PA, Rac1-DN/PA, and Rac1-C450A. A Schematic of laser stimulation of the bilateral BLA (left) and representative images of Rac1-C450A-mCherry, Rac1-PA-mCherry, and Rac1-DN/PA-mCherry expression in the BLA (right) (dotted lines, location of the BLA; scale bars, 100 μm). B Representative immunofluorescence images showing that few (2.49% ± 0.26%) neurons (NeuN+, green) are co-labeled with AAV-GFAP-mCherry, indicating that the expression of LOV-Rac1 is restricted to astrocytes (scale bars, 100 μm). C Diagram of the experiment timeline. Three weeks after virus injection, the mice received 15-min laser (473 nm) stimulation and were anesthetized immediately for Western blotting. D Laser stimulation of Rac1-PA increases the expression levels of p-cofilin, while laser stimulation of Rac1-DN/PA decreases its levels (Rac1-C450A: n = 5, Rac1-PA: n = 5, Rac1-DN/PA: n = 5; *P < 0.05, C450A vs PA, C450A vs DN/PA, one-way ANOVA).
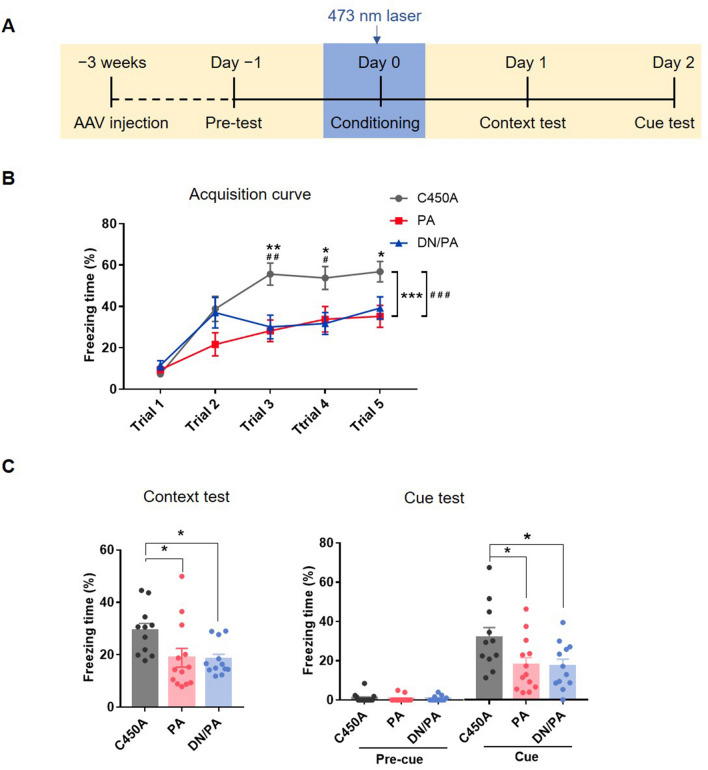
We bilaterally microinjected AAVs encoding Rac1-PA, Rac1-DN/PA, or Rac1-C450A driven by the mGFAP promotor into the BLA (Fig. 2A). After 3 weeks for recovery, each mouse was introduced into the conditioning chamber for 5 min without tone or footshock and the basal freezing level in the conditioning context were acquired. One day later, the mice received 5 CS-US pairing trials accompanied by laser stimulation (473 nm) in the bilateral BLA (Fig. 3A). The results showed that, compared to the Rac1-C450A control group, both photoactivation and photoinactivation of astrocytic Rac1 activity during conditioning attenuated the freezing levels during cue presentation (Fig. 3B; group effect: F(2,57) = 14.15, P < 0.001; time effect: F(4,228) = 21.13, P < 0.001; interaction: F(8,228) = 2.14, P < 0.05). After conditioning, the contextual and cued memory retention tests were performed in the following days without laser stimulation. The data showed that up-regulation or down-regulation of Rac1 activity in the BLA significantly decreased the freezing levels in both contextual and cued memory retention tests (Fig. 3C; context test: F(2,33) = 4.31, P < 0.05; cue test: F(2,33) = 3.85, P < 0.05). The results above suggest that either activation or inhibition of astrocytic Rac1 activity impairs fear memory learning, indicating that the normal activity of astrocytes in BLA is necessary for fear memory acquisition.
Fig. 3.
Increasing or decreasing astrocytic Rac1 activity in the BLA impairs fear memory acquisition. A Diagram of the experiment timeline. The AAV was injected 3 weeks before the behavioral tests. The laser stimulation (473 nm) was delivered 5 min before and during the 5 trials of CS-US pairing conditioning, then contextual and cued memory retention were tested 1 and 2 days later. B Compared to the Rac1-C450A group, laser activation of Rac1-PA and Rac1-DN/PA both attenuate the fear memory acquisition (Rac1-C450A: n = 20, Rac1-PA: n = 20, Rac1-DN/PA: n = 20; *P < 0.05, **P < 0.01, ***P < 0.001, C450A vs PA, #P < 0.05, ##P < 0.01, ###P < 0.001, C450A vs DN/PA, ANOVA with repeated measures and Bonferroni’s post hoc test). C Similarly, compared to the Rac1-C450A group, laser activation of Rac1-PA and Rac1-DN/PA both impaired contextual and cued fear memory (Rac1-C450A: n = 11, Rac1-PA: n = 13, Rac1-DN/PA: n = 12; *P < 0.05, C450A vs PA, C450A vs DN/PA, one-way ANOVA).
Disturbance of Astrocytic Rac1 Activity Decreases Neuronal Activity in the BLA During Fear Memory Acquisition
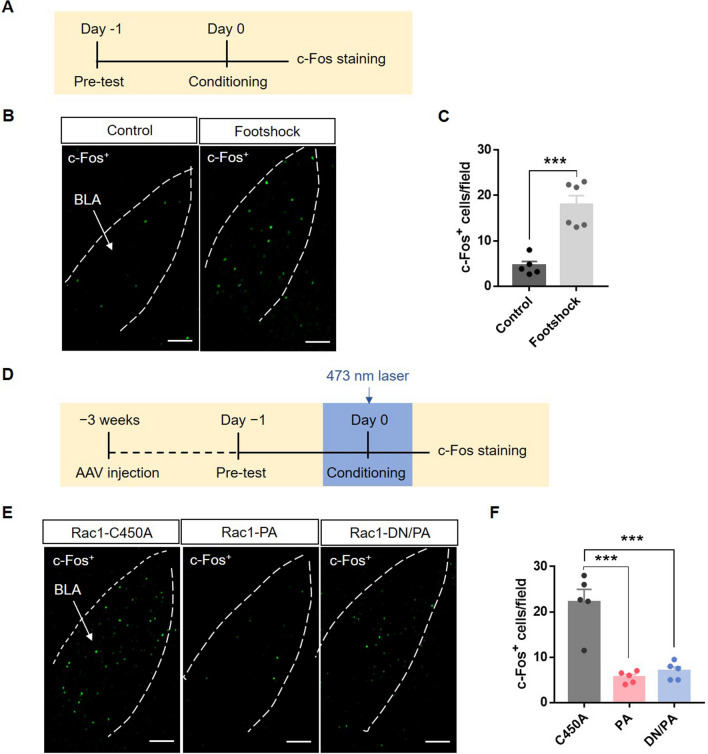
To explore the interaction between astrocytes and neurons, we tested the activation of BLA neurons after fear conditioning with and without optical stimulation of astrocytic Rac1 using c-Fos immunostaining (Fig. 4D). Immunofluorescence results showed that fear conditioning increased the c-Fos+ cell counts in the BLA (Fig. 4A–C, t(9) = 5.68, P < 0.001), consistent with previous reports. But after interfering with astrocytic Rac1 activity, the number of c-Fos+ neurons in the BLA decreased (Fig. 4E, F; F(2,12) = 27.15, P < 0.001). These results indicate that the activity of astrocytic Rac1 is involved in the acquisition of fear memory through the regulation of neuronal activity in the BLA. The abnormal activity of astrocytic Rac1 disrupts the well-balanced function of astrocytes and then impairs neuronal activity and memory acquisition in fear conditioning.
Fig. 4.
Optical stimulation of BLA astrocytic Rac1 decreases the neuronal activation induced by fear conditioning. A Diagram of the experiment timeline. Mice were put into the behavioral chamber, underwent footshock or not, and were perfused 90 min later. B Representative immunofluorescence images of BLA c-Fos+ neurons in mice with and without footshock (scale bars, 100 μm). C Quantitative analysis showing that footshock significantly increases c-Fos expression in the BLA (Control: n = 5, Footshock: n = 6; ***P < 0.001, Control vs Footshock, t-test). D Diagram of the experiment timeline. Three weeks after virus injection, the mice underwent 5 trials of CS-US paired conditioning with laser stimulation (473 nm) and were perfused 90 min later. E Representative immunofluorescence images showing c-Fos+ neurons in the BLA of mice in the Rac1-C450A, Rac1-PA, and Rac1-DN/PA groups (scale bars, 100 μm). F Quantitative analysis showing that laser stimulation of Rac1-PA and Rac1-DN/PA both significantly decrease c-Fos expression in the BLA (n = 5 in each group; ***P < 0.001, C450A vs PA, C450A vs DN/PA, one-way ANOVA).
Astrocytic Rac1 Activity in the BLA Regulates Fear Memory Retrieval
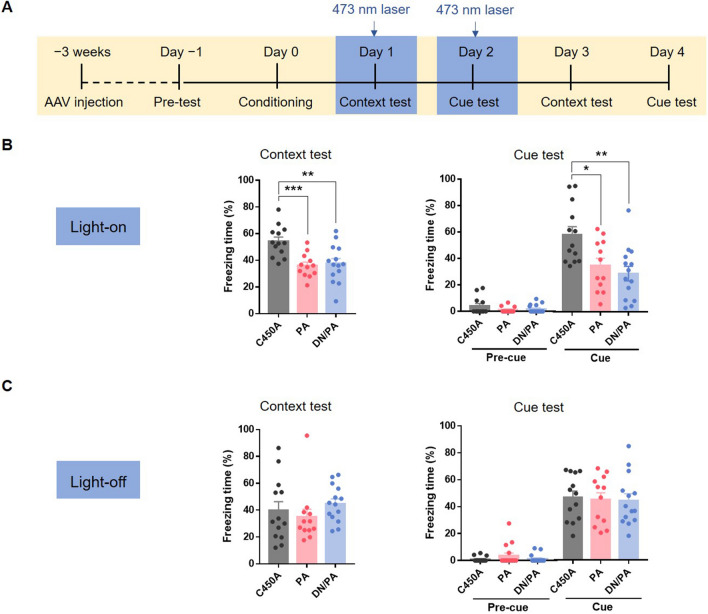
To determine whether the Rac1 activity of astrocytes in the BLA regulates fear memory retrieval, we bilaterally microinjected AAV-mGFAP-Rac1-PA-mCherry, AAV-mGFAP-Rac1-DN/PA-mCherry, or AAV-mGFAP-Rac1-C450A-mCherry into the BLA. After 3 weeks of recovery, each mouse underwent a 5-min pre-test and 5 CS-US paired conditioning without laser stimulation. For the contextual and cued memory retention tests, each mouse received 473-nm laser stimulation for 15 min before the tests and was put into the chamber immediately after laser delivery (Fig. 5A). Compared to the Rac1-C450A control group, up-regulation or down-regulation of astrocytic Rac1 activity in the BLA decreased the freezing levels induced by context and tone presentation during memory retention tests (Fig. 5B, context test: F(2,36) = 9.43, P < 0.001; cue test: F(2,36) = 7.508, P < 0.01), suggesting that astrocytic Rac1 activity in the BLA is critically involved in memory retrieval in fear conditioning.
Fig. 5.
Increasing or decreasing astrocytic Rac1 activity in the BLA impairs the retrieval of conditioned fear memory. A Diagram of the experiment timeline. Mice received 5 trials of CS-US paired conditioning 3 weeks after the AAV injection. Laser stimulation (473 nm) was delivered 15 min before and during the memory retention test, which was repeated without laser stimulation on days 3 and 4. B Compared to the Rac1-C450A group, laser stimulation of Rac1-PA and Rac1-DN/PA during tests both impair the contextual and cued fear memory retrieval (Rac1-C450A: n = 13, Rac1-PA: n = 12, Rac1-DN/PA: n = 14; *P < 0.05, **P < 0.01, ***P < 0.001, C450A vs PA, C450A vs DN/PA, one-way ANOVA). C Without laser stimulation, the mice in the Rac1-PA and Rac1-DN/PA groups showed no significant changes of freezing levels (Rac1-C450A: n = 13, Rac1-PA: n = 12, Rac1-DN/PA: n = 14; P >0.05, C450A vs PA, C450A vs DN/PA, one-way ANOVA).
To investigate whether the effects were transient or permanent, we then performed the contextual and cued memory retention tests again without laser stimulation. The results showed no difference between the Rac1-C450A group and the Rac1-PA group or the Rac1-DN/PA group (Fig. 5C; context test: F(2,36) = 0.85, P = 0.44; cue test: F(2,36) = 0.06, P = 0.94), suggesting that astrocytic Rac1 activity in the BLA transiently regulates fear memory retrieval.
These results indicate that both up-regulation and down-regulation of astrocytic Rac1 activity in the BLA affect fear memory retrieval. Moreover, the regulatory effect is transient, and not permanent.
Optical Stimulation of BLA Astrocytic Rac1 Decreases BLA Neuronal activation Induced by Memory Retrieval
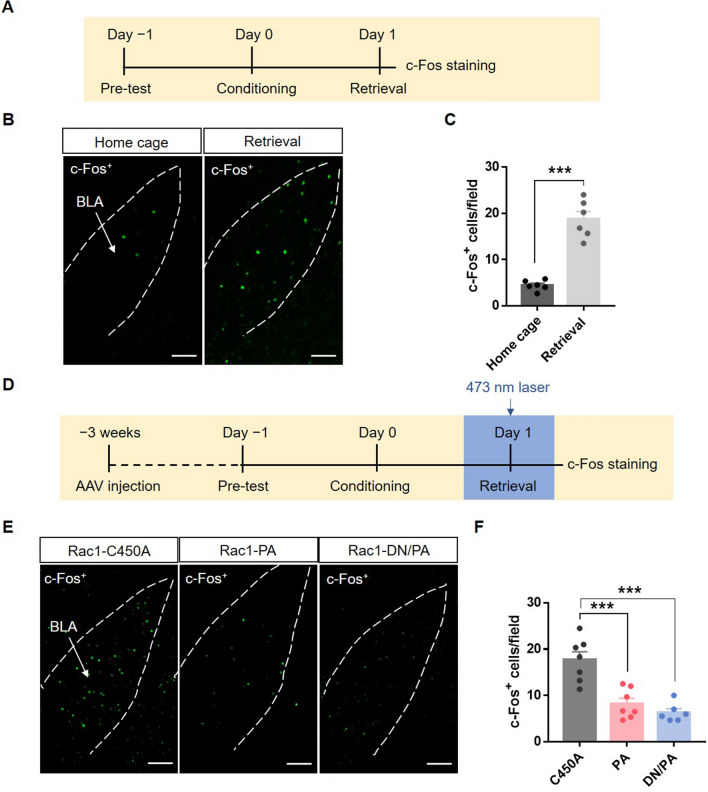
To confirm the regulation of neuronal activity by astrocytic Rac1 activity, we performed c-Fos immunostaining 90 min after contextual retrieval (Fig. 6D). Immunofluorescence results showed that the contextual memory retention test increased c-Fos+ cell counts in the BLA (Fig. 6A–C; t(10) = 8.33, P < 0.001), while up-regulation or down-regulation of astrocytic Rac1 activity reduced the number of c-Fos+ neurons in the BLA (Fig. 6E, F; F(2,17) = 19.84, P < 0.001). These results indicate that both up-regulation and down-regulation of astrocytic Rac1 activity in the BLA disrupt the normal function of astrocytes and impair the activation of neurons in BLA, thereby decreasing fear memory retrieval.
Fig. 6.
Optical stimulation of BLA astrocytic Rac1 inhibits neuronal activation induced by memory retrieval. A Diagram of the experiment timeline. Mice were placed in the behavior room, underwent contextual retrieval or not, and then were perfused 90 min later. B Representative images of c-Fos+ neurons in the BLA of mice with and without retrieval (scale bars, 100 μm). C Quantitative analysis showing that contextual retrieval significantly increases c-Fos expression in the BLA (Home cage: n = 6, Retrieval: n = 6; ***P < 0.001, Home cage vs Retrieval, t-test). D Diagram of the experiment timeline. Three weeks after virus injection, the mice underwent 5 trials of CS-US paired conditioning on day 0. After contextual retrieval with or without laser stimulation; 90 min later, the mice were perfused. E Representative images of c-Fos+ neurons in the BLA of mice in the Rac1-C450A, Rac1-PA, and Rac1-DN/PA groups (scale bars, 100 μm). F Quantitative analysis showing that laser stimulation of Rac1-PA and Rac1-DN/PA during memory retrieval decreases c-Fos expression in the BLA (Rac1-C450A: n = 7, Rac1-PA: n = 7, Rac1-DN/PA: n = 6; ***P < 0.001, C450A vs PA, C450A vs DN/PA, one-way ANOVA).
Discussion
Our results provide insights into the roles of BLA astrocytes in memory acquisition and retrieval in fear conditioning. Using the photo-stimulation approach, we manipulated astrocytic Rac1 activity in real time and found that both activation and inactivation of Rac1 in astrocytes impaired fear memory acquisition and retrieval, and decreased neuronal activity in the BLA.
Astrocytes, the largest population of neuroglial cells in the brain, have crucial structural, metabolic, and homeostatic functions [27–29]. In the adult brain, astrocytes communicate with neurons by the exocytosis of numerous molecules surrounding synapses. They release glutamate, ATP, GABA, D-serine, or endocannabinoids that regulate synaptic strength, such as long-term potentiation (LTP) and long-term depression (LTD), mechanisms of memory and learning [30]. They also maintain the homeostasis of neurotransmitters by recycling potassium ions and glutamate in the synaptic cleft [31, 32]. Recent studies have shown that the cognitive process requires the coordinated activity of astrocytes and neuron ensembles. Activation of astrocytes in the CeA induces cued fear memory extinction [33]. Chemogenetic activation of astrocytes in CA1 induces LTP, therefore enhancing memory acquisition [34]. Blocking the glutamate uptake into astrocytes in the prefrontal cortex impairs spatial memory [35]. Inhibiting the L-lactate production in astrocytes in the hippocampus suppresses LTP and disrupts the formation of long-term memory [36]. Here, we showed that both activation and inactivation of Rac1 activity in astrocytes decreased the number of activated neurons in the BLA and impaired fear memory acquisition and retrieval. This phenomenon may be caused by disturbing the balance between astrocytes and neurons: photo-activating Rac1 in astrocytes causes astrocytic expansion [23], and the extensive coverage of astrocytic lamellae might result in more glutamate reuptake into astrocytes [37]. The reduced neurotransmitter availability in synapses, in turn, impairs LTP expression [38]. The curbed function of neurons may lead to reduced responses to stimuli and finally suppress the acquisition and retrieval of conditioned fear memory. On the other hand, photo-inactivating Rac1 in BLA astrocytes causes flattening of astrocytes [23], and the limited coverage of astrocytic lamellae might result in less D-serine and L-lactate reaching synapses [39], which would reduce the activation of N-methyl-D-aspartate receptors and raise the threshold of LTP induction [36, 40]. The curbed function of neurons may also lead to reduced responses to stimuli and suppress the acquisition and retrieval of conditioned fear memory as well. Notably, the activation or inactivation of astrocytic Rac1 activity is not equivalent to activation or inactivation of astrocytic activity by stimulation of Gq-GPCRs or Gi-GPCRs. In conclusion, both up-regulating and down-regulating the activity of Rac1 may disturb the balance between astrocytes and neurons, and disrupt the normal function of neurons in the BLA, thus impairing the acquisition and retrieval of fear memory. Although the behavioral outcomes of the two operations were consistent, the concrete mechanisms may be distinct, which needs further exploration. This research indicates that the well-balanced function of astrocytes in the BLA is the basis of the neuronal activity and the acquisition and expression of fear memory.
Rac1 is a molecular switch of cell signal transduction and is involved in cytoskeletal protein remodeling. It also regulates cell migration, extension, polarity, and other functions [41, 42]. Studies have shown that activating Rac1 promotes spine formation, and inhibiting Rac1 activity decreases the stability of mature spines [43, 44]. In astrocytes, activating Rac1 causes expansion and inhibiting it flattens them [21]. In previous studies, Rac1 activity was usually regulated by permanent interventions, such as knockout, overexpression, sustained activation, and sustained suppression [45–47]. These methods likely lead to compensatory reactions caused by other Rho GTPases and reduce the reliability of experimental results. To optimize them, we constructed Rac1-PA and Rac1-DN/PA under the mGFAP promoter to manipulate the structure of astrocytes in real-time by photostimulation. Previous studies in our lab have shown that Rac1 knockout in BLA astrocytes results in facilitation of the acquisition of fear memory, but persistent inhibition of astrocytic Rac1 in the BLA has no apparent effect [21]. To explain this phenomenon further, we found that transient inhibition of astrocytic Rac1 activity in the BLA by AAV-Rac1-DN/PA impaired fear memory acquisition and retrieval. It is possible that diverse interventions lead to different inhibitory effects and permanent interventions may cause compensatory reactions or lead to other radical changes. Photostimulation of Rac1 activity is a good choice for regulating Rac1 activity in real time and avoiding compensatory reactions.
Thanks to the real-time effects of the interventions, we further explored whether the behavioral changes are reversible. We delivered photostimulation before memory retrieval and tested the expression of fear memory without light stimulation two days later. The results showed no significant difference between the Rac1-C450A and Rac1-PA or Rac1-DN/PA groups on fear expression without photostimulation (Fig. 5C), indicating that the inhibitory effect on memory retrieval was temporary. However, photostimulating Rac1 during conditioning not only impaired the acquisition of conditioned fear memory (showed by the decreased freezing level in the learning curve), but also decreased the freezing level in the retrieval of fear memory (Fig. 3C, D). These results suggest that the interventions during fear conditioning inhibit the formation of memory engram cells in the BLA and impair memory acquisition. Nevertheless, temporary stimulation during fear memory retrieval might temporarily suppress the activity of memory engram cells, and not induce permanent changes of the engrams in the BLA.
In conclusion, the present study demonstrates that astrocytic Rac1 activity in the BLA regulates fear memory acquisition and retrieval. Both up-regulation and down-regulation of astrocytic Rac1 activity disrupted the interaction between astrocytes and neurons, inhibited the activation of neurons in the BLA, and finally decreases the acquisition and retrieval of fear memory. The astrocytic Rac1 activity that regulates the balance between astrocytes and neurons might be a promising target for treating memory-related diseases, such as post-traumatic stress disorder.
Acknowledgements
This work was supported by Grants from the China Postdoctoral Science Foundation (BX20180070 and 2019M661347), and the National Natural Science Foundation of China (31930046 and 31771176).
Conflict of interest
The authors claim that there are no conflicts of interest.
Footnotes
Xiao-Cen Fan and Chao-Nan Ma contributed equally to this work.
Contributor Information
Xing Liu, Email: xingliu@fudan.edu.cn.
Lan Ma, Email: lanma@fudan.edu.cn.
References
- 1.Allen NJ, Barres BA. Neuroscience: Glia - more than just brain glue. Nature. 2009;457:675–677. doi: 10.1038/457675a. [DOI] [PubMed] [Google Scholar]
- 2.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 3.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 4.Allen NJ, Eroglu C. Cell biology of astrocyte-synapse interactions. Neuron. 2017;96:697–708. doi: 10.1016/j.neuron.2017.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez-Bayghen E, Ortega A. Glial glutamate transporters: new actors in brain signaling. IUBMB Life. 2011;63:816–823. doi: 10.1002/iub.536. [DOI] [PubMed] [Google Scholar]
- 6.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, et al. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 7.Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron. 2014;81:728–739. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savtchouk I, Volterra A. Gliotransmission: beyond black-and-white. J Neurosci. 2018;38:14–25. doi: 10.1523/JNEUROSCI.0017-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin C, Zhou LQ, Ma XT, Hu ZW, Yang S, Chen M, et al. Dual functions of microglia in ischemic stroke. Neurosci Bull. 2019;35:921–933. doi: 10.1007/s12264-019-00388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rose CR, Karus C. Two sides of the same coin: sodium homeostasis and signaling in astrocytes under physiological and pathophysiological conditions. Glia. 2013;61:1191–1205. doi: 10.1002/glia.22492. [DOI] [PubMed] [Google Scholar]
- 11.Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neurosci Biobehav Rev. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malleret G, Alarcon JM, Martel G, Takizawa S, Vronskaya S, Yin D, et al. Bidirectional regulation of hippocampal long-term synaptic plasticity and its influence on opposing forms of memory. J Neurosci. 2010;30:3813–3825. doi: 10.1523/JNEUROSCI.1330-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47:783–786. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Rodrigues SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi T, Duszkiewicz AJ, Morris RG. The synaptic plasticity and memory hypothesis: encoding, storage and persistence. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130288. doi: 10.1098/rstb.2013.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alberini CM, Cruz E, Descalzi G, Bessieres B, Gao V. Astrocyte glycogen and lactate: New insights into learning and memory mechanisms. Glia. 2018;66:1244–1262. doi: 10.1002/glia.23250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Logan S, Pharaoh GA, Marlin MC, Masser DR, Matsuzaki S, Wronowski B, et al. Insulin-like growth factor receptor signaling regulates working memory, mitochondrial metabolism, and amyloid-beta uptake in astrocytes. Mol Metab. 2018;9:141–155. doi: 10.1016/j.molmet.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Shen X, Dong J, Liu WC, Song M, Sun Y, et al. Inhibition of reactive astrocytes with fluorocitrate ameliorates learning and memory impairment through upregulating CRTC1 and synaptophysin in ischemic stroke rats. Cell Mol Neurobiol. 2019;39:1151–1163. doi: 10.1007/s10571-019-00709-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 20.Racchetti G, D'Alessandro R, Meldolesi J. Astrocyte stellation, a process dependent on Rac1 is sustained by the regulated exocytosis of enlargeosomes. Glia. 2012;60:465–475. doi: 10.1002/glia.22280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao Z, Tao Y, Guo X, Cheng D, Wang F, Liu X, et al. Fear conditioning downregulates Rac1 activity in the basolateral amygdala astrocytes to facilitate the formation of fear memory. Front Mol Neurosci. 2017;10:396. doi: 10.3389/fnmol.2017.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez LA, Klann E, Tejada-Simon MV. Translocation and activation of Rac in the hippocampus during associative contextual fear learning. Neurobiol Learn Mem. 2007;88:104–113. doi: 10.1016/j.nlm.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461:104–108. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Y, Zhu H, Liu Z, Chen X, Su X, Ma C, et al. A ventral CA1 to nucleus accumbens core engram circuit mediates conditioned place preference for cocaine. Nat Neurosci. 2019;22:1986–1999. doi: 10.1038/s41593-019-0524-y. [DOI] [PubMed] [Google Scholar]
- 25.Couto A, Mack NA, Favia L, Georgiou M. An apicobasal gradient of Rac activity determines protrusion form and position. Nat Commun. 2017;8:15385. doi: 10.1038/ncomms15385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan XC, Fu S, Liu FY, Cui S, Yi M, Wan Y. Hypersensitivity of prelimbic cortex neurons contributes to aggravated nociceptive responses in rats with experience of chronic inflammatory pain. Front Mol Neurosci. 2018;11:85. doi: 10.3389/fnmol.2018.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7:a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verkhratsky A, Nedergaard M. Physiology of astroglia. Physiol Rev. 2018;98:239–389. doi: 10.1152/physrev.00042.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ioannou MS, Jackson J, Sheu SH, Chang CL, Weigel AV, Liu H, et al. Neuron-astrocyte metabolic coupling protects against activity-induced fatty acid toxicity. Cell. 2019;177(1522–1535):e1514. doi: 10.1016/j.cell.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Chung WS, Allen NJ, Eroglu C. Astrocytes control synapse formation, function, and elimination. Cold Spring Harb Perspect Biol. 2015;7:a020370. doi: 10.1101/cshperspect.a020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breslin K, Wade JJ, Wong-Lin K, Harkin J, Flanagan B, Van Zalinge H, et al. Potassium and sodium microdomains in thin astroglial processes: A computational model study. PLoS Comput Biol. 2018;14:e1006151. doi: 10.1371/journal.pcbi.1006151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rose CR, Ziemens D, Untiet V, Fahlke C. Molecular and cellular physiology of sodium-dependent glutamate transporters. Brain Res Bull. 2018;136:3–16. doi: 10.1016/j.brainresbull.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Martin-Fernandez M, Jamison S, Robin LM, Zhao Z, Martin ED, Aguilar J, et al. Synapse-specific astrocyte gating of amygdala-related behavior. Nat Neurosci. 2017;20:1540–1548. doi: 10.1038/nn.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adamsky A, Kol A, Kreisel T, Doron A, Ozeri-Engelhard N, Melcer T, et al. Astrocytic activation generates de novo neuronal potentiation and memory enhancement. Cell. 2018;174(59–71):e14. doi: 10.1016/j.cell.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Bechtholt-Gompf AJ, Walther HV, Adams MA, Carlezon WA, Jr, Ongur D, Cohen BM. Blockade of astrocytic glutamate uptake in rats induces signs of anhedonia and impaired spatial memory. Neuropsychopharmacology. 2010;35:2049–2059. doi: 10.1038/npp.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pannasch U, Freche D, Dallerac G, Ghezali G, Escartin C, Ezan P, et al. Connexin 30 sets synaptic strength by controlling astroglial synapse invasion. Nat Neurosci. 2014;17:549–558. doi: 10.1038/nn.3662. [DOI] [PubMed] [Google Scholar]
- 38.Poskanzer KE, Yuste R. Astrocytes regulate cortical state switching in vivo. Proc Natl Acad Sci U S A. 2016;113:E2675–2684. doi: 10.1073/pnas.1520759113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, et al. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 40.Papouin T, Dunphy JM, Tolman M, Dineley KT, Haydon PG. Septal Cholinergic neuromodulation tunes the astrocyte-dependent gating of hippocampal NMDA receptors to wakefulness. Neuron. 2017;94(840–854):e847. doi: 10.1016/j.neuron.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Newey SE, Velamoor V, Govek EE, Van Aelst L. Rho GTPases, dendritic structure, and mental retardation. J Neurobiol. 2005;64:58–74. doi: 10.1002/neu.20153. [DOI] [PubMed] [Google Scholar]
- 42.Gordon-Weeks PR, Fournier AE. Neuronal cytoskeleton in synaptic plasticity and regeneration. J Neurochem. 2014;129:206–212. doi: 10.1111/jnc.12502. [DOI] [PubMed] [Google Scholar]
- 43.Tashiro A, Minden A, Yuste R. Regulation of dendritic spine morphology by the rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb Cortex. 2000;10:927–938. doi: 10.1093/cercor/10.10.927. [DOI] [PubMed] [Google Scholar]
- 44.Tashiro A, Yuste R. Regulation of dendritic spine motility and stability by Rac1 and Rho kinase: evidence for two forms of spine motility. Mol Cell Neurosci. 2004;26:429–440. doi: 10.1016/j.mcn.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Fan L, Lu Y, Shen X, Shao H, Suo L, Wu Q. Alpha protocadherins and Pyk2 kinase regulate cortical neuron migration and cytoskeletal dynamics via Rac1 GTPase and WAVE complex in mice. Elife 2018, 7: e35242. [DOI] [PMC free article] [PubMed]
- 46.Vaghi V, Pennucci R, Talpo F, Corbetta S, Montinaro V, Barone C, et al. Rac1 and rac3 GTPases control synergistically the development of cortical and hippocampal GABAergic interneurons. Cereb Cortex. 2014;24:1247–1258. doi: 10.1093/cercor/bhs402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Posada-Duque RA, Lopez-Tobon A, Piedrahita D, Gonzalez-Billault C, Cardona-Gomez GP. p35 and Rac1 underlie the neuroprotection and cognitive improvement induced by CDK5 silencing. J Neurochem. 2015;134:354–370. doi: 10.1111/jnc.13127. [DOI] [PMC free article] [PubMed] [Google Scholar]