Graphic Abstract
One new Daphniphyllum alkaloid, daphnioldhanol A (1), together with three known ones, were isolated from the stem part of Daphniphyllum angustifolium Hutch. Their structures were elucidated by spectroscopic methods and comparing with the literature data. Compound 2 is a new natural product, but known by synthesis as a racemate. Compound 1 exhibited week cytotoxic activity against Hela cell line with IC50 of 31.9 μM.

Supplementary Information
The online version contains supplementary material available at 10.1007/s13659-021-00309-w.
Keywords: Daphniphyllum angustifolium hutch, Secodaphnane-type, Daphnioldhanol A, Cytotoxic activity
Introduction
Alkaloids are a class of compounds with significant activities (with a variety of novel skeletons) that are widely found in nature [1–7]. Daphniphyllum alkaloids are a structurally diversified group of complex polycyclic natural products isolated from the Daphniphyllum genus [8]. Since these unique, versatile and complex nitrogen heterocyclic compounds exhibit a wide range of biological activities and are extremely challenging, they have aroused great interest in total synthesis and biosynthetic studies [9–16]. In recent years, quite a number of new Daphniphyllum alkaloids have been isolated and identified, and some of them possessed novel skeletons [17–20]. In our continued search for Daphniphyllum alkaloids with interesting skeletons [21–24], one new Daphniphyllum alkaloid, daphnioldhanol A (1), together with three known ones, were isolated from the stems of Daphniphyllum angustifolium Hutch (Fig. 1). Herein, the isolation, structural elucidation, and bioactivities of these compounds are reported.
Fig. 1.

The structures of compounds 1–4
Results and Discussion
Structure Elucidation of the Compounds
Daphnioldhanol A (1) was obtained as white amorphous powder. Its molecular formula, C32H48NO5, was established by positive HRESIMS at m/z 526.3534 [M+ H]+ (calcd 526.3534), with 10 degrees of unsaturation. The IR absorptions implied the presence of hydroxyl (3441 cm−1), and an imine moiety (1631 cm−1). The 13C NMR and DEPT data of 1 displayed 32 carbon signals (Table 1), due to three tetrasubstituted sp2 carbon atoms at lower field and 29 sp3 carbon atoms (5 × C, 6 × CH, 11 × CH2, 6 × CH3) at higher field. According to the molecular formula and relative NMR data, one CH group (δC 65.4, δH 2.95) was ascribed to those bearing an N-atom, while one quaternary C-atom (δC 84.4) were attributed to those bearing an O-atom. Additionally, three sp2 quaternary carbons were attributable to one lactone carbonyl (δC 180.2), one ester carbonyl (δC 172.2), and one iminium group (δC 168.4), while taking into account the three degrees of unsaturation. The remaining seven degrees of unsaturation were accounted for the presence of the heptacyclic system of 1.
Table 1.
1H and 13C NMR spectroscopic data for compound 1a (δ in ppm and J in Hz)
| No. | δC | δH (Mult. J) | No. | δC | δH (Mult. J) |
|---|---|---|---|---|---|
| 1 | 168.4 | – | 16a | 54.1 | 3.67 (dt, 14.4, 3.6) |
| 2 | 40.5 | 1.47 (o) | 16b | 2.50 (d, 15) | |
| 3a | 33.7 | 2.09 (m) | 17a | 43 | 2.89 (m) |
| 3b | 1.08 (m) | 17b | 2.23 (d, 4.2) | ||
| 4a | 38.4 | 2.06 (m) | 18 | 32.5 | 1.68 (m) |
| 4b | 1.61 (m) | 19 | 21.9 | 0.96 (d, 6.6) | |
| 5 | 38.7 | – | 20 | 22.4 | 1.02 (d, 6.6) |
| 6 | 55.2 | 2.73 (t, 9.0) | 21 | 27.3 | 1.13 (s) |
| 7 | 65.4 | 2.95 (s) | 22 | 58.1 | 1.86 (m) |
| 8 | 54.3 | – | 23 | 51.6 | – |
| 9 | 84.4 | – | 24 | 18.8 | 1.20 (s) |
| 10 | 54.2 | – | 25 | 180.2 | – |
| 11a | 35 | 2.64 (dd 12, 4.8) | 26 | 72.1 | 4.77 (d, 4.8) |
| 11b | 1.10 (m) | 27a | 26.9 | 1.85 (o) | |
| 12a | 30.3 | 1.85 (o) | 27b | 1.61 (o) | |
| 12b | 1.52 (dd, 11.4, 5.4) | 28a | 27 | 1.85 (o) | |
| 13a | 28.2 | 1.95 (m) | 28b | 1.61 (o) | |
| 13b | 1.69 (m) | 29 | 87.7 | – | |
| 14a | 27.9 | 1.77 (o) | 30 | 24.8 | 1.47 (o) |
| 14b | 1.17 (m) | 31 | 171.2 | – | |
| 15 | 44.4 | 1.77 (o) | 32 | 22 | 2.13 (s) |
aRecorded in Methanol-d4 at 800 MHz (1H) and 200 MHz (13C)
The 1H and 13C NMR spectra of 1 were closely related to those of the known compound Daphnioldhanine I [25], with the exception of the loss of signal for a CH in the latter and the addition of signal for quaternary carbon with a hydroxyl (δC 84.4), which were supported by the HMBC correlations of H-11/C-9, H-13/C-9, H-15/C-9, H-16a/C-9, H-17/C-9.
To determine the orientation of the hydroxyl at C-9, we compared the 13C NMR data of both 1 and daphnioldhanine I, which revealed that 9-OH substituent significantly shields the C-21 (5 ppm decrease) in the former. This indicated that the 9-OH in 1 should take a β-orientation. Moreover, the remaining relative configuration of 1 was elucidated from ROESY correlations as shown in computer-generated 3D drawing, which was the same as that of the daphnioldhanine I (Fig. 2).
Fig. 2.
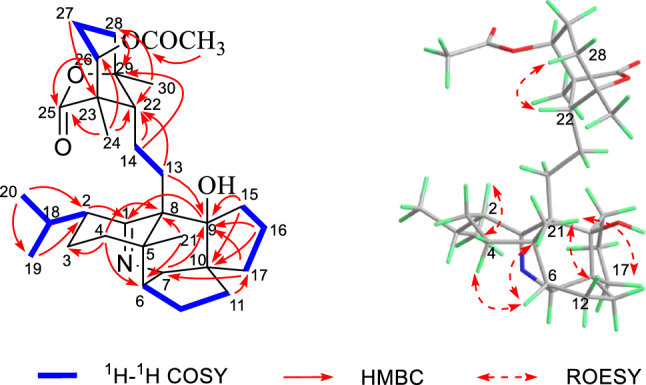
1H-1H COSY, key HMBC and ROESY correlations of 1
The known compounds were identified as (−)-nitrone 17 (2) [26], daphnilactone A (3) [27], dapholdhamine B (4) [28], respectively, by comparison of their spectroscopic data with those reported in the literature (Fig. 1). Compound 2 was obtained as white amorphous powder. MS analysis of 2 revealed a [2 M + H]+ peak at m/z 747. By comparison of its 1H and 13C NMR data with those of (±)-nitrone 17 in the literature, high similarity between them indicated that 2 shared the same structure as the latter. However, the compound 2 is a new chiral natural product with OR at − 31.75°, but known by synthesis is racemate.
A plausible biogenetic pathway for 1 and 2 was proposed as shown in Scheme 1. Biogenetically, both 1 and 2 should be the derivatives of secodaphnane-type alkaloid [13, 29], which might be originated from sequalene, as proposed from Heathcock [26]. Then, 1 and 2 might be formed via different pathway.
Scheme 1.
Plausible Biosynthetic Pathway of 1
Cytotoxic Activity
Both compounds 1 and 2 have been tested for their cytotoxicity against Hela, MCF-7, A549, MGC-803 and COLO-205 human cancer cell lines in vitro. The results indicated that 1 exhibited weak cytotoxic activity against Hela cell line with IC50 of 31.9 μM (Table 2).
Table 2.
Cytotoxic activity of Compound 1 against Hela, MCF-7, A549, MGC-803 and COLO-205 human cancer cell lines in vitro
| Human cancer cell lines | IC50 (μM) | |
|---|---|---|
| Compound 1 | Doxorubicin | |
| Hela | 31.9 | 0.77 |
| MCF-7 | > 76 | 1.57 |
| A549 | 52.2 | 1.92 |
| MGC-803 | 69.7 | 1.05 |
| COLO-205 | 71.8 | 2.23 |
Experimental
General Experimental Procedures
Optical rotations were measured with a Jasco P-1020 polarimeter. UV spectra were obtained using a Shimadzu UV-2401A spectrophotometer. A Tenor 27 spectrophotometer was used for IR spectra as KBr pellets. 1D and 2D NMR spectra were recorded on Bruker spectrometer with TMS as internal standard. HRESIMS was performed on a triple quadrupole mass spectrometer. Semi-preparative HPLC was performed on an Agilent 1100 liquid chromatograph with a Waters X-Bridge Prep Shield RP18 (10 × 150 mm) column. Column chromatography (CC) was performed using silica gel (100–200 mesh and 300–400 mesh, Qingdao Marine Chemical, Inc., Qingdao, P. R. China) and Sephadex LH-20 (40–70 μm, Amersham Pharmacia Biotech AB, Uppsala, Sweden).
Plant Material
The stems of Daphniphyllum Angustifolium used in this study was collected from Jinfo mountain, Chongqing, P. R. China, in October 2013, and botanically authenticated by professor Deng Hong-ping. A voucher specimen (KIBHAO2014012) was deposited in State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences.
Extraction and Isolation
Air-dried stems of Daphniphyllum Angustifolium (20 kg) were powdered and extracted with MeOH (24 h × 3) at room temperature, and the solvent was evaporated in vacuo. The MeOH extract was then partitioned between EtOAc and TFA/H2O at pH 3.0. Water-soluble materials, after being adjusted at pH 10.0 with saturated Na2CO3, were partitioned with CHCl3. CHCl3-soluble materials (100.6 g) was subjected to silica gel column chromatography (CC) and eluted with gradient CHCl3/MeOH to yield five fractions F-1–F-5. F-4 was repeatedly submitted to silica gel CC and Sephadex LH-20, then purified by HPLC to afford compounds 1 (1.2 mg) and 2 (2.0 mg). Accordingly, 3 (18.0 mg) was obtained from F-1; 4 (2.5 mg) was obtained from F-5.
Daphnioldhanol A (1)
Daphnioldhanol A (1): White amorphous powder; C32H47NO5; Positive HR-EI-MS at m/z 526.3534 [M + H] + (calcd. for C32H48NO5, 526.3527); [α]20D = + 9.88° (c = 0.54, MeOH); UV (MeOH) λmax (log ε) 265 (3.43) nm, 242 (3.56) nm, 215 (3.93) nm; IR: νmax (KBr) cm–1: 3440, 2928, 2869, 1772, 1743, 1713, 1631, 1383, 1226, 1057, 1028 cm–1.
Nitrone 17 (2)
(−)-Nitrone 17 (2): Colorless oil; C23H35NO3; ESI-MS (positive): m/z 747 [2 M + H] +; 1H NMR (CDCl3, 400 MHz) δH: 3.72 (3H, s), 1.58 (3H, s), 1.02 (1H, d, 6.16), 0.94 (3H, s), 0.85 (3H, d, 6.48); 13C NMR (CDCl3, 100 MHz) δC: 157.2 (C-1), 48.8 (C-2), 27.0 (C-3), 39.0 (C-4), 51.7 (C-5), 52.5 (C-6), 84.1 (C-7), 50.9 (C-8), 52.5 (C-9), 52.9 (C-10), 33.4 (C-11), 22.7 (C-12), 26.2 (C-13), 31.6 (C-14), 25.7 (C-15), 37.0 (C-16), 38.9 (C-17), 31.5 (C-18), 21.0 (C-19), 20.6 (C-20), 23.3 (C-21), 174.1 (C-22), 51.9 (C-23).
Cytotoxicity Assays
Cytotoxic activity of compound 1 against Hela, MCF-7, A549, MGC-803, and COLO-205 human cancer cell lines in vitro were measured using methylthiazoletetrazolium (MTT) assay [30]. Doxorubicin was used as a positive control.
Concluding Remarks
In conclusion, one new Daphniphyllum alkaloid, daphnioldhanol A (1), together with three known ones, were isolated from the stem part of D. angustifolium Hutch. Compound 1 exhibited week cytotoxic activity against Hela cell line.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 31770392 to YTD), and the Science and Technology Program of Yunnan Province (2018ZF013 to YTD). The authors are grateful to the analytical group of the Laboratory of Phytochemistry, Kunming Institute of Botany, Chinese Academy of Sciences, for recorded spectra.
Declarations
Conflict of interest
Authors declare that there is no conflict of interest.
Footnotes
Qing-Yun Lu and Jia-Hui Zhang have contributed equally to this work.
Contributor Information
Ying-Tong Di, Email: diyt@mail.kib.ac.cn.
Xiao-Jiang Hao, Email: haoxj@mail.kib.ac.cn.
References
- 1.Huo ZQ, Zhao Q, Zhu WT, Hao XJ, Zhang Y. Nat. Prod. Bioprospect. 2021;11:207–213. doi: 10.1007/s13659-020-00278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao MM, He HP, Gu YC, Zhang Q, Li XN, Zuo GY, Di YT, Yuan CM, Li SL, Zhang Y, Hao XJ. Nat. Prod. Bioprospect. 2013;3:29–32. doi: 10.1007/s13659-012-0095-z. [DOI] [Google Scholar]
- 3.Zhao YL, Su M, Shang JH, Wang X, Njateng GSS, Bao GL, Ma J, Sun QD, Yuan F, Wang JK, Luo XD. Nat. Prod. Bioprospect. 2020;10:77–88. doi: 10.1007/s13659-020-00237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Di YT, Liu HY, Li CS, Tan CJ, Zhang Q, Fang X, Li SL, Hao XJ. Helv. Chim. Acta. 2008;91:2153–2158. doi: 10.1002/hlca.200890232. [DOI] [Google Scholar]
- 5.Ye XW, Chai WY, Lian XY, Zhang ZZ. Nat. Prod. Res. 2017;31:1390–1396. doi: 10.1080/14786419.2016.1253079. [DOI] [PubMed] [Google Scholar]
- 6.Chattopadhyay AK, Hanessian S. Chem. Rev. 2017;117:4104–4146. doi: 10.1021/acs.chemrev.6b00412. [DOI] [PubMed] [Google Scholar]
- 7.Liu S, Zhang JH, Di YT, Dong JY, Hao XJ. Nat. Prod. Res. 2017;32:2165–2170. doi: 10.1080/14786419.2017.1371155. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi J, Ueno S, Morita H. J. Org. Chem. 2002;67:6546–6549. doi: 10.1021/jo0258204. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi J, Kubota T. Nat. Prod. Rep. 2009;26:936–962. doi: 10.1039/b813006j. [DOI] [PubMed] [Google Scholar]
- 10.Wu HF, Zhang XP, Ding LS, Chen SL, Yang JS, Xu XD. Planta Med. 2013;79:1589–1598. doi: 10.1055/s-0033-1351024. [DOI] [PubMed] [Google Scholar]
- 11.Tang XH, Luo RC, Ye MS, Tang HY, Ma YL, Chen YN, Wang XM, Lu QY, Liu S, Li XN, Yan Y, Yang J, Ran XQ, Fang X, Zhou Y, Yao YG, Di YT, Hao J. Org. Lett. 2021;23:262–267. doi: 10.1021/acs.orglett.0c03460. [DOI] [PubMed] [Google Scholar]
- 12.Denmark SE, Baiazitov RY. J. Org. Chem. 2006;71:593–605. doi: 10.1021/jo052001l. [DOI] [PubMed] [Google Scholar]
- 13.Wallace GA, Heathcock CH. J. Org. Chem. 2001;66:450–454. doi: 10.1021/jo001145r. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Di YT, Zhang Q, Mu SZ, Tan CJ. Org. Lett. 2009;11:5414–5417. doi: 10.1021/ol902262g. [DOI] [PubMed] [Google Scholar]
- 15.Li YH, Zhang Y, Peng LY, Li XN, Zhao QS, Li RT, Wu XD. Nat. Prod. Bioprospect. 2016;6:291–296. doi: 10.1007/s13659-016-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu YC, Zhang ZJ, Su J, Peng LY, Pan LT, Wu XD, Zhao QS. Nat. Prod. Bioprospect. 2017;7:405–411. doi: 10.1007/s13659-017-0140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang CR, Liu HB, Feng T, Zhu JY, Geng MY, Yue JM. J. Nat. Prod. 2009;72:1669–1672. doi: 10.1021/np9003799. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q, Di YT, Li CS, Fang X, Tan CJ, Zhang Z, He HP, Li SL, Hao XJ. Org. Lett. 2009;11:2357–2359. doi: 10.1021/ol9007958. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Di YT, He HP, Li SF, Lu Y, Gong NB, Hao XJ. Eur. J. Org. Chem. 2011;2011:4103–4107. doi: 10.1002/ejoc.201100414. [DOI] [Google Scholar]
- 20.Cao MM, Wang L, Zhang Y, He HP, Gu YC, Zhang Q, Li Y, Yuan CM, Li SL, Di YT. Fitoterapia. 2013;89:205–209. doi: 10.1016/j.fitote.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Yang TQ, Di YT, He HP, Zhang Q, Zhang Y, Hao XJ. Helv. Chim. Acta. 2011;94:397–403. doi: 10.1002/hlca.201000170. [DOI] [Google Scholar]
- 22.He T, Zhou Y, Wang YH, Mu SZ, Hao XJ. Helv. Chim. Acta. 2011;94:1019–1023. doi: 10.1002/hlca.201000390. [DOI] [Google Scholar]
- 23.Tan CJ, Wang YH, Di YT, He HP, Mu SZ, Li SF, Zhang Y, Hao XJ. Tetrahedron Lett. 2012;53:2588–2591. doi: 10.1016/j.tetlet.2012.03.021. [DOI] [Google Scholar]
- 24.Cao MM, Zhang Y, He HP, Li SF, Huang SD, Chen DZ, Tang GH, Li SL, Di YT, Hao XJ. J. Nat. Prod. 2012;75:1076–1082. doi: 10.1021/np200960z. [DOI] [PubMed] [Google Scholar]
- 25.Mu SZ, Wang JS, Yang XS, He HP, Li CS, Di YT, Wang Y, Zhang Y, Fang X, Huang LJ, Hao XJ. J. Nat. Prod. 2008;71:564–569. doi: 10.1021/np070512s. [DOI] [PubMed] [Google Scholar]
- 26.Heathcock CH, Joe D. J. Org. Chem. 1995;60:1131–1142. doi: 10.1021/jo00110a014. [DOI] [Google Scholar]
- 27.Sasaki K, Hirata Y. Tetrahedron Lett. 1972;13:1275–1278. doi: 10.1016/S0040-4039(01)84566-4. [DOI] [Google Scholar]
- 28.Zhang Y, Di YT, Mu SZ, Li CS, Zhang Q, Tan CJ, Zhang Z, Fang X, Hao XJ. J. Nat. Prod. 2009;72:1325–1327. doi: 10.1021/np900112d. [DOI] [PubMed] [Google Scholar]
- 29.Heathcock CH, Piettre S, Ruggeri RB, Ragan JA, Kath JC. J. Org. Chem. 1992;57:2554–2566. doi: 10.1021/jo00035a009. [DOI] [Google Scholar]
- 30.Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, VaigroWolff A. J. Natl. Cancer. 1991;I(83):757–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



