Neuropathic pain is a chronic pain caused by peripheral or central nervous system injury or diseases that affect the somatosensory nervous system. It is usually associated with abnormal sensations called dysesthesia and exaggerated pain from normally innocuous stimuli (allodynia) or noxious stimuli (hyperalgesia), and also aversive emotional responses [1, 2]. Neuropathic pain can be very difficult to treat, as classical analgesics like opioids produce inadequate pain relief and may cause tolerance and hyperalgesia, and even addiction after long-term use [3]. Thus, it is of critical importance to study the mechanisms of development, maintenance, and resolution of neuropathic pain. This study from Dr. Duan’s research team at Zhejiang University reveals a critical regulatory mechanism for neuropathic pain [4].
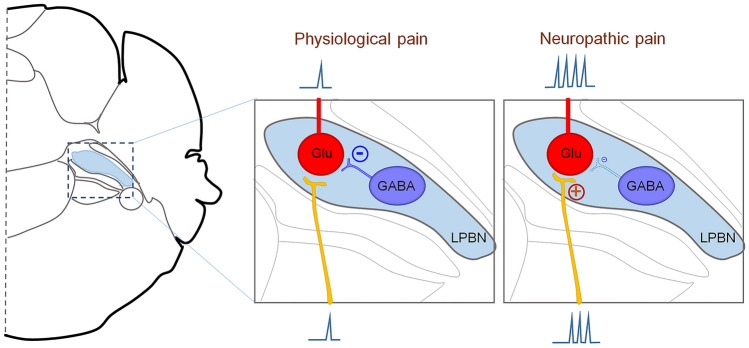
The spinoparabrachial tract is an ascending pathway that transmits spinal pain signals to the parabrachial nucleus, which further projects to the amygdala and other brain regions [5]. Recent studies have shown that the lateral parabrachial nucleus (LPBN) receives nociceptive inputs from projection neurons in the dorsal spinal cord [6, 7], as well as directly from the trigeminal ganglion [8]. However, the role of the LPBN in neuropathic pain is still elusive. Combining in-vivo calcium imaging, electrophysiological, optogenetic, and chemogenetic approaches, Sun et al. [4] demonstrated that the balance between glutamatergic and GABAergic LPBN neuronal activity modulates the initiation and maintenance of neuropathic pain. Furthermore, the GABAergic neurons in the LPBN gate control the sensitization of glutamatergic neurons that regulate the development and transmission of neuropathic pain (Fig. 1).
Fig. 1.
Glutamatergic neurons in the LPBN regulate both basal nociception and neuropathic pain. GABAergic neurons in the LPBN directly control the sensitization of glutamatergic neurons, which contribute to the development and maintenance of neuropathic pain.
To determine whether LPBN neurons are activated by neuropathic pain, Sun et al. [4] first profiled neuronal activation using c-Fos mapping and found that a large population of glutamatergic neurons but not GABAergic neurons in the LPBN were activated after common peroneal nerve (CPN) ligation. Further in-vivo calcium imaging experiments, by fiber photometry and miniaturized microscopy, indicated that CPN ligation increased the sensitivity of glutamatergic neurons, but not GABAergic neurons, in the LPBN to pinch or mechanical stimulation. Moreover, Sun et al. [4] demonstrated that optogenetic activation of VgluT2 neurons or CaMKIIα neurons, which represent approximately 90% of neurons in the LPBN, induced mechanical allodynia, thermal hyperalgesia, and place avoidance, mimicking neuropathic pain-like behaviors. On the other hand, optogenetic silencing of VgluT2 neurons or CaMKIIα neurons in the LPBN not only inhibited the basal sensitivity to mechanical and thermal stimulation in sham mice, but also induced place preference and alleviated the mechanical allodynia and heat hyperalgesia in mice with neuropathic pain. These results indicate that glutamatergic neurons in the LPBN are crucial for both relaying physiological pain and transmitting neuropathic pain.
Next, Sun et al. [4] illustrated that glutamatergic neurons in the LPBN receive direct monosynaptic innervation from local GABAergic neurons by using monosynaptic rabies virus tracing. They conducted elegant electrophysiological experiments to characterize the functional inhibitory synaptic inputs from GABAergic to glutamatergic neurons in the LPBN. Furthermore, they found that optogenetic activation of GABAergic neurons in the LPBN reversed the mechanical allodynia and heat hyperalgesia, and induced place preference in CPN-ligated mice, but not in sham-operated mice. Similarly, pharmacogenetic activation of GABAergic neurons in the LPBN had anti-allodynic effects in CPN-ligated mice, and these were blocked by the GABAA receptor chloride channel blocker picrotoxin. Surprisingly, Sun et al. also showed that optogenetic inhibition of GABAergic neurons, which represent approximately 10% of the neurons in the LPBN, activated a large population of glutamatergic neurons in the LPBN and induced neuropathic pain-like symptoms. These data suggest that GABAergic LPBN neurons critically participate in the homeostasis of pain sensation in the LPBN circuit and gate neuropathic pain transmission via local inhibition of glutamatergic neurons (Fig. 1).
Sun et al. [4] took further steps to test the effects of persistent activation of glutamatergic or GABAergic neurons in the LPBN. Their results showed that prolonged activation of glutamatergic neurons in the LPBN by pharmacogenetic approaches produced mechanical allodynia and a conditioned place aversion response. Notably, the mechanical allodynia lasted at least one month after withdrawal the pharmacogenetic activator. These data indicated that prolonged activation of glutamatergic neurons in the LPBN is sufficient to induce neuropathic pain-like behaviors. Conversely, prolonged activation of GABAergic neurons in the LPBN during the first week totally prevented the development of neuropathic pain after CPN ligation.
Taken together, Sun et al. [4] used multiple approaches to comprehensively evaluate the roles of LPBN circuits in modulating physiological and neuropathic pain, including the transmission and processing of both the sensory and emotional components. Previous studies demonstrated that peripheral and central sensitization, mainly in the secondary order neurons in the spinal dorsal horn, play important roles in its development and maintenance. Here Sun et al. [4] present the first evidence that sensitization of the third-order neurons in the LPBN is also essential in its development, suggesting that the LPBN is a key regulator of neuropathic pain. This study also provides a new strategy for neuropathic pain treatment by targeting LPBN neurons and circuits.
The study from Sun et al. opens several future directions that will be helpful for better understanding the LPBN circuits in pain, especially in chronic pain. Recent studies have identified several subpopulations of LPBN neurons, such as that tachykinin receptor 1- and dynorphin-expressing neurons play important roles in pain transmission [7, 9]. Chiang et al. demonstrated that neurons in spatially segregated regions of the LPBN collateralize to distinct targets and that activation of distinct efferents gives rise to separate components of the nocifensive response [9]. But the manner of encoding the sensory modality information, such as the mechanical-, heat-, cold-, touch-, and pain-related emotional aspects by LPBN neurons is still poorly understood. More studies focusing on the identification of detailed subpopulations of glutamatergic and GABAergic neurons in the LPBN, and their respective functions are warranted. In addition, it has been demonstrated that the LPBN participates in both ascending and descending pain pathways. Neurons in the LPBN receive nociceptive inputs from the trigeminal ganglion, nodose ganglion, and spinal projection neurons [6, 8], then their outputs project predominantly to the periaqueductal gray and ventromedial hypothalamus, central amygdala (CeA), bed nucleus of the stria terminalis (BNST), insular cortex, and medullary formation [8, 10, 11]. The LPBN neurons also receive inputs from several brain regions, such as the BNST and CeA [5, 12, 13], which are important for descending pain control. Future studies that aim to identify the upstream and downstream neuronal circuits that connect with LPBN neurons will be helpful to better understand the roles of LPBN neurons in pain modulation and provide new targets for chronic pain treatment.
Acknowledgements
This Research Highlight was supported by Duke University Anesthesiology Research Funds and the National Natural Science Foundation of China (31771162).
Contributor Information
Zilong Wang, Email: wangzl6@sustech.edu.cn.
Zhen-Zhong Xu, Email: xuzz@zju.edu.cn.
References
- 1.Calvo M, Davies AJ, Hebert HL, Weir GA, Chesler EJ, Finnerup NB, et al. The genetics of neuropathic pain from model organisms to clinical application. Neuron. 2019;104:637–653. doi: 10.1016/j.neuron.2019.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu XB, He LN, Jiang BC, Wang X, Lu Y, Gao YJ. Increased CXCL13 and CXCR5 in anterior cingulate cortex contributes to neuropathic pain-related conditioned place aversion. Neurosci Bull. 2019;35:613–623. doi: 10.1007/s12264-019-00377-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun L, Liu R, Guo F, Wen MQ, Ma XL, Li KY, et al. Parabrachial nucleus circuit governs neuropathic pain-like behavior. Nat Commun. 2020;11:5974. doi: 10.1038/s41467-020-19767-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiang MC, Bowen A, Schier LA, Tupone D, Uddin O, Heinricher MM. Parabrachial complex: a hub for pain and aversion. J Neurosci. 2019;39:8225–8230. doi: 10.1523/JNEUROSCI.1162-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi S, Hachisuka J, Brett MA, Magee AR, Omori Y, Iqbal NU, et al. Parallel ascending spinal pathways for affective touch and pain. Nature. 2020;587:258–263. doi: 10.1038/s41586-020-2860-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng J, Zhou H, Lin JK, Shen ZX, Chen WZ, Wang LH, et al. The parabrachial nucleus directly channels spinal nociceptive signals to the intralaminar thalamic nuclei, but not the amygdala. Neuron. 2020;107(909–923):e906. doi: 10.1016/j.neuron.2020.06.017. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez E, Sakurai K, Xu J, Chen Y, Toda K, Zhao S, et al. A craniofacial-specific monosynaptic circuit enables heightened affective pain. Nat Neurosci. 2017;20:1734–1743. doi: 10.1038/s41593-017-0012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiang MC, Nguyen EK, Canto-Bustos M, Papale AE, Oswald AM, Ross SE. Divergent neural pathways emanating from the lateral parabrachial nucleus mediate distinct components of the pain response. Neuron 2020. [DOI] [PubMed]
- 10.Palmiter RD. The parabrachial nucleus: CGRP neurons function as a general alarm. Trends Neurosci. 2018;41:280–293. doi: 10.1016/j.tins.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barik A, Thompson JH, Seltzer M, Ghitani N, Chesler AT. A brainstem-spinal circuit controlling nocifensive behavior. Neuron. 2018;100(1491–1503):e1493. doi: 10.1016/j.neuron.2018.10.037. [DOI] [PubMed] [Google Scholar]
- 12.Roeder Z, Chen Q, Davis S, Carlson JD, Tupone D, Heinricher MM. Parabrachial complex links pain transmission to descending pain modulation. Pain. 2016;157:2697–2708. doi: 10.1097/j.pain.0000000000000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raver C, Uddin O, Ji Y, Li Y, Cramer N, Jenne C, et al. An amygdalo-parabrachial pathway regulates pain perception and chronic pain. J Neurosci. 2020;40:3424–3442. doi: 10.1523/JNEUROSCI.0075-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]